Abstract
In the present study, the antiplatelet effects and mechanisms of a new synthetic compound YD-3 [1-benzyl-3(ethoxycarbonylphenyl)-indazole] were examined.
YD-3 inhibited the aggregation of washed rabbit platelets caused by thrombin (IC50=28.3 μM), but had no or little inhibitory effect on that induced by arachidonic acid, collagen, platelet-activating factor (PAF) or U46619. YD-3 also suppressed generation of inositol phosphates caused by thrombin. On the other hand, thrombin-induced fibrin formation was not affected by YD-3, indicating YD-3 does not inhibit the proteolytic activity of thrombin.
In washed human platelets, however, YD-3 had only mild inhibitory effect on the low concentration (0.05 u ml−1) of thrombin-induced human platelet aggregation, and did not affect that induced by higher concentrations (⩾0.1 u ml−1) of thrombin or SFLLRN, the protease-activated receptor 1 (PAR1) agonist peptide. By contrast, YD-3 inhibited both human and rabbit platelet aggregation elicited by trypsin with IC50 values of 38.1 μM and 5.7 μM, respectively.
YD-3, at 100 μM, had no effect on ristocetin-induced glycoprotein Ib (GPIb)-dependent aggregation of human platelets. In addition, platelets treated with chymotrypsin, which cleaves GPIb, enhanced rather than attenuated the inhibition of YD-3 on thrombin-induced human platelet aggregation. These data indicate that GPIb plays no role in the antiplatelet effect of YD-3.
In SFLLRN-desensitized human platelets, high concentration of thrombin (1 u ml−1) could still elicit intracellular Ca2+ mobilization, and the rise of [Ca2+]i was prevented by either leupeptin or YD-3.
Our results suggest that YD-3 inhibits a non-PAR1 thrombin receptor which mediates the major effect of thrombin in rabbit platelets, but in human platelets, this receptor function becomes significant only when the function of PAR1 has been blocked or attenuated.
Keywords: YD-3, thrombin, protease-activated receptors, platelets
Introduction
Thrombin is a trypsin-like serine protease playing a central role in both haemostasis and thrombosis (Hemker, 1994). In the blood coagulation pathway, thrombin is the final key enzyme, cleaving fibrinogen to form fibrin. Moreover, thrombin is the most potent agent to induce platelet activation and aggregation. These effects are thought to be critical for platelet-dependent arterial thrombosis in unstable angina and myocardial infarction. In addition to its activation of blood platelets, thrombin also acts on many different cells. Stimulation of vascular endothelial cells by thrombin results in the secretion of various vasoactive agents as well as expression of adhesion molecules (Tapparelli et al., 1993). When combined with the chemoattractant effect of thrombin on leukocytes and an ability to enhance cytokine production by such cells, the net result is an extravasation of leukocytes at sites of thrombin generation (Jones & Geczy, 1990; Colotta et al., 1994; Laposata et al., 1983). Thrombin also has a strong mitogenic effect on vascular smooth muscle cells and fibroblasts (McNamara et al., 1993; Chen et al., 1994b). These diverse cellular responses caused by thrombin may be involved in thrombotic, inflammatory and proliferative responses to mechanical injured vascular tissues or ruptured atherosclerotic plaques (Baykal et al., 1995; Harkar et al., 1995). Therefore, development of thrombin inhibitors or receptor antagonists would be of therapeutic interest in preventing these pathological events.
Thrombin's ability to activate cells depends upon its proteolytic activity and appear to be largely, if not exclusively, mediated by a family of G protein-coupled protease-activated receptors (PARs), for which PAR1 is the prototype (Vu et al., 1991a). Thrombin binds to and cleaves the exodomain of PAR1 to create a new amino terminus, which can then act as a tethered ligand to activate the receptor (Vu et al., 1991a,1991b; Chen et al., 1994a). The synthetic peptide SFLLRN corresponding to the first six residues of this new amino terminus, functions as a PAR1 agonist and mimics most cellular effects of thrombin (Dery et al., 1998; Coughlin, 1999). Recently, two additional PARs, termed PAR3 and PAR4, have also been identified as thrombin receptors (Ishihara et al., 1998; Xu et al., 1998; Kahn et al., 1998). By contrast, PAR2 is specifically activated by trypsin, but not by thrombin (Nystedt et al., 1994). Although PAR-1 is the major thrombin receptor in human platelets, the role of other PARs in the activation of platelets has been suggested. It is reported that both PAR1 and PAR4 contribute to human platelet activation at high concentrations of thrombin and that inhibition of both receptors is required to ablate thrombin-induced platelet aggregation (Kahn et al., 1998). By contrast, mouse platelets utilize PAR3 and PAR4, with no apparent role for PAR1. Defining the roles of the known PARs in diverse effects of thrombin in physiological and pathological states remains a challenge. Understanding the functions of these receptors will require studies of knockout and transgenic animals and development of selective antagonists and agonists.
In a screening test of antiplatelet activity of chemical compounds, we found YD-3, a newly synthetic indazole derivative, possessed inhibitory effects on the platelet aggregation. In the present study, we showed that YD-3 competitively inhibited thrombin-induced rabbit platelet aggregation with no or little inhibitory effect on that elicited by other platelet activators. The action of YD-3 is not due to inhibition of thrombin's proteolytic activity, since YD-3 does not affect thrombin-induced fibrinogen clotting. In contrast, YD-3 had different effects on thrombin's action in human platelets. Our data suggests that YD-3 inhibits thrombin-induced platelet activation through blockade of a novel protease-activated receptor that is distinct from the classic thrombin receptor PAR1.
Methods
Preparation of washed platelets
Blood anticoagulated with ethylenediaminetetraacetic acid (EDTA) was collected from New Zealand rabbits. Rabbit platelet suspension was prepared according to the procedure previously described (Wu et al., 1994). The platelets, after washing, were finally suspended in Tyrode's solution containing Ca2+ (1 mM), glucose (11.1 mM) and bovine serum albumin (3.5 mg ml−1) at a concentration of 3×108 platelets ml−1.
Human blood anticoagulated with acid citrate dextrose (ACD) was obtained from healthy human volunteers who had not taken drugs within the last 2 weeks. The platelet suspension was then prepared according to the washing procedure described previously (Wu et al., 1995). Platelets were finally suspended in Tyrode's solution containing Ca2+ (2 mM), glucose (11.1 mM) and bovine serum albumin (3.5 mg ml−1) at a concentration of 3×108 platelets ml−1.
In some experiments, platelets were incubated with chymotrypsin (10 u ml−1) for 30 min at 37°C in the presence of 2 μM prostaglandin E1. After washing twice, the chymotrypsin-treated platelets were finally suspended in Tyrode's solution.
Measurement of platelet aggregation
Platelet aggregation was measured turbidimetrically with a light-transmission aggregometer (Chrono-Log Co., U.S.A.) (Born, 1962). The platelet suspension was incubated with dimethyl sulphoxide (DMSO, vehicle) or YD-3 at 37°C for 3 min under a stirring condition (1200 r.p.m.) prior to the addition of the platelet activators. The extent of platelet aggregation was measured as the increase of light transmission at 5 min after the addition of inducers.
Measurement of the catalytic activity of thrombin and trypsin
Thrombin clotting activity was assayed as described by Hofmann et al. (1983). The clotting time of rabbit fibrinogen (2.5 mg ml−1) in Tyrode's solution containing 2 mM CaCl2 was measured after addition of thrombin (0.1 u ml−1 final concentration) preincubated at 37°C for 3 min with DMSO or YD-3.
The hydrolysis of benzoyl-DL-arginine-p-nitroanilid HCl (BAPNA) by trypsin was measured at 37°C in the buffer solution containing 50 mM Tris-HCl and 5 mM CaCl2, pH 8.2. Trypsin (10 u ml−1) which had been preincubated with DMSO or YD-3 was added to BAPNA (400 μg ml−1). Sixty-min after the addition of the enzyme, acetic acid (23%) was added to quench the reaction and the optical density was measured at 410 nm.
Labelling of membrane phospholipids and measurement of the production of [3H]-inositol monophosphate
Platelet membrane phospholipids were labelled with [3H]-inositol phosphate according to the method of Huang & Detwiler (1986). The reaction was then carried out at 37°C for 6 min with 1 ml of [3H]-inositol labelled-platelets under stirring. An equal volume of 10% (v v−1) trichloroacetic acid was added to stop the reaction. After centrifugation at 1000×g for 10 min, 1 ml of supernatant was pooled and trichloroacetic acid was removed by extracting with 5×2 volumes of diethylether. The acqueous phase was applied to a Dowex-1 ion exchange column for separation of inositol phosphates as described by Neylon & Summers (1987). All the experiments were carried out in the presence of 5 mM LiCl to inhibit inositol monophosphate phosphatase. Because the levels of inositol biphosphate and inositol trisphosphate were very low, inositol monophosphate was measured as an index of total inositol phosphates formation.
Measurement of intracellular Ca2+ mobilization
Platelets pelleted from platelet-rich plasma were resuspended in Ca2+-free Tyrode's solution, then incubated with fluo-3/AM (2 μM) and aspirin (100 μM) at 37°C for 30 min. In order to prevent leakage of dye, probenecid (2.5 mM) was added to the buffers throughout the experiments (Merritt et al., 1990). After washing twice, the fluo-3-loaded platelets were finally suspended in Ca2+-free Tyrode's solution at a concentration of 5×107 platelets ml−1. Fluorescence (Ex 505 nm, Em 530 nm) was measured with a fluorescence spectrophotometer (Model F4000; Hitachi, Tokyo, Japan) at 37°C.
Drugs
YD-3 [1-benzyl-3(ethoxycarbonylphenyl)-indazole; Figure 1] was synthesized based on the methods described previously (Yoshina & Kuo, 1978). Bovine α-thrombin was obtained from Parke–Davis Co. U.S.A. Collagen (type I, bovine Achilles tendon), platelet-activating factor (PAF, 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine), arachidonic acid, U46619 (9, 11-dideoxy-9α, 11α-methanoepoxy PGF2α), trypsin (type I, bovine pancreas), α-chymotrypsin (type IS, bovine pancreas) and fluo-3/AM (1-[2-amino-5-(2,7-dichloro-6-hydroxy-3-oxy-9-xanthenyl)-phenoxyl]-2-[2-amino-5-methylphenoxy]ethane-N,N,N′,N′-tetraacetic acid/acetoxymethyl ester) were obtained from Sigma Chemical Co. U.S.A. SFLLRN peptide and human von Willebrand factor (vWF) was purchased from Bachem Co., U.S.A. and Calbiochem Co., U.S.A., respectively. Myo-[2-3H]-inositol was purchased from Amersham Co. U.K. All other chemicals were purchased from Sigma Chemical Co. U.S.A.
Figure 1.
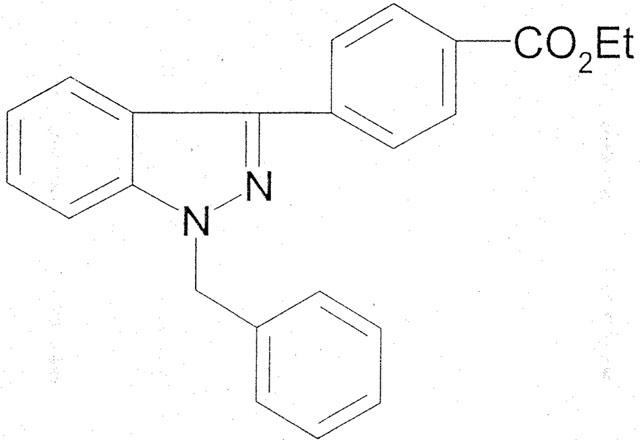
Chemical structure of YD-3.
Statistics
Results are expressed as the mean±standard error of the mean (s.e.mean) and comparisons were made using Student's t-test. A probability of 0.05 or less was considered significant.
Results
Effect of YD-3 on the aggregation of washed rabbit platelets
In washed rabbit platelets, thrombin (0.1 u ml−1), platelet-activating factor (PAF, 2 nM), collagen (10 μg ml−1), arachidonic acid (10 μM) and U46619 (1 μM, a stable thromboxane A2-mimetic) all elicited about 90% aggregation. YD-3 inhibited thrombin-induced platelet aggregation in a concentration-dependent manner with IC50 values 28.3±2.5 μM (Figure 2a). The inhibitory effect of YD-3 could be overcome by increasing the thrombin concentration (Figure 2b). However, YD-3 (100 μM) had no or little effect on platelet aggregation caused by other inducers (Figure 3).
Figure 2.
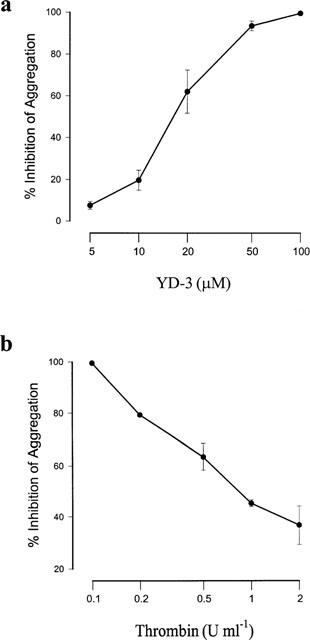
Inhibitory effects of YD-3 on thrombin-induced aggregation of rabbit platelets. (a) Washed rabbit platelets were preincubated with DMSO (0.5% control) or various concentrations of YD-3 at 37°C for 3 min, then thrombin (0.1 u ml−1) was added to trigger the aggregation. (b) Effect of YD-3 (100 μM) on rabbit platelet aggregation caused by different concentrations of thrombin. Percentages of inhibition are presented as mean±s.e.mean (n=6).
Figure 3.
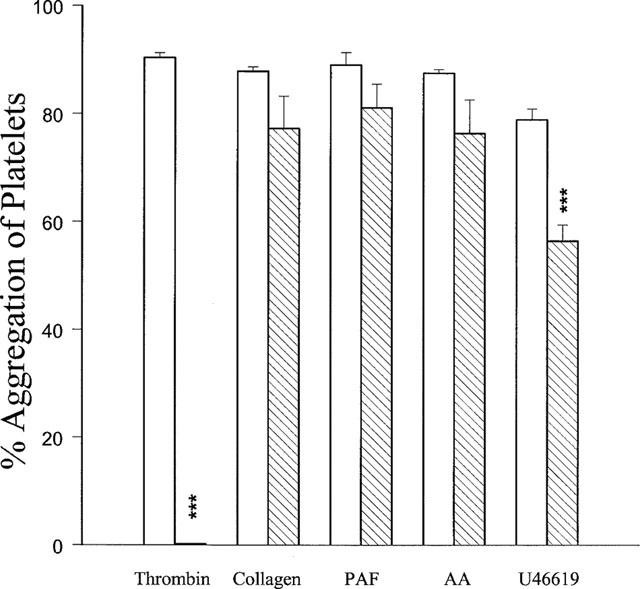
Effects of YD-3 on rabbit platelet aggregation induced by thrombin (0.1 u ml−1), collagen (10 μg ml−1), platelet-activating factor (PAF, 2 ng ml−1), arachidonic acid (AA, 10 μM) and U46619 (2 μM). Washed rabbit platelets were preincubated with DMSO (0.5%, control, open columns) or YD-3 (hatched columns, 50 μM versus thrombin-induced platelet aggregation, but 100 μM versus those caused by other inducers) at 37°C for 3 min, the inducer was then added to trigger the aggregation. Percentages of inhibition are presented as mean±s.e.mean (n=5–6). ***P<0.001 as compared with the respective control.
Effect of YD-3 on thrombin-induced fibrinogen clotting time
To assess whether the inhibitory effect of YD-3 on thrombin-induced platelet aggregation is via direct inhibition of thrombin proteolytic activity, we examined the effect of YD-3 on thrombin-induced fibrinogen clotting. YD-3, even at 100 μM, did not affect the thrombin (0.1 u ml−1)-induced fibrinogen clotting time (49.0±0.5 s vs control 52.7±4.3 s, n=6).
Effect of YD-3 on [3H]-inositol monophosphate formation in rabbit platelets
In [3H]-inositol-labelled rabbit platelets, thrombin (0.1 u ml−1) caused a 4.8±0.3 fold increase of [3H]-inositol monophosphate formation compared with the resting level. YD-3 (5–100 μM) concentration-dependently inhibited [3H]-inositol monophosphate formation elicited by thrombin (Figure 4). By contrast, PAF (2 nM)-induced [3H]-inositol monophosphate formation was not affected by 100 μM YD-3 (3.1 folds vs control 3.3 folds).
Figure 4.
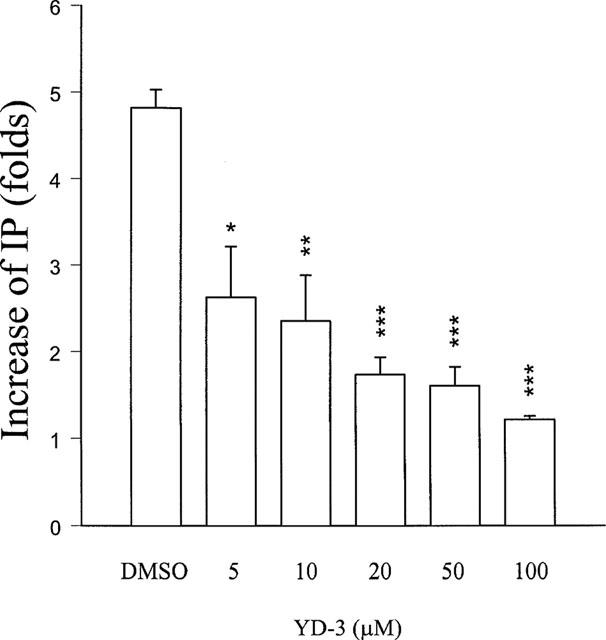
Inhibition of YD-3 on the formation of inositol monophosphate caused by thrombin in washed rabbit platelets. [3H]-Myoinositol-labelled platelets were incubated with DMSO (0.5%, control) or YD-3 (5–100 μM) at 37°C for 3 min, thrombin (0.1 u ml−1) was then added for another 6 min. All the experiments were carried out in the presence of LiCl (5 mM) and indomethacin (10 μM). Folds of the increase of IP are presented as mean±s.e.mean. *P<0.05, **P<0.01 and ***P<0.01 as compared with the control (n=3).
Effect of YD-3 on aggregation of washed human platelets
In washed human platelets, YD-3 (100 μM) only partially inhibited platelet aggregation induced by the lower concentration of thrombin (0.05 u ml−1), but not by higher concentrations (⩾0.1 u ml−1) of thrombin, U46619 (2 μM) or collagen (10 μg ml−1). Furthermore, YD-3 also had no effect on SFLLRN (5 μM)-induced platelet aggregation (Figure 5).
Figure 5.
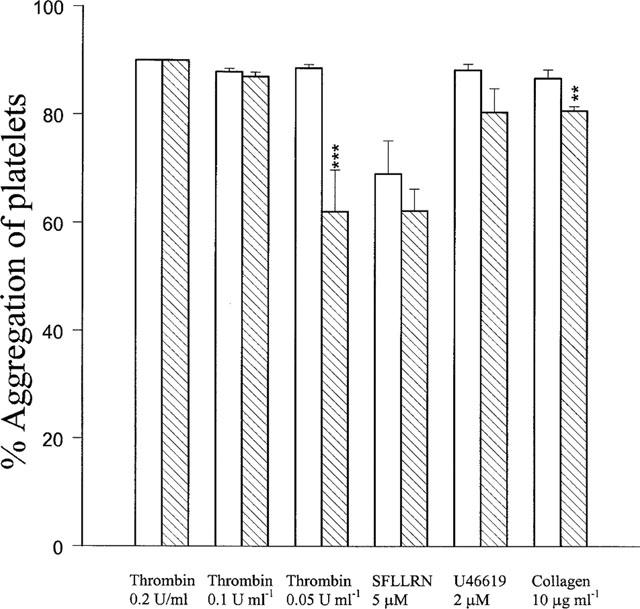
Effects of YD-3 on human platelet aggregation induced by thrombin (0.2, 0.1 and 0.05 u ml−1), SFLLRN peptide (5 μM), U46619 (2 μM) and collagen (10 μg ml−1). Washed human platelets were preincubated with DMSO (0.5%, control, open columns) or YD-3 (100 μM, hatched columns) at 37°C for 3 min, the inducer was then added to trigger the aggregation. Percentages of inhibition are presented as mean±s.e.mean (n=5–6). **P<0.01 and ***P<0.001 as compared with the respective control.
Trypsin, another serine protease, at the concentration of 10 u ml−1 elicited bout 90% aggregation of human platelets. YD-3 inhibited trypsin-induced platelet aggregation in a concentration-dependent manner with an IC50 value of 38.1±10.1 μM (Figure 6a). In washed rabbit platelets, YD-3 also had similar inhibitory effect on trypsin (5 u ml−1)-induced platelet aggregation with an IC50 value of 5.7±1.4 μM (Figure 6b). To elucidate whether the action of YD-3 is via direct inhibition of trypsin protease activity, the effect of YD-3 on the hydrolysis of BAPNA caused by trypsin was examined. YD-3, even at 100 μM, did not affect the amidolytic activity of trypsin (absorbance units at 410 nm: 0.160±0.004 vs control 0.164±0.0003, n=4).
Figure 6.
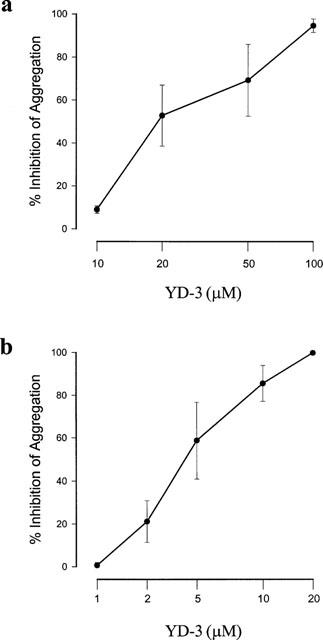
Effects of YD-3 on trypsin-induced platelet aggregation. Washed human platelets (a) or rabbit platelets (b) were incubated with DMSO (0.5%, control) or various concentrations of YD-3 at 37°C for 3 min. Trypsin (10 u ml−1 at human platelets, but 5 u ml−1 at rabbit platelets) was then added to trigger the aggregation. Percentages of inhibition are presented as mean±s.e.mean (n=5).
To assess the role of glycoprotein Ib (GPIb) in the antiplatelet activity of YD-3, the effect of YD-3 on ristocetin-induced vWF-GPIb-dependent platelet aggregation was determined. YD-3, at 100 μM, had no effect on platelet aggregation caused by ristocetin (Figure 7). By contrast, Aurin tricarboxylic acid (ATA, 10 μM), an inhibitor of the association of vWF and platelet GPIb, completely prevented ristocetin-induced platelet aggregation (Figure 7). In addition, platelets treated with chymotrypsin, which cleaves GPIb and PAR1, did not respond to 0.05 u ml−1 thrombin (data not shown), but still could be stimulated by 0.1 u ml−1 thrombin (Figure 8). In this condition, YD-3 (50 μM) completely prevented thrombin-induced platelet aggregation without affecting subsequent stimulation by U46619 (Figure 8).
Figure 7.
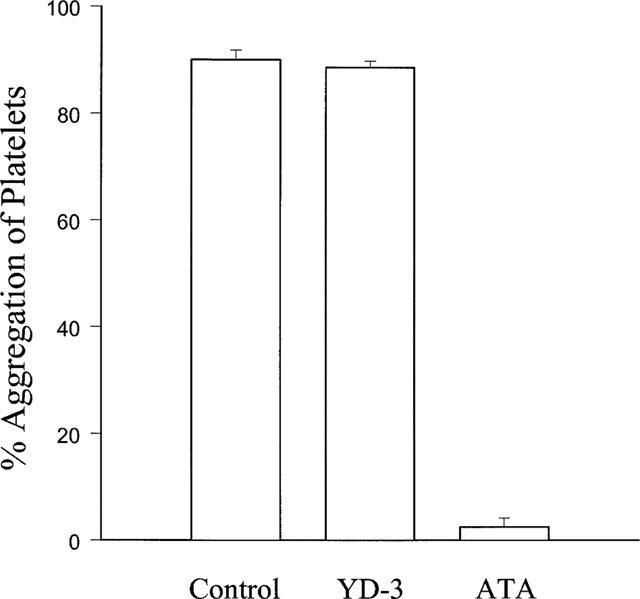
Effects of YD-3 and aurin tricarboxylic acid (ATA) on ristocetin-induced human platelet aggregation. Washed human platelets were incubated with DMSO (0.5%, control), YD-3 (100 μM) or ATA (10 μM) at 37°C for 3 min, platelet aggregation was then induced by the addition of ristocetin (600 μg ml−1) and von Willebrand factor (vWF, 5 μg ml−1). Percentages of aggregation are presented as mean±s.e.mean (n=6). ***P<0.001 as compared with the control.
Figure 8.
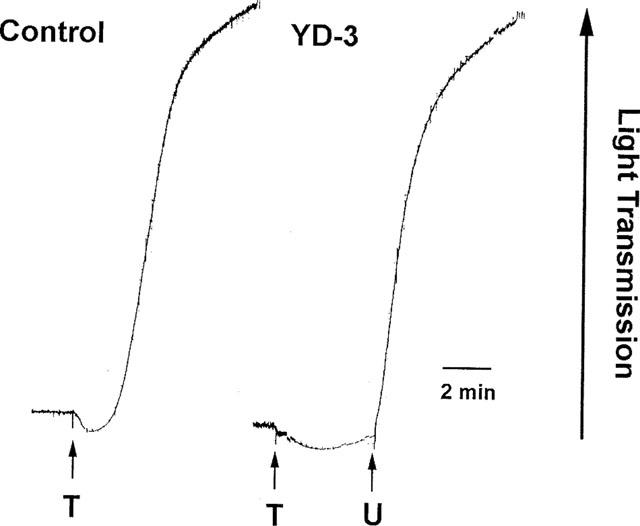
Effects of YD-3 on thrombin-induced aggregation of platelets pretreated with chymotrypsin. Human platelets were incubated with chymotrypsin (10 u ml−1) for 30 min at 37°C before rewashing, as described under Methods. Chymotrypsin-treated platelets were incubated with DMSO (0.5%, control) or YD-3 (50 μM) at 37°C for 3 min, platelet aggregation was then induced by the addition of thrombin (T, 0.1 u ml−1) and/or U46619 (U, 2 μM).
Effect of YD-3 on thrombin- and trypsin-induced Ca2+ mobilization in human platelets
Fluo-3-loaded platelets were used to measure the effect of YD-3 on thrombin- and trypsin-induced intracellular Ca2+ mobilization. To exclude the involvement of endoperoxides/thromboxane A2 and ADP and eliminate repletion of internal Ca2+ pool due to agonist-induced calcium influx through plasma membrane, all measurements were performed using aspirinated platelets incubated in the presence of 0.5 u ml−1 apyrase and 1 m M EGTA. Since apyrase markedly inhibited Ca2+ mobilization elicited by trypsin, however, in the trypsin experiment apyrase was withdrawn. YD-3 (20 μM) markedly prevented trypsin-induced Ca2+ mobilization with little effect on thrombin- or SFLLRN-induced Ca2+ mobilization. Incubation of platelets with 100 μM SFLLRN in unstirred condition for 10 min rendered them refractory to subsequent challenge by a very high concentration (200 μM) of SFLLRN indicating desensitization of PAR1 in these cells. The SFLLRN-desensitized platelets failed to respond to 0.1 u ml−1 thrombin, but still could elicit significant increases of intracellular Ca2+ concentration with 1 u ml−1 thrombin. Both leupeptin (100 μM, an inhibitor of serine protease) and YD-3 (20 μM) completely inhibited 1 u ml−1 thrombin induced Ca2+ mobilization in SFLLRN-desensitized platelets (Figure 9).
Figure 9.
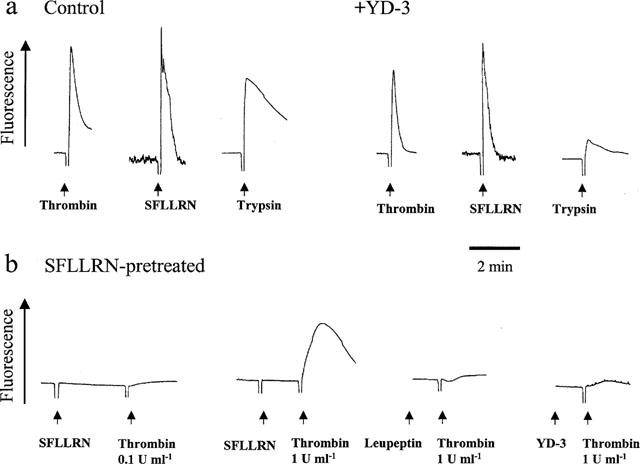
Effects of YD-3 on intracellular calcium mobilization in human platelets. (a) Fluo-3-loaded human platelets were incubated with DMSO (0.5%, control) or YD-3 (20 μM) at 37°C for 3 min, the inducer was then added to trigger the increase of [Ca2+]i. (b) Washed human platelet was preincubated with SFLLRN peptide (100 μM) in unstirred condition for 10 min. These platelets were then challenged with SFLLRN (200 μM) or thrombin in the absence or presence of leupeptin (100 μM) or YD-3 (20 μM). All measurements were performed using aspirinated platelets incubated in the presence of apyrase and EGTA (except the trypsin experiment in which apyrase was withdrawn).
Discussion
In the present work, we showed that YD-3, a low-molecular weight, non-peptide-derived compound, competitively inhibited thrombin-induced rabbit platelet aggregation. The action of YD-3 is not due to inhibition of thrombin's proteolytic activity, since YD-3 does not affect thrombin-induced fibrinogen clotting. In contrast, YD-3 had no or little inhibitory effect on platelet aggregation elicited by PAF, collagen, arachidonic acid and U46619. Furthermore, YD-3 selectively prevented phosphoinositide breakdown caused by thrombin, a very early event closed to G-protein-coupling receptor activation. These results suggest that YD-3 may interfere with thrombin-elicited rabbit platelet activation at the receptor level.
Interestingly, in contrast to rabbit platelets, YD-3 had only a mild inhibitory effect on the low concentration (0.05 u ml−1) of thrombin-induced human platelet aggregation, but did not affect that induced by higher concentrations (⩾0.1 u ml−1) of thrombin or SFLLRN, the PAR1 agonist peptide. These data suggest that YD-3 inhibits a rabbit platelet thrombin receptor, which is distinct from PAR1, the major receptor in human platelets. Kinlough-Rathbone et al. (1993) and Connolly et al. (1994) have reported that rabbit, rat, hamster and dog platelets lack a functional response to PAR1 agonist peptides that fully aggregate human platelets, despite a full response of these non-human platelets to human thrombin. Moreover, the thrombin responses of platelets from PAR1 knockout and wild-type mice were indistinguishable (Connolly et al., 1996). These observations suggest protease-activated receptors other than PAR1 may be involved in the stimulatory effect of thrombin in these cells. The pharmacological discrepancy of YD-3 on rabbit and human platelets also provide definitive evidence for the presence of different platelet thrombin receptors on these two species.
Although PAR1 has been shown to be the major thrombin receptor in human platelets, there is a lot of evidence which indicates that PAR1-independent mechanisms may exist. PAR1 agonist peptides, in contrast to thrombin itself, are not full agonists for activation of human platelets in the absence of platelet-derived secondary mediators (Lau et al., 1994; Kramer et al., 1995). In addition, platelets totally desensitized to very high concentration of PAR1 agonist peptides remained responsive to thrombin (Lau et al., 1994). Antibodies against two different regions of PAR1 inhibited human platelet activation by low, but not high, concentrations of thrombin (Hung et al., 1992; Brass et al., 1992). Moreover, two additional thrombin receptors: PAR3 and PAR4 have been identified in human and mouse platelets (Scase et al., 1997; Kahn et al., 1998). Apart from protease-activated receptors, GPIb has been suggested to be a positive effector of PAR1-driven platelet activation or a receptor for thrombin capable of activating platelets in its own right (Greco & Jamieson, 1991). Therefore, we first wanted to assess the role of GPIb in the antiplatelet activity of YD-3. Our results showed that YD-3 did not affect ristocetin-induced GPIb-dependent human platelet aggregation and chymotrypsin, which removes GPIb, enhances rather than attenuates YD-3's action on thrombin-elicited aggregation of human platelet. Thus, GPIb appears to play no role in the antiplatelet effect of YD-3.
On the other hand, there are several lines of evidence suggesting that YD-3 is capable of inhibiting a non-PAR1 protease-activated receptor in human platelets. First YD-3 can inhibit both human and rabbit platelet aggregation caused by another serine protease trypsin. Besides PAR2, trypsin is also capable of activating PAR1, PAR3 and PAR4 with different efficiency compared to thrombin (Dery et al., 1998). Therefore, it is possible that different types of PAR have differential proportions in mediating platelet activation elicited by trypsin and thrombin. The discrepancy of YD-3's effect on thrombin- and trypsin-induced human platelet aggregation implies that YD-3 inhibits a protease-activated receptor which plays a more important role in human platelet activation caused by trypsin than in that by thrombin. Second, chymotrypsin has been reported to cleave and impair PAR1 as well as GPIb (Okumara et al., 1978; Vouret-Craviari et al., 1995). Our results showed that pretreatment of human platelets with chymotrypsin selectively enhanced the inhibitory effect of YD-3 on thrombin-, but not U46619-induced platelet aggregation. Third, in PAR1-desensitized human platelets, we showed that the high concentration of thrombin still could elicit intracellular calcium mobilization and the rise of calcium was abolished by either serine protease inhibitor leupeptin or YD-3. These results strongly indicate involvement of a non-PAR1 protease-activated receptor in thrombin-induced human platelet activation and, this receptor could be blocked by YD-3. Since rabbit platelets fail to respond to the PAR1 agonist peptide and, thrombin-induced rabbit platelet aggregation is inhibited by YD-3, the non-PAR1 thrombin receptor may also exist in rabbit platelets and may be analogous to that in human platelets. Taken together, we suggest that YD-3 inhibits a non-PAR1 thrombin receptor mediating the major effect of thrombin in rabbit platelets, but in human platelets, this receptor function becomes significant only when the activity of PAR1 has been blocked or attenuated.
In summary, YD-3 selectively inhibits rabbit platelet aggregation and phosphoinoisitide breakdown caused by thrombin without affecting thrombin's proteolytic activity. In human platelets, YD-3 inhibits thrombin-induced platelet activation only when PAR1 activity is impaired. Our results suggest that YD-3 could interfere with platelet activation elicited by thrombin through blockade of a non-PAR1 thrombin receptor. The novel action of YD-3 may be useful for investigation of functional role of PARs-mediated signalling in platelets or other cells. In addition, the chemical structure of YD-3 is totally distinct from existent thrombin receptor antagonists, thus YD-3 may be as a lead compound for development of the new class of thrombin receptor antagonist.
Acknowledgments
This work was supported by grants from National Science Council of Taiwan (NSC 89-2320-B127-001).
Abbreviations
- AA
arachidonic acid
- ACD
acid citrate dextrose
- ATA
aurin tricarboxylic acid
- BAPNA
benzoyl-DL-arginine-p-nitroanilid HCl
- DMSO
dimethyl sulphoxide
- GPIb
glycoprotein Ib
- PAF
platelet-activating factor
- PAR
protease-activated receptor
- U46619
9, 11-dideoxy-9α, 11α-methanoepoxy PGF2α
- vWF
von Willebrand factor
- YD-3
1-benzyl-3(ethoxycarbonylphenyl)-indazole
References
- BAYKAL D., SCHMEDTJE J.F., Jr, RUNGE M.S. Role of the thrombin receptor in restenosis and atherosclerosis. Am. J. Cardiol. 1995;75:82B–87B. doi: 10.1016/0002-9149(95)80019-o. [DOI] [PubMed] [Google Scholar]
- BORN G.V.R. Quantitative investigations into the aggregation of blood platelets. J. Physiol. 1962;162:67–72. [Google Scholar]
- BRASS L.F., VASSALLO R.R., Jr, BELMONTE E., AHUJA M., CICHOWSKI K., HOXIE J.A. Structure and function of the human platelet thrombin receptor. Studies using monoclonal antibodies directed against a defined domain within the receptor N terminus. J. Biol. Chem. 1992;267:13795–13798. [PubMed] [Google Scholar]
- CHEN J., ISHII M., WANG L., ISHII K., COUGHLIN S.R. Thrombin receptor activation: confirmation of the intramolecular tethered liganding hypothesis and discovery of an alternative intramolecular liganding mode. J. Biol. Chem. 1994a;269:16041–16045. [PubMed] [Google Scholar]
- CHEN Y.H., POUYSSEGUR J, , COURTNEIDGE S.A., OBBERGHEN-SCHILLING E.V. Activation of Src family kinase activity by G protein-coupled thrombin receptor in growth-responsive fibroblasts. J. Biol. Chem. 1994b;269:27372–27377. [PubMed] [Google Scholar]
- COLOTTA F., SCIACCA F.L., SIRONI M., LUINI W., RABIET M.J., MANTOVANI A. Expression of monocyte chemotactic protein-1 by monocytes and endothelial cells exposed to thrombin. Am. J. Pathol. 1994;144:975–985. [PMC free article] [PubMed] [Google Scholar]
- CONNOLLY A.J., ISHIHARA H., KAHN M.L., FARESE R.V., COUGHLIN S.R. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1996;381:516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- CONNOLLY T.M., CONDRA C., FENG D.M., COOK J.J., STRANIERI M.T., REILLY C.F., NUTT R.F., GOULD R.J. Species variability in platelet and other cellular responsiveness to thrombin receptor-derived peptides. Thromb. Haemost. 1994;72:627–633. [PubMed] [Google Scholar]
- COUGHLIN S.R. How the protease thrombin talks to cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- GRECO N.J., JAMIESON G.A. High and moderate affinity pathways for α-thrombin-induced platelet activation. Proc. Soc. Exp. Biol. Med. 1991;198:792–799. doi: 10.3181/00379727-198-43321d. [DOI] [PubMed] [Google Scholar]
- HARKAR L.A., HANSON S.R., RUNGE M.S. Thrombin hypothesis of thrombus generation and vascular lesion formation. Am. J. Cardiol. 1995;75:12B–17B. doi: 10.1016/0002-9149(95)80004-c. [DOI] [PubMed] [Google Scholar]
- HEMKER H.C.Thrombin generation, an essential step in haemostasis and thrombosis Haemostatis and Thrombosis 1994Edinburgh, U.K.: Churchill Livingstone; 477–490.eds. Bloom A.L., Forbes, C.D., Thomas, D.P., Tuddenham, E.G.D. [Google Scholar]
- HOFFMAN H., DUMAREY C., BON C. Blood coagulation induced by Bothrops atrox venom identification and properties of a factor X activator. Biochimie. 1983;65:201–210. doi: 10.1016/s0300-9084(83)80085-6. [DOI] [PubMed] [Google Scholar]
- HUNG D.T., VU T.-K., WHEATON V.I., ISHII K., COUGHLIN S.R. Cloned platelet thrombin receptor is necessary for thrombin-induced platelet activation. J. Clin. Invest. 1992;89:1350–1353. doi: 10.1172/JCI115721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG E.M., DETWILER T.C. The effect of lithium on platelet phosphoinositide metabolism. Biochem. J. 1986;236:895–901. doi: 10.1042/bj2360895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIHARA H., ZENG D., CONNOLLY A.J., TAM C., COUGHLIN S.R. Antibodies to protease-activated receptor 3 inhibit activation of mouse platelets by thrombin. Blood. 1998;91:4152–4157. [PubMed] [Google Scholar]
- KAHN M.L., ZHENG Y.W., HUANG W., BIGORNIA V., ZENG D., MOFF S., FARESE R.V., Jr, TAM C., COUGHLIN S.R. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- JONES A., GECZY C.L. Thrombin and factor Xa enhance the production of interleukin-1. Immunology. 1990;71:236–241. [PMC free article] [PubMed] [Google Scholar]
- KINLOUGH-RATHBONE R.L., RAND M.L., PACKHAM M.A. Rabbit and rat platelets do not respond to thrombin receptor peptides that activate human platelets. Blood. 1993;82:103–106. [PubMed] [Google Scholar]
- KRAMER R.M., ROBERTS E.F., HYSLOP P.A., UTTERBACK B.G., HUI K.Y., JAKUBOWSKI J.A. Differential activation of cytosolic phospholipase A2 by thrombin and thrombin receptor agonist peptide in human platelets. J. Biol. Chem. 1995;270:14816–14823. doi: 10.1074/jbc.270.24.14816. [DOI] [PubMed] [Google Scholar]
- LAPOSATA M., DOVNARSKY D.K., SHIN H.S. Thrombin-induced gap formation in confluent endothelial cell monolayers in vitro. Blood. 1983;62:549–556. [PubMed] [Google Scholar]
- LAU L.F., PUMIGLIA K., COTE Y.P., FEINSTEIN M.B. Thrombin-receptor agonist peptides, in contrast to thrombin itself, are not full agonists for activation and signal transduction in human platelets in the absence of platelet-derived secondary mediators. Biochem. J. 1994;303:391–400. doi: 10.1042/bj3030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNAMARA C.A., SAREMBOCK I.J., GIMPLE L.W., FENTON J.W., II, COUGHLIN S.R., OWENS G.K. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J. Clin. Invest. 1993;91:94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRITT J.E., MCCARTHY S.A., DAVIES M.P.A., MOORES K.E. Use of fluo-3 to measure cytosolic Ca2+ in platelets and neutrophils. Biochem. J. 1990;269:513–519. doi: 10.1042/bj2690513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase-activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEYLON C.B., SUMMERS R.J. Stimulation of α1-adrenoceptors in rat kidney mediates increased inositol phospholipid hydrolysis. Br. J. Pharmacol. 1987;91:367–376. doi: 10.1111/j.1476-5381.1987.tb10291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUMURA T., HASITZ M., JAMIESON G.A. Platelet glycocalicin. Interaction with thrombin and the role as thrombin receptor of the platelet surface. J. Biol. Chem. 1978;253:3435–3443. [PubMed] [Google Scholar]
- SCASE T.J., HEATH M.F., EVANS R.J. Cloning of PAR3 cDNA from human platelets, and human erythroleukemic and human promonocytic cell lines. Blood. 1997;90:2113–2114. [PubMed] [Google Scholar]
- TAPPARELLI C., METTERNICH R., EHRHARDT C., COOK N.S. Synthetic low-molecular weight thrombin inhibitors: molecular design and pharmacological profile. Trends Pharmacol. Sci. 1993;14:366–376. doi: 10.1016/0165-6147(93)90095-2. [DOI] [PubMed] [Google Scholar]
- VOURET-CRAVIARI V., GRALL D., CHAMBARD J.C., RASMUSSEN U.B., POUYSSEGUR J., VAN OBBERGHEN-SCHILLING E. Post-translational and activation-dependent modification of the G protein-coupled thrombin receptor. J. Biol. Chem. 1995;270:8367–8372. doi: 10.1074/jbc.270.14.8367. [DOI] [PubMed] [Google Scholar]
- VU T.-K.H., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991a;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- VU T.-K.H., WHEATON V.I., HUNG D.T., CHARO I., COUGHLIN S.R. Domains specifying thrombin-receptor interaction. Nature. 1991b;353:674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- WU C.C., KO F.N., KUO S.C., LEE F.Y., TENG C.M. YC-1 inhibited human platelet aggregation through NO-independent activation of soluble guanylate cyclase. Br. J. Pharmacol. 1995;116:1973–1978. doi: 10.1111/j.1476-5381.1995.tb16400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU C.C., KO F.N., WU T.S., TENG C.M. Antiplatelet effects of Clausine-D isolated from Clausena excavata. Biochem. Biophys. Acta. 1994;1201:1–6. doi: 10.1016/0304-4165(94)90142-2. [DOI] [PubMed] [Google Scholar]
- XU W.F., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., FORSTER D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. USA. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHINA S., KUO S.C. Studies on heterocyclic compounds: XXXV. Synthesis of fuo[3,3-C]pyrazole derivatives. (2) Electrophilic substitution of 1,3-diphenylfuro[3,2-C]pyrazole. Yakugaku Zasshi. 1978;98:204–208. doi: 10.1248/yakushi1947.98.2_204. [DOI] [PubMed] [Google Scholar]


