Abstract
The effect of ethanol on the function of P2X4 receptors expressed in Xenopus oocytes was studied using two-electrode voltage-clamp recording.
The amplitude of current activated by 1 μM ATP was decreased by ethanol in a concentration-dependent manner over the concentration range 1–500 mM. The concentration of ethanol that produced 50% inhibition (IC50) of current activated by 1 μM ATP was 58 mM.
Ethanol inhibition of ATP-activated current was not dependent on membrane potential from −60 to +20 mV, and ethanol did not change the reversal potential of ATP-activated current.
Ethanol, 50 mM, shifted the ATP concentration-response curve to the right, increasing the EC50 for ATP from 9.1 to 16.0 μM, but did not reduce the maximal response to ATP.
The results suggest that ethanol may inhibit P2X4 receptors by decreasing the apparent affinity of the binding site for ATP.
Since the P2X4 receptor is the most abundant P2X subunit in the brain, these receptors could be important effectors of ethanol action in the central nervous system.
Keywords: ATP, ethanol, ion channel, receptor, purinoceptor, oocyte
Introduction
P2X receptors are ligand-gated membrane ion channels that are activated by extracellular ATP. These receptor-channels have received recent attention due to their potential importance in the central and peripheral nervous systems. P2X receptors have been reported to mediate excitatory synaptic transmission in the medial habenula (Edwards et al., 1992; 1997) and locus coeruleus (Nieber et al., 1997) of rat brain, rat hippocampus (Pankratov et al., 1998), rat spinal cord (Bardoni et al., 1997), and in tissue culture of autonomic ganglion neurons (Evans et al., 1992; Silinsky et al., 1992; Galligan & Bertrand, 1994). In addition, P2X receptors have been reported to mediate excitatory responses in a number of types of neurons including sensory (Krishtal et al., 1983; Bean, 1990; Li et al., 1993b; 1997; Khakh et al., 1995), autonomic (Fieber & Adams, 1991; Cloues et al., 1993; Nakazawa, 1994; Khakh et al., 1995; Zhong et al., 1998), and brain (Tschöpl et al., 1992; Ueno et al., 1992; Shen & North, 1993).
Currently, seven P2X receptor subunits, designated P2X1 to P2X7, have been identified (Buell et al., 1996a; MacKenzie et al., 1999). These subunits have been found to be widely distributed in the central nervous system, including cerebral cortex, hippocampus, thalamus, hypothalamus, midbrain, cerebellum, and spinal cord, and in sensory and autonomic ganglia in the peripheral nervous system (Collo et al., 1996). Each of these subunits can form ATP-activated homomeric cation channels when expressed in Xenopus oocytes or cell lines.
P2X receptors in bullfrog dorsal root ganglion (DRG) neurons have been found to be inhibited by pharmacological concentrations of ethanol (Li et al., 1993a; 1998a; Weight et al., 1999). Although the amino acid sequence, molecular structure, and subunit composition of P2X receptors in amphibian DRG have not been identified, this ethanol-sensitive P2X receptor almost certainly does not belong to the fast desensitizing type of P2X receptors, such as the P2X3 receptor, as the P2X receptor in bullfrog DRG neurons exhibits little or no desensitization to ATP. One of the possibilities is that it belongs to the P2X4 type, which desensitizes more slowly than P2X3 receptors and is the most abundant P2X subunit expressed in the brain (Collo et al., 1996). Therefore, in the present study, we investigated the effect of ethanol on the function of P2X4 receptors expressed in Xenopus oocytes. Some of this work has been presented previously in preliminary form (Li et al., 1998b).
Methods
Preparation of cRNA and expression of receptors were performed as described previously (Xiong et al., 1999b). Briefly, cRNA was synthesized in vitro from a linearized cDNA template using T7 RNA polymerase in the presence of the cap analogue 7 mGpppG, and was injected into Xenopus oocytes using a pressurized microinjection device (PV 800 Pneumatic Picopump, World Precision Instruments, Sarasota, FL, U.S.A.). Mature Xenopus laevis frogs were anaesthetized by immersion in water containing 3-aminobenzoic acid ethyl ester (2 g l−1). Oocytes were excised, mechanically isolated into clusters of four to five oocytes, and shaken in a water bath in two changes of 0.2% collagenase A in a solution containing (in mM): NaCl 83, KCl 2, MgCl2 1 and HEPES 5 (pH 7.4) for 1 h each. Each oocyte was injected with a total of 10 ng of RNA in 50 nl of diethylpyrocarbonate-treated water and was incubated at 17°C for 2–5 days in Modified Barth's Saline (MBS) containing sodium pyruvate (2 mM), penicillin (10,000 U l−1), streptomycin (10 mg l−1), gentamycin (50 mg l−1) and theophylline (0.5 mM). The care and use of animals in this study was approved by the Animal Care and Use Committee of the National Institute on Alcohol Abuse & Alcoholism (Protocol No. LMCN-SP-05) in accordance with National Institutes of Health Guidelines.
Two-electrode voltage-clamp recording was performed at room temperature using a Geneclamp Amplifier (Axon Instruments Inc., Foster City, CA, U.S.A.). Oocytes were placed in a recording chamber and impaled with two sharp microelectrodes filled with 3 M KCl. Electrode tip resistances were in the range 0.5–1.5 MΩ. Oocytes were voltage-clamped at −70 mV, except as indicated. Currents were recorded on a pen recorder (Model RS3400, Gould Inc., Valley View, OH, U.S.A.). Oocytes were constantly superfused at the rate of ∼2.5 ml min−1 with bathing medium containing (in mM): NaCl 95, KCl 2, CaCl2 2, HEPES 5 (pH 7.4). Solutions of ATP (added as the sodium salt) were prepared daily in extracellular medium. Solutions were delivered by gravity flow from a 0.5 mm silica tube connected to a 7-barrel manifold. Solutions superfusing the oocytes were changed via manually-switched solenoid valves. At least 5 min was allowed to elapse between agonist applications. All of the drugs used in these experiments were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.), except suramin and pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), which were purchased from Research Biochemicals International (Natick, MA, U.S.A.), and ethanol, which was purchased from Aldrich Chemical Company (Milwaukee, WI, U.S.A.).
Current amplitudes reported are peak values, and average values are expressed as mean±s.e.mean, with n equal to the number of cells studied. Data were statistically compared using Student's t-test or analysis of variance (ANOVA), as noted. Statistical analysis of concentration-response data was performed using the nonlinear curve-fitting program ALLFIT (DeLean et al., 1978), which uses an ANOVA procedure. Values reported for concentrations yielding 50% inhibition (IC50) or 50% of maximal effect (EC50) and slope factor (n) are those obtained by fitting the data to the equation:
where x and y are concentration and response, respectively, and Emax is the maximal response.
Results
It has previously been reported that purinoceptor antagonists suramin and PPADS have little or no effect on ATP-activated current in HEK293 cells mediated by P2X4 receptors cloned from rat superior cervical ganglion (Buell et al., 1996b). Figure 1 illustrates the ATP-activated inward current in Xenopus oocytes expressing P2X4 receptors cloned from rat superior cervical ganglion and the effect of purinoceptor antagonists suramin, PPADS and reactive blue 2 on that current in our experiments. As can be seen, the inward current activated rapidly upon ATP application, showed slow desensitization, and decayed quickly upon removal of ATP. Moreover, suramin, PPADS, or reactive blue 2, at a concentration of 100 μM, had little or no effect on the current activated by ATP. Similar results were observed in five other oocytes expressing P2X4 receptors (Student's t-test; P>0.5). Suramin, PPADS or reactive blue 2 alone (10–100 μM) did not activate current in any tested Xenopus oocytes expressing P2X4 receptors (data not shown, n=5). In uninjected oocytes, ATP, at concentrations up to 500 μM, did not evoke detectable current (n=6, data not shown).
Figure 1.
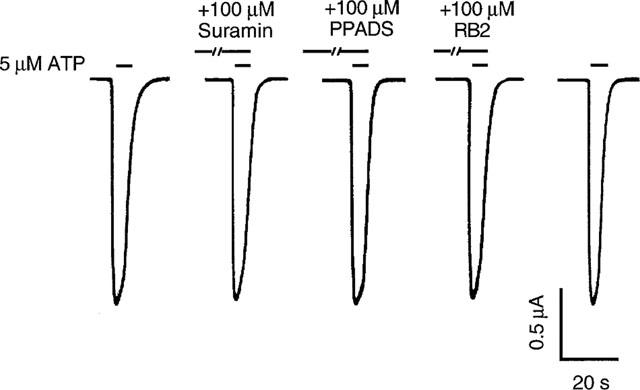
Effect of suramin, PPADS and reactive blue 2 on ATP-activated current mediated by P2X4 receptors expressed in Xenopus oocytes. Records of current activated by 5 μM ATP in the absence and the presence of 100 μM suramin, PPADS or reactive blue 2 (RB2). Records are sequential current traces (from left to right) obtained from a single oocyte. Solid bar above each record indicates time of agonist application in the absence or presence of antagonists, as labelled. Suramin and reactive blue 2 were preapplied for 1 min, and PPADS was preapplied for 8 min.
Figure 2 illustrates the effect of ethanol on ATP-activated current mediated by P2X4 receptors. As shown in Figure 2a, the amplitude of current activated by 1 μM ATP was markedly decreased in the presence of 50 and 100 mM ethanol, and completely recovered after ethanol washout. On average, 50 and 100 mM ethanol decreased the amplitude of current activated by 1 μM ATP by 48±37% (n=16) and 65±23% (n=12), respectively. As shown in Figure 2b, ethanol inhibition of current activated by 1 μM ATP exhibited a clear concentration-dependence over the concentration range, 1–500 mM. The calculated ethanol concentration that produced 50% inhibition (IC50) was 58±4.5 mM, the apparent Hill coefficient for ethanol inhibition of ATP-activated current was 1.0±0.2, and the maximal effect was 100% inhibition. Over the concentration range 1–500 mM, application of ethanol alone did not activate detectable current (n=8, data not shown).
Figure 2.
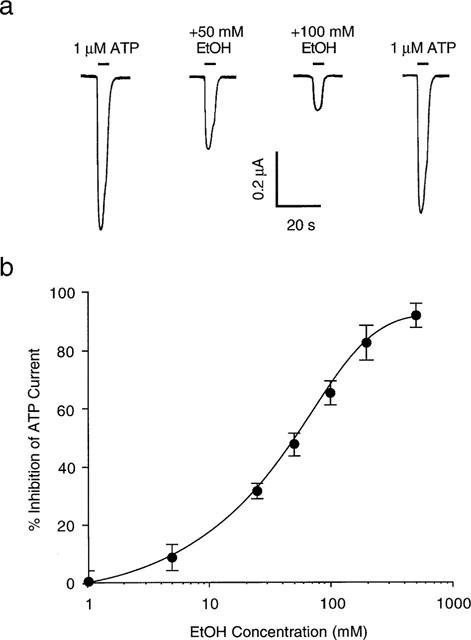
Effect of ethanol on ATP-activated current. (a) Current activated by application of 1 μM ATP before, during, and after application of 50 and 100 mM ethanol (EtOH). Records are sequential current traces (from left to right) obtained from a single oocyte. (b) Graph plotting average percentage inhibition of the amplitude of current activated by 1 μM ATP as a function of ethanol concentration. Each point is the average of 4–16 cells; error bars indicate s.e.mean. The curve shown is the best fit of the data to the equation in the Methods. Values obtained were: IC50=58±4.5 mM; n=1.0±0.2; and Emax=100%.
To evaluate the mechanism involved in the inhibitory effect of ethanol on ATP-activated current, we tested the effect of membrane potential on ethanol inhibition of ATP-activated current by activating current with ATP at various membrane voltages in the absence and presence of ethanol. As illustrated in Figure 3a, the average inhibition of ATP-activated current by 50 mM ethanol did not differ significantly at membrane holding potentials between −60 and +20 mV (ANOVA, P>0.25; n=5). In addition, as can be seen from the current-voltage relationship for current activated by 1 μM ATP, in the absence and presence of 50 mM ethanol (Figure 3b), ethanol did not change the reversal potential of ATP-activated current (−1±3 mV in the absence vs 0±4 mV in the presence of 50 mM ethanol; Student's t-test; P>0.1; n=5).
Figure 3.
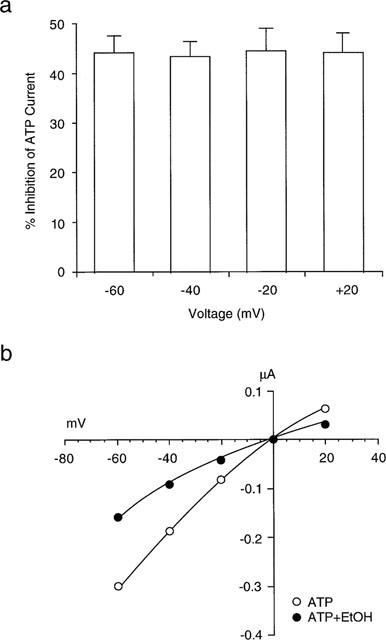
Effect of membrane potential on inhibition of ATP-activated current by ethanol. (a) Bar graphs showing average percentage inhibition by 50 mM ethanol of current activated by 1 μM ATP at membrane potentials from −60 to +20 mV. Current was activated by ATP at various membrane voltages in the absence and presence of ethanol. The percentage inhibition of ATP-activated current by 50 mM EtOH was not significantly different at the holding potentials shown (ANOVA, P>0.25; n=5). (b) Current-voltage relationship (I-V plot) showing the amplitude of current activated by 1 μM ATP as a function of membrane potential, in the absence and presence of 50 mM ethanol in a single oocyte. Note that ethanol did not alter the reversal potential of ATP-activated current.
The mechanism of the inhibitory effect of ethanol on ATP-activated current was evaluated further by studying the effect of ethanol on current activated by different concentrations of ATP. The records in Figure 4a show current activated by 0.5 μM ATP before, during, and after application of 50 mM ethanol (upper series), and current activated by 30 μM ATP under the same conditions in the same cell (lower series). On average, 50 mM ethanol decreased the amplitude of the current activated by 0.5 and 30 μM ATP by 86±3% (n=5) and 15±6% (n=6), respectively. Figure 4b illustrates that ethanol shifted the ATP concentration-response curve to the right in a parallel manner. In the absence of ethanol, ATP had an EC50 value of 9.1±0.4 μM and a slope factor of 1.0±0.1. Ethanol, 50 mM, increased the EC50 value of ATP to 16.0±0.4 μM (ANOVA, P<0.01), but did not alter the slope factor (1.0±0.1) or Emax (1.0±0.01) of the curve (P>0.5).
Figure 4.
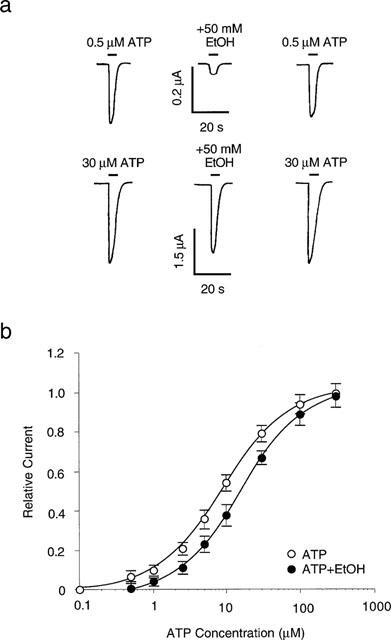
Effect of ATP concentration on ethanol inhibition of ATP-activated current. (a) Records showing currents activated by 0.5 μM ATP (upper traces) and 30 μM ATP (lower traces), before, during, and after application of 50 mM ethanol in a single oocyte. (b) Graph plotting the relative amplitude of ATP-activated current in the absence and the presence of 50 mM ethanol as a function of ATP concentration. Amplitude is normalized to the current activated by 300 μM ATP in the absence of ethanol. Each data point is the average current from 5–8 cells. The solid curves are the best fits of the data to the equation described in the Methods section. Ethanol significantly increased the EC50 for ATP from 9.1±0.4 μM in the absence of ethanol to 16.0±0.4 μM in the presence of 50 mM ethanol (ANOVA, P<0.01).
Discussion
P2X4 receptors have been cloned from rat brain (Bo et al., 1995; Séguéla et al., 1996; Soto et al., 1996), mouse brain (Townsend-Nicholson et al., 1999), human brain (Garcia-Guzman et al., 1997), rat superior cervical ganglion (Buell et al., 1996b), rat pancreatic islets (Wang et al., 1996), and stomach (Dhulipala et al., 1998). In most cases, P2X4 receptors can be readily distinguished from the other known P2X receptors because the purinoceptor antagonists, suramin, PPADS and reactive blue 2, have little or no effect on ATP-activated current mediated by P2X4 receptors (Bo et al., 1995; Buell et al., 1996b; Soto et al., 1996). In the present study, we found that suramin, PPADS, and reactive blue 2, at a concentration of 100 μM, did not antagonize ATP-activated current in Xenopus oocytes expressing P2X4 receptors cloned from rat superior cervical ganglion (Buell et al., 1996b). We also found that the EC50 value of ATP-activated current was 9.1 μM when P2X4 receptors were expressed in Xenopus oocytes, which is similar to the value of 10 μM observed previously for this receptor expressed in HEK293 cells (Buell et al., 1996b). Thus, these observations indicate that the receptors activated by ATP in our experiments have the characteristics of the P2X4 receptors cloned from rat superior cervical ganglion and expressed in HEK293 cells. It should be noted, however, that the EC50 value of ATP for P2X4 receptors cloned from a mouse brain cDNA library was reported to be 1.7 μM (Townsend-Nicholson et al., 1999) and the EC50 value of ATP for P2X4 receptors cloned from a rat pancreatic islet cDNA library was reported to be 63 μM (Wang et al., 1996). The reason for these differences in EC50 values is not known.
The results reported here show that ATP-activated current mediated by P2X4 receptors can be inhibited by ethanol in a concentration-dependent manner over the concentration range 1–500 mM, and the concentration that produced 50% inhibition (IC50) of current activated by 1 μM ATP was 58 mM. Additionally, 1–500 mM ethanol alone did not activate detectable current, indicating that the observed effect of ethanol was not due to an activation of outward current by ethanol.
Ethanol could inhibit the current mediated by P2X4 receptors by any of several mechanisms. For example, ethanol might alter the ion permeance ratio of ATP-gated channels. We found, however, that ethanol did not alter the reversal potential of current activated by ATP, and thus this mechanism does not appear to account for the ethanol-induced inhibition of ATP-activated current that was observed in the present study. Ethanol could also inhibit ATP-activated current by decreasing the number of channels gated by ATP, but this mechanism would be expected to be reflected in a decrease in the maximal response to ATP, which was not observed. In addition, another type of inhibitory action, open-channel block, appears to be unlikely, as this mechanism would also be expected to decrease the maximal response to ATP, and this was not observed. In addition, although a voltage-dependent ion channel block would not be expected because ethanol is not charged, ethanol might induce a conformational change in the channel protein that could result in voltage-dependence (Magleby & Stevens, 1972). This possibility seems unlikely since voltage-dependence of ethanol action was not observed. Finally, ethanol could decrease the apparent affinity of the receptor for ATP. Our observation that ethanol shifted the ATP concentration-response curve to the right in a parallel manner, increasing the EC50 for ATP, is consistent with an action of ethanol to decrease the apparent affinity of the P2X4 receptor for agonist. Moreover, this observation agrees well with previous findings for ethanol inhibition of P2X receptors in bullfrog dorsal root ganglion neurons (Li et al., 1993a; 1998a). This action of ethanol on ATP-gated P2X receptor-channels contrasts with the observations on ethanol inhibition of another ligand-gated ion channel, N-methyl-D-aspartate (NMDA) receptors. Ethanol inhibition of NMDA receptors is associated with a decrease in maximal response to agonist (Emax), without a change in EC50 (Peoples et al., 1997).
Of the known P2X receptor subunits, the P2X4 subunit has been found to be the most abundant P2X subunit in brain. Northern blot analysis and in situ hybridization studies have revealed extensive distribution of P2X4 mRNA in rat brain with particularly strong expression in the hippocampus, Purkinje cells of the cerebellum, and spinal cord motoneurons (Bo et al., 1995; Collo et al, 1996; Séguéla et al., 1996; Soto et al., 1996). In addition, the P2X4 receptor and another major central subunit, P2X6,- have highly overlapping mRNA distribution at both the regional and the cellular level (Collo et al., 1996). The coexpression of P2X4 and P2X6 subunits in Xenopus oocytes generates a novel heteromeric ATP receptor (Lê et al., 1998), which is another candidate for ethanol action in the central nervous system (Xiong et al., 1999a). Moreover, in recent preliminary experiments, pharmacological concentrations of ethanol have been found to inhibit ATP-activated current in freshly isolated adult rat hippocampal neurons (Li & Weight, 1999). Thus, our observations suggest that P2X receptors could be important effectors of ethanol action in the central nervous system.
Acknowledgments
We thank Dr Gary Buell for providing the cDNA for the P2X4 subunit.
Abbreviations
- DRG
dorsal root ganglion
- NMDA
N-methyl-D-aspartate
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid
- RB2
reactive blue 2
References
- BARDONI R., GOLDSTEIN P.A., LEE C.J., GU J.G., MACDERMOTT A.B. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J. Neurosci. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAN B.P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J. Neurosci. 1990;10:1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BO X., ZHANG Y., NASSAR M., BURNSTOCK G., SCHOEPFER R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- BUELL G., COLLO G., RASSENDREN F. P2X receptors: an emerging channel family. Eur. J. Neurosci. 1996a;8:2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- BUELL G., LEWIS S., COLLO G., NORTH R.A., SURPRENANT A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996b;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- CLOUES R., JONES S., BROWN D.A. Zn2+ potentiates ATP-activated currents in rat sympathetic neurons. Pflügers Arch. 1993;424:152–158. doi: 10.1007/BF00374606. [DOI] [PubMed] [Google Scholar]
- COLLO G., NORTH R.A., KAWASHIMA E., MERLO-PICH E., NEIDHART S., SURPRENANT A., BUELL G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELEAN A., MUNSON P.J., RODBARD D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am. J. Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- DHULIPALA P.D., WANG Y.X., KOTLIKOFF M.I. The human P2X4 receptor gene is alternatively spliced. Gene. 1998;207:259–266. doi: 10.1016/s0378-1119(97)00647-1. [DOI] [PubMed] [Google Scholar]
- EDWARDS F.A., GIBB A.J., COLQUHOUN D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;395:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- EDWARDS F.A., ROBERTSON S.J., GIBB A.J. Properties of ATP receptor-mediated synaptic transmission in the rat medial habenula. Neuropharmacology. 1997;36:1253–1268. doi: 10.1016/s0028-3908(97)00127-5. [DOI] [PubMed] [Google Scholar]
- EVANS R., DERKACH V., SURPRENANT A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- FIEBER L.A., ADAMS D.J. Adenosine triphosphate-evoked currents in cultured neurons dissociated from rat parasympathetic cardiac ganglia. J. Physiol. 1991;434:239–256. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLIGAN J.J., BERTRAND P.P. ATP mediates fast synaptic potentials in enteric neurons. J. Neurosci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-GUZMAN M., SOTO F., GOMEZ-HERNANDEZ J.M., LUND P.-E., STÜHMER W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol. Pharmacol. 1997;51:109–118. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- KHAKH B.S., HUMPHREY P.P.A., SURPRENANT A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurons. J. Physiol. 1995;484:385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHTAL O.A., MARCHENKO S.M., PIDOPLICHKO V.I. Receptor for ATP in the membrane of mammalian sensory neurons. Neurosci. Lett. 1983;35:41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- LÊ K.-T., BABINSKI K., SÉGUÉLA P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J. Neurosci. 1998;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C., AGUAYO L., PEOPLES R.W., WEIGHT F.F. Ethanol inhibits a neuronal ATP-gated ion channel. Mol. Pharmacol. 1993a;44:871–875. [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., LI Z., WEIGHT F.F. Zn2+ potentiates excitatory action of ATP on mammalian neurons. Proc. Natl. Acad. Sci. U.S.A. 1993b;90:8264–8267. doi: 10.1073/pnas.90.17.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., WEIGHT F.F. Inhibition of ATP-activated current by zinc in dorsal root ganglion neurons of bullfrog. J. Physiol. 1997;505:641–653. doi: 10.1111/j.1469-7793.1997.641ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., WEIGHT F.F. Ethanol-induced inhibition of a neuronal P2X purinoceptor by an allosteric mechanism. Br. J. Pharmacol. 1998a;123:1–3. doi: 10.1038/sj.bjp.0701599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C., XIONG K.-M., WEIGHT F.F. Inhibition by ethanol of P2X4 purinoceptors expressed in Xenopus oocytes. Alcohol. Clin. Exp. Res. (Abstracts) 1998b;22:5A. [Google Scholar]
- LI C., WEIGHT F.F. Inhibition by ethanol of ATP-activated current in freshly isolated adult rat hippocampal neurons. Alcohol. Clin. Exp. Res. (Abstracts) 1999;23:7A. [Google Scholar]
- MACKENZIE A.B., SURPRENANT A., NORTH R.A. Functional and molecular diversity of purinergic ion channel receptors. Ann. N.Y. Acad. Sci. 1999;868:716–729. doi: 10.1111/j.1749-6632.1999.tb11351.x. [DOI] [PubMed] [Google Scholar]
- MAGLEBY K.L., STEVENS C.F. The effect of voltage on the time course of end-plate currents. J. Physiol. 1972;223:151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAZAWA K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J. Neurosci. 1994;14:740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIBER K., POELCHEN W., ILLES P. Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurons of the rat. Br. J. Pharmacol. 1997;122:423–430. doi: 10.1038/sj.bjp.0701386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANKRATOV Y., CASTRO E., MIRAS-PORTUGAL M.T., KRISHTAL O.A. Purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur. J. Neurosci. 1998;10:3898–3902. doi: 10.1046/j.1460-9568.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- PEOPLES R.W., WHITE G., LOVINGER D.M., WEIGHT F.F. Ethanol inhibition of N-methyl-D-aspartate-activated current in mouse hippocampal neurons: whole-cell patch-clamp analysis. Br. J. Pharmacol. 1997;122:1035–1042. doi: 10.1038/sj.bjp.0701483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SÉGUÉLA P., HAGHIGHI A., SOGHOMONIAN J.-J., COOPER E. A novel neuronal P2X ATP receptor ion channel with widespread distribution in the brain. J. Neurosci. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN K.-Z., NORTH R.A. Excitation of rat locus coeruleus neurons by adenosine 5′-triphosphate: ionic mechanism and receptor characterization. J. Neurosci. 1993;13:894–899. doi: 10.1523/JNEUROSCI.13-03-00894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILINSKY E.M., GERZANICH V., VANNER S.M. ATP mediates excitatory synaptic transmission in mammalian neurons. Br. J. Pharmacol. 1992;106:762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOTO F., GARCIA-GUZMAN M., GOMEZ-HERNANDEZ J.M., HOLLMANN M., KARSCHIN C., STÜHMER W. P2X4: An ATP-activated iontropic receptor cloned from rat brain. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOWNSEND-NICHOLSON A., KING B.F., WILDMAN S.S., BURNSTOCK G. Molecular cloning, functional characterization and possible cooperativity between the murine P2X4 and P2X4a receptors. Mol. Brain. Res. 1999;64:246–254. doi: 10.1016/s0169-328x(98)00328-3. [DOI] [PubMed] [Google Scholar]
- TSCHÖPL M., HARMS L., NÖRENBERG W., ILLES P. Excitatory effects of adenosine 5′-triphosphate on rat locus coeruleus neurons. Eur.J. Pharmacol. 1992;213:71–77. doi: 10.1016/0014-2999(92)90234-u. [DOI] [PubMed] [Google Scholar]
- UENO S., HARATA N., INOUE K., AKAIKE N. ATP-gated current in dissociated rat nucleus solitarii neurons. J. Neurophysiol. 1992;68:778–785. doi: 10.1152/jn.1992.68.3.778. [DOI] [PubMed] [Google Scholar]
- WANG C.Z., NAMBA N., GONOI T., INAGAKI N., SEINO S. Cloning and pharmacological characterization of a fourth P2X receptor subtype widely expressed in brain and peripheral tissues including various endocrine tissues. Biochem. Biophys. Res. Commun. 1996;220:196–202. doi: 10.1006/bbrc.1996.0380. [DOI] [PubMed] [Google Scholar]
- WEIGHT F.F., LI C., PEOPLES R.W. Alcohol action on membrane ion channels gated by extracellular ATP (P2X receptors) Neurochem. Int. 1999;35:143–152. doi: 10.1016/s0197-0186(99)00056-x. [DOI] [PubMed] [Google Scholar]
- XIONG K.M., LI C., WEIGHT F.F. Differential ethanol sensitive to P2X4 and P2X6 receptors expressed in Xenopus oocytes. Alcohol. Clin. Exp. Res. (Abstracts) 1999a;23:8A. [Google Scholar]
- XIONG K., PEOPLES R.W., MONTGOMERY J., CHIANG Y.-S., STEWART R., WEIGHT F.F., LI C. Differential modulation by divalent cations of P2X2 and P2X4 receptor function. J. Neurophysiol. 1999b;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- ZHONG Y., DUNN P.M., XIANG Z., BO X., BURNSTOCK G. Pharmacological and molecular characterization of P2X receptors in rat pelvic ganglion neurons. Br. J. Pharmacol. 1998;125:771–781. doi: 10.1038/sj.bjp.0702118. [DOI] [PMC free article] [PubMed] [Google Scholar]


