Abstract
Microcystin-LR, a specific and effective inhibitor of serine/threonine phosphatases type 1/2A which does not permeate cells, was used to distinguish intracellular and extracellular effects of phosphatase inhibitors on insulin secretion by RINm5F cells.
Incubation of intact RINm5F cells with microcystin-LR (0.1–2 μM) almost doubled basal insulin release at 3 mM glucose but left maximal insulin release induced by KCl (30 mM) unaffected.
In parallel, there was an increase in cytosolic Ca2+ by up to half maximum, which could be suppressed by the Ca2+-channel blocker D600.
In contrast, microcystin-LR incubation of intact cells did not affect phosphatase activity but significantly reduced phosphatase activity when used in cellular fractions.
From these data we conclude that microcystin-LR could affect Ca2+-channels and insulin release by inhibiting an extracellular phosphatase-like activity.
Keywords: Serine/threonine phosphatase, phosphatase inhibition, microcystin-LR, insulin release, insulin-secreting cells, cytosolic calcium
Introduction
Protein phosphatases play an important role in the regulation of cellular functions (Schonthal, 1998). Apart other mechanisms, their contribution to the phosphorylation state of calcium channels seems to play a crucial role in the regulation of the channel function (Levitan, 1994). This is indicated by studies with heart myocytes, in which increased phosphorylation of calcium channels or associated proteins caused by an inhibition of protein phosphatases enhanced channel activity (Kameyama et al., 1986; Hescheler et al., 1987). In pancreatic β-cells, the entry of calcium through voltage-dependent Ca2+-channels is a key event in the stimulus-secretion cascade (Wollheim et al., 1996). In these cells, a regulatory effect of intracellular phosphorylation (Arkhammar et al., 1994) and dephosphorylation on calcium-channel function (Haby et al., 1994; Ammon et al., 1996) has also been shown. However, the use of membrane-permeating inhibitors of the serine/threonine phosphatases like okadaic acid gave contradictory results, either suggesting an increase in [Ca2+]i (Haby et al., 1994) or a decrease in [Ca2+]i and insulin secretion (Tamagawa et al., 1992; Ammon et al., 1996). With the discovery of microcystin-LR as an inhibitor of protein phosphatases 1/2A (Matsushima et al., 1990), which does not permeate through cell membranes, another specific tool to study the role of phosphatases in cell function became available. To further clarify the role of phosphatases in the regulation of cellular calcium-metabolism, we evaluated the effect of microcystin-LR on protein phosphatase activity, cytosolic calcium-concentration ([Ca2+]i) and insulin release in the insulin-secreting clonal cell-line RINm5F.
Methods
Chemicals
RPMI 1640, penicillin G and streptomycin were obtained from Biochrom/Seromed (Berlin D). Bovine serum albumin (BSA), tris(hydroxymethyl)-aminomethane (TRIS), EGTA (ethylene glycol-bis[β-aminoethylether]-N,N,N′,N′-tetraacetic acid) and trypsin inhibitor from soybean were from Sigma (Deisenhofen, Germany). Microcystin-LR, foetal calf serum, glycogen phosphorylase b and phosphorylase kinase were supplied by Life Technologies (Eggenstein, Germany). Fura-2-acetoxymethyl ester (fura-2 AM) was obtained from Molecular Probes (Eugene, OR, U.S.A.). [33P-γ]-ATP was from Amersham Buchler (Braunschweig, Germany). All other chemicals, in the purest grade available, were from E. Merck AG (Darmstadt, Germany).
Cell culture
Cells of the clonal rat insulinoma cell line RINm5F (Gazdar et al., 1980) were cultured in 175-cm2 flasks (Greiner, Nürtingen, Germany) at 37°C in a water saturated atmos-phere gassed with an air-CO2-mixture (95 : 5). The RPMI 1640-NaHCO3 medium supplemented with 10% foetal calf serum, 11.1 mM glucose, 100 IU penicillin G and streptomycin (100 μg ml−1) was used as culture medium (Trier et al., 1988). The cells were subcultured weekly and the medium was renewed after 4 days of culture.
Phosphorylase a phosphatase activity
To determine the effect of microcystin-LR on phosphatase 1 and 2A in the different cell fractions phosphorylase a has been used as a substrate. Therefore, phosphorylase a was obtained by phosphorylation of glycogen-phosphorylase b using [32P-γ]-ATP and phosphorylase-kinase, as reported earlier (Ammon et al., 1996).
For the assay, RINm5F cells were washed three times with TRIS/EGTA (20 mM/0.1 mM) buffer, harvested after trypsinization and homogenized in TRIS/EGTA buffer with a dounce homogenizer (10 strokes). To prevent protease activities, 0.2 mg ml−1 trypsin inhibitor from soybean was added before homogenization. Protein phosphatase activity was measured in the homogenates of RINm5F cells by incubation for 10 min at 30°C with [33P]-labelled phosphorylase a as a substrate and subsequent determination of the release of [33P]i (Cohen et al., 1988). Protease activities were excluded using the method described by Antoniw & Cohen (1976). Microcystin-LR was added to homogenates or fractions immediately before the assay. In order to find out if microcystin-LR permeates in sufficient amounts to inhibit phosphatase activities, intact cells were incubated with microcystin-LR for 60 min in a Krebs-Ringer-HEPES buffer containing 0.5% BSA. At the end of the incubation period the cells were washed five times, harvested, homogenized and assayed for phosphorylase a phosphatase activity after fractionation.
Subcellular fractionation procedure
The cells were fractionated according to a method of Salers et al. (1991). All procedures were undertaken at 4°C. The cells were homogenized as described above. The crude homogenate was centrifuged at 600×g for 5 min to remove the nuclei and intact cells. The pellet was resuspended and homogenized again to disrupt remaining cells and centrifuged at 600×g for 5 min. The pooled two supernatants were centrifuged at 20,000×g for 20 min. The resulting supernatant was centrifuged again for 60 min at 105,000×g. The 105,000×g pellet was resuspended, homogenized and pelleted as above. The two 105,000×g supernatants were pooled and referred to as the cytosolic fraction. The 600×g and 105,000×g pellets were resuspended in 500 μl buffer each and referred to as the nuclear and membrane fraction, respectively.
Cytosolic calcium [Ca2+]i
Cells being attached to coverslips after overnight culture were incubated with 2 μM fura-2-acetomethoxymethylester for 30 min at 37°C in a modified Krebs-Ringer-HEPES-buffer containing 1 mM Ca2+, 2.8 mM glucose and 4% BSA (Pralong et al., 1990). After washing the cells three times with the experimental buffer they were placed in a perifusion chamber (volume 250 μl) on a temperature-controlled microscopic stage (35–37°C) and perifused with the experimental media. All experiments were performed at a constant superfusion rate. Solution changes were completed within 45 s.
[Ca2+]i was determined with a dual excitation system (excitation wavelength 340/380 nm, emission wavelength 510 nm) based on a photomultiplier tube (Seefelder Mess-technik, Seefeld and Uhl, München, Germany). Small clusters of several cells were analysed for [Ca2+]i by monitoring the fluorescence ratio for the two excitation wavelengths at a sampling rate of 2 Hz using a microcomputer, as reported earlier (Ammon et al., 1996).
Insulin secretion
RINm5F cells were seeded in multiwells (Greiner, Nürtingen, Germany) and grown for 3 days in culture to reach approximately half-confluency. For the experiments, the cells were washed three times with Krebs-Ringer-HEPES buffer containing 2.8 mM glucose and 0.5% BSA. Incubation was performed at 37°C in 1 ml buffer. For kinetic experiments, RINm5F cells were preincubated with microcystin-LR for 10 min before addition of the stimulus. Insulin was quantified by radioimmunoassay (Biermann, Bad Nauheim, Germany) using rat insulin as a standard. Statistical evaluation of multiple comparisons was done by ANOVA followed by Newman-Keuls-test or by calculating the slopes by linear regression and comparing these slopes by ANOVA followed by Bonferroni's Multiple Comparison Test. Values marked with different symbols differ significantly (P<0.05).
Results
Phosphorylase a phosphatase activity
To study the effect of microcystin-LR on phosphorylase a phosphatase activity in RINm5F cells, intact cells were incubated for 60 min in the presence or absence of 2 μM microcystin-LR. After washing and homogenization, phosphorylase a phosphatase activity remained unchanged by the microcystin-LR-incubation when compared to the controls (Table 1).
Table 1.
Effect of microcystin-LR on phosphatase activity of intact cells and cellular fractions
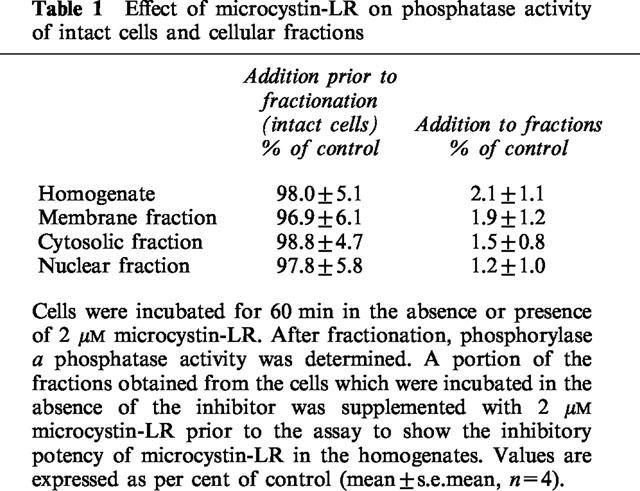
To verify the competence of microcystin-LR as an inhibitor of phosphorylase a phosphatase activity, the homogenate and an aliquot of each cellular fraction (membrane, cytosolic, nuclear) were incubated with 2 μM microcystin-LR for 10 min. Here, phosphorylase a phosphatase activity was nearly completely inhibited compared to the respective controls in the absence of microcystin-LR (Table 1).
Cytosolic calcium
Stimulation of RINm5F cells by increasing the extracellular concentration of KCl from 4.8 to 30 mM for 1 min caused a steep rise of [Ca2+]i which was followed by a decline. Adding microcystin-LR (0.75–2 μM) for 10 min in the absence of other stimulators induced a concentration-dependent rise in basal [Ca2+]i (Figure 1). However, the subsequent addition of KCl (to 30 mM) led to an unchanged elevation of [Ca2+]i in all cases (Figure 1).
Figure 1.
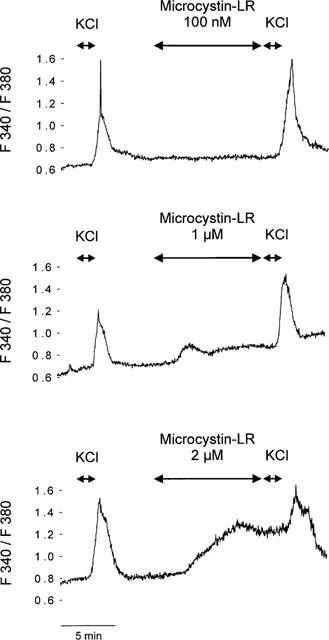
Effect of microcystin-LR (100 nM–2 μM) on [Ca2+]i determined by fluorescence measurements. The cells were initially stimulated by an increase in KCl from 4.8 to 30 mM for a 1 min period, as indicated by the arrow. After a washout period (400 s) 100 nM (upper panel); 1 μM (mid panel) or 2 μM (lower panel) microcystin LR was added for 10 min in the respective concentrations as indicated by the arrows. After the end of the microcystin-LR-incubation period the cells were again depolarized by rising KCl from 4.8 to 30 mM for 1 min. Each trace is a representative of five independent experiments.
Incubation of the cells with 50 μM of the calcium-channel blocker D 600 prior (5 min) to the addition of microcystin-LR (1 μM) completely inhibited the microcystin-LR-induced rise of [Ca2+]i. A subsequent stimulation with KCl (30 mM) was completely suppressed too. After a wash-out period of at least 250 s the response of the cells to a depolarization started to recover (Figure 2).
Figure 2.
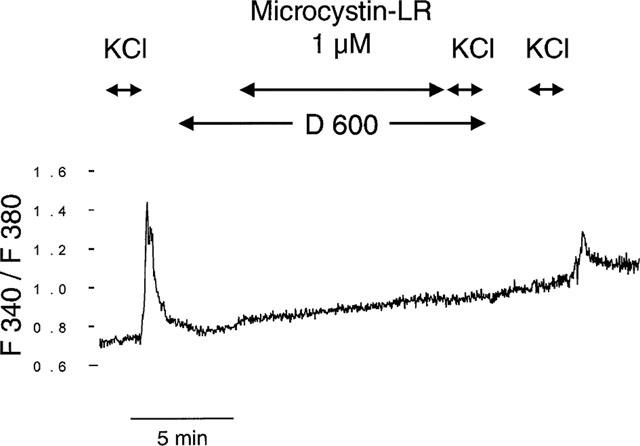
Effect of microcystin-LR (1 μM) on [Ca2+]i in the presence of a Ca2+-channel blocker. The cells were initially stimulated by an increase in KCl from 4.8 to 30 mM for a 1 min period, as indicated by the arrow. After a washout period (2 min) the cells were incubated with 50 μM D 600. The subsequent addition of 1 μM microcystin-LR was as ineffective on [Ca2+]i as a following depolarization with KCl (30 mM). After a washout period of 4 min the cells responded again to the KCl-stimulus (30 mM). The trace is a representative of five independent experiments.
Effect of microcystin-LR on insulin secretion
Incubation of RINm5F cells in the presence of 2 μM microcystin-LR showed a significant increase in basal insulin release. However, depolarization-induced (KCl 30 mM) insulin release remained unchanged in the presence of microcystin-LR (2 μM) (Figure 3). At a fixed incubation time of 30 min, the concentration-dependent increase in insulin release induced by microcystin-LR was significantly (P<0.05) reduced by 50 μM of the calcium-channel-blocker D600 (Figure 4).
Figure 3.
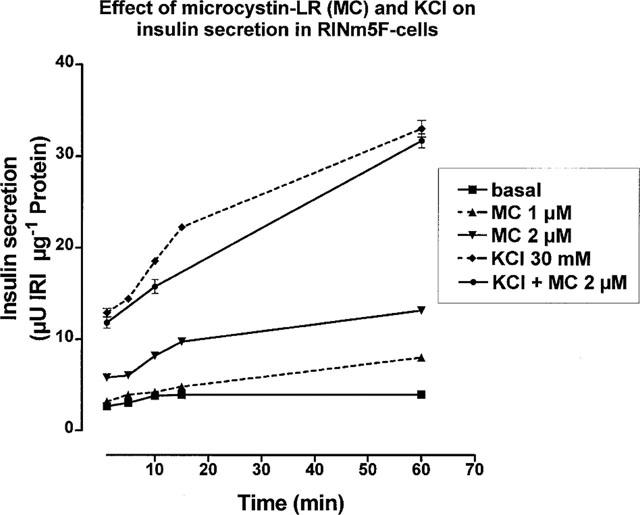
Time course of the effect of microcystin-LR on insulin release. Incubations were performed in microtiter plates and samples taken after 2, 8, 10, 15 and 60 min of incubation. Microcystin-LR-treated cells were preincubated for 10 min prior to stimulation. Additions as indicated in the figure. IRI (ordinate axis): immuno-reactive insulin. Mean±s.d., n=3–4. For values without given s.d. the size of the symbols in the figure exceeded the error bars.
Figure 4.
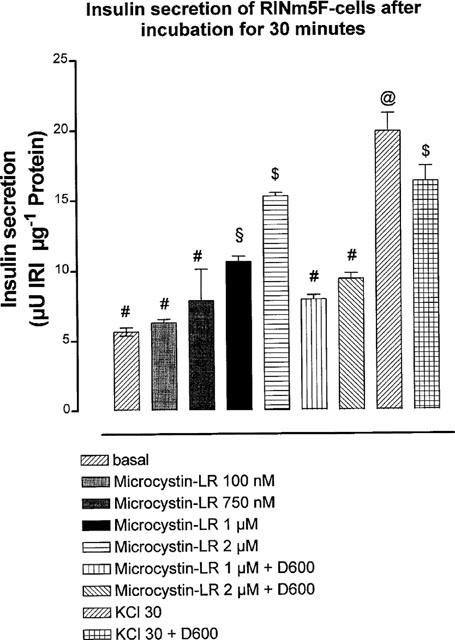
Effect of D600 on microcystin-LR-induced insulin secretion by RINm5F cells. After preincubation of 10 min with microcystin-LR (0.1–2 μM) the cells were incubated for 30 min with microcystin-LR both in the presence or absence of D600 (50 μM), as indicated in the figure. IRI (ordinate axis): immunoreactive insulin. Mean±s.e.mean, n=3–4. ANOVA, Newman-Keuls-test. Values marked with different symbols (#,$,&;@) are significantly different (P<0.05).
Discussion
Protein phosphatases (PP) have been classified by Shenolikar & Nairn (1991) into five major types (PP-1, PP-2A, PP-2B, PP-2C and PP-3), depending on their substrate specificity, sensitivity to phosphatase inhibitor proteins (inhibitor-1, inhibitor-2), and their dependence on divalent cations (Ca2+, Mg2+). Only PP-1 and PP-2A, but not the other phosphatases possess significant activity when phosphorylase a, a phosphorylated enzyme of glycogen metabolism, is used as a substrate (Ingebritsen & Cohen, 1983).
Microcystin-LR is able to inhibit phosphatases type 1 and 2A of insulin secreting cells almost completely, supporting its role as a blocker of these phosphatases (Cartus et al., 1998). This was reported from other tissues before (Sjöholm et al., 1993; Honkanen et al., 1994). However, in insulin secreting cells the effect of microcystin-LR could only be achieved in cell homogenates. Microcystin-LR was without effect on phosphatase 1/2A activity after incubation of intact cells, being in contrast to other inhibitors like okadaic acid (Ammon et al., 1996). An uptake of microcystin-LR into the insulin-secreting cells is therefore unlikely, this has been confirmed by findings in neuroblastoma cells (Laidley et al., 1997) but is in contrast to liver cells, where the presence of a carrier-transport-system for microcystin was reported (Dawson, 1998). In intact cells, the action of microcystin-LR led, however, to a concentration-dependent increase in [Ca2+]i. In contrast to the rapid depolarization induced by KCl, the microcystin-effect occurred slower and did not reach the maximum as with KCl. The treatment with microcystin-LR did not damage the cells since a normal depolarization-induced rise in [Ca2+]i was still present.
Insulin secretion is thought to be the consequence of a rise in the [Ca2+]i (Wollheim & Pozzan, 1984; Wollheim et al., 1996). As seen for [Ca2+]i, microcystin-LR induced insulin secretion in a time- and concentration-dependent manner and did not reach the maximal effect as obtained with KCl.
The fact, that both did not act synergistically supports the view that microcystin-LR may act by the same way as KCl and due to the already maximal stimulation could not further increase the secretion. However, it might also be true that the amount of insulin released by microcystin-LR-treatment is just too small to be detected beside the large amount released by KCl.
The underlying mechanism of the increase in [Ca2+]i and insulin release seems to be the opening of voltage-dependent Ca2+-channels since both effects could be inhibited by a blocker of the voltage-dependent Ca2+-channel, D600. For voltage-dependent Ca2+-channels, a dependence of their function on the dephosphorylation was shown earlier (Hescheler et al., 1987; Frace & Hartzell, 1993). In addition, phosphorylation-sites on the channel protein have been reported before (Bouron et al., 1995). In our hands, dephos-phorylation in membrane-fractions of insulin-secreting cells is sensitive to an inhibition by microcystin-LR (Table 1). We therefore conclude that a dephosphorylation system related to the calcium-channel function may be accessible from the extracellular surface. The activity of this extracellular accessible dephosphorylation system is sufficient to modulate calcium-channels and insulin release even though its contribution to the total activity of the membrane related phosphatase activity was indistinguishable.
The existence of an extracellular action site for phosphatase 1/2A inhibitors could also explain why at low concentrations okadaic acid, a membrane permeable inhibitor of phosphatase 1 and 2A, increases [Ca2+]i (Haby et al., 1994) and insulin release (Haby et al., 1994; Tamagawa et al., 1992) but at higher concentrations reduces [Ca2+]i (Ammon et al., 1996) and secretion (Tamagawa et al., 1992; Ammon et al., 1996). This may be due to an extracellular action of the low but an additional intracellular action of the higher concentrations. As shown previously (Honkanen et al., 1994; Lin & Chu, 1994), microcystin-LR posesses high specificity for phosphatases 1 or 2A. This provides further evidence that the extracellular action site of microcystin-LR to open voltage-dependent Ca2+-channels may be of a phosphatase type 1 or 2A. However, an additional drug-specific effect different from the inhibition of the serine/threonine phosphatases type 1 or 2A can't be ruled out.
Acknowledgments
This work was supported in part by a research grant from the Deutsche Diabetes Gesellschaft. Part of the work was presented as an abstract at the 32nd Annual Meeting of the European Association for the Study of Diabetes, Vienna, 1996.
Abbreviations
- ANOVA
analysis of variance
- ATP
adenosine trisphosphate
- BSA
bovine serum albumin
- EGTA
ethylene glycol-bis[β-aminoethylether]-N,N,N′,N′-tetraacetic acid
- IRI
immunoreactive insulin
- MC
microcystin-LR
- PP
protein phosphatase
- RINm5F
insulin secreting rat insulinoma cell line
- TRIS
tris(hydroxymethyl)-aminomethane
References
- AMMON H.P.T., HEURICH R.O., KOLB H.-A., LANG F., SCHAICH R., DREWS G., LEIERS T. Phosphatase inhibitor okadaic acid blocks KCl-depolarization-induced rise of cytosolic calcium of rat insulinoma cells (RINm5F) Naunyn-Schmiedeberg's Arch Pharmacol. 1996;354:95–101. doi: 10.1007/BF00178708. [DOI] [PubMed] [Google Scholar]
- ANTONIW J.F., COHEN P. Separation of two phosphorylase kinases from rabbit skeletal muscle. Eur. J. Biochem. 1976;68:45–54. doi: 10.1111/j.1432-1033.1976.tb10763.x. [DOI] [PubMed] [Google Scholar]
- ARKHAMMAR P., JUNTTI-BERGGREN L., LARSSON O., WELSH M., NANBERG E., SJÖHOLM A., KÖHLER M., BERGGREN P.O. Protein kinase C modulates the insulin secretory process by maintaining a proper function of the β-cell voltage-activated calcium channels. J. Biol. Chem. 1994;269:2743–2749. [PubMed] [Google Scholar]
- BOURON A., SOLDATOV N.M., REUTER H. The βi-subunit is essential for modulation by protein kinase C of an human and a non-human L-type Ca2+ channel. FEBS Lett. 1995;377:159–162. doi: 10.1016/0014-5793(95)01327-x. [DOI] [PubMed] [Google Scholar]
- CARTUS T., HEURICH R.O., DREWS G., AMMON H.P.T. Distribution of protein phosphatases type 1 and 2A in RINm5F cells. Regul. Pept. 1998;77:77–81. doi: 10.1016/s0167-0115(98)00043-3. [DOI] [PubMed] [Google Scholar]
- COHEN P., ALEMANY S., HEMMINGS B.A., RESINK T.J., STRÅLFORS P., TUNG H.Y.-L. Protein phosphatase-1 and protein phosphatase-2A from rabbit skeletal muscle. Meth. Enzymol. 1988;159:390–408. doi: 10.1016/0076-6879(88)59039-0. [DOI] [PubMed] [Google Scholar]
- DAWSON R.M. The toxicology of microcystins. Toxicon. 1998;36:953–962. doi: 10.1016/s0041-0101(97)00102-5. [DOI] [PubMed] [Google Scholar]
- FRACE A.M., HARTZELL H.C. Opposite effects of phosphatase inhibitors on L-type calcium and delayed rectifier currents in frog cardiac myocytes. J. Physiol. (Lond.) 1993;472:305–326. doi: 10.1113/jphysiol.1993.sp019948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAZDAR A.F., CHICK W.L., OIE H.K., SIMS H.L., KING D.L., WEIR G.C., LAURIS V. Continuous, clonal, insulin- and somatostatin-secreting cell lines established from a transplantable rat islet cell tumor. Proc. Natl. Acad. Sci. U.S.A. 1980;77:3519–3523. doi: 10.1073/pnas.77.6.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABY C., LARSSON O., ISLAM M.S., AUNIS D., BERGGREN P.O., ZWILLER J. Inhibition of serine/threonine protein phosphatases promotes opening of voltage-activated L-type Ca2+ channels in insulin-secreting cells. Biochem. J. 1994;298:341–346. doi: 10.1042/bj2980341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESCHELER J., KAMEYAMA M., TRAUTWEIN W., MIESKES G., SÖLING H.-D. Regulation of the cardiac calcium channel by protein phosphatases. Eur. J. Biochem. 1987;165:261–266. doi: 10.1111/j.1432-1033.1987.tb11436.x. [DOI] [PubMed] [Google Scholar]
- HONKANEN R.E., CODISPOTI B.A., TSE K., BOYNTON A.L. Characterization of natural toxins with inhibitory activity against serine/threonine protein phosphatases. Toxicon. 1994;32:339–350. doi: 10.1016/0041-0101(94)90086-8. [DOI] [PubMed] [Google Scholar]
- INGEBRITSEN T.S., COHEN P. The protein phosphatases involved in cellular regulation. 1. Classification and substrate specificities. Eur. J. Biochem. 1983;132:255–261. doi: 10.1111/j.1432-1033.1983.tb07357.x. [DOI] [PubMed] [Google Scholar]
- KAMEYAMA M., HESCHELER J., MIESKES G., TRAUTWEIN W. The protein-specific phosphatase 1 antagonizes the β-adrenergic increase of the cardiac Ca current. Pflügers Arch. 1986;407:461–463. doi: 10.1007/BF00652635. [DOI] [PubMed] [Google Scholar]
- LAIDLEY C.W., COHEN E., CASIDA J.E. Protein phosphatase in neuroblastoma cells: [3H]cantharidin binding site in relation to cytotoxicity. J. Pharmacol. Exp. Ther. 1997;280:1152–1158. [PubMed] [Google Scholar]
- LEVITAN I.B. Modulation of ion channels by protein phosphorylation and dephosphorylation. Ann. Rev. Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- LIN J.R., CHU F.S. In vitro neutralization of the inhibitory effect of microcystin-LR to protein phosphatase 2A by antibody against the toxin. Toxicon. 1994;32:605–613. doi: 10.1016/0041-0101(94)90208-9. [DOI] [PubMed] [Google Scholar]
- MATSUSHIMA R., YOSHIZAWA S., WATANABE M.F., HARADA K., FURUSAWA M., CARMICHAEL W.W., FUJIKI H. In vitro and in vivo effects of protein phosphatase inhibitors, microcystin-LR and nodularin, on mouse skin and fibroblasts. Biochem. Biophys. Res. Commun. 1990;171:867–874. doi: 10.1016/0006-291x(90)91226-i. [DOI] [PubMed] [Google Scholar]
- PRALONG W.-F., BARTLEY C., WOLLHEIM C.B. Single islet β-cell stimulation by nutrients: Relationship between pyridine nucleotides, cytosolic Ca2+ and secretion. EMBO J. 1990;9:53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALERS P., OUAFIK L.H., GIRAUD P., DUTOUR A., MALTESE J.Y., OLIVER C. Evidence for pyroglutamyl peptidase I and prolyl endopeptidase activities in the rat insulinoma cell line RINm5F: lack of relationship with the TRH metabolism. Mol. Cell. Biochem. 1991;106:15–24. doi: 10.1007/BF00231184. [DOI] [PubMed] [Google Scholar]
- SHENOLIKAR S., NAIRN A.C.Protein phosphatases: recent progress Advances in Second Messenger and Phosphoprotein Research 199123New York: Raven Press; 1–121.ed. Greengard, P. & Robinson, J.A. Vol [PubMed] [Google Scholar]
- SCHONTHAL A.H. Role of PP2A in intracellular signal transduction pathways. Front. Biosci. 1998;3:D1262–D1273. doi: 10.2741/A361. [DOI] [PubMed] [Google Scholar]
- SJÖHOLM A., HONKANEN R.E., BERGGREN P.O. Characterization of serine/threonine phosphatases in RINm5F insulinoma cells. Biosci. Rep. 1993;13:349–358. doi: 10.1007/BF01150479. [DOI] [PubMed] [Google Scholar]
- TAMAGAWA T., IGUCHI A., UEMURA K., MIURA H., NONOGAKI K., ISHIGURO T., SAKAMOTO N. Effects of the protein phosphatase inhibitors okadaic acid and calyculin A on insulin release from rat pancreatic islets. Endocrinol. Japon. 1992;39:325–339. doi: 10.1507/endocrj1954.39.325. [DOI] [PubMed] [Google Scholar]
- TRIER T.K., KÜHN M., AMMON H.P.T. Adenylate cyclase in membrane fractions of RIN-A2-cells: Studies with forskolin, NaF, GppNHp and NEM. Cell. Biochem. Funct. 1988;6:131–135. doi: 10.1002/cbf.290060208. [DOI] [PubMed] [Google Scholar]
- WOLLHEIM C.B., POZZAN T. Correlation between cytosolic free Ca2+ and insulin release in an insulin-secreting cell line. J. Biol. Chem. 1984;259:2262–2267. [PubMed] [Google Scholar]
- WOLLHEIM C.B., LANG J., REGAZZI R. The exocytotic process of insulin secretion and its regulation by Ca2+ and G-proteins. Diabetes Rev. 1996;4:276–297. [Google Scholar]


