Abstract
Anti-inflammatory non steroidal drugs releasing NO (NO-NSAIDs) are a new class of anti-inflammatory drugs to which has been added an NO-releasing moiety. These compounds have been shown to retain the anti-inflammatory, analgesic and antipyretic activity of the parent compound but to be devoid of gastrointestinal (GI) toxicity.
Freund's adjuvant (FA) arthritis was induced in rats by a single intraplantar injection into the right hindpaw of 100 μl of mycobacterium butirricum (6 mg ml−1). The effect of equimolar doses of naproxen (1, 3 and 10 mg kg−1) and NO-naproxen (1.5, 4.5 and 16 mg kg−1) was evaluated using two dosage regimen protocols: (i) preventive, starting oral administration of the drugs at the time of induction of arthritis and for the following 21 days (day 1–21); (ii) therapeutic, starting oral administration of the drugs 7 days after adjuvant injection and for the following 14 days (day 7–21).
Hindpaw swelling (days 3, 7, 11, 14, 17, 21) and nociception (days 15 and 21) were measured. On day 22 rats were sacrificed, draining lymph nodes were removed and T cells isolated. In vitro proliferation of T cells following stimulation with concanavalin A (0.5–5 μg ml−1) was measured using a tritiated thymidine incorporation assay. IL-2 receptor expression on T cells was measured by FACS analysis.
Naproxen and NO-naproxen showed similar activity in reducing oedema formation in the non-injected (controlateral) hindpaw. Both drugs showed anti-nociceptive effect. NO-naproxen was anti-nociceptive at a dose of 4.5 mg kg−1 while naproxen showed the same extent of inhibition only at a dose of 10 mg kg−1.
T cells were isolated and characterized by FACS analysis. Stimulation of isolated T cells with concanavallin A in vitro caused a significant increase in thymidine uptake. NO-naproxen at a dose of 4.5 mg kg−1 inhibited T cell proliferation to the same extent as 10 mg kg−1 of naproxen.
Inhibition of T cell proliferation was well correlated with reduced IL-2 receptor expression on T cells. In addition, NO-naproxen reduced both IL-1β and TNFα plasma levels whilst naproxen reduced IL-1β levels only.
In conclusion, both naproxen and NO-naproxen reduce inflammation and nociception associated with arthritis. In addition NO-naproxen interferes to a larger extent with cellular mechanism involved in T cell activation in rat adjuvant arthritis indicating that introduction of the NO moiety in the naproxen structure increases the effect at the level of the immune system.
Keywords: Freund's adjuvant arthritis, rat, naproxen, draining lymph nodes, proliferation, IL-2, pain
Introduction
Rats injected into the hindpaw with a mixture of Mycobacterium butirricum emulsified in light mineral oil develop a severe polyarthritis which shares some features in common with human rheumatoid arthritis (RA), such as swelling of the extremities, cartilage degradation, loss of joint function and lymphocyte infiltration into diseased joints. This model was originally described more than 30 years ago (Pearson et al., 1961; Pearson, 1963) and is still the most widely used assay to identify chemical agents having a potential therapeutic efficacy in RA. Furthermore, this model allows evaluation of analgesic activity of antirheumatic drugs in pathologically induced pain. Indeed, it has been shown that between days 14 and 21 following arthritis induction, a clear hyperalgesic response is present both in the injected and in the controlateral (non injected) hindpaw. This hyperalgesic response has been shown to be reduced by non-steroidal anti-inflammatory drugs (NSAIDs).
NSAIDs are the mainstay in the treatment of pain and inflammation. Patients with diseases such as rheumatoid arthritis are prescribed large amount of NSAIDs. However, one of the major contraindications to NSAID therapy are the GI side effects, which can cause ulceration as well as GI bleeding. Recently, we have shown that a nitrosylated derivative of naproxen, NO-naproxen, retains the therapeutic effects of the native compound, without causing GI damage (Davies et al., 1997). The effect of NO-naproxen, as well as of other members of this family of NO-releasing compounds, has been attributed to COX inhibition and NO released (Wallace & Cirino, 1994).
Nitric oxide is produced constitutively by endothelial nitric oxide synthase or by neuronal nitric oxide synthase. Moreover, a large amount of nitric oxide is produced by inducible nitric oxide synthase upon stimulation with bacterial products and cytokines, including IFN-γ, TNFα and IL-1 (Moncada & Higgs, 1993; Feldman et al., 1996). As such, NO has emerged as an important endogenous mediator of inflammation and immune response (McCartney-Francis et al., 1993; Stefanovic-Racic et al., 1994a). NO is produced by rodent synoviocytes and macrophages, by rodent and human neutrophils, chondrocytes and mast cells and by human synovial tissues obtained from RA and ostheoarthritic patients (Charles et al., 1993; Barnes & Liew, 1994; Stefanovic-Racic et al., 1994b; McInnes et al., 1996). Up until now, there has been no evidence of the beneficial effect of NO-NSAIDs on immunologic inflammation; however, a member of this class of compounds, NO-flurbiprofen, has been shown to interfere with iNOS induction (Cirino et al., 1996).
In order to evaluate whether NO-NSAIDs could affect also immunocompetent cells involved in inflammation, in the present study we evaluated the effect of NO-naproxen and naproxen on an experimental model of immunologic inflammation.
Methods
Induction of Freund's adjuvant arthritis
Male, Lewis rats (120–140 g) were housed in propylene cages with food and water ad libitum. The light cycle was automatically controlled and the room temperature thermostatically regulated to 21±1°C. Prior to the start of the experiment, animals were housed under these conditions for 6–8 days. Arthritis was induced by injecting into the right hindpaw 100 μl of Mycobacterium butirricum (6 mg ml−1) suspended in complete adjuvant. Hindpaw volume was measured by the mean of an hydroplethysmometer (Ugo Basile, Italy) immediately before arthritis induction (basal value) and 3, 7, 11, 14, 17, 21 days thereafter, for both the injected and controlateral hindpaw. Hindpaw swelling is expressed as increase in hindpaw volume in ml calculated by subtracting the basal value from the hindpaw volume measured at all times considered. Either naproxen (1, 3 and 10 mg kg−1) or equimolar doses of NO-naproxen (1.5, 4.5 and 16 mg kg−1) were dissolved in dimethylsulfoxide (DMSO) and suspended in carboxymethylcellulose (CMC) 0.2% w v−1. Drugs were given daily by oral gavage to rats following two regimen of dosing: (i) preventive, from day 1 to 21 and (ii) therapeutic, from day 7 to 21. Control groups received the same volume of DMSO plus CMC 0.2% w v−1 by oral gavage. Higher doses of naproxen could not be tested due to the elevated toxicity (we were not allowed by the veterinarian to study the effect of 30 mg kg−1 of naproxen).
Randall Selitto assay
Randall Selitto assay was performed on both hindpaws at day 15 and 21 (Randall & Selitto, 1957). Briefly, pressure was applied through a tip to the plantar surface of the hindpaw at a constant rate using an analgesiometer (Ugo Basile, Comerio, Italy) to the point at which the animal struggled, squealed or attempted to bite. The test was run by an observer unaware of the treatment. The force (expressed in grams) at which the animal began to struggle was assumed to represent the nociceptive threshold and served as the end point.
T cell proliferation assay
Draining lymph nodes (DLN) were removed from controlateral or injected hindpaws of Freund's adjuvant (FA)-treated rats at the end of each experiment at day 22 as previously described (Ianaro et al., 1994). A cell suspension was obtained, counted and resuspended at 2.5× 106 cells ml−1 and dispensed at 100 μl well−1 in 96-well flat-bottomed plates. Concanavallin A (0.5 or 5 μg ml−1) was added to each well and the cultures incubated at 37°C in an atmosphere of 5% CO2, for 24, 48 and 72 h. Cultures in triplicates were pulsed with 1 μCi well−1 [3H]-thymidine for the final 6 h of incubation then harvested and counted in a β scintillation counter (Beckman).
Cell FACS analysis
T cells obtained as described above, were suspended in RPMI medium containing 5% v v−1 foetal calf serum (FCS) and 1% w v−1 glutamine and incubated for 30 min at 4°C with primary antibody (1 : 50 dilution). Each cell suspension was tested with the following panel of mouse anti-rat monoclonal antibodies: anti-CD4, anti-CD8 and anti-CD25 [IL-2 receptor (IL-2R)]. Cells were then incubated with the FITC-conjugated second antibody (1 : 100 dilution) for 30 min at 4°C, washed twice and resuspended in PBS/formaldehyde (0.5% v v−1). Control samples were stained with the FITC-conjugated second antibody (1 : 100 dilution). Stained cells were analysed on FACScan cytoflurimeter (Becton Dickinson, San Jose, CA, U.S.A.). Cells were gated using forward versus side scatter to exclude dead cells and debris.
Cytokine, IL-1β and TNFα plasma levels
Cytokine levels were assayed in plasma samples using a rat IL-1β ELISA kit (Endogen, U.S.A.) and TNFα ELISA kit (Genzyme, Cambridge, MA, U.S.A.) following the manufacturer's instructions. TNFα detection limit is 25 pg ml −1, inter/intra assay variation is <10%. Cross reactivity with TNFα is 100%, TNFβ <0.01%; TNFrI<0.01%; TNFrII <0.01%. IL-1β detection limit is 30 pg ml−1; inter/intra assay variation is <10%. Cross reactivity with IL-1β 100%. IL-1α <0.01% and IL-2 <0.01%.
Statistical analysis
Data are expressed as mean±s.e.mean. (n=6–10) and statistical significance was evaluated by using ANOVA followed by Dunnett's test. For the statistical determination a statistical computerised package, INSTAT (Graphpad Software, U.S.A.) was used. A value of P<0.05 was considered significant.
Drugs
Mycobacterium butirricum, Freund's adjuvant (DIFCO), concanavalin A (ConA) were obtained from Sigma (Milan, Italy). Rabbit anti-rat CD4, CD8 and CD25 (IL-2R) monoclonal antibodies were from Pharmingen (San Diego, CA, U.S.A.). Fluorescein isothiocyanate (FITC)-conjugated anti-rabbit monoclonal antibody was purchased from Sigma (Milan, Italy). Culture medium was RPMI containing 10% v v−1 foetal calf serum, L-glutamine (2 mM), penicillin (100 u ml−1), streptomycin (100 μg ml−1), 2-mercaptoethanol (50 μM) were purchased from Sigma (Milan, Italy). [3H]-thymidine was purchased from Amersham (Milan, Italy). Naproxen was obtained from Sigma (Milan, Italy) NO-naproxen was a generous gift of NicOx SA Sophia Antipolis (France).
Results
Adjuvant arthritis
Swelling in the controlateral hindpaw was significantly reduced by naproxen 10 mg kg−1 and by NO-naproxen 16 mg kg−1 using both regimens of dosing (day 1–21 and 7–21). Controlateral hindpaw volume at day 14 following the dosing regimen day 1–21 was: 0.62±0.05 ml for control, 0.43±0.04 ml for naproxen 10 mg kg−1 (n=10; P<0.05) and 0.39±0.04 ml for NO-naproxen 16 mg kg−1 (n=10; P<0.05). NO-naproxen (1.5 mg kg−1) and naproxen (1 mg kg−1) did not inhibit oedema formation and paw volume at day 14 i.e. 0.54±0.09 ml (n=6; P>0.05) and 0.60±0.08 ml (n=6; P>0.05) for naproxen and NO-naproxen respectively. Similarly, at day 14 following the regimen dosing day 7–21, the hindpaw volume of the controlateral hindpaw in control rats was 0.65±0.04 ml while that of naproxen and NO-naproxen treated animals was 0.43±0.08 ml (n=10; P<0.05) and 0.35±0.04 ml−1 (n=10; P<0.05), respectively. The doses of NO-naproxen 1.5 mg kg−1 and naproxen 1 mg kg−1 were ineffective and the hindpaw volumes achieved were 0.58±0.05 ml (n=6; P>0.05) and 0.60±0.07 ml (n=6; P>0.05), respectively.
Randall Selitto pain assay
As shown in Figure 1a, treatment with naproxen and NO-naproxen from day 1–21 caused an increase in the threshold of nociception of arthritic rats in the injected hindpaw compared to control group. The NO-naproxen effect was present both at day 15 and 21, while naproxen resulted active only at day 15 and at the highest dose tested (10 mg kg−1). The effect in controlateral hindpaw is summarized in Figure 1b.
Figure 1.
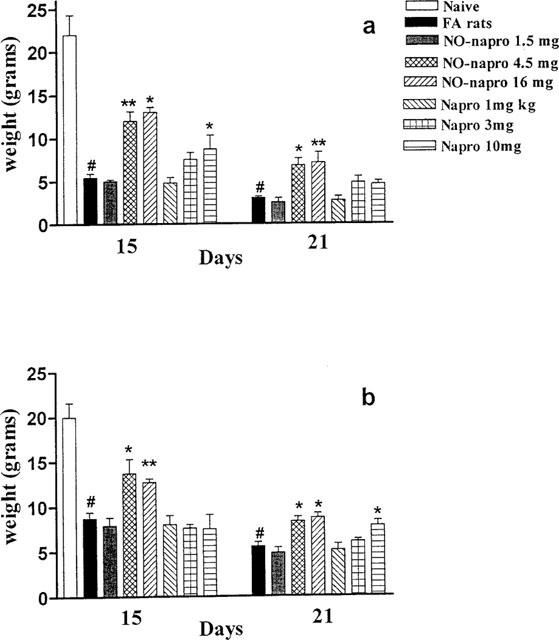
(a) Effect of naproxen and NO-naproxen on nociception following oral treatment from day 1–21. The Randall Selitto assay was performed on day 15 and 21 after induction of arthritis on the injected hindpaw. (b) The effect of naproxen and NO-naproxen on nociception following oral treatment from day 1–21. The Randall Selitto assay was performed on day 15 and 21 after induction of arthritis on the controlateral hindpaw (not injected). *P<0.05; **P<0.01 significantly different versus FA rats. #P<0.001 significantly different versus naïve animals.
Figure 2a shows the analgesic effect in the injected hindpaw following treatment from day 7–21 with both naproxen and NO-naproxen. NO-naproxen caused analgesia at the doses of 4.5 and 16 mg kg−1 whilst naproxen was active only at the highest dose used (10 mg kg−1). In the controlateral hindpaw, following treatment from day 7–21, the analgesic effect was not significant with both NO-naproxen and naproxen (Figure 2b). Naproxen and NO-naproxen at doses of 1 mg kg−1 and 1.5 mg kg−1 respectively were ineffective in both protocols.
Figure 2.
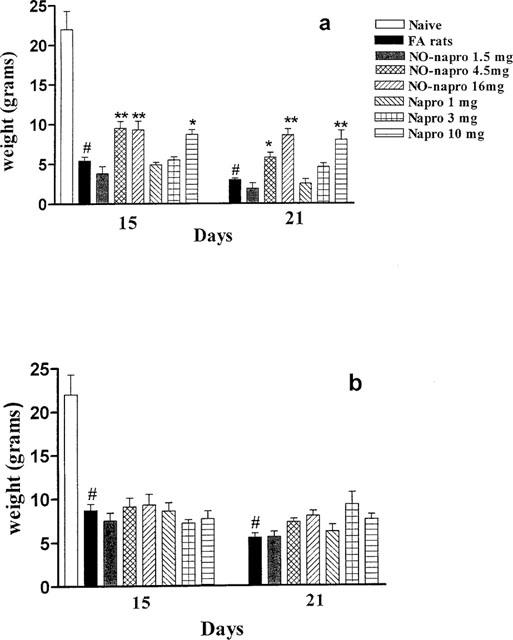
(a) Effect of naproxen and NO-naproxen on nociception in the injected hindpaw following oral treatment from day 7–21. The Randall & Selitto assay was performed on day 15 and 21 after induction of arthritis. (b) The lack of effect of naproxen and NO-naproxen on nociception perception following oral treatment from day 7–21 in the controlateral hindpaw (not injected). The Randall & Selitto assay was performed on day 15 and 21 after induction of arthritis. *P<0.05; **P<0.01 significantly different versus FA rats. #P<0.001 significantly different versus naïve.
TNFα and IL-1β plasma levels
Increased plasma levels of IL-1β and TNFα observed in arthritic rats at day 21 were significantly reduced following treatment with NO-naproxen (16 mg kg−1) only at the regimen dosing 1–21 (Figure 3a). Conversely, following treatment with naproxen (10 mg kg−1), at the same dosing regimen, there was a significant reduction in IL-1β plasma levels only (Figure 3b).
Figure 3.
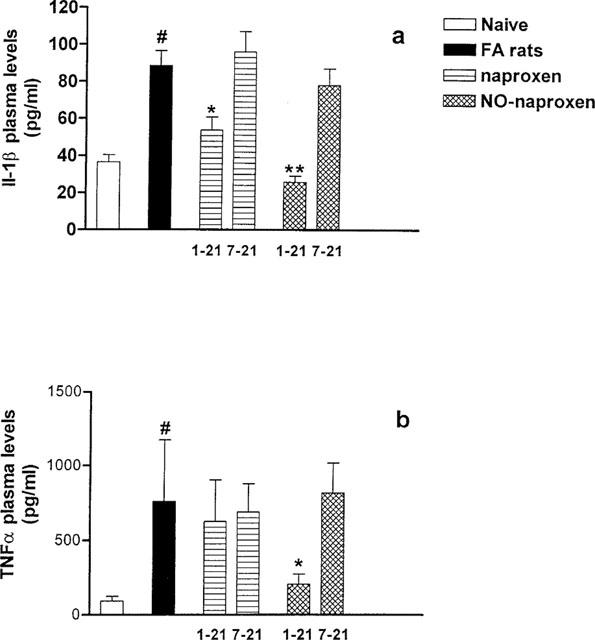
(a) Effect of naproxen (10 mg kg−1) and NO-naproxen (16 mg kg−1) following oral treatment either from day 1–21 or from day 7–21 on IL-1β plasma levels. (b) Effect of naproxen (10 mg kg−1) and NO-naproxen (16 mg kg−1) following oral treatment either from day 1–21 or from day 7–21 on TNFα plasma levels. *P<0.05; **P<0.01 significantly different versus FA rats. #P<0.001 significantly different versus naïve animals.
FACS analysis
FACS analysis of cells isolated from peripheral DLN showed that the cells isolated were T lymphocytes (99.2%). T cells were composed of 58.3±4% (n=6) CD4+ and 42.3±4.6% (n=6) CD8+. At the point of evaluation taken in this study, e.g. at day 21, there was no difference in CD4+ and CD8+ ratio present in naïve vs arthritic rats. The values for the CD4+ and CD8+ in T cells isolated from FA rats were 60.5±4.4% (n=6) and 39.5±3.4% (n=6; P>0.05) respectively.
Thymidine uptake assay
T cells were incubated for 24, 48 and 72 h with ConA (0.5–5 μg ml−1). The dose response preliminary study showed that maximal labelled thymidine accumulation was achieved for DLN T cells isolated from lymph nodes of the injected hindpaw at the 48 h point and at 72 h for DLN T cells isolated from lymph nodes of the controlateral hindpaw using a dose of ConA of 5 μg ml−1. Thus ConA at a dose of 5 μg ml−1 and the two time points were selected for the study. As shown in Figure 4a naproxen, following treatment from day 1–21, caused a dose dependent decrease in labelled thymidine uptake following in vitro stimulation with ConA of DLN T cells isolated from the controlateral hindpaw. Conversely NO-naproxen (4.5 mg kg−1) inhibited T cell proliferation to the same extent as naproxen (10 mg kg−1) (Figure 4a). Following treatment from day 7–21 (Figure 4b) DLN T cells isolated from the controlateral hindpaw of rats treated with naproxen showed an inhibition only at the highest dose tested (10 mg kg−1). Similarly NO-naproxen significantly reduced DLN T cells proliferation only at 16 mg kg−1.
Figure 4.
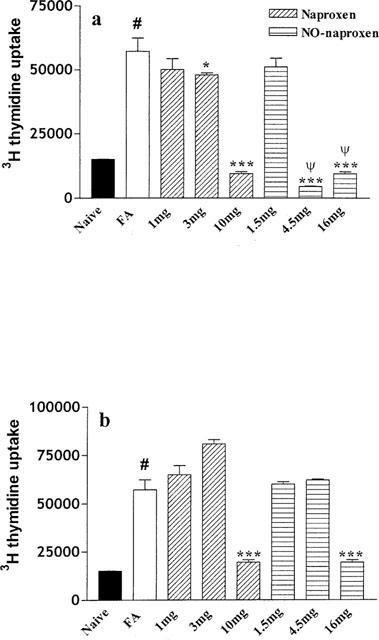
(a) Effect of naproxen (1, 3 and 10 mg kg−1) and NO-naproxen (1.5, 4.5 and 16 mg kg−1) following treatment from day 1–21 on in vitro proliferation of T cells isolated from controlateral hindpaw DLN. *P<0.05; ***P<0.001, significantly different versus FA rats. #P<0.001 significantly different versus naïve. ψP<0.001 significantly different versus naproxen 3 and 10 mg kg−1. (b) Effect of naproxen (1, 3 and 10 mg kg−1) and NO-naproxen (1.5, 4.5 and 16 mg kg−1) following treatment from day 7–21 on in vitro proliferation of T cells isolated from controlateral hindpaw. ***P<0.001 significantly different versus FA rats. #P<0.001 significantly different versus naïve animals.
A similar study was conducted on DLN T cells isolated from the injected hindpaw as shown in Figure 5. Naproxen as well as NO-naproxen following treatment from day 1–21 (Figure 5a) significantly reduced thymidine uptake. However, NO-naproxen caused a more marked inhibition of T cell proliferation when compared to naproxen. Following treatment from day 7–21 (Figure 5b), naproxen caused thymidine uptake inhibition only at the highest dose (10 mg kg−1) while NO-naproxen was active at both 4.5 and 16 mg kg−1.
Figure 5.
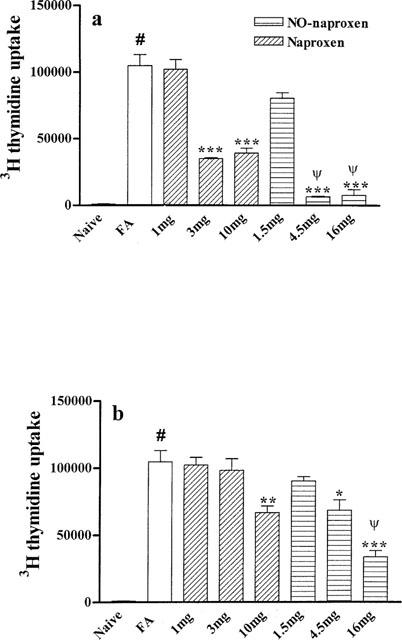
(a) Effect of naproxen (1, 3 and 10 mg kg−1) and NO-naproxen (1.5, 4.5 and 16 mg kg−1) following treatment from day 1–21 on in vitro proliferation of T cells isolated from injected hindpaw DLN. ***P<0.001 significantly different versus FA rats. #P<0.001 significantly different versus naïve animals. ψP<0.01 NO-naproxen 4.5 and 16 mg kg−1 significantly different vs naproxen 3 and 10 mg kg−1, respectively. (b) Effect of naproxen (3 and 10 mg kg−1) and NO-naproxen (4.5 and 16 mg kg−1) following treatment from day 7–21 on in vitro proliferation of T cells isolated from injected hindpaw DLN. *P<0.05, **P<0.01, ***P<0.001 significantly different versus FA rats. #P<0.001 significantly different versus naïve animals. ψP<0.01 significantly different versus naproxen 10 mg kg−1.
IL-2 receptor (CD25 evaluation)
The IL-2 receptor evaluation was performed on day 21 e.g. on DLN T cells isolated at the end of both treatments (day 1–21 and 7–21) with both naproxen (10 mg kg−1) and NO-naproxen (16 mg kg−1). As shown in Figure 6a, following FA administration there was a clear increase in the per cent of IL-2R positive cells, significantly prevented by treatment with both naproxen and NO-naproxen given at dosage regimen 1–21. However, when the data were expressed as mean intensity fluorescence (MIF %), that can be interpreted as a measure of the receptor number expressed on the cell, both the therapeutic (day 7–21) and the preventive treatment (day 1–21) with both NO-naproxen and naproxen significantly reduced receptor expression on cell surface. (Figure 6b). Surprisingly the treatment with NO-naproxen (day 1–21) reduced receptor expression levels significantly below the control value (Figure 6b).
Figure 6.
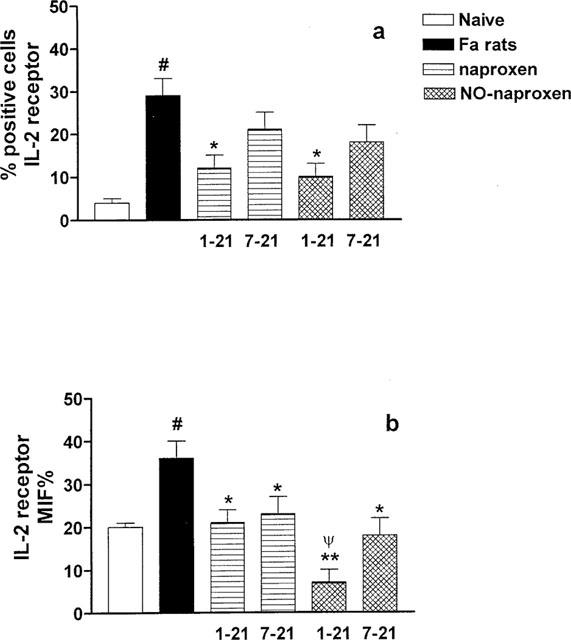
(a) Effect of naproxen (10 mg kg−1) and NO-naproxen (16 mg kg−1) on CD25 expression on T cell surface. *P<0.01 significantly different versus FA rats; #P<0.01 significantly different versus naïve. (b) Effect of naproxen (10 mg kg−1) and NO-naproxen (16 mg kg−1) on CD25 expressed as MIF %. *P<0.05 **P<0.01 significantly different versus FA rats. #P<0.01 significantly different versus naïve. ψP<0.01 significantly different versus naproxen 1–21.
Discussion
Nonsteroidal antiinflammatory drugs are the most widely used drugs in the treatment of pain and inflammation associated with several immunological and non-immunological diseases. However, their use is strongly limited by adverse GI effects. Recently, NO-NSAIDs, a novel class of anti-inflammatory drugs, presenting a nitric oxide releasing moiety, have been shown to retain the classic therapeutic profile of native compounds, such as the ability to inhibit inflammatory response, nociception and fever, but to spare the GI tract (Wallace & Cirino, 1994; Davies et al., 1997; Wallace et al., 1997). This particular profile has been attributed to the ability of these compounds to release discrete amounts of nitric oxide.
Inducible nitric oxide synthase (iNOS) has been proposed to be at the interface between the innate and the adaptive immune system; there is evidence that NO produced by activated macrophages can restrict T cell expansion (Gregory et al., 1993, Wei et al., 1995). NSAIDs have been shown to reduce spleen lymphocyte proliferation and, in particular, indomethacin has been shown to inhibit T cell proliferation (Waltz et al., 1971; Bayer & Beavan, 1979; Seng et al., 1990).
The observation that T cells infiltrate the rheumatoid synovium was originally made by van Boxel & Paget in 1975 and since then T cell activation has been widely studied and recognized as a key feature of RA (Firstein & Zvaifler 1990; Panayi et al., 1992; Van den Berg, 1998). Nearly 45% of T cells, following a brief transit of about 30 min in the bloodstream, are carried from the blood directly to the spleen where they reside for approximately 5 h. However, an almost equal number of lymphocytes (42%) leave the spleen to go to the lymph nodes where they reside for about 12 h. On the basis of the above observation, we have studied T cell activation using T cells isolated from peripheral draining lymph nodes of arthritic rats. Rat adjuvant arthritis is believed to be the result of a sequence of immunopathologic events involving sensitization to antigen, proliferation of immunocompetent cells, cellular hypersensitivity and mediator release. This cascade of events allows a distinction between a primary and a secondary inflammatory response. To characterize the pharmacological profile of these drugs we have used two regimen of dosing, one prophylactic (day 1–21) and a second therapeutic (day 7–21), by starting the treatment the same day of FA induction (day 1) or 7 days thereafter, respectively. Under our experimental conditions, both NO-naproxen and naproxen not only reduced the secondary inflammation but also modulated T cell proliferative response. T cells isolated from FA rats, once challenged with ConA in vitro, showed a significant increase in proliferation as measured by the labelled thymidine incorporation assay. Interestingly, while thymidine incorporation by T cells isolated from FA rats injected hindpaw was over 100,000 c.p.m., for the controlateral hindpaw it was around 60,000 c.p.m., already underscoring a difference in activation between T cells derived from lymph nodes interested either in the primary (injected hindpaw) or secondary lesion (controlateral hindpaw). In T cells, isolated from either the injected hindpaw or controlateral hindpaw, about 90% of inhibition of proliferation induced by ConA was achieved with a dose of NO-naproxen of 4.5 mg kg−1, whilst naproxen showed comparable activity only at the highest dose tested (10 mg kg−1). These data imply that both NO-naproxen and naproxen modulate the secondary lesion T cell-dependent response in a therapeutic fashion, and suggest that the effect of both drugs is dependent upon a direct action on immunocompetent cells and not a consequence of inhibition of the primary inflammatory response.
Naïve T cells, upon activation, have to re-enter the cell cycle and rapidly divide. Their proliferation and differentiation is driven by interleukin-2. To further characterize the mechanism of action of drugs studied, we have evaluated whether the reduction in T cell proliferation was paralleled by a down regulation of interleukin-2 receptor expression on cell surface. Our results clearly show that in arthritic rats, the increased T cell activation was paralleled by increased IL-2R expression on the cell surface as compared to naïve rats. Following treatment with either naproxen or NO-naproxen, IL-2R expression on the T cell surface was significantly reduced. Interestingly, when data were expressed as mean intensity fluorescence percentage (MIF %), which can be interpreted as a measure of the number of receptors expressed on the cells, we found that both treatment regimens (day 1–21 and 7–21) with either naproxen or NO-naproxen reduced the number of IL-2 receptors expressed. In addition, treatment with NO-naproxen from day 1–21 caused a reduction of MIF % (e.g. receptor number) to IL-2R levels present in T cells of naïve animals.
Production of IL-1β and TNFα is known to be a key feature in rheumatoid arthritis. In particular specific antibodies to TNFα or its receptors have been shown to be effective in patients with active RA (Kavanaugh, 1998; Moreland, 1998). We found that NO-naproxen at a dose of 16 mg kg−1 (regimen dosing 1–21) reduced significantly both TNFα and IL-1β plasma levels in FA rats whilst naproxen (10 mg kg−1) significantly reduced IL-1β but not TNFα levels.
It has been shown that macrophages regulate T cell function through NO production (Wei et al., 1995; Klimiuk et al., 1999). Thus, it is likely that the increased effect on T cells of NO-naproxen could be due to the introduction of the NO moiety. In addition introduction of the NO-moiety did not modify the pharmacological profile of the compound on classical parameters such as inflammation and pain. Indeed, both naproxen and NO-naproxen, tested at equimolar doses, have the same ability to inhibit oedema formation. Concerning nociception, both NO-naproxen and naproxen following treatment from day 1–21 reduced nociception in both injected (primary lesion) and controlateral hindpaw (secondary lesion). Interestingly, NO-naproxen at the dose of 4.5 mg kg−1 showed an analgesic effect comparable to naproxen 10 mg kg−1.
These data cannot be due to a different pharmacokinetic profile since it has been shown that following oral administration of NO-naproxen, plasma levels of parent compound, naproxen, are lower than those achieved after oral administration of an equimolar dose of naproxen (Davies et al., 1997).
In conclusion our data demonstrate that naproxen and its nitrosylated derivative, NO-naproxen, reduce nociception and inflammation associated to arthritis. Both compounds interfere with T cell activation, a key event for the spreading of this disease. In addition NO-naproxen, has a comparable anti-inflammatory effect but interferes to a larger extent with cellular mechanism involved in T cell activation in rat adjuvant arthritis.
Abbreviations
- RA
rheumatoid arthritis
- NSAID
non-steroidal anti-inflammatory drug
- DMSO
dimethylsulfoxide
- CMC
carboxymethylcellulose
- DLN
draining lymph nodes
- FA
Freund's adjuvant
- NO-naproxen
nitric oxide releasing naproxin
- TNFα
tumour necrosis factor α
- IL-1β
interleukin 1β
- ConA
concanavalin A
- MIF
mean intensity fluorescence
References
- BARNES P.J., LIEW F.Y. Nitric oxide and asthmatic inflammation. Immunol. Today. 1994;16:128–130. doi: 10.1016/0167-5699(95)80128-6. [DOI] [PubMed] [Google Scholar]
- BAYER B.M., BEAVEN M.B. Evidence that indomethacin reversibly inhibits cell growth in the G1 phase of the cell cycle. Biochem. Pharmacol. 1979;28:441–443. doi: 10.1016/0006-2952(79)90112-6. [DOI] [PubMed] [Google Scholar]
- CHARLES I.G., PALMER R.J., HICKERY M.S., BAYLISS M.T., CHUBB A.P., HALL V.S., MOSS D.W., MONCADA S. Cloning, characterization and expression of a cDNA encoding an inducible nitric oxide synthase from human chondrocytes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11419–11423. doi: 10.1073/pnas.90.23.11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRINO G., WHEELER-JONES C.P.D., WALLACE J.L., DEL SOLDATO P., BAYDOUN A.R. Inhibition of inducible nitric oxide synthase expression by novel nonsteroidal anti-inflammatory derivatives with gastrointestinal-sparing properties. Br. J. Pharmacol. 1996;117:1421–1426. doi: 10.1111/j.1476-5381.1996.tb15301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES N.M., ROSETH A.G., APPLEYARD C.B., MCKNIGHT W., DEL SOLDATO P., CALIGNANO A., CIRINO G., WALLACE J.L. NO-naproxen vs naproxen: ulcerogenic, analgesic and anti-inflammatory effects. Aliment. Pharm. Ther. 1997;11:69–79. doi: 10.1046/j.1365-2036.1997.115286000.x. [DOI] [PubMed] [Google Scholar]
- FELDMANN M., BRENNAN F.M., MAINI R.N. Role of cytokines in rheumatoid arthritis. Ann. Rev. Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- FIRSTEIN G.S., ZVAIFLER N.J. How important are T cells in chronic rheumatoid synovitis. Arthritis Rheum. 1990;33:768–773. doi: 10.1002/art.1780330602. [DOI] [PubMed] [Google Scholar]
- GREGORY S.H., WING E.J., HOFFMAN R.A., SIMONS R.L. Reactive nitrogen intermediates suppress the primary immunologic response to listeria. J. Immunol. 1993;150:2901–2909. [PubMed] [Google Scholar]
- IANARO A., O'DONNEL C.A., DI ROSA M., LIEW F.Y. A nitric oxide inhibitor reduces inflammation, down-regulates inflammatory cytokines and enhances IL-10 production in carrageenin-induced oedema in mice. Immunol. 1994;82:370–375. [PMC free article] [PubMed] [Google Scholar]
- KAVANAUGH A.F. Anti-tumor necrosis factor-alpha monoclonal antibody therapy for rheumatoid arthritis. Rheum Dis. Clin. North. Am. 1998;24:593–614. doi: 10.1016/s0889-857x(05)70028-4. [DOI] [PubMed] [Google Scholar]
- KLIMIUK P.A., YANG H., GORONZY J.J., WEYAND C.M. Production of cytokines and metalloproteinase in rheumatoid synovitis is T cell dependent. Clin. Immunol. 1999;90:65–78. doi: 10.1006/clim.1998.4618. [DOI] [PubMed] [Google Scholar]
- MCCARTNEY-FRANCIS N., ALLEN J.B., MIZEL D.E., ALBINA Q., XIE Q.-W. Suppression of arthritis by an inhibitor of nitric oxide synthase. J. Exp. Med. 1993;178:749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCINNES I.B., LEUNG B.P., FIELD M., WEI X.Q., HUANG F.-P., STURROCK R.D., KINNINMONTH A., WEIDNER J., MUMFORD R., LIEW F.Y. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J. Exp. Med. 1996;184:1519–1524. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., HIGGS A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993;29:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- MORELAND L.W. Soluble tumour necrosis factor receptor (p75) fusion protein (ENBREL) as a therapy for rheumatoid arthritis. Rheum. Dis. Clin. North. Am. 1998;24:579–691. doi: 10.1016/s0889-857x(05)70027-2. [DOI] [PubMed] [Google Scholar]
- PANAYI G.S., LANCHBURY J.S., KINGSLEY G.H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthr. Rheum. 1992;35:729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- PEARSON C.M. Experimental joint disease. Observations on adjuvant-induced arthritis. J. Chron. Dis. 1963;16:863–874. doi: 10.1016/0021-9681(63)90136-x. [DOI] [PubMed] [Google Scholar]
- PEARSON C.M., WAKSMAN B.H., SHARP J.T. Studies on arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. J. Exp. Med. 1961;113:485–509. doi: 10.1084/jem.113.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL L.O., SELITTO J.A. Method for measurement analgesic activity on inflamed tissue. Arch. Int. Pharmacodyn. 1957;111:233–249. [PubMed] [Google Scholar]
- SENG G.F., BENENSOHN J., BAYER B.M. Changes in T and B lymphocyte proliferative responses in adjuvant-arthritic rats: antagonism by indomethacin. Eur. J. Pharmacol. 1990;178:267–273. doi: 10.1016/0014-2999(90)90105-f. [DOI] [PubMed] [Google Scholar]
- STEFANOVIC-RACIC M., MEYERS K., MESCHTER C., COFFEY J.W., HOFFMAN R.A., EVANS C.H. N-monomethyl arginine, an inhibitor of nitric oxide synthase, suppresses the development of adjuvant arthritis in rats. Arthr. Rheum. 1994a;37:1062–1069. doi: 10.1002/art.1780370712. [DOI] [PubMed] [Google Scholar]
- STEFANOVIC-RACIC M., STADLER J., GEORGESCU G.I., EVANS C. Nitric oxide synthesis and its regulation by rabbit synoviocytes. J. Rheumatol. 1994b;21:1892–1898. [PubMed] [Google Scholar]
- VAN BOXEL J.A., PAGET S.A. Predominantly T cell infiltrate in rheumatoid synovial membranes. N. Engl. J. Med. 1975;293:517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- VAN DEN BERG W.B.Role of T cells in arthritis: lessons from animal models T cells in Arthritis 1998Basel: Birkhauser Verlag; 75–92.eds. Miossec P, van den Berg W.B. & Firstein GS, pp [Google Scholar]
- WALLACE J.L., CIRINO G. Gastrointestinal-sparing NSAIDs on the horizon. Trends Pharmacol. Sci. 1994;15:405–406. doi: 10.1016/0165-6147(94)90083-3. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., CALIGNANO A., CIRINO G. Potential of new NSAIDs as analgesics. Pain Rev. 1997;4:230–237. [Google Scholar]
- WALTZ D.T., DIMARTINO M.J., MISHER A. Adjuvant-induced arthritis in rats. II Drug effects on physiological, biochemical and immunologic parameters. J. Pharmacol. Exp. Ther. 1971;178:223–231. [PubMed] [Google Scholar]
- WEI X.-Q., CHARLES I.G., SMITH A., URE J., FENG G.J., HUANG F.P., XU D., MULLER W., MONCADA S., LIEW F.Y. Altered immune response in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]


