Abstract
SB-258585 (4-Iodo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulphonamide) is a high affinity ligand at 5-HT6 receptors. It displays over 100 fold selectivity for the 5-HT6 receptor over all other 5-HT receptors tested so far. SB-258585 has been radiolabelled, to high specific activity, for its characterization as a 5-HT6 receptor selective radioligand.
[125I]-SB-258585 bound, with high affinity, to a single population of receptors in a cell line expressing human recombinant 5-HT6 receptors. Kinetic and saturation binding experiments gave pKD values of 9.01±0.09 and 9.09±0.02, respectively.
In membranes derived from rat or pig striatum and human caudate putamen, [125I]-SB-258585 labelled a single site with high levels (>60%) of specific binding. Saturation analysis revealed pKD values of 8.56±0.07 for rat, 8.60±0.10 for pig and 8.90±0.02 for human. Bmax values for the tissues ranged from 173±23 and 181±25 fmol mg−1 protein in rat and pig striatum, respectively, to 215±41 fmol mg−1 protein in human caudate putamen.
The pKi rank order of potency for a number of compounds, determined in competition binding assays with [125I]-SB-258585, at human caudate putamen membranes was: SB-271046>SB-258585>SB-214111>methiothepin>clozapine>5-Me-OT>5-HT>Ro 04-6790>mianserin>ritanserin=amitriptyline>5-CT>mesulergine. Similar profiles were obtained from pig and rat striatal membranes and recombinant 5-HT6 receptors; data from the latter correlated well with [3H]-LSD binding.
Thus, [125I]-SB-258585 is a high affinity, selective radioligand which can be used to label both recombinant and native 5-HT6 receptors and will facilitate further characterization of this receptor subtype in animal and human tissues.
Keywords: 5-HT6 receptors, SB-258585, SB-271046, radioligand binding
Introduction
5-Hydroxytryptamine (5-HT) exerts a wide variety of physiological and behavioural effects through actions on multiple receptor subtypes. These receptors have been classified by structural, functional and pharmacological criteria into seven distinct receptor classes (5-HT1-7) (Hoyer et al., 1994; Hoyer & Martin, 1996).
The rat and human 5-HT6 receptors have been cloned and characterized (Monsma et al., 1993; Ruat et al., 1993; Kohen et al., 1996). More recently, a splice variant of the human 5-HT6 receptor has been identified and although mRNA for the truncated variant was detected in caudate and substantia nigra, in vitro studies have demonstrated that it is non-functional (Olsen et al., 1999). 5-HT6 receptors couple positively to adenylyl cyclase when expressed in cell lines (Monsma et al., 1993; Ruat et al., 1993; Kohen et al., 1996; Boess et al., 1997). Functionally coupled endogenous 5-HT6-like receptors have been described in mouse neuroblastoma derived cell lines (Conner & Mansour, 1990; Unsworth & Molinoff, 1994), primary cultures of mouse striatal neurons (Sebben et al., 1994) and in pig striatal membranes (Schoeffter & Waeber, 1994) suggesting that native 5-HT6 receptors are also positively coupled to adenylyl cyclase.
In the rat brain relatively high levels of 5-HT6 receptor mRNA are detected in the striatum, olfactory tubercle, nucleus accumbens, cerebral cortex and hippocampus (Monsma et al., 1993; Ruat et al., 1993; Ward et al., 1995). 5-HT6 receptor mRNA expression is not affected by a selective lesion of serotonergic neurons (Gerard et al., 1996), suggesting a post-synaptic localization of the receptors. This has subsequently been demonstrated by light and electron microscopic studies using a 5-HT6 receptor antiserum (Gerard et al., 1997), where, both in the striatum and hippocampus, the 5-HT6 receptor-like immunoreactivity was associated with dendritic processes (Gerard et al., 1997). Localization of 5-HT6 receptors to both basal ganglia and limbic structures suggests that this receptor subtype may participate in the serotonergic control of motor function, mood-dependent behaviour, depression and cognition. 5-HT6 receptor function has been addressed in a number of recent studies using antisense oligonucleotides to reduce the number of receptors (Bourson et al., 1995; Yoshioka et al., 1998) and selective 5-HT6 receptor antagonists (Sleight et al., 1998; Bourson et al., 1998). These studies suggest that the 5-HT6 receptor may be involved in the modulation of cholinergic neuronal function (Bourson et al., 1995; 1998; Sleight et al., 1998; Bentley et al., 1999) and in increased 5-HT release induced by conditioned fear stress (Yoshioka et al., 1998). A study with the recently described selective 5-HT6 receptor antagonist, 5-Chloro-3-methyl-benzo [b]thiophene-2-sulphonic acid (4-methoxy-3-piperazin-1-yl-phenyl)-amide (SB-271046) (Bromidge et al., 1999; Routledge et al., 1999) has confirmed the putative link between 5-HT6 receptors and cognitive function (Rogers et al., 1999).
Prior to the recent introduction of [3H]-4-amino-N-(2,6 bis-methylamino-pyridine-4-yl)-benzene sulphonamide ([3H]-Ro 63-0563) (Boess et al., 1998) and [125I]-SB-258585 (4-Iodo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulphonamide; structure shown in Figure 1) (Hirst et al., 1999 and the present study), there have been no selective radioligands for the 5-HT6 receptor and studies have relied on non-selective radioligands, including radiolabelled 5-HT and D-lysergic acid diethylamide (LSD). In the present study we describe the characterisation of [125I]-SB-258585, a novel, selective 5-HT6 receptor antagonist which binds to both recombinant and native 5-HT6 receptors with high affinity and displays high levels of specific binding. A preliminary account of the data presented here has been published in abstract form (Hirst et al., 1999; Minton et al., 1999).
Figure 1.
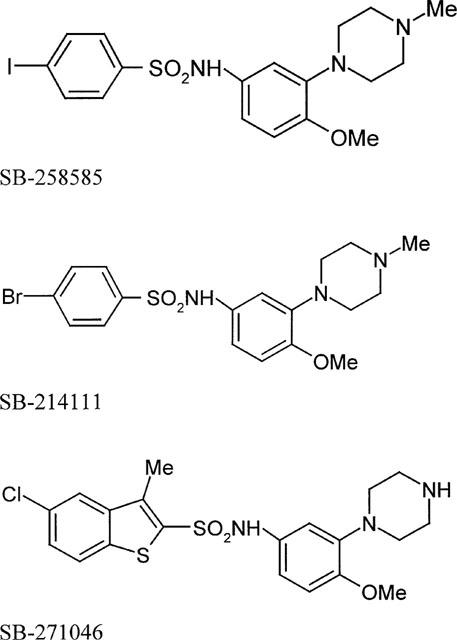
Chemical structures of SB-258585, 4-Iodo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulphonamide, SB-214111, 4-Bromo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulphonamide and SB-271046, 5-Chloro-3-methyl-benzo[b]thiophene-2-sulphonic acid (4-methoxy-3-piperazin-1-yl-phenyl)-amide.
Methods
Preparation of membranes
Cloned receptors
HeLa cells stably transfected with cDNA coding for the human 5-HT6 receptor were obtained, under a licensing agreement, from Dr D. Sibley (National Institute of Health, Bethesda, MD, U.S.A.). The cells were grown in Dulbecco' modified Eagle' medium (DMEM) containing 5% foetal bovine serum and were routinely treated with 5 mM sodium butyrate 24 h prior to harvesting. The cells were harvested in phosphate buffered saline (PBS) containing 0.1 mM EDTA and pelleted by centrifugation (1000×g), the supernatant was discarded and the pellets were stored at −80°C prior to membrane preparation. For preparation of membranes, cell pellets were homogenized in ice-cold 50 mM Tris-HCl buffer (pH 7.7 at 25°C), for approximately 20 s, using a Kinematic Ultra-Turrax homogenizer. The homogenates were centrifuged at 35,000×g for 20 min and the resulting pellet was re-homogenized and incubated at 37°C for 15 min. Following two further centrifugation steps (as above) the membranes were finally resuspended (approximately 4 mg protein ml−1) and stored at −80°C until use.
Native tissue
Striatal tissue from adult rats (Sprague-Dawley, 200–250 g, Charles River, U.K.), adult pigs (from a local abattoir: Dalehead Foods, Linton, U.K.) and human caudate putamen tissue (from three non-identifiable patients aged 64–76 years, whose cause of death was non-neurological, from Resource, Institute of Neurology, London, U.K. approved by a local ethics committee) were homogenized and prepared exactly as described above for the HeLa cells.
Radioligand binding
Studies using membranes of cells stably transfected with the 5-HT6 receptor were carried out in a buffer containing 20 mM HEPES, 3 mM MgCl2 and 2 mM ascorbate (pH 7.4). A number of experiments were carried out using the Tris based buffer, described below, with no apparent differences (data not shown). Studies using membranes derived from rat, pig or human brain tissues were carried out in a buffer containing 50 mM Tris-HCl, 10 μM pargyline, 5 mM MgCl2, 5 mM ascorbate and 0.5 mM EDTA (pH 7.4).
Binding assays consisted of 50 μl of displacing compound or buffer, 400 μl of membrane suspension (corresponding to approximately 15 μg protein well−1 for the recombinant cells and 60 μg protein well−1 for the brain tissue) and 50 μl of [125I]-SB-258585 (specific activity, 2000 Ci mmol−1). [125I]-SB-258585 was used at a concentration of 0.1 nM.
Association rates were determined by incubation of membranes with radioligand at 37°C for time periods from 0.5–120 min before termination of the experiment by filtration (described below). Dissociation rates were determined by pre-incubating membranes with [125I]-SB-258585 for 45 min at 37°C, 10 μM methiothepin was then added to initiate dissociation and the experiment was terminated by filtration after incubating for 0.5–120 min. For saturation analysis, membranes were incubated with 0.1 nM [125I]-SB-258585 and unlabelled SB-258585 to give 12 final ligand concentrations ranging from approximately 0.1–25 nM, for 45 min at 37°C. In competition binding experiments, 10 concentrations of the competing ligands were tested (concentration range:0.03 nM–1 μM and 0.3 nM–10 μM) at a final [125I]-SB-258585 concentration of 0.1 nM. Saturation and competition studies, using [3H]-LSD as a radioligand, were also undertaken using membranes from HeLa cells expressing human recombinant 5-HT6 receptors. In saturation studies eight concentrations of [3H]-LSD were used (final concentrations of approximately 0.05–10 nM), whereas a single concentration of 2 nM was used in competition binding experiments.
Incubations with [125I]-SB-258585 and [3H]-LSD were for 45 min at 37°C (except for the kinetic studies). Non-specific binding was measured in the presence of 10 μM methiothepin. The experiments were terminated by rapid filtration through Whatman GF/B filters, pre-treated with 0.3% (v v−1) polyethyleneimine (PEI), and washed with 6–9 ml of ice cold buffer. Radioactivity was determined by gamma spectrometry using a Packard Cobra II gamma counter or by liquid scintillation spectrometry using a Packard 2700 liquid scintillation counter.
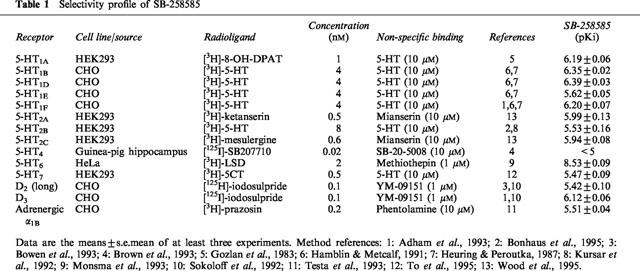
To determine the relative receptor selectivity of SB-258585, binding studies were also carried out on human cloned 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT1F, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT7, dopaminergic D2 and D3 and adrenergic α1B receptors and to native 5-HT4 receptors. Brief details of the cell line, radioligands used and the compounds included to define non-specific binding and references to the methodologies are shown in Table 1.
Table 1.
Selectivity profile of SB-258585
Protein concentrations were determined using the Bradford assay method (Bio-Rad protein assay kit, Bio-Rad, York, U.K.) using bovine serum albumin as a standard.
Data analysis
Association and dissociation studies were analysed using GRAFIT (Erithacus Software Ltd., Staines, U.K.). In saturation binding studies, KD and Bmax values were calculated using Radlig and LIGAND (Biosoft, Cambridge, U.K.) (Munson & Rodbard, 1980; McPherson, 1997). The concentration of drug inhibiting specific radioligand binding by 50% (IC50) was determined by iterative curve fitting (Bowen & Jerman, 1995). pKi values (the negative log10 of the molar Ki) for receptor binding were then calculated from the IC50 values as described by Cheng & Prusoff (1973) using the KD values determined in the saturation binding studies. Data are expressed as the mean±s.e.mean of at least three separate experiments.
Materials
SB-271046 (5-Chloro-3-methyl-benzo[b]thiophene-2-sulphonic acid (4-methoxy-3-piperazin-1-yl-phenyl)-amide), SB-214111 (4-Bromo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulphonamide), SB-258585 (4-Iodo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulphonamide) and Ro 04-6790, 4-amino-N-(2,6 bis-methylamino-pyrimidin-4-yl)-benzene sulphonamide were synthesized by SmithKline Beecham Pharmaceuticals (Harlow, U.K.); the chemical structures of SB-258585 and SB-214111 are shown in Figure 1. [125I]-SB-258585 was prepared at SmithKline Beecham (Synthetic Isotope Chemistry) by reaction of the tributyltin derivative of SB-258585 with chloramine-T and sodium [125I]-iodide. Methiothepin mesylate, clozapine, ritanserin, mianserin hydrochloride, 5-carboxamidotryptamine maleate (5-CT) and mesulergine hydrochloride were purchased from Research Biochemicals Inc. (Natick, MA, U.S.A.). 5-hydroxytryptamine creatine sulphate (5-HT), 5-methoxytryptamine hydrochloride (5-Me-OT), amitriptyline hydrochloride and pargyline were purchased from Sigma (Poole, U.K.). Cell culture reagents were obtained from Life Technologies Ltd. (Paisley, U.K.). All other reagents were obtained from Sigma or Merck-BDH (Lutterworth, U.K.) and were of analytical grade.
Results
Receptor binding profile of SB-258585
To determine the relative receptor selectivities of SB-258585, binding studies were carried out on a number of receptors, as detailed in Table 1. SB-258585 had the highest affinity for the 5-HT6 receptor (pKi=8.53) with greater than 100 fold selectivity over all other receptors investigated. The compound had only modest affinity for 5-HT1D receptor (pKi=6.39), lower affinities were seen with the 5-HT1B (pKi=6.35), 5-ht1F (pKi=6.20), 5-HT1A (pKi=6.19) and the dopamine D3 (pKi=6.12) receptors. SB-258585 had pKi values less than 6 at all other receptors examined (Table 1).
[125I]-SB-258585 binding to human recombinant 5-HT6 receptors
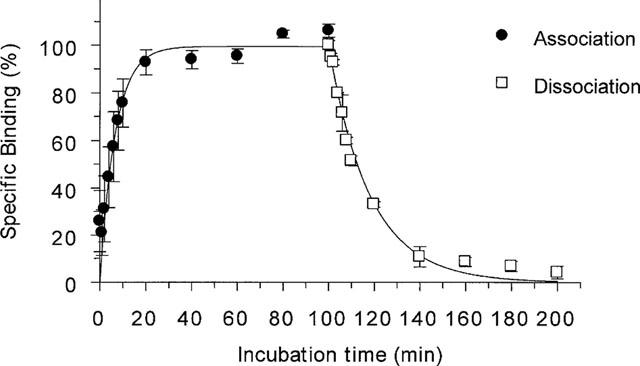
Specific [125I]-SB-258585 binding to human recombinant 5-HT6 receptors reached equilibrium within 45 min (Figure 2). Kinetic analysis of the binding revealed a monophasic apparent association rate kobs of 0.07±0.014 min−1. This binding was reversible and dissociation rates were determined in separate experiments by the addition of 10 μM methiothepin, giving a monophasic dissociation curve with a rate constant (k−1) of 0.065±0.004 min−1 (Figure 2). The calculated association rate (k1) was 0.066±0.01 min−1 nM−1 which gave a derived dissociation constant (KD=k−1/k1) of 0.98 nM. Saturation analysis of [125I]-SB-258585 binding to human recombinant 5-HT6 receptors in HeLa cell membranes revealed a single binding site (Figure 3) with a KD of 0.80±0.05 nM and a Bmax of 6.1±0.95 pmol mg−1 protein. At a radioligand concentration of 0.1 nM, the concentration used in both kinetic and competition binding experiments, specific binding represented 97±0.2% of total binding. Saturation analysis of [3H]-LSD binding to the same membranes gave a KD of 1.5±0.1 nM and a Bmax of 3.9±0.8 pmol mg−1 protein (data not shown, n=4).
Figure 2.
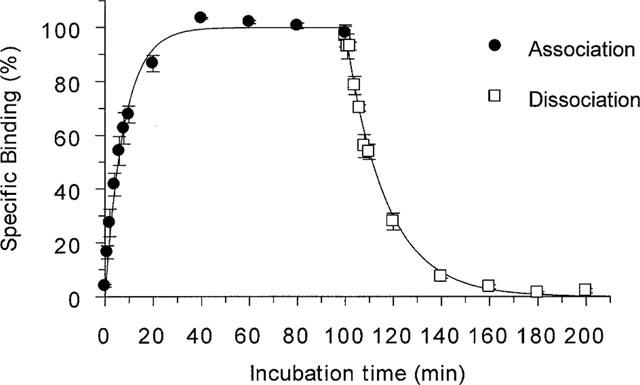
Association and dissociation kinetics of 0.1 nM [125I]-SB-258585 binding to human recombinant 5-HT6 receptors at 37°C. The apparent association rate (kobs) was 0.072±0.014 min−1. Dissociation rate was determined in separate experiments by the addition of 10 μM methiothepin, giving a monophasic dissociation curve with a rate constant (k−1) of 0.065±0.004 min−1. The calculated association rate (k1) was 0.066±0.01 min−1 nM−1 and the derived KD was 0.98 nM. Data points represent the means±s.e.mean of 3–5 independent experiments, each performed in triplicate.
Figure 3.
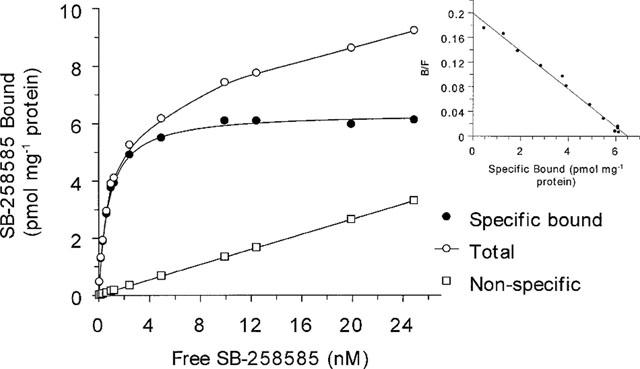
Saturation and Scatchard analysis of specific [125I]-SB-258585 binding to human recombinant 5-HT6 receptors. Membranes were incubated with 0.1 nM [125I]-SB-258585 and unlabelled SB-258585 to give final ligand concentrations of approximately 0.1–25 nM for 45 min at 37°C. Non-specific binding was determined in the presence of 10 μM methiothepin. The data shown are from one of five similar experiments, each performed in triplicate. Average KD, Bmax and standard error values are given in Table 3.
Pharmacological profile of [125I]-SB-258585 and [3H]-LSD binding to human recombinant 5-HT6 receptors
A comparison of the affinity of previously characterized serotonergic agonists and antagonists and novel 5-HT6 receptor selective antagonists to compete with [125I]-SB-258585 and [3H]-LSD binding to human recombinant 5-HT6 receptors is shown in Table 2 and Figure 4. The rank order of affinity to inhibit [125I]-SB-258585 binding to human recombinant 5-HT6 receptors, SB-271046>SB-258585>methiothepin>SB-214111>clozapine>5-Me-OT>Ro-04-6790>mianserin>ritanserin>amitriptyline>5-HT>5-CT>mesulergine, was similar to that determined with [3H]-LSD in the same cells (Table 2). This is shown in Figure 5 where a plot of the pKi values determined in the competition binding experiments with both radioligands gave a correlation coefficient, r, of 0.99.
Table 2.
Pharmacological profile of [125I]-SB-258585 and [3H]-LSD binding to human recombinant 5-HT6 receptors
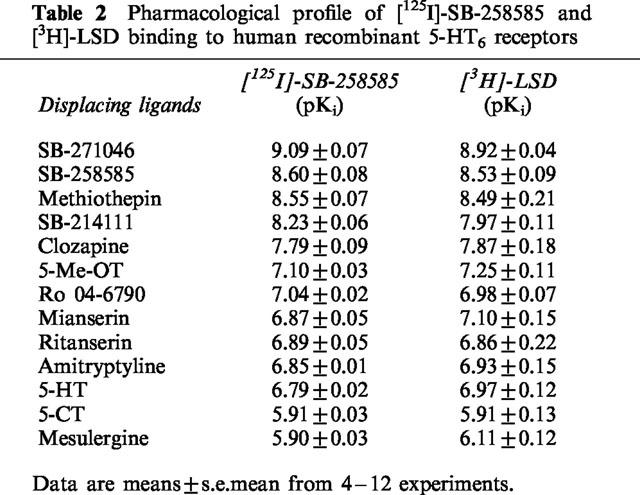
Figure 4.
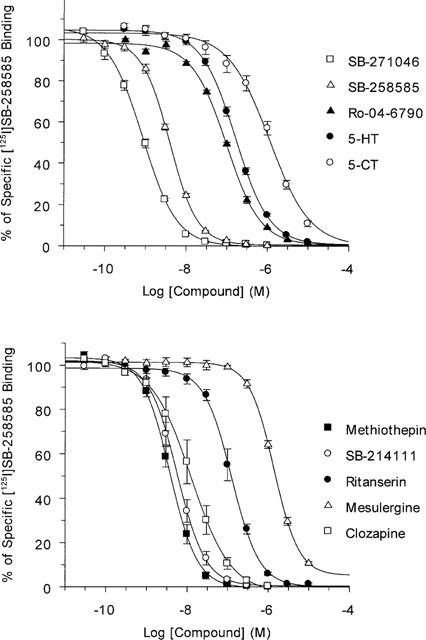
Pharmacological profile of [125I]-SB-258585 binding to human recombinant 5-HT6 receptors. Competition experiments with SB-271046, SB-258585, Ro 04-6790, 5-HT, 5-CT, methiothepin, clozapine, SB-214111, ritanserin and mesulergine were performed as described in the Methods section. Data points represent the means±s.e.mean of 4–12 independent experiments. Average pKi and standard error values are given in Table 2.
Figure 5.
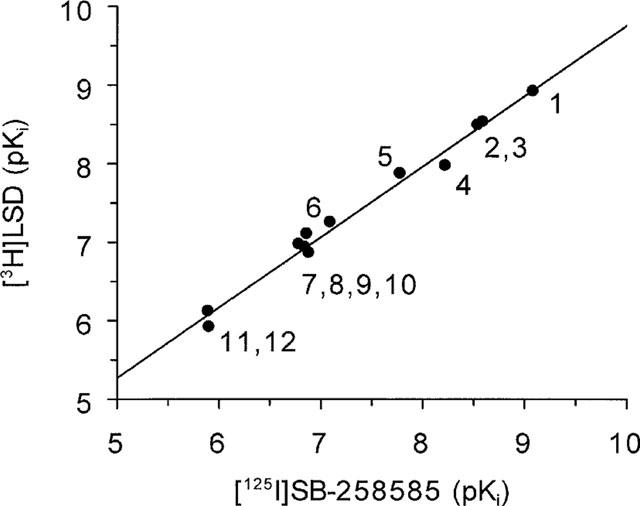
Correlation plot of pKi values for 12 compounds inhibiting [125I]-SB-258585 or [3H]-LSD binding to human recombinant 5-HT6 receptors. 1: SB-271046, 2: SB-258585, 3: methiothepin, 4: SB-214111, 5: clozapine, 6 : 5-Me-OT, 7: ritanserin, 8: mianserin, 9: amitriptyline, 10 : 5-HT, 11 : 5-CT, 12: mesulergine. Correlation coefficient, r,=0.99 and slope=0.90.
[125I]-SB-258585 binding to rat and pig striatal membranes and to human caudate putamen membranes
Specific [125I]-SB-258585 binding to human caudate putamen membranes reached equilibrium within 45 min (Figure 6). Kinetic analysis of the binding revealed a monophasic apparent association rate kobs of 0.064±0.002 min−1. This binding was reversible and dissociation rates, determined as described above, gave a monophasic dissociation curve with a rate constant (k−1) of 0.057±0.006 min−1 (Figure 6). The calculated association rate (k1) was 0.07±0.04 min−1 nM−1 and the derived dissociation constant (KD=k−1/k1) was 0.80 nM. Figure 7 shows representative saturation curves and Scatchard analyses of specific [125I]-SB-258585 binding to rat and pig striatal membranes and human caudate putamen membranes. 0.1 nM [125I]-SB-258585 labelled a single binding site, with high levels of specific binding: 59.7±0.9%, 65.5±0.7% and 67.5±1.8%, in rat, pig and human membranes, respectively. However, at higher concentrations of radioligand the specific signal decreased, for example, in the human caudate putamen membranes the specific binding was 39% at 0.5 nM and 25% at 1 nM. The binding affinities (KD) and capacities (Bmax) for each of the tissues, together with data from the recombinant cell line expressing human 5-HT6 receptors are given in Table 3. All three tissues displayed similar 5-HT6 receptor affinities for [125I]-SB-258585 and similar receptor densities.
Figure 6.
Association and dissociation kinetics of 0.1 nM [125I]-SB-258585 binding to human caudate putamen membranes at 37°C. The apparent association rate constant (kobs) was 0.064±0.002 min−1. Dissociation rate was determined in separate experiments by the addition of 10 μM methiothepin, giving the monophasic dissociation rate constant (k−1) of 0.057±0.006 min−1. The calculated association rate (k1) was 0.07±0.04 min−1 nM−1 and the derived KD was 0.80 nM. Data points represent the means±s.e.mean of three independent experiments, each performed in triplicate.
Figure 7.
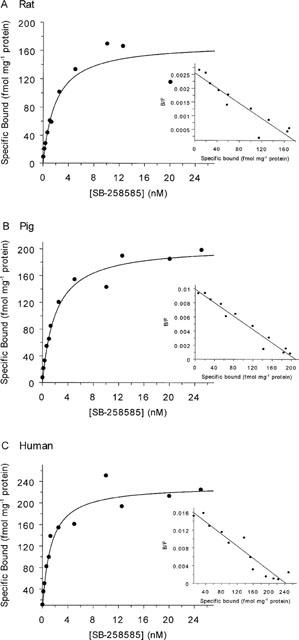
Saturation and Scatchard analysis of specific [125I]-SB-258585 binding to rat striatal membranes (A), pig striatal membranes (B) and human caudate putamen membranes (C). Membranes were incubated with 0.1 nM [125I]-SB-258585 and unlabelled SB-258585 to give final ligand concentrations of approximately 0.1–25 nM for 45 min at 37°C. Non-specific binding was determined in the presence of 10 μM methiothepin. The data shown are from one of 3–4 similar experiments, each performed in triplicate. Average KD, Bmax and standard error values are given in Table 3.
Table 3.
[125I]-SB-258585 binding to striatal membranes and human recombinant 5-HT6 receptors
Pharmacological profile of [125I]-SB-258585 binding to rat and pig striatal membranes and to human caudate putamen membranes
Competition binding analysis was used to characterize the [125I]-SB-258585 binding sites in rat and pig striatal membranes and to human caudate putamen membranes (Figure 8 and Table 4). The same 12 compounds, used to profile the radioligand binding to recombinant receptors, were used in the experiments with native tissues. The pKi rank order of potency at human caudate putamen membranes was SB-271046>SB-258585>SB-214111>methiothepin>clozapine>5-Me-OT>5-HT>Ro-04-6790>mianserin>ritanserin=amitriptyline>5-CT>mesulergine. Similar values were obtained from rat and pig striatal membranes (Table 4). A comparison of the pKi values determined using human recombinant 5-HT6 receptors and human caudate putamen membranes revealed a statistically significant correlation, r=0.98, (Figure 9), indicating that the native tissue [125I]-SB-258585 binding site had a pharmacological profile consistent with that of the 5-HT6 receptor.
Figure 8.
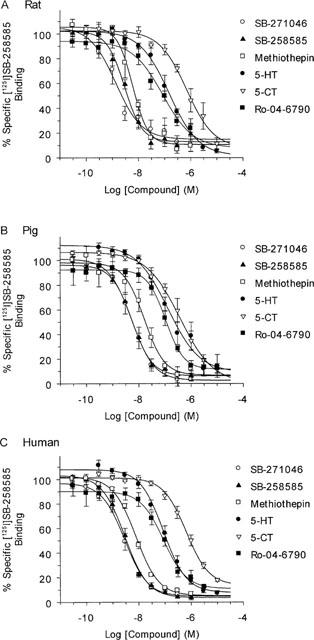
Pharmacological profile of [125I]-SB-258585 binding to rat striatal membranes (A), pig striatal membranes (B) and human caudate putamen membranes (C). Competition experiments with SB-271046, SB-258585, methiothepin, 5-HT, 5-CT and Ro 04–6790 were performed as described in the Methods section. Data points represent the means±s.e.mean of 4–12 independent experiments. Average pKi and standard error values are given in Table 4.
Table 4.
Pharmacological profile of [125I]-SB-258585 binding to rat striatal, pig striatal and human caudate putamen membranes
Figure 9.
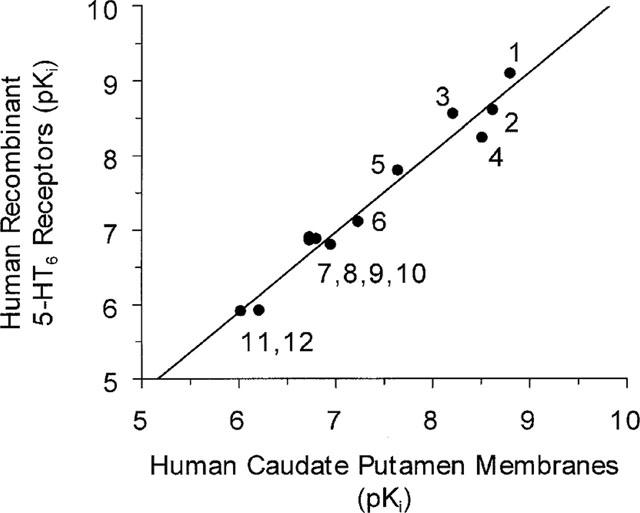
Correlation plot of pKi values for 12 compounds inhibiting [125I]-SB-258585 binding to human recombinant 5-HT6 receptors and human caudate putamen membranes. 1: SB-271046, 2: SB-258585, 3: methiothepin, 4: SB-214111, 5: clozapine, 6 : 5-Me-OT, 7: ritanserin, 8: mianserin, 9: amitriptyline, 10 : 5-HT, 11 : 5-CT, 12: mesulergine. Correlation coefficient, r,=0.98 and slope=1.08.
Discussion
This study has shown that [125I]-SB-258585 is a high affinity, reversible radioligand at 5-HT6 receptors. SB-258585 is a potent antagonist at 5-HT6 receptors (Bromidge et al., 1999) and has greater than 100 fold selectivity for 5-HT6 receptors over 10 other 5-HT receptors investigated. The structurally related compound, SB-271046, was shown to be more than 200 fold selective for the human 5-HT6 receptor as compared to 55 other receptors, enzymes and ion channels (Routledge et al., 1999) and SB-271046 fully displaced specific [125I]-SB-258585 binding in the striatal membranes. Taken together these data suggest that [125I]-SB-258585 is a highly selective radioligand.
Kinetic analyses, demonstrating rapid rates of association and dissociation, and saturation binding studies show that [125I]-SB-258585 binds with sub-nanomolar affinity (KD=0.80–0.98 nM) to a single binding site in membranes prepared from HeLa cells recombinantly expressing human 5-HT6 receptors. The number of binding sites labelled with [125I]-SB-258585 in this cell line (6.1 pmol mg−1 protein) is slightly higher than that determined with a non-selective agonist radioligand, [3H]-LSD (3.9 pmol mg−1 protein), which could be explained by an antagonist radioligand labelling all the receptors compared to the agonist radioligand which selectively binds to receptors in their high affinity state. However, there is no suggestion of multiple affinity states from either [3H]-LSD saturation studies (data not shown) nor from agonist displacement curves in competition binding experiments using [125I]-SB-258585 where the Hill coefficient for 5-HT, 5-CT and 5-Me-OT were 1.04±0.01, 1.05±0.07 and 0.97±0.02, respectively. Hence the minor discrepancy in the number of binding sites detected by the two different radioligands may be due to experimental variation as a result of the different approaches used in generating the saturation isotherms; increasing concentrations of [3H]-LSD, from 0.05–10 nM, and a single concentration of [125I]-SB-258585 (0.1 nM) and unlabelled SB-258585 to give 12 final ligand concentrations ranging from approximately 0.1–25 nM.
The pharmacological profile of [125I]-SB-258585, determined in competition binding studies, at human 5-HT6 receptors stably expressed in HeLa cells correlated closely to the profile determined with [3H]-LSD (r=0.99). This profile was also consistent with previous studies on cloned rat (Monsma et al., 1993; Boess et al., 1997) and human (Kohen et al., 1996) 5-HT6 receptors using [125I]-LSD, [3H]-LSD or [3H]-5-HT and also with a more recent study which reported on the characterization of a selective 5-HT6 receptor radioligand, [3H]-Ro 63-0563 (Boess et al., 1998).
In the majority of studies to date, non-selective radioligands, including [3H]-5-HT, [3H]-LSD, [125I]-LSD and [3H]-clozapine, have been used to label 5-HT6 receptors. In simple, recombinant systems these ligands are adequate (Monsma et al., 1993; Kohen et al., 1996; Boess et al., 1997), however their lack of selectivity has precluded definitive studies on 5-HT6 receptors in native tissues. For example, studies investigating the effects of antisense oligonucleotides on 5-HT6 receptor expression (Bourson et al., 1995; Yoshioka et al., 1998) have used [3H]-LSD binding. Addition of compounds to prevent binding of [3H]-LSD to other receptors may have resulted in an under or over estimation in the number of 5-HT6 receptors. [3H]-clozapine has also been used to label 5-HT6 receptors in rat brain (Glatt et al., 1995). However under the conditions employed this radioligand was shown to label at least two populations of receptors, one of which was probably a muscarinic receptor (Glatt et al., 1995). [3H]-Ro 63-0563 selectively binds to 5-HT6 receptors in recombinant cell lines (Boess et al., 1998). However, in binding assays, using [3H]-Ro 63-0563, with rat and pig striatal membranes there are relatively high levels of non-specific binding (70–90%) (Boess et al., 1998), which could make detailed investigations of 5-HT6 receptor localization and pharmacology difficult, particularly in human brain tissues, where post-mortem delays and protein degradation could reduce this small specific binding signal further.
High levels of 5-HT6 mRNA have been demonstrated in rat striatum by Northern blot analysis (Monsma et al., 1993; Ruat et al., 1993), in situ hybridization (Ruat et al., 1993; Ward et al., 1995) and by reverse transcriptase-polymerase chain reaction (RT–PCR) (Gerard et al., 1996). 5-HT6 receptor-like immunoreactivity has also been shown, in addition to other regions, in the rat striatum (Gerard et al., 1997) and 5-HT6 receptor binding sites have been identified in rat and pig striatal membranes (Boess et al., 1998). Based on these previous studies, we used striatal membranes to investigate and characterize 5-HT6 receptor binding sites in native brain tissue. The amount of specific binding observed in rat and pig striatal membranes and in human caudate putamen membranes, at a radiolabel concentration of 0.1 nM, was 59.7±0.9%, 65.5±0.7% and 67.5±1.8%, respectively. These values are considerably higher than those reported with [3H]-Ro 63-0563, which were 10–20% and 20–30% for rat and pig tissue, respectively (Boess et al., 1998). The higher specific binding with [125I]-SB-258585 allowed a detailed characterization of radioligand binding in the rat and pig striatal membranes and in human caudate putamen membranes. Kinetic studies on specific [125I]-SB-258585 binding to human caudate putamen membranes gave results which were comparable to those obtained for the human 5-HT6 receptors expressed in a HeLa cell line. In the human caudate putamen membranes the binding was high affinity, saturable and reversible and the KD value determined from kinetic studies was 0.80 nM. In equilibrium binding studies, [125I]-SB-258585 labelled a single binding site in the native tissues with a KD of 1.3 nM for human caudate putamen membranes and 2.8 nM for rat and pig striatal membranes, which correlated well with the affinity estimate from the kinetic studies. The number of binding sites labelled with [125I]-SB-258585 in the native tissues was in the region of 170–215 fmol mg−1 protein, depending on the species (Table 3), a value similar to that previously determined with [3H]-Ro 63-0563 in pig striatal membranes (Boess et al., 1998).
The pharmacological profile, determined in the present study with both previously characterized serotonergic agonists and antagonists and novel 5-HT6 receptor selective antagonists, did not show any notable differences between rat, pig or human membranes (Table 4), suggesting that the pharmacology of the 5-HT6 receptor is conserved between species. The profile was also similar to that obtained for the human 5-HT6 receptor stably expressed in a cell line (Figure 9). Collectively these data confirm that [125I]-SB-258585 is selectively labelling 5-HT6 receptors in native tissues. These results provide the first data describing the pharmacology of the 5-HT6 receptor in membranes prepared from human caudate putamen.
Several studies have described a clear association of 5-HT6 receptors (Ruat et al., 1993; Monsma et al., 1993; Ward et al., 1995; Kohen et al., 1996; Gerard et al., 1997) with both the basal ganglia and the limbic system, suggesting potential roles for this receptor in control of motor function, mood dependent behaviour, depression and cognition. Furthermore, the high affinity of classical and atypical antipsychotics (Monsma et al., 1993; Roth et al., 1994; Kohen et al., 1996), together with the localization of the receptor in cortical and limbic areas suggests that 5-HT6 receptors may play a role in the pathophysiology of schizophrenia. Targeting 5-HT6 receptors may offer the potential of antipsychotic activity, without the propensity for extrapyramidal side effects associated with dopamine D2 receptor blockade. [125I]-SB-258585 will be a useful tool in studying 5-HT6 receptor density and distribution in animal models of disease and in human brains from patients suffering from schizophrenia and Alzheimer' disease. Preliminary studies have demonstrated that [125I]-SB-258585 is also a suitable ligand for autoradiographic labelling of 5-HT6 receptors in brain sections (Roberts et al., 1999).
In conclusion, we have described the characterization of a radioligand, [125I]-SB-258585, which selectively binds to recombinant and native 5-HT6 receptors with high affinity. A comparison of the pharmacological profile of [125I]-SB-258585 binding to human recombinant 5-HT6 receptors and native tissues demonstrates the similarity in 5-HT6 receptor pharmacology between species. This is the first radioligand binding study to characterize and quantify 5-HT6 receptors in human brain tissue.
Abbreviations
- CHO
chinese hamster ovary
- 5-CT
5-carboxamidotryptamine
- HEK
human embryonic kidney
- HEPES
N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]
- 5-HT
5-hydroxytryptamine
- LSD
D-lysergic acid diethylamide
- 5-Me-OT
5-methoxytryptamine
- 8-OH-DPAT
8-hydroxy-2-(di-N-propylamino)-tetralin
- Ro 04-6790
4-amino-N-(2,6 bis-methylamino-pyrimidin-4-yl)-benzene sulphonamide
- Ro 63-05636
4-amino-N-(2,6 bis-methylamino-pyridine-4-yl)-benzene sulphonamide
- SB-214111
4-Bromo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzensulphonamide
- SB-258585
4-Iodo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulphonamide
- SB-271046
5-Chloro-3-methyl-benzo[b]thiophene-2-sulphonic acid (4-methoxy-3-piperazin-1-yl-phenyl)-amide
References
- ADHAM N., KAO H.T., SCHECHTER L.E., BARD J., OLSEN M., URQUHART D., DURKIN M., HARTIG P.R., WEINSHANK R.L., BRANCHEK A. Cloning of another human serotonin receptor (5 HT1F): A fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc. Natl. Acad. Sci. USA. 1993;90:408–412. doi: 10.1073/pnas.90.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENTLEY J.C., BOURSON A., BOESS F.G., FONE K.C., MARSDEN C.A., PETIT N., SLEIGHT A.J. Investigation of stretching behaviour induced by the selective 5-HT6 receptor antagonist, Ro 04-6790, in rats. Br. J. Pharmacol. 1999;126:1537–1542. doi: 10.1038/sj.bjp.0702445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOESS F.G., MONSMA F.J., CAROLO C., MEYER V., RUDLER A., ZWINGELSTEIN C., SLEIGHT A.J. Functional and radioligand binding characterization of rat 5-HT6 receptors stably expressed in HEK293 cells. Neuropharmacol. 1997;36:713–720. doi: 10.1016/s0028-3908(97)00019-1. [DOI] [PubMed] [Google Scholar]
- BOESS F.G., RIEMER C., BOS M., BENTLEY J., BOURSON A., SLEIGHT A.J. The 5-hydroxytryptamine6 receptor-selective radioligand [3H]Ro 63-0563 labels 5-hydroxytryptamine receptor binding sites in rat and porcine striatum. Mol. Pharmacol. 1998;54:577–583. doi: 10.1124/mol.54.3.577. [DOI] [PubMed] [Google Scholar]
- BONHAUS D.W., BACH C., DESOUZA A., SALAZAR F.H.R., MATSUOKA B.D., ZUPPAN P., CHAN H.W., EGLEN R.M. The pharmacology and distribution of human2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br. J. Pharmacol. 1995;115:622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOURSON A., BOESS F.G., BOS M., SLEIGHT A.J. Involvement of 5-HT6 receptors in nigro-striatal function in rodents. Br. J. Pharmacol. 1998;125:1562–1566. doi: 10.1038/sj.bjp.0702230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOURSON A., BORRONI E., AUSTIN R.H., MONSMA F.J., SLEIGHT A.J. Determination of the role of the 5-ht6 receptor in the rat brain: a study using antisense oligonucleotides. J. Pharmacol. Exp. Ther. 1995;274:173–180. [PubMed] [Google Scholar]
- BOWEN W.P., COLDWELL M.C., HICKS F.R., RILEY G.J. Further characterisation of human D2 and D3 dopamine receptors: GppNHp shifts are explained by the presence of more than one binding site in each clone. Br. J. Pharmacol. 1993;108:277P. [Google Scholar]
- BOWEN W.P., JERMAN J.C. Nonlinear regression using spreadsheets. Trends in Pharmacol. Sci. 1995;16:413–417. doi: 10.1016/s0165-6147(00)89091-4. [DOI] [PubMed] [Google Scholar]
- BROMIDGE S.M., BROWN A.M., CLARKE S.E., DODGSON K., GAGER T., GRASSAM H.L., JEFFREY P.M., JOINER G.F., KING F.D., MIDDLEMISS D.N., MOSS S.F., NEWMAN H., RILEY G., ROUTLEDGE C., WYMAN P. 5-Chloro-N-(4-methoxy-3-piperazin-1-yl-phenyl)-3-methyl-2-benzothiophenesulfonamide (SB-271046): a potent, selective, and orally bioavailable 5-HT6 receptor antagonist. J. Med. Chem. 1999;42:202–205. doi: 10.1021/jm980532e. [DOI] [PubMed] [Google Scholar]
- BROWN A.M., YOUNG T.J., PATCH T.L., CHEUNG C.W., KAUMANN A.J., GASTER L., KING F.D. [125I]-SB 207710, a potent, selective radioligand for 5-HT4 receptors. Br. J. Pharmacol. 1993;110:10P. [Google Scholar]
- CHENG Y.C., PRUSSOF W.H. Relationship between inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;92:881–894. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CONNER D.A., MANSOUR T.E. Serotonin receptor-mediated activation of adenylate cyclase in the neuroblastoma NCB 20: a novel 5-hydroxytryptamine receptor. Mol. Pharmacol. 1990;37:742–751. [PubMed] [Google Scholar]
- GERARD C., EL MESTIKAWY S., LEBRAND C., ADRIEN J., RUAT M., TRAIFFORT E., HAMON M., MARTRES M.P. Quantitative RT-PCR distribution of serotonin 5-HT6 receptor mRNA in the central nervous system of control or 5,7-dihydroxytryptamine-treated rats. Synapse. 1996;23:164–173. doi: 10.1002/(SICI)1098-2396(199607)23:3<164::AID-SYN5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- GERARD C., MARTRES M.P., LEFEVRE K., MIQUEL M.C., VERGE D., LANFUMEY L., DOUCET E., HAMON M., EL MESTIKAWY S. Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res. 1997;746:207–219. doi: 10.1016/s0006-8993(96)01224-3. [DOI] [PubMed] [Google Scholar]
- GLATT C.E., SNOWMAN A.M., SIBLEY D.R., SNYDER S.H. Clozapine: selective labeling of sites resembling 5HT6 serotonin receptors may reflect psychoactive profile. Mol. Med. 1995;1:398–406. [PMC free article] [PubMed] [Google Scholar]
- GOZLAN H., EL MESTIKAWY S., PICHAT L., GLOWINSKI J., HAMON M. Identification of presynaptic serotonin autoreceptors using a new ligand, [3H]PAT. Nature. 1983;305:140–142. doi: 10.1038/305140a0. [DOI] [PubMed] [Google Scholar]
- HAMBLIN M.W., METCALF M.A. Primary structure and functional characterization of a human 5-HT1D-type serotonin receptor. Mol. Pharmacol. 1991;40:143–148. [PubMed] [Google Scholar]
- HEURING R.E., PEROUTKA S.J. Characterization of a novel 3H-5 hydroxytryptamine binding site subtype in bovine brain membranes. J. Neurosci. 1987;7:894–903. doi: 10.1523/JNEUROSCI.07-03-00894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRST W.D., MINTON J.A.L., BROMIDGE S.M., ROUTLEDGE C., MIDDLEMISS D.N., PRICE G.W. [125I]SB-258585 - A selective antagonist radioligand for 5-HT6 receptors. Br. J. Pharmacol. 1999;127:23P. [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- HOYER D., MARTIN G.R. Classification and nomeclature of 5-HT receptors: A comment on current issues. Behav. Brain Res. 1996;73:263–268. doi: 10.1016/0166-4328(96)00109-x. [DOI] [PubMed] [Google Scholar]
- KOHEN R., METCALF M.A., KHAN N., DRUCK T., HEUBNER K., LACHOWICZ J.E., MELTZER H.Y., SIBLEY D.R., ROTH B.L., HAMBLIN M.W. Cloning, characterisation, and chromosomal localisation of a human 5-HT6 serotonin receptor. J. Neurochem. 1996;66:47–56. doi: 10.1046/j.1471-4159.1996.66010047.x. [DOI] [PubMed] [Google Scholar]
- KURSAR J.D., NELSON D.L., WAINSCOTT D.B., COHEN M.L., BAEZ M. Molecular cloning, functional expression, and pharmacological characterization of a novel serotonin receptor (5-hydroxytryptamine2F) from rat stomach fundus. Mol. Pharmacol. 1992;42:549–557. [PubMed] [Google Scholar]
- McPHERSON G.A. KELL: A collection of radioligand binding analysis programs. Cambridge, UK: Biosoft; 1997. [Google Scholar]
- MINTON J.A.L., HIRST W.D., BROMIDGE S.M., ROUTLEDGE C., MIDDLEMISS D.N., PRICE G.W. Characterisation of [125I]SB-258585 binding to 5-HT6 receptors in native tissues. Br. J. Pharmacol. 1999;128:157P. doi: 10.1038/sj.bjp.0703458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONSMA F.J., JR, SHEN Y., WARD R.P., HAMBLIN M.W., SIBLEY D.R. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol. Pharmacol. 1993;43:320–327. [PubMed] [Google Scholar]
- MUNSON P.J., RODBARD D. Ligand: A versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- OLSEN M.A., NAWOSCHIK S.P., SCHURMAN B.R., SCHMITT H.L., BURNO M., SMITH D.L., SCHECHTER L.E. Identification of a human 5-HT6 receptor variant produced by alternative splicing. Mol. Brain Res. 1999;64:255–263. doi: 10.1016/s0169-328x(98)00338-6. [DOI] [PubMed] [Google Scholar]
- ROBERTS J.C., HIRST W.D., REAVILL C., PATEL S., ROUTLEDGE C., LESLIE R.A. Autoradiographic localisation of the 5-HT6 receptor in the CNS of rats using [125I]SB-258585. Br. J. Pharmacol. 1999;128:156P. [Google Scholar]
- ROGERS D.C., ROBINSON T.L., QUILTER C.A., HUNTER A.J., ROUTLEDGE C., HAGAN J.J. Cognitive enhancement effects of the selective 5-HT6 antagonist SB-271046. Br. J. Pharmacol. 1999;127:22P. [Google Scholar]
- ROTH B.L., CRAIGO S.C., CHOUDHARY M.S., ULUER A., MONSMA F.J., SHEN Y., MELTZER H.Y., SIBLEY D.R. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharmacol. Exp. Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- ROUTLEDGE C, PRICE G.W., BROMIDGE S.M., MOSS S.F., NEWMAN H., RILEY G., GAGER T., BROWN A.M., LIGHTOWLER S., MIDDLEMISS D.N. Characterisation of SB-271046, a potent and selective 5-HT6 receptor antagonist. Br. J. Pharmacol. 1999;127:21P. doi: 10.1038/sj.bjp.0703457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., ARRANG J.M., TARDIVEL-LACOMBE J., DIAZ J., LEURS R., SCHWARTZ J.C. A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem. Biophys. Res. Comm. 1993;193:268–276. doi: 10.1006/bbrc.1993.1619. [DOI] [PubMed] [Google Scholar]
- SCHOEFFTER P., WAEBER C. 5-Hydroxytryptamine receptors with a 5-HT6 receptor-like profile stimulating adenylyl cyclase activity in pig caudate membranes. Naunyn Schmiedebergs Arch. Pharmacol. 1994;350:356–360. doi: 10.1007/BF00178951. [DOI] [PubMed] [Google Scholar]
- SEBBEN M., ANSANAY H., BOCKAERT J., DUMUIS A. 5-HT6 receptors positively coupled to adenylyl cyclase in striatal neurones in culture. Neuroreport. 1994;5:2553–2557. doi: 10.1097/00001756-199412000-00037. [DOI] [PubMed] [Google Scholar]
- SLEIGHT A.J., BOESS F.G., BOS M., LEVET-TRAFIT B., RIEMER C., BOURSON A. Characterization of Ro 04-6790 and Ro 63-0563: potent and selective antagonists at human and rat 5-HT6 receptors. Br. J. Pharmacol. 1998;124:556–562. doi: 10.1038/sj.bjp.0701851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOKOLOFF P., ANDRIEUX M., BESANCON R., PILON C., MARTRES M.-P., GIROS B., SCHWARTZ J.C. Pharmacology of human dopamine D3 receptor expressed in mammalian cell lines: comparison with D2 receptor. Eur. J. Pharmacol. 1992;225:331–337. doi: 10.1016/0922-4106(92)90107-7. [DOI] [PubMed] [Google Scholar]
- TESTA R., GUARNERI M., IBBA M., STRADA G., POGGESI E., TADDEI C., SIMONAZZI I., LEONARDI A. Characterisation of α1-adrenoceptor subtypes in prostate and prostatic urethra of rat, rabbit, dog and man. Eur. J. Pharmacol. 1993;249:307–315. doi: 10.1016/0014-2999(93)90527-o. [DOI] [PubMed] [Google Scholar]
- TO Z.P., BONHAUS D.W., EGLEN R.M., JAKEMAN L.B. Characterization and distribution of putative 5-HT7 receptors in guinea-pig brain. Br. J. Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNSWORTH C.D., MOLINOFF P.B. Characterization of a 5-hydroxytryptamine receptor in mouse neuroblastoma N18TG2 cells. J. Pharmacol. Exp. Ther. 1994;269:246–255. [PubMed] [Google Scholar]
- WARD R.P., HAMBLIN M.W., LACHOWICZ J.E., HOFFMAN B.J., SIBLEY D.R., DORSA D.M. Localization of serotonin subtype 6 receptor messenger RNA in the rat brain by in situ hybridization histochemistry. Neuroscience. 1995;64:1105–1111. doi: 10.1016/0306-4522(94)00439-c. [DOI] [PubMed] [Google Scholar]
- WOOD M.D., GLEN A., GAGER T.L., BLACKBURN T.P., LEE J.A., SUTIPHONG J.A., KUMAR C., CAREY J.E., ROBINSON J.H. Comparison of 3H-mesulergine binding to the cloned rat, mouse and human 5-HT2C receptor and of 3H-ketanserin binding to the cloned human 5-HT2A receptor. Pharmacol. Comun. 1995;5:109–116. [Google Scholar]
- YOSHIOKA M., MATSUMOTO M., TOGASHI H., MORI K., SAITO H. Central distribution and function of 5-HT6 receptor subtype in the rat brain. Life Sci. 1998;62:1473–1477. doi: 10.1016/s0024-3205(98)00092-7. [DOI] [PubMed] [Google Scholar]


