Abstract
The effects of adenosine and adenine on the gating of native sheep cardiac ryanodine receptor (RyR) channels were investigated. By examining the mechanisms underlying channel activation and by using comparative molecular field analysis (CoMFA) we have investigated the structural features of adenine-based ligands involved in channel activation.
In the presence of 10 μM cytosolic Ca2+, adenosine and adenine both activate the channel but only to a level approximately 10 and 20% respectively of that of ATP indicating that both are partial agonists of low efficacy.
Adenosine was able to antagonize the ATP-induced increase in open probability (Po) as expected for a partial agonist of low efficacy at the ATP sites on the cardiac RyR.
GTP (100 μM–10 mM) had no effect on channel gating indicating that the adenine ring structure is important for agonist activity at the ATP-sites on RyR.
CoMFA revealed an extremely strong correlation between the structural features of the five ATP analogues and the ability to increase (Po). Our model indicates that the high efficacy of ATP results primarily from the large electrostatic field established by the ionized phosphate groups. Reducing the number of phosphate groups lowers the strength of this field, leading to ligands with lower efficacy. In addition, steric interactions between the α-phosphate and ribose moieties and the RyR are correlated with low Po.
Keywords: ATP, adenosine, adenine, ryanodine receptor, cardiac excitation-contraction coupling, Ca2+-release, sarcoplasmic reticulum, CoMFA
Introduction
In cardiac cells, Ca2+ entering via L-type Ca2+ channels has been shown to be the mechanism triggering the opening of RyR channels during excitation-contraction (EC) coupling (Beuckelmann & Wier, 1988; Nabauer et al., 1989; Bers, 1991). Single channel studies demonstrate, however, that in the absence of other activating ligands, cytosolic Ca2+ alone is a poor activator of RyR and does not increase Po above 0.5. To produce full channel activation the presence of micromolar cytosolic Ca2+ plus a second ligand is required (Ashley & Williams, 1990; Sitsapesan & Williams, 1994; Zahradníková & Zahradník, 1995; Sitsapesan, 1998). These results indicate that although a rise in cytosolic [Ca2+] is the trigger that initiates the opening of cardiac RyR channels under physiological conditions, sufficient activation of RyR to support EC-coupling is unlikely to be achieved without the synergistic action of Ca2+ and at least one other activating ligand. ATP is a likely candidate for such a role as it is a very effective activator of RyR channels (Smith et al., 1985; Kermode et al., 1998; Sitsapesan, 1998) and is present in high millimolar quantities in muscle cells. ATP, therefore, may play a pivotal physiological role in potentiating the effects of cytosolic Ca2+ and thus allowing RyR channels to open over the full range of open probabilities (0–1). Support for this hypothesis has been recently shown in experiments in isolated cardiac myocytes where a reduction in intracellular [ATP] led to a reduction in sarcoplasmic reticulum (SR) Ca2+-release (O'Neill & Smith, 2000).
In cardiac muscle it is known that intracellular ATP levels may be depleted under conditions of myocardial ischaemia, leading to the build up of adenine-based metabolites (for example, Allen & Orchard, 1987; Allue et al., 1996). In particular, millimolar quantities of adenosine are produced as ATP is metabolized during ischaemia and there is evidence that adenosine levels may accumulate intracellularly rather than being released from intact cells by the membrane purine transporter (Bratten & Cobbold, 1998). It is important therefore to establish the affinity and efficacy of adenosine and related compounds at the cardiac RyR. We can then predict if these compounds will compete effectively with ATP for the same sites on RyR and affect Ca2+-release from the sarcoplasmic reticulum (SR). In earlier work investigating the effects of ATP, ADP and AMP, we demonstrated that reducing the number of phosphates caused a marked lowering of the affinity of the compounds for RyR and also reduced the ability of the ligands to open the channel (Kermode et al., 1998; Ching et al., 1999). In the present study we examined the effects of adenine and adenosine on the gating of single cardiac RyR channels to investigate the importance of phosphate groups and the ribose group for activity at the cardiac RyR.
The predicted role of adenine-based compounds as regulators of cardiac RyR function confirm the importance of understanding the interactions of these ligands with their binding sites on RyR. We have therefore used comparative molecular field analysis (CoMFA) to correlate the chemical structure of adenine-based compounds with their ability to activate the cardiac ryanodine receptor. The CoMFA offers several advantages in seeking an understanding of the mechanism by which ligands binding to the ATP binding site are able to modulate RyR channel function. A few of these are listed here. Firstly, with CoMFA (Cramer & Patterson, 1988) we are able to quantitatively evaluate the effect of structural changes on biological activity. Secondly, CoMFA is robust and can be used to predict the behaviour of novel ligands. Thirdly, CoMFA can be used to provide a functional map of the RyR nucleotide-binding site.
We find that adenine and adenosine have significantly higher affinities for the ATP sites on RyR than either AMP or ADP but both ligands display partial agonist behaviour. Adenosine in particular has low efficacy and, at 10 μM cytosolic Ca2+, is a very effective antagonist of ATP. The CoMFA of the relationship between the structure of the ATP analogues and their ability to open the channel indicates the steric and electrostatic interactions between the ligand and RyR that promote or impede channel opening.
Methods
Preparation of SR membrane vesicles and planar lipid bilayer methods
Heavy SR membrane vesicles were prepared from sheep hearts as previously described (Sitsapesan et al., 1991) and rapidly frozen and stored in liquid nitrogen. Vesicles were fused with planar phosphatidylethanolamine lipid bilayers as previously described (Sitsapesan et al., 1991). The vesicles fused in a fixed orientation such that the cis chamber corresponded to the cytosolic space and the trans chamber to the SR lumen. The trans chamber was held at ground and the cis chamber held at potentials relative to ground. After fusion, the cis chamber was perfused with 250 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES), 125 mM tris(hydroxymethyl)aminomethane (Tris) and 10 μM free Ca2+ buffered with EGTA and CaCl2, pH 7.2. The trans chamber was perfused with 250 mM glutamic acid and 10 mM HEPES and the pH was brought to 7.2 with Ca(OH)2 (free [Ca2+] approximately 50 mM). Experiments were performed at room temperature (22±2°C). The free [Ca2+] and pH of the solutions were determined at 22°C using a Ca2+ electrode (Orion 93–20) and Ross-type pH electrode (Orion 81–55) as previously described (Sitsapesan et al., 1991). Additions of ATP, adenosine and adenine were made to the cis chamber and the free [Ca2+] was maintained at 10 μM at all ligand concentrations.
Data acquisition and analysis
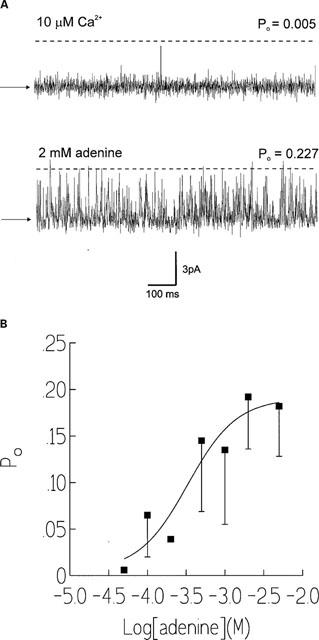
Single channel recordings were displayed on an oscilloscope and recorded on digital audio tape (DAT). Steady-state recordings were carried out at 0 mV. At this holding potential, Ca2+ current flows in the luminal to cytosolic direction. The current recordings were filtered at 0.5 kHz (−3 dB) and digitized at 2 kHz using Satori (Intracel, Cambridge, U.K.). Channel open probability (Po) and the lifetimes of the open and closed events were determined over 3 min of recording using the method of 50% threshold analysis (Colquhoun & Sigworth, 1983). When more than one channel was incorporated into the bilayer, average Po was calculated according to the formula:
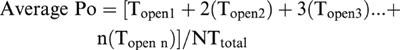 |
where Topen1, Topen2, Topen3 are the times in the first, second and third open channel levels respectively, Ttotal is the total recording time and N is the number of channels in the bilayer. The number of channels in the bilayer was determined by adding EMD 41000 (a caffeine analogue that maximally activates the channels) at the end of each experiment. Lifetime analysis was carried out only when a single channel was incorporated into the bilayer. Events <1 ms in duration were not fully resolved and were excluded from lifetime analysis. Lifetimes accumulated from 3-min steady-state recordings were stored in sequential files and displayed in noncumulative histograms. Individual lifetimes were fitted to a probability density function (pdf) by the method of maximum likelihood (Colquhoun & Sigworth, 1983) according to the equation:
with areas a and time constants τ. A missed events correction was applied as described by Colquhoun & Sigworth (1993). A likelihood ratio test (Blatz & Magleby, 1986) was used to compare fits to up to four exponentials by testing twice the difference in loge (likelihood) against the chi-squared distribution at the 1% level. Single-channel current amplitudes were measured from digitized data using manually controlled cursors. All Po values are quoted as mean±s.e.mean where n⩾4. For n=3, standard deviation (s.d.) is given.
Comparative molecular field analysis
Comparative molecular field analysis (CoMFA) is a three-dimensional quantitative structure-activity relationship technique (Cramer & Patterson, 1988). Briefly, the Lennard-Jones (steric) potentials and electrostatic potentials are measured at the points in a three dimensional array around each member of a series of compounds. Changes in these potentials are correlated with changes in the biological properties of the molecules using the method of partial least squares. We used SYBYL6.6 (Tripos Associates, St. Louis, MO, U.S.A.) and the Tripos force field for all of the calculations reported in this communication. The structures of ATP and analogues were correlated with the ability of these molecules to maximally activate the sheep cardiac RyR in the presence of 10 μM cytosolic Ca2+. The conformation of ATP and analogues was based on the conformation of the bound nucleotide analogue (inhibitor) in Saccharomyces cerevisiae adenylate kinase (PDB ID: 1aky) (Abele & Schulz, 1995). The conformation of the compounds in the enzyme binding site was determined by molecular mechanics and molecular dynamics while keeping the peptide backbone conformation rigid. These minimized molecules were removed from the protein and used for CoMFA after alignment by the adenine ring. Cross-validation was used to test for predictive ability of the model. A cross-validated correlation coefficient of 1.0 indicates no deviation between predicted and measured biological properties; a value of 0.0 indicates a random correlation between structure and biological activity. A cross-validated correlation coefficient of 0.3 indicates a less than 5% probability that the relationship between structure and activity is due to chance (Clark & Cramer, 1993; Clark et al., 1990). If the cross-validated correlation coefficients are sufficiently high, partial least squares is used to build a model using all of the tested compounds. This final correlation coefficient can be interpreted analogously to a conventional correlation coefficient.
Materials
ATP, adenosine and adenine were 99% pure (Sigma, Poole, U.K.). EMD 41000 was a gift from Merck (64271 Darmstadt, Germany). Phosphatidylethanolamine was purchased from Avanti Polar Lipids, Birmingham, AL, U.S.A.). Solutions were prepared using MilliQ deionized water (Millipore, Harrow, U.K.) and filtered through a Millipore membrane filter (pore size 0.45 μm) before use. Other chemicals were AnalaR or the best equivalent grade from BDH or Sigma (Poole, U.K.).
Results
The effects of adenosine on RyR gating
Under control ionic conditions the cytosolic [Ca2+] was maintained at 10 μM. At this [Ca2+], the Po of cardiac RyR channels is very low and the gating is characterized by brief opening events which are often not fully resolved. A typical example of Ca2+-activated channel openings is shown in Figure 1A (top trace). Addition of adenosine (10 μM to 5 mM) to the cytosolic channel side increased Po. Optimal activation of RyR was achieved at approximately 1 mM adenosine which increased Po from 0.07±0.03 to 0.11±0.06 (s.e.mean; n=4). Further increases in adenosine concentration did not lead to further increases in Po. The relationship between Po and [adenosine] is shown in Figure 1B. The figure clearly demonstrates that, in the presence of 10 μM cytosolic Ca2+, adenosine is a very ineffective activator of the channel and even high millimolar levels cannot fully activate the channel. This is in contrast to the effect of ATP under identical conditions which can almost fully activate the channel (Po in the presence of 2 mM ATP is approximately 0.9) (Kermode et al., 1998; Ching et al., 1999). Adenosine is therefore acting as a partial agonist at the cardiac RyR with very low efficacy. Figure 1A shows that even the maximally effective concentration of adenosine increased Po by increasing the frequency of channel openings with very little effect on the duration of openings.
Figure 1.
(A) Effects of adenosine on a representative channel. The holding potential is 0 mV. In the top trace, the channel is activated solely by 10 μM Ca2+. In the lower trace, cytosolic adenosine (2 mM) has been added. The arrows and dotted lines illustrate the closed and open channel levels respectively. (B) Relationship between [adenosine] and Po. s.e.mean for ⩾n=4 are shown. The EC50 value and Hill coefficient were 0.27 mM and 1.7 respectively.
Competition of adenosine with ATP
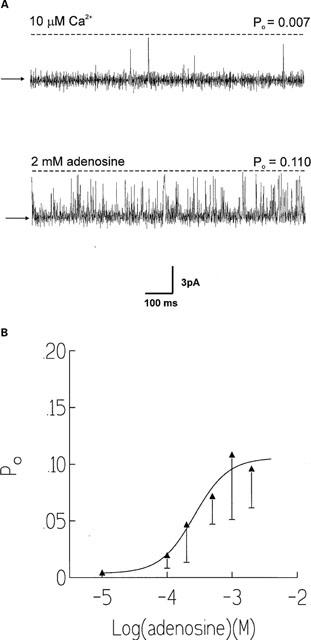
If adenosine is indeed acting as a partial agonist of low efficacy at the ATP sites on RyR then it would be expected to antagonize the effects of ATP. Figure 2 provides evidence that adenosine and ATP do compete for the same binding sites. Figure 2A illustrates a representative experiment in which two channels have incorporated into the bilayer. In the top trace the channels have been activated by 1 mM ATP in the presence of 10 μM cytosolic Ca2+. Subsequent addition of adenosine (1 mM) causes a marked lowering of Po (bottom trace). Figure 2B summarizes the effectiveness of 100 μM and 1 mM adenosine at reducing the activation of RyR caused by 1 mM ATP. 100 μM and 1 mM adenosine inhibits the activation caused by 1 mM ATP by approximately 57% (n=3) and approximately 74% (n=3) respectively. From these inhibition data, we can estimate the inhibition constant for adenosine. If one assumes simple competition for the ATP binding site, the apparent adenosine inhibition constant is 0.2 mM, which is consistent with the experimentally determined EC50 value of adenosine.
Figure 2.
The effects of adenosine on channels already activated by 1 mM ATP. (A) A typical experiment in which two channels have incorporated into the bilayer and are activated by ATP (1 mM) in the presence of 10 μM cytosolic Ca2+ (top trace). Subsequent addition of 1 mM adenosine reduces Po (lower trace). The arrows illustrate the zero current level and the dotted lines illustrate the open channel levels. (B) Histogram illustrating the reduction in Po that occurs after addition of 100 μM and 1 mM adenosine to channels already activated by 1 mM ATP. Po is plotted as a percentage of the Po in the presence of 1 mM ATP which is taken to be 100%. s.e.mean from three experiments shown.
The effects of adenine on RyR gating
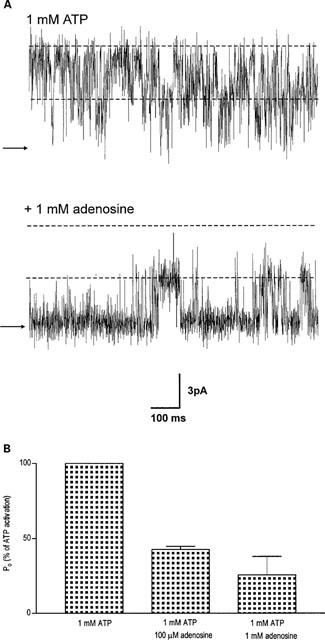
The ability of adenine to activate the channel in the presence of 10 μM cytosolic free Ca2+ is shown in Figure 3A for a typical single channel. Adenine was slightly more effective than adenosine at increasing Po and optimal activation of the channel was observed at approximately 2 mM adenine and was 0.19±0.06 (s.e.mean; n=5). Figure 3B illustrates the adenine dose-response curve. As was observed with adenosine, it can be seen from Figure 3A, that increasing concentrations of adenine appeared to increase Po predominantly by increasing the frequency of channel openings and no large increases in mean open time were observed. In control conditions with Ca2+ as the sole activating ligand, the mean open and closed lifetimes were 0.76±0.31 ms and 78.28±68.04 ms (s.d.; n=3) respectively. Addition of 500 μM adenine resulted in mean open and closed times of 2.18±0.88 ms and 3.51±0.39 ms respectively. Figure 4A illustrates the changes in Po and mean open and closed lifetimes that occur in a representative channel as the concentration of adenine is increased. The figure demonstrates that there is very little change in the duration of open events compared to the large changes in mean closed time and Po. A similar relationship between Po and the mean open and closed lifetimes was observed for adenosine. Figure 4B illustrates that the mechanism for ATP activation of the cardiac RyR is very different. Not only does ATP increase Po to much higher levels than adenine (optimal activation =0.95 with 2 mM ATP compared with 0.24 with 5 mM adenine) but a dose-dependent increase in mean open lifetimes is observed together with the dose-dependent reduction in mean closed times. Analysis of the open and closed lifetime distributions confirms our understanding of the mechanisms underlying adenine activation of RyR and can be seen in Figure 5. In the presence of 10 μM cytosolic Ca2+, the best fit to the open and closed lifetime distributions was obtained with two and three exponentials respectively indicating that there are at least two open and three closed channel states (Figure 5A). Addition of a maximally effective concentration of adenine (2 mM; Figure 5B) led to a slight redistribution of the open events towards the longer time constant but no increase in the open time constants was observed. In contrast, the closed time constants were much shorter after addition of adenine. Most of the closed lifetimes occurred to the shortest time constant and the third longest time constant could no longer be resolved.
Figure 3.
(A) Effects of adenine on a typical single cardiac RyR channel. A single channel has incorporated into the bilayer and the holding potential is 0 mV. In the top trace, the channel is activated solely by 10 μM Ca2+. Subsequent addition of 2 mM cytosolic adenine is shown in the following trace. The arrows and dotted lines illustrate the closed and open channel levels respectively. (B) Relationship between [adenine] and Po. s.e.mean for ⩾n=4 are shown. The EC50 value and Hill coefficient were 0.31 mM and 1.8 respectively.
Figure 4.
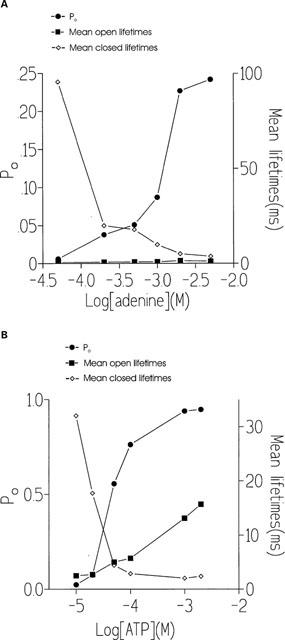
Effect of adenine (A) and ATP (B) on Po, mean open lifetimes and mean closed lifetimes from typical RyR channels.
Figure 5.
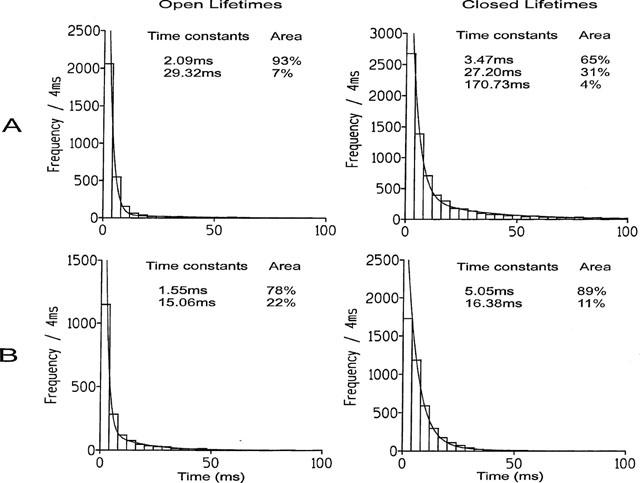
Open and closed lifetime distributions and probability density functions (pdfs) for a representative channel activated by (A) 10 μM cytosolic Ca2+ and (B) 10 μM Ca2+ plus 2 mM adenine. The best fits to the data were obtained by the method of maximum likelihood and the resulting time constants and corresponding percentage areas are shown.
The effects of GTP on RyR gating
Cytosolic additions of GTP (100 μM–10 mM) failed to produce any observable effects on the Po of the cardiac RyR. For example, Po was 0.029±0.013 (s.e.mean; n=7) in the absence of GTP and 0.029±0.017 and 0.035±0.035 (s.e.mean; n=5) in the presence of 2 mM and 10 mM GTP respectively. These results are in line with previous reports that GTP cannot stimulate the release of [45Ca2+] from isolated SR membrane fractions (Meissner, 1984; Morii & Tonomura, 1983) indicating that the purine ring system may be essential for agonist activity at the ATP site on the sheep cardiac RyR.
Comparison of the effects of adenine compounds on cardiac RyR gating
The activating effects of adenosine and adenine on the native sheep cardiac RyR in the present study can be compared with the effects of ATP, ADP and AMP reported in our earlier papers (Kermode et al., 1998; Ching et al., 1999) as identical experimental conditions were used. In addition, we regularly check the ATP-sensitivity of RyR from different SR preparations. Figure 6 compares the EC50 values and the maximum Po achievable at 10 μM Ca2+, under the above experimental conditions, for ATP, ADP, AMP, adenosine and adenine. The figure demonstrates that as one or two phosphate groups are removed from ATP to give ADP and AMP, there is a dramatic reduction in both the affinity of the compound (evidenced by the EC50 value) and the ability of the compound to activate the channel (maximum Po). Having no phosphate groups (adenosine) or removal of the ribose group (adenine) produces a marked increase in affinity but appears to cause only a slight improvement in the ability of the compound to open the channel. The efficacy of adenosine is only slightly greater than that of AMP and therefore the addition of a single phosphate group to the 5′-carbon of the ribose ring does not significantly alter the effectiveness of the ligand once bound to the ATP sites on the cardiac RyR. A striking difference between the effects of AMP and adenosine, however, were found when comparing the EC50 values. Half-maximal activation of the channel was obtained at approximately 3 mM with AMP (Ching et al., 1999) and at approximately 0.3 mM with adenosine. The affinity of adenosine is therefore more similar to that of ATP (EC50=0.22 mM) (Kermode et al., 1998).
Figure 6.
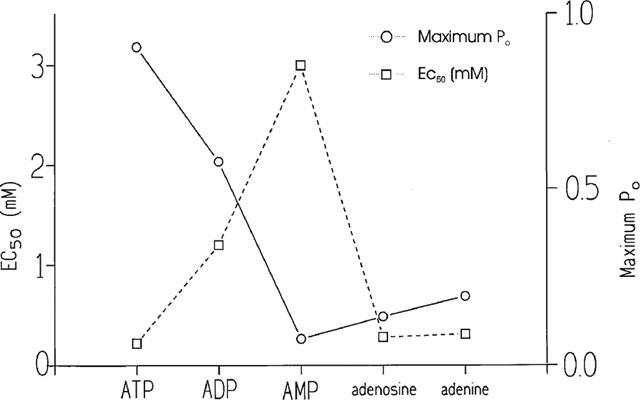
Comparison of the affinity and efficacy of ATP, ADP, AMP, adenosine and adenine. The affinity (EC50 value) of the ligands for the cardiac RyR is shown by the squares and the efficacy of the ligands once bound (maximum Po achievable under the experimental conditions used) is shown by the circles.
To examine the possible reasons for the differences in affinity and efficacy exhibited by the various adenine-based compounds we have compared the mean open and closed lifetimes that occurred at optimal channel activation by each ligand and the data is shown in Figure 7. The figure demonstrates that the ability of ATP to increase open lifetime duration is much greater (approximately 10 times or greater) than that of any other compound. ATP is also the most effective at reducing the duration of closed lifetimes although adenine and adenosine are also very effective in this respect. Comparison of Figures 6 and 7 reveals trends that are suggestive of the possible underlying mechanisms responsible for high affinity and efficacy. There is an obvious similarity in the trend for efficacy (maximum Po attainable; Figure 6) and the ability of the ligand to increase open lifetime duration (Figure 7). There is also a remarkable similarity in the trend for affinity (EC50; Figure 6) and the ability to reduce closed lifetime durations (Figure 7). Not entirely unexpectedly therefore, these results indicate that the affinity of the adenine-based compounds for the cardiac RyR is primarily related to the ability of the ligand to bind to the closed channel state and reduce closed lifetime durations. In contrast, the efficacy of the ligand appears to depend to a great extent on the ability of the ligand to stabilize the open channel state. This may result from a slow dissociation rate of the ligand from the open state or the ability of the ligand to induce transitions to a long open state.
Figure 7.
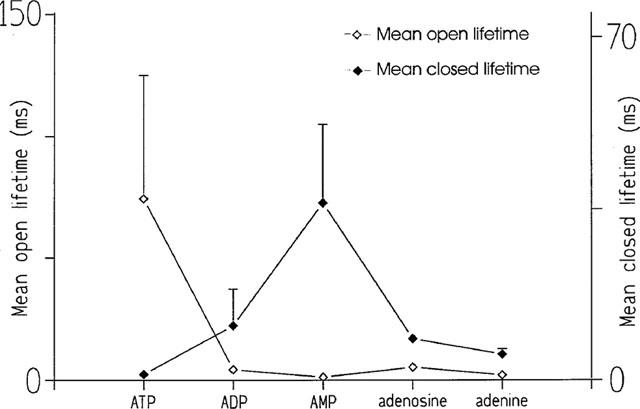
Comparison of the ability of ATP, ADP, AMP, adenosine and adenine to affect the mean open lifetimes and mean closed lifetimes of the cardiac RyR.
Another obvious difference in the mechanism of channel activation by the different ligands can be highlighted by comparing the Hill coefficients for activation. The Hill coefficients were 1.5 for ATP, 0.97 for ADP and 0.78 for AMP (Kermode et al., 1998; Ching et al., 1999) whereas for adenine and adenosine the Hill coefficients were 1.8 and 1.7 respectively.
CoMFA
Here we report the results of a CoMFA of the relationship between the structure of the ATP analogues and the ability to increase the Po of the channel. Po is a property of the RyR-ligand complex and therefore these relationships depend on the interaction energy between ligand and RyR in the complex and do not reflect the affinity of the ligand for the RyR. We found an extremely strong correlation between structural changes in the ATP analogues and changes in Po. The cross-validated correlation coefficient was 0.56 indicating the high predictive reliability of the model presented here. The final correlation coefficient was 0.997. Changes in electrostatic field account for 64% of the total correlation between structure and function. However, steric factors are important and account for 36% of the total correlation. Inclusion of GTP (which failed to increase Po) in the CoMFA drops the cross-validated correlation coefficient to −0.45, indicating that GTP-RyR interactions are qualitatively different from those of the adenine series. We interpret the drastic change in correlation to indicate that GTP fails to modulate Po because it fails to bind to RyR at the concentrations used.
In Figure 8 the contours reflect the locations where changes in analogue structure are most strongly correlated to changes in Po; ATP is shown to orient the reader to the location of the contours. The correlation between changes in electrostatic charge and changes in Po are shown in the lower panels of Figure 8. The wire frame in the right panel encloses the region where increases in negative electrostatic potential are most strongly correlated with increases in Po. This region is centred to the right of the γ-phosphate. Because of the unequal charge distribution between atoms (e.g., the oxygens and the phosphates) the effect of electrostatic field is complex. In the left panel, the wire frame encloses the region where increasing positive electrostatic potential is most strongly correlated with increasing Po. Note that the major correlation between electric field and Po is located in a diffuse area around the phosphate groups.
Figure 8.
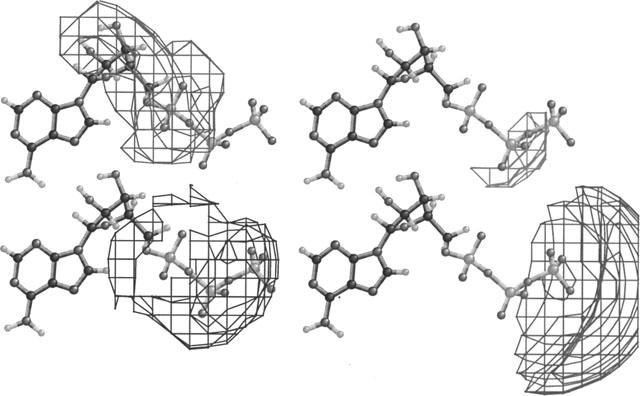
Relationship between structure and Po. The wireframes enclose the regions in space where changes in structure are most strongly correlated with changes in Po. A ball-and-stick representation of ATP is shown as a guide to the eye. Lower left: region where increases in positive electrostatic potential correlate with increases in Po. Lower right: region where increases in negative electrostatic potential correlate with increased Po. Upper left: region where increases in steric bulk correlate with decreases in Po. Upper right: region where increases in steric bulk correlate with increases in Po.
In contrast, the steric correlations are less diffuse and primarily oppose the opening of the RyR channel (Figure 8, upper panels). The right-hand panel shows the region accounting for the major part of the correlation between increased steric bulk and increased Po. This region is confined to the region between the β- and γ-phosphate groups. The left panel shows the much larger correlation between increased steric bulk and decreased Po. These loci include the α-phosphate and the ribose.
Discussion
A surprising outcome of the single channel experiments performed in this study was that removing the single phosphate from the 5′ carbon on the ribose group of AMP caused an approximately 10 fold improvement in affinity coupled with only a slight improvement in the efficacy of the ligand. Thus, adenosine and adenine are partial agonists at the cardiac RyR but exhibit similar affinity for RyR as ATP. Figures 6 and 7 indicate that the affinity of adenine-based compounds for the cardiac RyR is related to the ability of the ligand to reduce closed lifetimes. As all the ligands share the capacity to reduce the closed lifetime durations it is reasonable to assume that they bind to the closed channel state/s, presumably a Ca2+-bound state since adenine nucleotides are virtually without effect in the absence of activating cytosolic [Ca2+] (Kermode et al., 1998; Ching et al., 1999). An additional parameter that must be considered is the cooperative nature of the increases in Po induced by the adenine-based compounds. The dose-response curves to ATP, adenosine and adenine indicate positively cooperative behaviour and suggest that at least two molecules bind to RyR to produce increases in Po. In contrast, ADP activation appears not to show cooperativity whereas AMP activation indicates negative cooperativity. It appears that when a molecule of AMP binds, the resulting conformational change in RyR is such that it is more difficult for the next molecule to bind. In our earlier papers we speculated that a Hill coefficient >1 might be related to the ability of the ligand to bind to the open state of the channel in addition to the closed state (Kermode et al., 1998; Ching et al., 1999). This appeared reasonable when the results for ATP, ADP and AMP were considered, especially in the light of the effects of increasing cytosolic [Ca2+] on AMP activation of the channel. Increasing cytosolic [Ca2+] from 10 to 65 μM led to increases in the affinity and efficacy of AMP coupled with an increase in the Hill coefficient from 0.78 to 1.5 and in the ability of AMP to increase the duration of open lifetimes. This theory does not appear to hold true, however, for adenosine and adenine. At 10 μM cytosolic free [Ca2+], adenine and adenosine are relatively ineffective at increasing open lifetimes and Po but both compounds have Hill coefficients of approximately 2 indicating positive cooperativity. A simple explanation is that multiple adenosine and adenine molecules bind to the closed channel states at 10 μM Ca2+. In contrast to adenine and adenosine, ATP causes marked increases in the duration of channel openings (Figure 4B) in a concentration-dependent manner and this appears to be crucial for a ligand to exhibit high efficacy (or the ability to increase Po to high levels). If increasing concentrations of ATP (or other ligands) produce increases in open times this suggests that the duration of open times is not solely dependent on the dissociation rate of ATP (or other ligands) from the open states. Therefore the reason that ATP increases open lifetimes more than other ligands used in this study is not simply because the dissociation rate of ATP from the open channel state is slower than that of other ligands; ATP-dependent transitions to an additional long open state must occur. This is in agreement with lifetime analysis of ATP-activated RyR that demonstrates the induction of a third long open state by ATP (Kermode et al., 1998). Understanding the agonist actions of adenine nucleotides is complicated by the fact that the effects are strongly Ca2+-dependent. It is likely, for example, that Ca2+-dependent transitions between the various closed and open channel states will vary with different adenine ligands bound to the channel which in turn will affect subsequent adenine-ligand binding interactions. The complex interactions of Ca2+ and ATP analogues with the channel therefore requires much further experimentation before a full understanding of adenine-dependent activation of the cardiac RyR can be achieved.
The CoMFA provides us with a functional map of the interactions between the ligand and the RyR nucleotide-binding site. The large electrostatic field established by the ionized phosphate groups provides most of the energy required to increase the Po of the RyR channel. This electrostatic field overcomes the steric antagonism of the α-phosphate and ribose groups that tend to stabilize the closed form of the RyR channel. Complex and opposing interactions between a ligand and its receptor are not surprising as not all ligand-receptor interactions are favourable. It is important to recall that, because of the nature of the measurement of Po, these correlations between structure and Po represent the energy of interaction between ligand and RyR in the complex and do not reflect those excess energies related to the affinity of the ligands for the RyR. The phosphoryl groups of the nucleotides provide a dipolar field that stabilizes the open conformation of the RyR channel. As the strength of this field is diminished by decreasing the number of phosphates, the magnitude of Po is reduced. The results suggest that electrostatic interactions are particularly important in determining the conformational state of the channel. The CoMFA map is consistent with the notion that several amino acids dispersed over a large region of the nucleotide binding site are involved in transducing the electrostatic potential into a conformation change in the RyR. This is in contrast to a model where the formation of a single ionic bond (salt-link) is the primary factor in the enhanced Po. The CoMFA map shows us that most of the energy of steric interactions between nucleotide and RyR actually promote the closed state (or at least provide an obstacle to induction of the open state). However, this effect is confined to discrete regions of the nucleotide binding site–that is the loci which interact with the α-phosphate and the ribose. Removal of these groups from the activating ligands actually enhances the ability of the ligand to induce the open state of the channel. This insight provides an explanation as to why AMP is a less potent inducer than one would expect based on the number of phosphate groups and why adenine is a better inducer of the open state than either AMP or adenosine. According to this model, the adenine group provides little energy that can be used to drive the RyR into an open state. However, as demonstrated by the use of GTP, the adenine group is essential for nucleotide binding and provides the energy required for molecular recognition of the correct modulator of RyR channel function. In a future publication, we will provide a more detailed analysis of the interactions at the nucleotide-binding site.
An important finding of the present study is that adenosine has an affinity for the cardiac RyR which is similar to that of ATP but an efficacy which is much lower. Our single channel studies also demonstrate that adenosine is very effective at antagonizing the effects of ATP; 100 μM adenosine produces more than 50% inhibition of the activation caused by 1 mM ATP. Thus, the potential in vivo regulatory effects of adenosine at cytosolic [Ca2+] ⩽10 μM cannot be ignored. This surprising action of adenosine would be most likely to affect SR Ca2+-release under conditions of myocardial ischaemia when intracellular ATP levels are depleted and millimolar quantities of adenosine are produced (for example, Allen & Orchard, 1987; Allue et al., 1996; Bratten & Cobbold, 1998).
By combining single channel studies with CoMFA we have been able to explore the mechanisms underlying the affinity and efficacy of ligands which interact at the ATP binding sites on the cardiac RyR and suggest a model to explain the molecular interactions between the ligand and RyR which lead to an increase in Po.
Acknowledgments
This study was supported by The British Heart Foundation.
Abbreviations
- CoMFA
comparative molecular field analysis
- DAT
Digital Audio Tape
- HEPES
N′-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
probability density function
- Po
open probability
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- Tris
tris(hydroxymethyl)-methylamine
References
- ABELE U., SCHULZ G.E. High-resolution structures of adenylate kinase from yeast ligated with inhibitor Ap5A, showing the pathway of phosphoryl transfer. Protein Sci. 1995;4:1262–1271. doi: 10.1002/pro.5560040702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLEN D.G., ORCHARD C.H. Myocardial contractile function during ischemia and hypoxia. Circulation Research. 1987;60:153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- ALLUE I., GANDELMAN O., DEMENTIEVA E., UGAROVA N., COBBOLD P. Evidence for rapid consumption of millimolar concentrations of cytoplasmic ATP during rigor-contracture of metabolically compromised single cardiomyocytes. Biochem. J. 1996;319:463–469. doi: 10.1042/bj3190463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHLEY R.H., WILLIAMS A.J. Divalent cation activation and inhibition of single calcium release channels from sheep cardiac sarcoplasmic reticulum. J. Gen. Physiol. 1990;95:981–1005. doi: 10.1085/jgp.95.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERS D.M. Excitation-contraction coupling and cardiac contractile force. Dordrecht: Kluwer; 1991. pp. 119–148. [Google Scholar]
- BEUCKELMANN D.J., WIER W.G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J. Physiol. 1988;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLATZ A.L., MAGLEBY K.L. A quantitative description of 3 modes of activity of fast chloride channels from rat skeletal muscle. J. Physiol. 1986;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRATTEN C.D.T., COBBOLD P.H. Single-cell measurements of purine release using a micromachined electroanalytical sensor. Analyt. Chem. 1998;70:1164–1170. doi: 10.1021/ac970982z. [DOI] [PubMed] [Google Scholar]
- CHING L.L., WILLIAMS A.J., SITSAPESAN R. AMP is a partial agonist at the sheep cardiac ryanodine receptor. Brit. J. Pharmacol. 1999;127:161–171. doi: 10.1038/sj.bjp.0702491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK M., CRAMER R.D., III The probability of chance correlation using partial least squares (PLS) Quant. Struct. -Act. Relat. 1993;12:137–145. [Google Scholar]
- CLARK M., CRAMER R.D., III, JONES D.M., PATTERSON D.E., SIMEROTH P.E. Comparative molecular field analysis (CoMFA): Towards its use with 3D-structural databases. Tetrahedron Comp. Method. 1990;3:47–60. [Google Scholar]
- COLQUHOUN D., SIGWORTH F.J.Fitting and statistical analysis of single-channel recording Single-channel recording 1983New York & London: Plenum; 191–263.eds. Sakmann, B. & Neher, E. pp [Google Scholar]
- CRAMER R.D., III, PATTERSON D.E. Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J. Am. Chem. Soc. 1988;110:5959–5967. doi: 10.1021/ja00226a005. [DOI] [PubMed] [Google Scholar]
- KERMODE H., WILLIAMS A.J., SITSAPESAN R. The interactions of ATP, ADP and inorganic phosphate with the sheep cardiac ryanodine receptor. Biophys. J. 1998;74:1296–1304. doi: 10.1016/S0006-3495(98)77843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISSNER G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J. Biol. Chem. 1984;259:2365–2374. [PubMed] [Google Scholar]
- MORII H., TONOMURA Y. Ca2+-induced Ca2+ release from fragmented sarcoplasmic reticulum. J. Biochem. 1983;93:1271–1285. doi: 10.1093/oxfordjournals.jbchem.a134261. [DOI] [PubMed] [Google Scholar]
- NABAUER M., CALLEWAERT G., CLEEMANN L., MORAD M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989;244:800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- O'NEILL S.C., SMITH G.L. The effect of reduced ATP concentrations on frequency and amplitude of spontaneous release of Ca2+ in rat permeabilised ventricular myocytes. Biophys. J. 2000;78:439A. [Google Scholar]
- SITSAPESAN R.Regulation of ryanodine receptor channel gating The Structure and Function of Ryanodine Receptors 1998London: Imperial College Press; eds. Sitsapesan, R. & Williams, A.J. [Google Scholar]
- SITSAPESAN R., MONTGOMERY R.A.P., MACLEOD K.T., WILLIAMS A.J. Sheep cardiac sarcoplasmic reticulum calcium release channels: modification of conductance and gating by temperature. J. Physiol. 1991;434:469–488. doi: 10.1113/jphysiol.1991.sp018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SITSAPESAN R., WILLIAMS A.J. Gating of the native and purified cardiac SR Ca2+-release channel with monovalent cations as permeant species. Biophys. J. 1994;67:1484–1494. doi: 10.1016/S0006-3495(94)80622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J.S., CORONADO R., MEISSNER G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985;316:446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- ZAHRADNÍKOVÁ A., ZAHRADNÍK I. Description of modal gating of the cardiac calcium release channel in planar lipid membranes. Biophys. J. 1995;69:1780–1788. doi: 10.1016/S0006-3495(95)80048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


