Abstract
Aminobisphosphonates (aminoBPs) are potent inhibitors of bone resorption. However, they cause undesirable inflammatory reactions, including fever, in humans. Intraperitoneal injection of aminoBPs into mice also induces inflammatory reactions, including a prolonged elevation of the activity of the histamine-forming enzyme, histidine decarboxylase (HDC). Because interleukin-1 (IL-1) is a typical pyrogen and a strong inducer of HDC, we examined whether aminoBPs induce inflammatory reactions in mice deficient in genes for both IL-1α and IL-1β (IL-1-KO mice).
In control mice, aminoBPs induced an elevation of HDC activity and other inflammatory reactions (enlargement of the spleen, atrophy of the thymus, exudate in the thorax and increase in granulocytic cells in the peritoneal cavity). These responses were all weak or undetectable in IL-1-KO mice.
We have previously shown that lipopolysaccharides (LPSs) from Escherichia coli and Prevotella intermedia (a prevalent gram-negative bacterium both in periodontitis and endodontal infections) are capable of inducing HDC activity in various tissues in mice. In control mice treated with an aminoBP, the LPS-induced elevations of serum IL-1 (α and β) and tissue HDC activity were both markedly augmented. However, such an augmentation of HDC activity was small or undetectable in IL-1-KO mice.
These results, taken together with our previous findings (i) suggest that IL-1 is involved in the aminoBP-induced inflammatory reactions and (ii) lead us to think that under some conditions, inflammatory reactions induced by gram-negative bacteria might be augmented in patients treated with an aminoBP.
In this study, we also obtained a result suggesting that IL-1-deficiency might be compensated by a second, unidentified, mechanism serving to induce HDC in response to LPS when IL-1 is lacking.
Keywords: Histidine decarboxylase, histamine, interleukin-1, bisphosphonates, lipopolysaccharide
Introduction
Bisphosphonates (BPs) with a nonhydrolysable P-C-P structure are analogues of pyrophosphonate. BPs strongly inhibit bone resorption and many derivatives of BPs have been developed for use against diseases involving enhanced bone resorption, such as Paget' disease, tumoral osteolysis, tumoral hypercalcaemia, osteoporosis and rheumatoid arthritis (Rodan & Fleisch, 1996; Bonjour et al., 1994; Geddes et al., 1994). Among these derivatives, the aminobisphosphonates (aminoBPs) exhibit particularly potent activity levels (Bonjour et al., 1994; Geddes et al., 1994). However, undesirable inflammatory reactions occur in human patients following administration of aminoBPs, including an increase in acute-phase proteins, fever (in 10–50% of treated patients) and, when they are given orally, gastrointestinal disturbances (Adami et al., 1987; Sauty et al., 1996; Thiébaud et al., 1997; Fleisch, 1997). In addition, inflammatory side effects have been reported in the field of ophthalmology (Siris, 1993; Macarol & Frauenfelder, 1994).
BPs bind irreversibly to hydroxyapatite in bone, in which they accumulate during repeated administration. In addition, aminoBPs, but not non-aminoBPs, are deposited in significant amounts in the liver and spleen in mice (Mönkkönen et al., 1989). We found that in mice, a single intraperitoneal injection at an experimental dose (larger than those used clinically in mg/kg terms) of any one of the aminoBPs tested induced a prolonged elevation of the activity of histidine decarboxylase (HDC, the histamine-forming enzyme) in the liver, spleen, lung and bone (tibia, femur and mandible), resulting in an increase in histamine (Endo et al., 1993; Funayama et al., 2000). This effect was not induced by non-aminoBPs. In addition, aminoBPs induced hypertrophy of the spleen, atrophy of the thymus, ascites, accumulation of exudate in the thorax and an increase in the number of granulocytic cells in the peritoneal exudate (Endo et al., 1993). Repeated daily injections of small doses of aminoBPs also resulted in an elevation of HDC activity (Endo et al., 1993), and weakly injections of small doses exacerbated experimental arthritis in mice (Nakamura et al., 1996).
Some years ago, we found that a macrophage cell line, P388D1, can produce two IL-1-like factors capable of inducing HDC in various tissues when injected into mice (Endo et al., 1986). More recent studies have shown that HDC can be induced by the recombinant cytokines IL-1(α and β), TNF(α and β), G-CSF, GM-CSF and IL-3 (Endo et al., 1986; 1992b; Endo, 1989; Lebel et al., 1990; Schneider et al., 1990; Dy et al., 1993). In mice, recombinant IL-1 and TNF both induce HDC activity in a number of tissues (Endo et al., 1995; 1998) but G-CSF, GM-CSF and IL-3 induce it only in haematopoietic organs (spleen and bone marrow).
Endotoxin, a mixture of constituents from the cell walls of gram-negative bacteria, is involved in inflammatory reactions following bacterial infection. The lipopolysaccharide (LPS) component of endotoxin is thought to play important roles in these reactions. The structure of the O-antigen of a typical LPS–as found in Enterobacteriaceae (Esherichia coli, Salmonella and Klebsiella)–is made of repeating units of oligosaccharide (Reitschel et al., 1994). Black-pigmented bacteria, such as Porphyromonas gingivalis and Prevotella intermedia, are the dominant gram-negative bacteria in the periodontal pockets of patients with periodontitis (Zambon et al., 1994). These bacteria are also prevalent in endodontal and periapical infections (Baumgartner & Falkler, 1991; Bae et al., 1997), suggesting their contribution to the development of periodontitis and osteomyelitis (Dahlén & Möller, 1992). Interestingly, LPSs from some black-pigmented bacteria (such as P. intermedia) lack the repeating oligosaccharide structure seen in typical Enterobacteriaceae LPSs (Takada et al., 1990). In addition, the biological activities of oral bacterial LPSs are not the same as those of enterobacterial LPSs (Wilson, 1995; Endo et al., 1997). We have shown that an E. coli-LPS induces a marked elevation of HDC activity in various mouse tissues (Endo et al., 1995). Recently we showed that a P. intermedia LPS also has the ability to induce HDC activity in various tissues in mice, including the mandible, although its potency was much smaller than that of E. coli LPS (Endo et al., 1997; Funayama et al., 2000).
The elevation of HDC activity by IL-1 or by E. coli LPS occurs through the formation of HDC-mRNA (Kikuchi et al., 1997). Because various LPSs can stimulate the production of the cytokines mentioned above, it is thought that the cytokines may mediate the elevation of HDC activity induced by LPSs. Among these cytokines, IL-1 seems to play a key role in the induction of HDC because (i) IL-1 induces HDC in various organs as well as haematopoietic organs; (ii) IL-1 is particularly potent at inducing HDC activity, the effective dose being 100–1000 times less than that of TNF (Endo, 1989); (iii) IL-1 augments the HDC-inducing effect of TNF (Endo, 1989); (iv) in terms of time kinetics, the LPS-induced production of IL-1 corresponds well to the elevation of HDC activity (Endo & Kumagai, 1998); (v) alendronate, a typical aminoBP, augments both the LPS-induced production of IL-1 and the LPS-induced elevation of HDC activity (Sugawara et al., 1998); and (vi) dichloromethylene bisphosphonate (clodronate), a typical non-aminoBP, reduces the ability of aminoBPs to augment both the production of IL-1 and the elevation of HDC activity induced by LPS (Endo et al., 1999).
In the present study, therefore, we examined the role of IL-1 in the inflammatory actions of aminoBPs, using mice deficient in genes for both IL-1α and IL-1β (IL-1-KO mice). In addition, since, as mentioned above, the LPS-induced production of IL-1 and elevation of HDC activity are both augmented in aminoBP-treated mice, the combined effect of an aminoBP plus LPS from either E. coli or P. intermedia was also examined.
Methods
IL-1-deficient and control mice
Homozygous BALB/cA mice deficient in both IL-1α and IL-1β (IL-1α/β double-knockout mice, IL-1-KO mice), originally produced by Iwakura and his co-workers (Horai et al., 1998), were bred in our laboratory. The deficiency of the genes of IL-1α and IL-1β was confirmed by Northern blot hybridization analysis of LPS-stimulated peritoneal macrophages prepared from the IL-1-KO mice (Horai et al., 1998). The IL-1-KO mice were fertile, and the pups were born healthy, although previous studies have suggested that IL-1 is important in gonadotropin release, oogenesis, testosterone synthesis and in the maintenance of pregnancy (Hurwitz et al., 1991; De et al., 1992). After birth, they developed normally in terms of their increase in body weight (Horai et al., 1998). Control BALB/cA mice (6–7 weeks old) were obtained from the facility for experimental animals in Tohoku University. Males of both the control and IL-1-KO strains were used in the present study. All experiments complied with the Guidelines for Care and Use of Laboratory Animals in Tohoku University.
Reagents
4-Amino-1-hydroxybutylidene-1,1-bisphosphonic acid (AHBuBP, alendronate) was synthesized by us. Cycloheptylaminomethylene bisphosphonate (CHAMBP, incadronate) and 3-(N-methyl-N-pentyl)amino-1-hydroxypropane-1,1-diphosphonic acid (MP-AHPrBP, ibandronate) were provided by Yamano-uchi Pharmaceutical Co. (Tokyo, Japan) and Boehringer Mannheim (Mannheim, Germany), respectively. Each bisphosphonate was dissolved in sterile saline and the pH adjusted to 7.0 with NaOH or HCl. A lipopolysaccharide (LPS) from Escherichia coli O55:B5 prepared by Boivin' method was obtained from Difco Laboratories (Detroit, MI, U.S.A.). An LPS from Prevotella intermedia ATCC 25611 (P. intermedia) which was prepared by the method of Galanos et al. (1969) (phenol-chloroform-petroleum ether extraction) was kindly provided by Dr H. Takada (Takada et al., 1990). Each LPS was dissolved in sterile saline. The aminoBPs were injected intraperitoneally (i.p.) and the LPSs intravenously (i.v.). Each of the test solutions was injected in a volume of 0.1 ml per 10 g body weight.
Assay of HDC activity
HDC activity was assayed by a previously described method (Endo et al., 1992a). HDC activity in the liver, lung and spleen is expressed as nmol of histamine formed in 1 h by the enzyme contained in 1 g of each tissue (nmol h−1 g−1). HDC activity in the mandible was also expressed as the activity in 1 g of tissue, because the entire bone was assayed for HDC activity.
Determination of cytokines in the serum
Blood was collected directly into test tubes following decapitation. Serum was recovered by centrifugation at 2000× g at 4°C, then stored at −80°C until used. The IL-1α and IL-1β in the serum were assayed using ELISA kits (Endogen, Cambridge, MA, U.S.A.), the assay procedures being performed exactly as described by the manufacturer. The amount of each cytokine is expressed as pg per ml serum.
Determination of exudate in thorax
After the thorax had been opened with scissors, the exudate present in the thoracic cavity was absorbed using small pre-weighed pieces of filter paper. The amount of exudate present was measured as the increase in the weight of the filter paper.
Determination of the number of cells in the peritoneal exudate
Cells from the peritoneal exudate were obtained as follows. Sterile saline (10 ml) was injected into the peritoneal cavity of ether-anaesthetized mice, and the cavity was massaged. Then, a suspension of cells in saline (5 ml) was recovered using a syringe and the number of cells in the suspension was counted after appropriate dilution.
Data analysis
Experimental values are given as mean±standard deviation (s.d.). The statistical significance of differences was analysed using an unpaired t-test after ANOVA, P values less than 0.05 being considered to indicate significance.
Results
Effects of aminoBPs on HDC activity in control and IL-1-KO mice
Since the maximal elevation of HDC activity (as well as of other inflammatory reactions) occurs 3–4 days after an injection of a given aminoBP (Endo et al., 1993), the effects of three aminoBPs on HDC activity in various tissues were examined 3 days after their injection into control BALB/cA and IL-1-KO BALB/cA mice (Figure 1). Like AHBuBP, two other aminoBPs (CHAMBP and MP-AHPrBP) also elevated HDC activity in liver, lung and spleen in control BALB/cA mice. However, in IL-1-KO mice, such an elevation was not detected with any of these agents (Figure 1).
Figure 1.
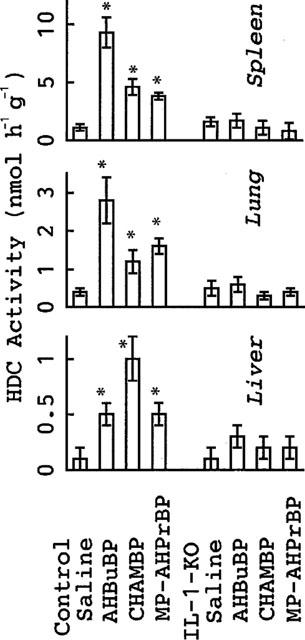
Effects of aminoBPs on HDC activity in control and IL-1-KO mice. Control BALB/cA mice or IL-1-KO BALB/cA mice were sacrificed 3 days after an injection of AHBuBP, CHAMBP, MP-AHPrBP (each at 40 μmol kg−1, i.p.) or saline. Each value is the mean±s.d. from four mice. *P<0.05 vs saline group.
Inflammatory reactions to aminoBPs in control and IL-1-KO mice
In addition to their effect on HDC activity, the three aminoBPs all induced hypertrophy of the spleen, an accumulation of exudate in the thorax, atrophy of the thymus and an increase in the number of granulocytic cells in the peritoneal cavity (Figure 2). None of these inflammatory reactions were induced by aminoBPs in IL-1-KO mice (Figure 3).
Figure 2.
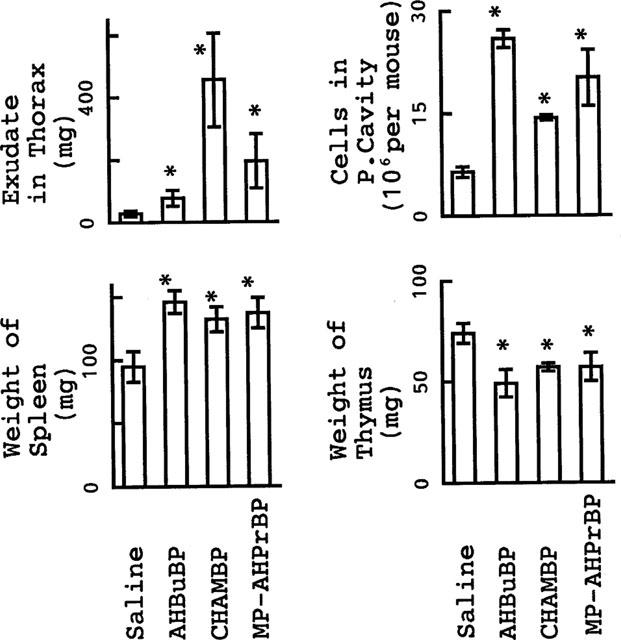
Inflammatory reactions induced by aminoBPs in control BALB/cA mice. Mice were sacrificed 3 days after an injection of AHBuBP, CHAMBP, MP-AHPrBP (each at 40 μmol kg−1, i.p.) or saline. Each value is the mean±s.d. from four mice. *P<0.05 vs saline group.
Figure 3.
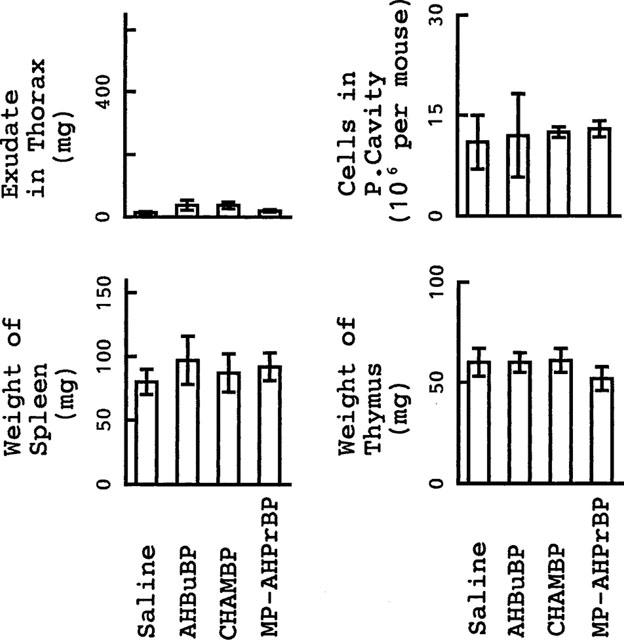
Inflammatory reactions induced by aminoBPs in IL-1-KO BALB/cA mice. Mice were sacrificed 3 days after an injection of AHBuBP, CHAMBP, MP-AHPrBP (each at 40 μmol kg−1, i.p.) or saline. Each value is the mean±s.d. from four mice. *P<0.05 vs saline group.
Effects of AHBuBP on HDC activity and serum IL-1 in control BALB/cA mice
As reported earlier (Sugawara et al., 1998; Endo et al., 1999), in control BALB/cA mice the E. coli LPS-induced elevation of HDC activity was markedly augmented in animals treated with AHBuBP (Figure 4). In such AHBuBP-injected mice, the E. coli LPS-induced elevation of the serum levels of both IL-1α and IL-1β was also markedly augmented (Figure 5). In the absence of LPS, the IL-1α and IL-1β in the serum were each below the detectable level in both saline- and AHBuBP-injected mice (see the values at time 0 in Figure 5). In this study, we confirmed that neither IL-1α nor IL-1β are detectable in the serum of IL-1-KO mice (whether AHBuBP-injected or not) even after injection of E. coli LPS (data not shown).
Figure 4.
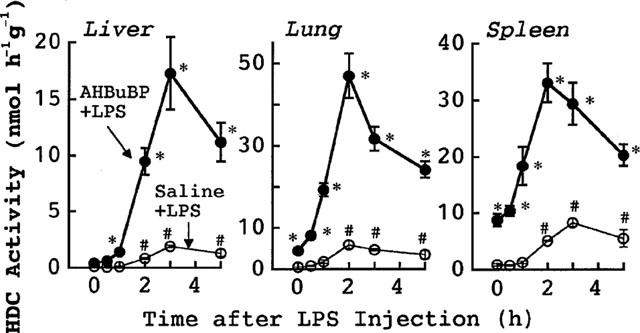
Effects of AHBuBP on the E. coli LPS-induced elevation of HDC activity in control BALB/cA mice. Control BALB/cA mice were injected with saline or AHBuBP (40 μg kg−1, i.p.). Three days later, the same mice were injected with E. coli LPS (0.1 mg kg−1, i.v.), then killed at the times indicated. Each value is the mean±s.d. from four mice. *P<0.05 vs saline+LPS group. #P<0.05 vs time 0.
Figure 5.
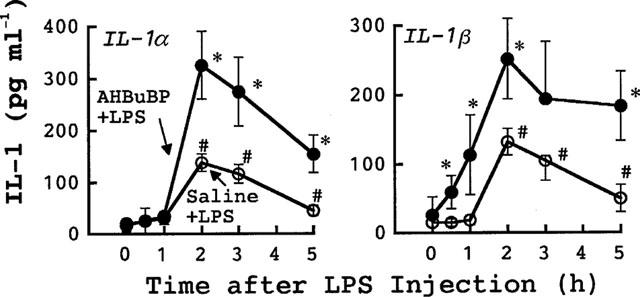
Effects of AHBuBP on the E. coli LPS-induced elevation in the serum levels of IL-1α and IL-1β in control BALB/cA mice. The blood of each mouse used for Figure 4 was assayed for IL-1α and IL-1β. Each value is the mean±s.d. from four mice. *P<0.05 vs saline+LPS group. #P<0.05 vs time 0.
The magnitudes of the elevations of both HDC activity and serum IL-1 shown in the present study are markedly higher than those reported in our previous paper (Sugawara et al., 1998). This is due to that in the previous study LPS was injected intraperitoneally, but in the present study LPS was injected intravenously.
Effects of AHBuBP on HDC activity in IL-1-KO mice
In control BALB/cA mice, as mentioned in the above sections, E. coli LPS elevated HDC activity in all the tissues tested (liver, spleen and lung) (Figure 6). AHBuBP itself also produced a significant elevation of HDC activity in these tissues (P<0.05 vs S+S). In AHBuBP-treated mice, injection of the LPS produced a marked augmentation of HDC elevation. In IL-1-KO mice, the augmentation of HDC activity induced by AHBuBP plus E. coli LPS was small or not detected: there was only a small augmentation in the lung but not in the liver and spleen (Figure 6).
Figure 6.
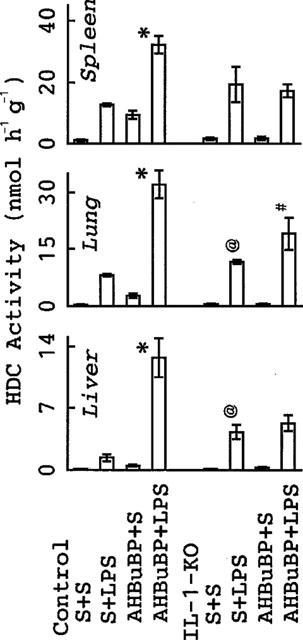
Effects of AHBuBP on the E. coli LPS-induced elevation of HDC activity in IL-1-KO BALB/cA mice. Control and IL-1-KO BALB/cA mice were injected with saline (S) or AHBuBP (40 μmol kg−1, i.p.). Three days later, they were injected with E. coli LPS (0.1 mg kg−1, i.v.) or saline and killed 4 h thereafter. Each value is the mean±s.d. from four mice. *P<0.05 vs S+LPS-injected control BALB/cA mice. #P<0.05 vs S+LPS-injected IL-1-KO BALB/cA mice. @P<0.05 vs corresponding value (S+LPS) in control BALB/cA mice.
Before this experiment, we expected that the LPS-induced elevation of HDC activity seen in control BALB/cA mice would be small or undetectable in IL-1-KO mice. However, contrary to our expectations we found that E. coli LPS induced a more elevation of HDC activity in IL-1-KO mice; indeed, in the liver and lung this elevation was significantly greater than that seen in control BALB/cA mice.
Effects of aminoBPs on the elevation of HDC activity and production of IL-1 induce by P. intermedia LPS in control and IL-1-KO mice
The effects of P. intermedia LPS were essentially the same as those of E. coli LPS. Thus, as observed in the two experiments shown in Figure 7, P. intermedia LPS elevated HDC activity in the mandible of control mice. The HDC elevation induced by P. intermedia LPS in IL-1-KO mice was higher than that induced in control mice. AminoBPs (AHBuBP and MP-AHPrBP) also elevated HDC activity in the mandible in control mice but not in IL-1-KO mice. A combination of an aminoBP with P. intermedia LPS led to an augmented elevation of HDC activity in control mice but not in IL-1-KO mice. Although serum IL-1β in control mice was not elevated by either P. intermedia LPS or MP-AHPrBP at the doses used, it was markedly elevated by a combination of the two (Figure 8).
Figure 7.
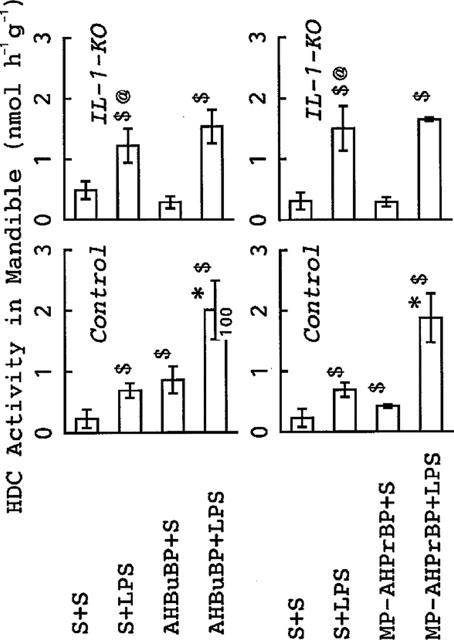
Effects of AHBuBP and MP-AHPrBP on the P. intermedia LPS-induced elevation of HDC activity in the mandible of control and IL-1-KO BALB/cA mice. The data from two experiments are shown in this figure. In each experiment, some control and IL-1-KO BALB/cA mice were injected with saline (S) and others with AHBuBP (40 μmol kg−1, i.p.) (left panels) or MP-AHPrBP (40 μmol kg−1, i.p.) (right panels). Three days later, they were injected with P. intermedia LPS (0.1 mg kg−1 i.v.) or saline and killed 4 h thereafter. Each value is the mean±s.d. from four mice $P<0.05 vs S+S. *P<0.05 vs S+LPS and aminoBP+S. @P<0.05 vs corresponding value (S+LPS) in control BALB/cA mice.
Figure 8.
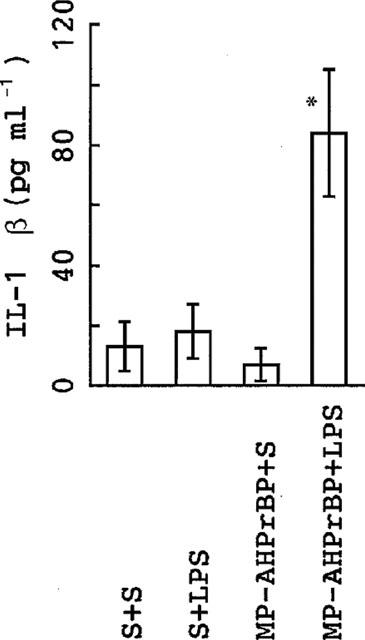
Effects of MP-AHPrBP on the P. intermedia LPS-induced elevation of serum IL-1β in control BALB/cA mice. The mice were injected with saline (S) or MP-AHPrBP (40 μmol kg−1, i.p.). Three days later, they were injected with P. intermedia LPS (0.1 mg kg−1, i.v.) or saline and killed 2 h thereafter. Each value is the mean±s.d. from four mice. *P<0.001 vs other groups.
Discussion
In the present study, we found that while all the three aminoBPs tested (40 μmol kg−1, 13–15 mg kg−1) induced an elevation of HDC activity and other inflammatory reactions (enlargement of the spleen, exudate in the thorax, increased number of cells in the peritoneal exudate and atrophy of the thymus) in control BALB/cA mice, these responses were all very weak or undetectable in IL-1-KO BALB/cA mice. Moreover, while in control mice treatment with AHBuBP (a typical aminoBP) strongly augmented the LPS-induced elevations of both serum IL-1 (α and β) and tissue HDC activity, in IL-1-KO mice this augmentation was much smaller or undetectable. The inflammatory reactions induced by two other aminoBPs were also small or undetectable in IL-1-KO mice. In our earlier study (Sugawara et al., 1998), we found that spleen cells, bone marrow cells and peritoneal exudate cells taken from mice given AHBuBP spontaneously produced a significant amount of IL-1β and that they produced a larger amount of IL-1β in response to LPS. Taken together with these published results, our present findings suggest that IL-1 (α and β subtypes) is responsible for both the elevation of HDC activity and the inflammatory reactions induced by aminoBPs. In this study, we unexpectedly found that the LPS-induced elevation of HDC activity was higher in IL-1-KO mice than in control BALB/cA mice. In the following sections, we discuss these results.
Effects of aminoBPs on the production of proinflammatory cytokines
Recently, Thiébaud et al. (1997) showed, firstly, that AHPrBP (pamidronate) increased the in vitro production of IL-6 and TNFα by intact blood cells taken from healthy volunteers and, secondly, that the serum levels of IL-1β, TNFα and IFNγ in patients showed a tendency to increase after a single intravenous dose of this agent (60 mg per patient). Then, Pietschmann et al. (1998) found that the in vitro production of IL-1β, TNFα and IFNγ and the expression of CD54 (intracellular adhesion molecule-1, ICAM-1) by mononuclear cells from human peripheral blood were all increased by AHBuBP. Although IL-6 and IFNγ have no detectable ability to elevate HDC activity, TNFα does have this property (Endo et al., 1992b). In addition, IL-1 and TNF act synergistically to elevate HDC activity (Endo, 1989), and IL-1β, TNFα and IFNγ are all pyrogens. Taken together, these results are consistent with the idea that in addition to IL-1, other proinflammatory cytokines may also contribute to the inflammatory action of aminoBPs.
Unfortunately, the literature on the in vitro production of proinflammatory cytokines contains many apparent inconsistencies. For example, Evéquoz et al. (1985) reported that aminoBPs had no significant effect on the production of an IL-1-like substance by bone marrow cells, while Sansoni et al. (1995) found that AHBuBP decreased the production of IL-1, TNF and IL-6 by activated human blood mononuclear cells. According to Sanders et al. (1998), IL-6 production by foetal rat limb bone cultured with IL-1β was enhanced by AHBuBP and a similar enhancement occurred in an osteoblast cell line (UMR-106), but Giuliani et al. (1998) reported that AHBuBP inhibited IL-6 production in an osteoblastic cell line (MG-63) stimulated by IL-1+TNF. Further, Pennanen et al. (1995) reported that at higher concentrations, pamidronate (AHPrBP) inhibited the LPS-induced production of IL-1β by a macrophage cell line, RAW 264, and that the production of IL-6 was enhanced by lower concentrations of AHPrBP. By contrast, using the same experimental system, they recently reported that ibandronate (MP-AHPrBP) did not inhibit but instead markedly enhanced the LPS-induced secretion of IL-1β (Mönkkönen et al., 1998). On the basis of the data in the literature, it is doubtful whether the effects of aminoBPs in these in vitro experiments can explain the in vivo inflammatory actions of aminoBPs.
Relevance to clinical side effects of aminoBPs
Single intraperitoneal injections of alendronate and pamidronate produce dose-dependent elevations of HDC activity in mice at 5–40 μmol kg−1 (1.5–12 mg kg−1) (Endo et al., 1993). There are some clinical data obtained from single intravenous injections of aminoBPs in human patients. In these studies, 10–60 mg per patient (about 0.2–1.2 mg kg−1) of alendronate or pamidronate were slowly infused into patients (with metastatic bone diseases, tumour-induced hypercalcaemia, Paget' disease or osteoporosis) (Adami et al., 1987; Sauty et al., 1996; Thiébaud et al., 1997). Although the doses used in our animal experiments are larger than those used in the clinical trials in mg kg−1 terms, it should be noted that these clinical doses of aminoBPs were sufficient to produce inflammatory responses (fever, acute phase responses or serum elevation of proinflammatory cytokines) in many patients. Repeated oral administration of pamidronate or dimethylpamidronate (200–600 mg per patient per day) also produces fever (Schweitzer et al., 1995; Harinck et al., 1987). Repeated oral administration of aminoBPs in human patients, even at low doses, produces gastrointestinal disturbances (Fleisch, 1997). These results suggest that human patients might be more sensitive than mice to the inflammatory actions of aminoBPs.
It should also be noted that BPs bind irreversibly to hydroxyapatite in bone, in which they accumulate during repeated administration (Geddes et al., 1994). However, Mönkkönen et al. (1989) showed that in addition to its accumulation in bone, a large percentage of any administered alendronate is deposited in the spleen and liver of mice for more than 48 h, although it disappears rapidly from the blood. Four successive daily injections of a small dose of alendronate (0.32 μmol kg−1 per day or 0.1 mg kg−1 per day) into mice also resulted in an elevation of HDC activity in the liver (Endo et al., 1993), while weekly injections of 1.6 μmol kg−1 of incardronate of 8 μmol kg−1 of alendronate exacerbated experimental arthritis in mice (Nakamura et al., 1996). In the latter study, we unexpectedly found a destruction of the bone around the joints of the arthritic mice treated with aminoBPs, although there was a strong inhibition of physiological bone resorption. In the present study, we found that the ability of P. intermedia LPS to elevate HDC activity in the mandible was also markedly augmented in mice treated with alendronate (AHBuBP) or ibandronate (MP-AHPrBP). Previously, we observed that the alendronate-induced elevation of HDC activity is augmented in mice injected with inducers of experimental arthritis (type II collagen plus Freund' complete adjuvant) (Nakamura et al., 1996). These results lead us to think that under some conditions, the inflammatory actions of aminoBPs might be augmented in patients with inflammatory diseases such as periodontal diseases or rheumatoid arthritis. Furthermore, it is also probable that the inflammatory actions of LPS might be augmented in patients treated with an aminoBP.
Mechanisms underlying the inflammatory action of aminoBPs
Over the last 30 years, thousands of papers have reported the inhibitory effect of BPs on bone resorption. Today, BPs, are widely used as therapeutic agents and account for nearly $1 billion of the global pharmaceutical market (Rawls, 1998). Unfortunately, however, there is still only a limited understanding of their mode of action. Amin et al. (1992; 1996) found that aminoBPs, but not non-aminoBPs, inhibit cholesterol biosynthesis in rat liver in vitro and ex vivo and also in mouse macrophage J774 cells. Recent studies by other groups have confirmed their findings and it is supposed that the inhibitory effects of aminoBPs on bone resorption are due to their inhibitory effects on the formation of mevalonate-derived intermediates in the cholesterol biosynthesis pathway (such as farnesyl diphosphate and geranylgeranyl diphosphate) (Fisher et al., 1999; Van Beek et al., 1999a,1999b,1999c; Martin et al., 1999; Keller & Fliesler, 1999). These intermediates play important roles in cell physiology: prenylation (farnesylation and geranylgeranylation) of various proteins, including G proteins, are involved in the regulation of a variety of cell functions (Glomset et al., 1992; Olkkonen & Stenmark, 1997). In addition, they are also involved in the operation of nuclear hormone receptors (Forman et al., 1997). The enzyme system producing mevalonate-derived intermediates is thought to be present widely in eucaryotic cells. If aminoBPs were selectively incorporated into osteoclasts alone, their effects might be restricted to bone. However, if they are in fact also incorporated into other cells such as macrophages and endothelial cells, a variety of unidentified responses might be expected to occur systemically. Indeed, aminoBPs induce apoptosis through protein prenylation in macrophage-like J774 cells (Benford et al., 1999). As the abilities of aminoBPs to induce HDC are roughly parallel to their inhibitory actions on bone resorption (Endo et al., 1993), the biochemical mechanism underlying the aminoBPs-stimulated in vivo production of IL-1 by macrophages or endothelial cells might be similar to their inhibitory actions on osteoclasts.
Is IL-1 involved in the LPS-induced elevation of HDC activity?
Our present in vivo study has clarified the involvement of IL-1 in the inflammatory actions of aminoBPs. However, it raises another interesting problem. As described in Introduction, we started out with the idea that IL-1 may play an important role in the induction of HDC by LPS in normal mice. However, contrary to our expectations the LPS-induced elevation of HDC activity was not diminished in IL-1-KO mice; in fact, it was enhanced. This result might be explained in one of two ways: either (i) IL-1 does not contribute at all to the LPS-induced elevation of HDC activity in normal mice or (ii) IL-1 does contribute to the HDC elevation in normal mice, but in IL-1-KO mice a different mechanism(s) may operate to induce HDC activity. Although the present study cannot pretend to solve this problem, we shall briefly discuss it in the following paragraphs.
First, as described in Introduction, IL-1 seems to be a key cytokine in the induction of HDC. Nevertheless, if the first possibility is true, we must conclude either that LPS directly stimulates the cells in which HDC is induced or that a factor(s) other than IL-1 mediates the induction of HDC. At present, we cannot exclude either of these possibilities. However, possibility (i) does not offer any clue as to why the elevation of HDC activity by LPS was greater in IL-1-KO mice than in control mice. To us, it seems unlikely that IL-1 does not contribute at all to the induction of HDC by LPS.
If the second possibility were true, it would imply that a second mechanism for the LPS-induced elevation of HDC activity may be present in IL-1-KO mice and may effectively compensate for the lack of IL-1 in these animals. As described in Introduction, other cytokines such as TNF and haematopoietic cytokines (GM-CSF, G-CSF and IL-3) have the ability to elevate HDC activity. However, Horai et al. (1998) found no difference in the in vitro production of TNF between IL-1-deficient mice and control mice. Furthermore, we found no significant difference in the LPS-induced elevation of serum TNFα levels between control mice and IL-1-KO mice (data not shown). In mice deficient in IL-1β converting enzyme, the production of TNFα is suppressed or not changed (Li et al., 1995; Kuida et al., 1995; Fantuzzi et al., 1996; Alheim et al., 1997). Therefore, the involvement of TNFα in any second HDC-inducing mechanism seems unlikely. The involvement of the haematopoietic cytokines may also be excluded, because they elevate HDC activity only in the spleen and bone marrow, not in the liver and lung. Although Kozak et al. (1995) reported that the febrile response to LPS was reduced in IL-1β-deficient mice, Alheim et al. (1997) found that IL-1β-deficient mice were hyperresponsive to LPS in terms of fever development. The reason for this discrepancy is not yet clear, but the latter finding is consistent with there being a mechanism compensating for the lack of IL-1 in the deficient strain.
A few years ago, Okamura et al. (1995) discovered a new proinflammatory cytokine, IL-18, which was initially identified as an IFNγ-inducing factor. Interestingly, several similarities have been found between IL-8 and IL-1 in terms of structure, biological activity and signalling pathway (Bazan et al., 1996; Torigoe et al., 1997; Dinallero et al., 1998). We found that IL-18 is capable of elevating HDC activity with a time kinetic similar to that of IL-1 (Yamaguchi et al., 2000). Therefore, one speculative idea is that, with respect to HDC induction, IL-18 may compensate for the lack of IL-1 in IL-1-KO mice given LPS. Clearly, additional experiments will be needed to establish whether this might explain how HDC can be fully induced by LPS in IL-1-KO mice.
Conclusion, proposal and problems remained to be clarified
In conclusion, IL-1 is involved in the action by which aminoBPs induce both HDC activity and many other inflammatory reactions in mice. IL-1 has been shown to be implicated in the development of various diseases in which the immune system is involved, for example rheumatoid arthritis (Alvaro-Gracia et al., 1991), diabetes (André-Schmutz et al., 1999), gastric ulcer (Noach et al., 1994; Endo & Kumagai, 1998) and various infectious diseases, including periodontitis (Nair et al., 1996). For this reason, and assuming that our results can be extrapolated to humans, when aminoBPs are used clinically it should be remembered that these agents have the ability to promote the production of IL-1.
Finally, it would be interesting to know what mechanism(s) is involved in the stimulation of IL-1 production by aminoBPs. In our previous study, we found that dichloromethylene bisphosphonate (clodronate), a BP derivative without an amino group, can suppress the inflammatory action of aminoBPs (Endo et al, 1999). We feel that this finding may provide a clue to the nature of the above mechanism(s). In addition, as proposed in our previous paper, a combined clinical use of an aminoBP and a non-aminoBP might be a useful regimen in that it might produce much weaker inflammatory side effects than use of an aminoBP alone (Endo et al., 1999), although it is also possible that the strong inhibitory action of aminoBPs on bone resorption might be decreased by non-aminoBPs. The present study also raises the interesting, but as yet unanswered question, as to whether IL-1α/β-deficiency can be compensated by a second mechanism that does not involve IL-1 yet is capable of mediating the inflammatory responses to LPS or gram-negative bacteria.
Acknowledgments
This work was supported in part by a grant-in-aid for Scientific Research from the Ministry of Education of Japan (No. 05671567).
Abbreviations
- AHBuBP
4-amino-1-hydroxybutylidene-1,1-bisphosphonate
- aminoBP
aminobisphosphonate
- BP
bisphosphonate
- CHAMBP
cycloheptylaminomethylene bisphosphonate
- CSF
colony-stimulating factor
- G
granulocyte
- HDC
histidine decarboxylase
- IL
interleukin
- KO
knockout
- LPS
lipopolysaccharide
- M
macrophage
- MP-AHPrBP
3-(N-methyl-N-pentyl) amino-1-hydroxypropane-1,1-bisphosphonate
- TNF
tumour necrosis factor
References
- ADAMI S., BHALLA A.K., DORIZZI R., MONTESANI F., ROSINI S., SALVAGNO G., LO CASCIO V. The acute phase response after bisphosphonate administration. Calcif. Tissue. Int. 1987;41:326–331. doi: 10.1007/BF02556671. [DOI] [PubMed] [Google Scholar]
- ALHEIM K., CHAI Z., FANTUZZI G., HASANVAN H., MALINOWSKY D., DI SANTO E., GHEZZI P., DINARELLO C.A. Hyperresponsive febrile reactions to interleukin(IL)-1α and IL-1β, and altered brain cytokine mRNA and serum cytokine levels, in IL-1β-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2681–2686. doi: 10.1073/pnas.94.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALVARO-GRACIA J.M., ZVAIFLER N.J., BROWN C.B., KAUSHANSKY K., FIRESTEIN G.S. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocytes-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-α. J. Immunol. 1991;146:3365–3371. [PubMed] [Google Scholar]
- AMIN D., CORNELL S.A., GUSTAFSON S.K., NEEDLE S.J., ULLRICH J.W., BILDER G.E., PERRONE M.H. Bisphosphonates used for the treatment of bone disorders inhibit squalene synthase and cholesterol biosynthesis. J. Lipid Res. 1992;33:1657–1663. [PubMed] [Google Scholar]
- AMIN D., CORNELL S.A., PERRONE M.H., BILDER G.E. 1-Hydroxy-3-(methylpentylamino)-propylidene-1,1-bisphosphonic acid as a potent inhibitor of squalene synthase. Drug Res. 1996;46:759–762. [PubMed] [Google Scholar]
- ANDRÉ-SCHMUTZ I., HINDELANG C., BENOIST C., MATHIS D. Cellular and molecular changes accompanying the progression from insulitis to diabetes. Eur. J. Immunol. 1999;29:245–255. doi: 10.1002/(SICI)1521-4141(199901)29:01<245::AID-IMMU245>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- BAE K-S., BAUMGARTNER J.C., SHEARER T.R., DAVID L.L. Occurrence of Prevotella nigrescens and Prevotella intermedia in infections of endodontic origin. J. Endodont. 1997;23:620–623. doi: 10.1016/S0099-2399(97)80173-2. [DOI] [PubMed] [Google Scholar]
- BAUMGARTNER J.C., FALKLER W.M. Bacteria in the apical 5 mm of infected root canals. J. Endodont. 1991;17:380–383. doi: 10.1016/s0099-2399(06)81989-8. [DOI] [PubMed] [Google Scholar]
- BAZAN J.F., TIMANS J.C., KASTELEIN R.A. A new defined interleukin-1. Nature. 1996;379:591. doi: 10.1038/379591a0. [DOI] [PubMed] [Google Scholar]
- BENFORD H.L., FRITH J.C., AURIOLA S., MÖNKKÖNEN J., ROGERS M.J. Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol. Pharmacol. 1999;56:131–140. doi: 10.1124/mol.56.1.131. [DOI] [PubMed] [Google Scholar]
- BONJOUR J.P., RIZZOLI R., AMMANN P., CHEVALLEY T.Bisphosphonates in clinical medicine Bone and Mineral Research 19948Amsterdam: Elsevier Science BV; 205–264.ed. Heersche, N.M. & Kanis, J.K. pp [Google Scholar]
- DAHLÉN G., MÖLLER Å.J. R.Microbiology of endodontic infections Contemporary Oral Microbiology and Immunology 1992St. Louis: Mosby Year Book; 444–475.ed. Slots, J. & Taubman, M.A. pp [Google Scholar]
- DE M., SANFORD T.H., WOOD G.W. Detection of interleukin-1, interleukin-6, and tumor necrosis factor α in the uterus during the second half of pregnancy in the mouse. Endocrinol. 1992;131:14–20. doi: 10.1210/endo.131.1.1611993. [DOI] [PubMed] [Google Scholar]
- DINARELLO C.A., NOVICK D., PUREN A.J., FANTUZZI G., SHAPIRO L., MÜHL H., YOON D.-Y., REZINIKOV L.L., KIM S.-H., RUBINSTEIN M. Overview of interleukin-18: more than an interferon-γ inducing factor. J. Leukocyte Biol. 1998;63:658–664. [PubMed] [Google Scholar]
- DY M., MACHAVOINE F., LEBEL B., ICHIKAWA A., GASTINEL L.N., SCHNEIDER E. Interleukin 3 promotes histamine synthesis in hematopoietic progenitors by increasing histidine decarboxylase mRNA expression. Biochem. Biophys. Res. Commun. 1993;192:167–173. doi: 10.1006/bbrc.1993.1396. [DOI] [PubMed] [Google Scholar]
- ENDO Y. Induction of histidine and ornithine decarboxylase activities in mouse tissues by recombinant interleukin-1 and tumor necrosis factor. Biochem. Pharmacol. 1989;38:1287–1292. doi: 10.1016/0006-2952(89)90335-3. [DOI] [PubMed] [Google Scholar]
- ENDO Y., KIKUCHI T., NAKAMURA M., SHINODA H. Determination of histamine and polyamines in calcified tissues of mice: contribution of mast cells and histidine decarboxylase to the amount of histamine in the bone. Calcif. Tissue Int. 1992a;51:67–71. doi: 10.1007/BF00296220. [DOI] [PubMed] [Google Scholar]
- ENDO Y., KIKUCHI T., TAKEDA Y., NITTA Y., RIKIISHI H., KUMAGAI K. GM-CSF and G-CSF stimulate the synthesis of histamine and putrescine in the hematopoietic organs in vivo. Immunol. Lett. 1992b;33:9–14. doi: 10.1016/0165-2478(92)90086-4. [DOI] [PubMed] [Google Scholar]
- ENDO Y., KUMAGAI K. Induction by interleukin-1, tumor necrosis factor and lipopolysaccharide of histidine decarboxylase in the stomach and prolonged accumulation of gastric acid. Br. J. Pharmacol. 1998;125:842–848. doi: 10.1038/sj.bjp.0702108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENDO Y., NAKAMURA N., KIKUCHI T., SHINODA H., TAKEDA K., NITTA Y., KUMAGAI K. Aminoalkylbisphosphonates, potent inhibitors of bone resorption, induce a prolonged stimulation of histamine synthesis and increase macrophages, granulocytes, and osteoclasts in vivo. Calcif. Tissue Int. 1993;52:248–254. doi: 10.1007/BF00298728. [DOI] [PubMed] [Google Scholar]
- ENDO Y., NAKAMURA M., NITTA Y., KUMAGAI K. Effect of macrophage depletion on the induction of histidine decarboxylase by lipopolysaccharide, interleukin-1 and tumor necrosis factor. Br. J. Pharmacol. 1995;114:187–193. doi: 10.1111/j.1476-5381.1995.tb14924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENDO Y., SHIBAZAKI M., NAKAMURA M., KOSUGI H. Inhibition of inflammatory actions of aminobisphosphonates by dichloromethylene bisphosphonate, a non-aminobisphosphonate. Br. J. Pharmacol. 1999;126:903–910. doi: 10.1038/sj.bjp.0702367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENDO Y., SHIBAZAKI M., NAKAMURA M., TAKADA M. Contrasting effects of lipopolysaccharides (endotoxins) from oral black-pigmented bacteria and Enterobacteriaceae on platelets, a major source of serotonin, and on histamine-forming enzyme in mice. J. Infec. Dis. 1997;175:1404–1412. doi: 10.1086/516473. [DOI] [PubMed] [Google Scholar]
- ENDO Y., SUZUKI R., KUMAGAI K. Macrophages can produce factors capable of inducing histidine decarboxylase, a histamine-forming enzyme, in vivo in the liver, spleen, and lung of mice. Cell. Immunol. 1986;97:13–22. doi: 10.1016/0008-8749(86)90370-9. [DOI] [PubMed] [Google Scholar]
- ENDO Y., TABATA T., KURODA H., TADANO T., MATSUSHIMA K., WATANABE M. Induction of histidine decarboxylase in skeletal muscle in mice by electrical stimulation, prolonged walking and interleukin-1. J. Physiol. 1998;509:587–598. doi: 10.1111/j.1469-7793.1998.587bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVÉQUOZ V., TRECHSEL U., FLEISCH H. Effect of bisphosphonates on production of interleukin 1-like activity by macrophages and its effect on rabbit chondrocytes. Bone. 1985;6:439–444. doi: 10.1016/8756-3282(85)90221-2. [DOI] [PubMed] [Google Scholar]
- FANTUZZI G., ZHENG H., FAGGIONI R., BENIGNI F., GHEZZI P., SIPE J.D., SHAW A.R., DINARELLO C.A. Effect of endotoxin in IL-1β-deficient mice. J. Immunol. 1996;157:291–296. [PubMed] [Google Scholar]
- FISHER J.E., ROGERS M.J., HALASY J.M., LUCKMAN S.C., HUGHES D.E., MASARACHIA P.J., WESOLOWSKI G., RUSSELL R.G.G., RODAN G.A., RESZKA A.A. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclasts formation, bone resorption, and kinase activation in vitro. Proc. Natl. Acad. Sci. U.S.A. 1999;96:133–138. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCH H.A. Bisphosphonates: preclinical aspects and use in osteoporosis. Annal. Med. 1997;29:56–62. doi: 10.3109/07853899708998743. [DOI] [PubMed] [Google Scholar]
- FORMAN B.M., RUAN B., CHEN J., SCHROEPFER G.J., Jr, EVANS R.M. The orphan nuclear receptor LXRα is positively and negatively regulated by distinct products of mevalonate metabolism. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10588–10593. doi: 10.1073/pnas.94.20.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUNAYAMA H., MAYANAGI H., TAKADA H., ENDO Y.Elevation of histidine decarboxylase activity in the mandible of mice by Prevotella intermedia lipopolysaccharide and its augmentation by an aminobisphosphonate Arch. Oral Biol. 2000. in press [DOI] [PubMed]
- GALANOS C., LÜDERITZ O, WESTPHAL O. A new method for the extraction of R lipopolysaccharides. Eur. J. Biochem. 1969;9:245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- GEDDES A.D., D'OUZA S.M., EBENTINO F.H., KENNETH J.L.Bisphosphonates: structure-activity relationships and therapeutic implicates Bone and Mineral Research/8 1994Amsterdam: Elsevier Science BV; 265–306.ed. Heersche, N.M. & Kanis, J.K. pp [Google Scholar]
- GIULIANI N., PEDTAZZONI M., PASSERI G., GIRASOLE G. Bisphosphonates inhibits IL-6 production by human osteoblast-like cells. Scand. J. Rheumatol. 1998;27:38–41. doi: 10.1080/030097498441155. [DOI] [PubMed] [Google Scholar]
- GLOMSET J.A., GELB M.H., FARNSWORTH C.C. Geranylgeranylated proteins. Biochem. Soc. Trans. 1992;20:479–484. doi: 10.1042/bst0200479. [DOI] [PubMed] [Google Scholar]
- HARINCK H.I.J., PAPAPOULOS S.E., BLANKSMA H.J., MOOLENAAR A.J., WERMEIJ P., BIJVOET O.L.M. Paget' disease of bone: early and late responses to three modes of treatment with aminohydroxypropylidene bisphosphonate (APD) Br. Med. J. 1987;295:1301–1305. doi: 10.1136/bmj.295.6609.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORAI R., ASANO M., SUDO K., KANUKA H., SUZUKI M., NISHIHARA M., TAKAHASHI M., IWAKURA Y. Production of mice deficient in genes for interleukin(IL)-1α, IL-1β, IL-1α/β, and IL-1 receptor antagonist shows that IL-1β is crucial in turpentine-induced fever development and glucocorticoid secretion. J. Exp. Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURWITZ A., PAYNE D.W., PACKMAN J.N., ANDREANI C.L., RESNICK C.E., HERNANDEZ E.R., ADASHI E.Y. Cytokine-mediated regulation of ovarian function: interleukin-1 inhibits gonadotropin-induced androgen biosynthesis. Endocrinol. 1991;129:1250–1256. doi: 10.1210/endo-129-3-1250. [DOI] [PubMed] [Google Scholar]
- KELLER K., FLIESLER S.J. Mechanism of aminobisphosphonate action: characterization of alendronate inhibition of isoprenoid pathway. Biochem. Biophys. Res. Commun. 1999;266:560–563. doi: 10.1006/bbrc.1999.1849. [DOI] [PubMed] [Google Scholar]
- KIKUCHI H., WATANABE M., ENDO Y. Induction by interleukin-1 (IL-1) of the mRNA of histidine decarboxylase, the histamine-forming enzyme, in the lung of mice in vivo and the effect of actinomycin D. Biochem. Pharmacol. 1997;53:1383–1388. doi: 10.1016/s0006-2952(96)00887-8. [DOI] [PubMed] [Google Scholar]
- KOZAK W., ZHENG H., CONN C.A., SOSZYNSKI D., VAN DER PLOEG L.H.T., KLUGER M.J. Thermal and behavioural effects of lipopolysaccharide and influenza in interleukin-1β-deficient mice. Am. J. Physiol. 1995;269:R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- KUIDA K., LIPPKE J.A., KU G., HARDING M.W., LIVINGTON D.J., SU M.S.-S., FLAVELL R.A. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- LEBEL B., SCHNEIDER E., PIQUET-PELLORCE C., MACHAVOINE F., KINDER V., LUFFAU G., DY M. Antigenic challenge of immunized mice induces endogenous production of IL-3 that increases histamine synthesis in hematopoietic organs. J. Immunol. 1990;145:1222–1226. [PubMed] [Google Scholar]
- LI P., ALLEN H., BANERJEE S., FRANKLIN S., HERZOG L., JOHNSTON C., MCDOWELL J., PASKIND M., RODMAN L., SALFELD J., TOWNE E., TRACEY D., WARDWELL S., WEI F.-Y., WONG W., KAMEN R., SESHARDRI T. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- MACAROL V., FRAUENFELDER F.T. Pamidronate disodium and possible ocular adverse drug reactions. Am. J. Ophthamol. 1994;118:220–224. doi: 10.1016/s0002-9394(14)72902-2. [DOI] [PubMed] [Google Scholar]
- MARTIN M.B., ARNOLD W., HEATH H.T., III, URBINA J.A., OLDFIELD E. Nitrogen-containing bisphosphonates as carbocation transition state analogs for isoprenoid biosynthesis. Biochem. Biophys. Res. Commun. 1999;263:754–758. doi: 10.1006/bbrc.1999.1404. [DOI] [PubMed] [Google Scholar]
- MÖNKKÖNEN J., KOPONEN H.-M., YLITALO P. Comparison of the distribution of three bisphosphonates in mice. Pharmacol. Toxicol. 1989;65:294–298. doi: 10.1111/j.1600-0773.1990.tb00750.x. [DOI] [PubMed] [Google Scholar]
- MÖNKKÖNEN J., SIMILÄ J., ROGERS M.J. Effects of tiludronate and ibandronate on the secretion of proinflammatory cytokines and nitric oxide from macrophages in vitro. Life Sciences. 1998;62:PL 95–PL 02. doi: 10.1016/s0024-3205(97)01178-8. [DOI] [PubMed] [Google Scholar]
- NAIR S.P., MEGHJI S., WILSON M., REDDI K., WHITE P., HENDERSON B. Bacterially induced bone resorption: mechanisms and misconceptions. Infect. Immun. 1996;64:2371–2380. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMUA M., ANDO T., MASATAKA A., KUMAGAI K., ENDO Y. Contrast between effects of aminobisphosphonates and non-aminobisphosphonates on collagen-induced arthritis in mice. Br. J. Pharmacol. 1996;119:205–212. doi: 10.1111/j.1476-5381.1996.tb15972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOACH L.A., BOSMA N.B., JANSEN J., HOEK F.J., VAN DEVENTER S.J.H., TYTGAT G.N. Mucosal tumor necrosis factor-α, interleukin-1β, and interleukin-8 in patients with Helicobacter pylori infection. Scand. J. Gastroenterol. 1994;29:425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- OKAMURA H., TSUTSUI H., KOMATSU T., YUTSUDO M., HAKURA A., TANIMOTO T., TORIGOE K., OKURA T., NUKADA Y., HATTORI K., AKITA K., NAMBA M., TANABE F., KONISHI K., FUKUDA S., KURIMOTO M. Cloning of a new cytokine that induces IFNγ production by T-cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- OLKKONEN N.V.M., STENMARK H. Role of Rab GTPase in membrane traffic. Int. Rev. Cytol. 1997;176:1–85. doi: 10.1016/s0074-7696(08)61608-3. [DOI] [PubMed] [Google Scholar]
- PENNANEN N., LAPINJOKI S., URTTI A., MÖNKKÖNEN J. Effect of liposomal and free bisphosphonates on the IL-1β, IL-6 and TNFα secretion from RAW 264 cells in vitro. Pharmaceutical Res. 1995;12:916–922. doi: 10.1023/a:1016281608773. [DOI] [PubMed] [Google Scholar]
- PIETSCHMANN P., STOHLAWETZ P., BROSCH S., STEINER G., SMOLEN J.S., PETERLIK M. The effect of alendronate on cytokine production, adhesion molecule expression, and transendothelial migration of human peripheral blood mononuclear cells. Calcif. Tissue Int. 1998;63:325–330. doi: 10.1007/s002239900535. [DOI] [PubMed] [Google Scholar]
- RAWLS R. Dazzling phosphorus: international gathering of phosphorus chemists highlights medical applications, novel bonding of versatile element. Chem. Eng. News. 1998;76:38–40. [Google Scholar]
- REITSCHEL E.T., KIRIKAE T., URICH S., MAMAT U., SCHMIDT G., LOPPNOW H., ULMER A.J., ZÄHRINGER U., SEYDEL U., PADOVA F.D., SCHREIER M., BRADE H. Bacterial endotoxin: molecular relationships to structure, to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- RODAN G.A., FLEISCH H.A. Bisphosphonates: mechanism of action. J. Clin. Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDERS J.L., TARJAN G., FOSTER S.A., STERN P.H. Alendronate/interleukin-1β cotreatment increases interleukin-6 in bone and UMR-106 cells: dose dependence and relationship to antiresorptive effect of alendronate. J. Bone Min. Res. 1998;13:786–792. doi: 10.1359/jbmr.1998.13.5.786. [DOI] [PubMed] [Google Scholar]
- SANSONI P., PASSERI G., FAGNONI F., MOHAGHEGHPOUR N., SNELL G., BRIANTI V., ENGLEMAN E.G. Inhibition of antigen-presenting cell function by alendronate in vitro. J. Bone Min. Res. 1995;10:1719–1725. doi: 10.1002/jbmr.5650101115. [DOI] [PubMed] [Google Scholar]
- SAUTY A., PECHERSTORFER M., ZIMMER-ROTH I., FIORONI P., JUILLERAT L., MARKERT M., LUDWIG H., LEUENBERGER P., BURCKHARDT P., THIÉBAUD D. Interleukin-6 and tumor necrosis factor α levels after bisphosphonates treatment in vitro and in patients with malignancy. Bone. 1996;18:133–139. doi: 10.1016/8756-3282(95)00448-3. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER E., PIQUET-PELLORCE C., DY M. New role for histamine in interleukin-3-induced proliferation of hematopoietic stem cells. J. Cell. Physiol. 1990;143:337–343. doi: 10.1002/jcp.1041430218. [DOI] [PubMed] [Google Scholar]
- SCHWEITZER D.H., RUIT M.O.V.D., PRUIJM G.V.D., LÖWIK C.W.G.M., PAPAPOULOS S.E. Interleukin-6 and the acute phase response during treatment of patients with Paget' disease with the nitrogen-containing bisphosphonate dimethylaminohydroxypropylidene bisphosphonate. J. Bone Mineral Res. 1995;10:956–962. doi: 10.1002/jbmr.5650100617. [DOI] [PubMed] [Google Scholar]
- SIRIS E. Bisphosphonateas and iritis. Lancet. 1993;2:436–437. doi: 10.1016/0140-6736(93)93029-z. [DOI] [PubMed] [Google Scholar]
- SUGAWARA S., SHIBAZAKI M., TAKADA H., KOSUGI K., ENDO Y. Contrasting effects of an aminobisphosphonate, a potent inhibitor of bone resorption, on lipopolysaccharide-induced production of interleukin-1 ad tumour necrosis factor α in mice. Br. J. Pharmacol. 1998;125:735–740. doi: 10.1038/sj.bjp.0702151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKADA H., HIRAI H., FUJIWARA T., KOGA T., OGAWA T., HAMADA H. Bacteriodes lipopolysaccharides (LPS) induce anaphylactoid and lethal reactions in LPS-responsive and -nonresponsive mice primed with muramyl dipeptide. J. Infect. Dis. 1990;162:428–434. doi: 10.1093/infdis/162.2.428. [DOI] [PubMed] [Google Scholar]
- THIÉBAUD D., SAUTY A., BURCKHARDT P., LEUENBERGER P., SITZLER L., GREEN J.R., KANDRA A., ZIESCHANGE J., IBARRA, DE PALACIOS P. An in vitro and in vivo study of cytokines in the acute-phase response associated with bisphosphonates. Calcif. Tissue Int. 1997;61:386–392. doi: 10.1007/s002239900353. [DOI] [PubMed] [Google Scholar]
- TORIGOE K., USHIO S., OKURA T., KOBAYASHI S., TANIAI M., KUNIKATA T., MURAKAMI T., SANOU O., KOJIMA H., FUJII M., OHTA T., IKEDA M., IKEGAMI H., KURIMOTO M. Purification and characterization of the human interleukin-18 receptor. J. Biol. Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- VAN BEEK E., LÖWIK C., VAN DER PLUIJM G., PAPAPOULOS S. The role of geranylgeranylation in bone resorption and its suppression by bisphosphonates in fetal bone explants in vitro: a clue to the mechanism of action of nitrogen-containing bisphosphonatyes. J. Bone Miner. Res. 1999a;14:722–729. doi: 10.1359/jbmr.1999.14.5.722. [DOI] [PubMed] [Google Scholar]
- VAN BEEK E., PIETERMAN E., COHEN L., LÖWIK C., PAPAPOULOS S. Nitrogen-containing bisphosphonates inhibit isopentenyl pyrophosphate isomerase/farnesyl pyrophosphate synthase activity with relative potencies corresponding to their antiresorptive potencies in vitro and in vivo. Biochem. Biophys. Res. Commun. 1999b;255:491–494. doi: 10.1006/bbrc.1999.0224. [DOI] [PubMed] [Google Scholar]
- VAN BEEK E., PIETERMAN E., COHEN L., LÖWIK C., PAPAPOULOS S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem. Biophys. Res. Commun. 1999c;264:108–111. doi: 10.1006/bbrc.1999.1499. [DOI] [PubMed] [Google Scholar]
- WILSON M. Biological activities of lipopolysaccharides from oral bacteria and their relevance to the pathogenesis of periodontitis. Sci. Prog. 1995;78:19–34. [PubMed] [Google Scholar]
- YAMAGUCHI K., MOTEGI K., KURIMOTO M., ENDO Y.Induction of the activity of the histamine-forming enzyme, histidine decarboxylase, in mice by IL-18 and by IL-18 plus IL-12 Inflammation Res. 2000. in press [DOI] [PubMed]
- ZAMBON J.J., GROSSI S., DUNFORD R., HARASZTHY V.I., PREUS H., GENCO R.J.Epidemiology of subgingival bacterial pathogens in periodontal disease Molecular Pathogenesis of Periodontal Disease 1994Washington DC: ASM Press; 3–12.ed. Genco, R.J., Hamada, S., Lehner, T., McGhee, J. & Mergenhagen, S. pp [Google Scholar]


