Abstract
Sulphonylurea drugs have been shown to protect against hypoxic damage in isolated proximal tubules of the kidney. In the present study we investigated whether these drugs can protect against hypoxic damage in a whole kidney preparation.
Tolbutamide (200 μM) and glibenclamide (10 μM) were applied to the isolated perfused rat kidney prior to changing the gassing from oxygen to nitrogen for 30 min.
Hypoxic perfusions resulted in an increased fractional excretion of glucose (FE % glucose 14.3±1.5 for hypoxic perfusions vs 4.9±1.6 for normoxic perfusions, mean±s.e.mean, P<0.05), which could be completely restored by 200 μM tolbutamide (5.7±0.4 for tolbutamide vs 14.3±1.5 for untreated hypoxic kidneys, P<0.01). Furthermore, tolbutamide reduced the total amount of LDH excreted in the urine (220±100 mU for tolbutamide vs 1220±160 mU for untreated hypoxic kidneys, P<0.01). Comparable results were obtained with glibenclamide (10 μM).
In agreement with the effect on functional parameters, ultrastructural analysis of proximal tubules showed increased brush border preservation in tolbutamide treated kidneys compared to untreated hypoxic kidneys.
We conclude that glibenclamide and tolbutamide are both able to reduce hypoxic damage to proximal tubules in the isolated perfused rat kidney when applied in the appropriate concentrations.
Keywords: Kidney, ischaemia, hypoxia, sulphonylurea drugs, glibenclamide, tolbutamide, ATP-regulated potassium channels
Introduction
The predominant site of ischaemic renal damage in humans, experimental animals and isolated kidneys perfused with an erythrocyte-containing medium, is the proximal tubule (Lieberthal et al., 1988; Brady et al., 1998; Venkatachalam et al., 1978). Damage to this site has morphologically been characterized by blebbing of the apical membrane, loss of brush border membrane, cytosolic vacuoles and swelling of mitochondria. Eventually this results in focal cellular loss leaving a denuded basement membrane and leading to cast formation in the more distal segments of the nephron (Brady et al., 1998; Spencer et al., 1991; Molitoris, 1991; Venkatachalam et al., 1978; Glaumann & Trump, 1975; Mergner et al., 1976). Isolated kidneys, perfused with an oxygenated cell-free medium develop a characteristic damage of the medullary thick ascending limb of Henle (mTAL) with sparing of the proximal tubule (Brezis et al., 1984).
At the cellular level hypoxic damage to proximal tubules is accompanied by a rapid fall of intracellular ATP and potassium levels, while sodium, chloride and calcium accumulate (Mason et al., 1981; Burke & Schrier, 1996). This ionic imbalance has been attributed to impaired functioning of the basolateral Na/K-ATPase, but the contribution of a potassium conductance in the cellular loss of this ion cannot be ruled out. An ATP-dependent potassium channel (K-ATP) has been described at the basolateral membrane of rabbit proximal tubules, which regulates the recycling of potassium (Tsuchiya et al., 1992; Mauerer et al., 1998a). This channel is a likely mediator of hypoxia-induced potassium loss as it is regulated by intracellular ATP levels; a decline in ATP results in an increased open probability of the channel. Another general characteristic is that these channels can be blocked by oral antidiabetic sulphonylureas, such as tolbutamide and glibenclamide. However, the potency of these sulphonylurea drugs varies widely between different tissues (Ashcroft & Ashcroft, 1990). K-ATP channels of the pancreatic β-cell and excitable cells such as neurones, and cardiac and smooth muscle cells, are heteromultimers composed of an inward rectifying potassium channel (Kir 6.0) and a sulphonylureum receptor protein (SUR), a member of the ATP binding cassette (ABC) superfamily (Babenko et al., 1998). Epithelial K-ATP channels, including renal tubular K-ATP channels, seem to make up a different class of channels reflected by their much lower affinities for sulphonylurea drugs (Ashcroft & Ashcroft, 1990; Quast, 1996). Although recent reports describe the expression of SUR in mouse and rat kidney, including proximal tubules, its contribution to the regulation of basolateral potassium conductance is at present unknown (Beesley et al., 1999; Tanemoto et al., 2000).
An important role for K-ATP channels in the pathophysiology of hypoxic damage is well established in cerebral and cardiac ischaemia (Smits et al., 1996; Hearse, 1995; Takaba et al., 1997; Heurteaux et al., 1993). To our knowledge the only data available on the function of K-ATP channels in renal ischaemia come from a study by Reeves & Shah (1994), who showed that hypoxic damage to isolated rabbit proximal tubules, as assessed by measurement of DNA damage and LDH leakage, could be reduced by 500 μM glibenclamide. However, the effect of sulphonylurea in a whole kidney could be very different, because such a preparation comprises tubular and vascular structures, both of which contain K-ATP channels. We investigated whether sulphonylurea drugs, applied in concentrations capable of inhibiting tubular epithelial K-ATP channels, can protect against hypoxic injury in whole organ kidney experiments. In the present study we found such a protective effect for both tolbutamide and glibenclamide in the isolated perfused rat kidney.
Methods
Materials
Pluronic F-108 was obtained from BASF (Arnhem, The Netherlands). All other chemicals were of analytical grade and obtained either from Sigma (St. Louis, MO, U.S.A) or Merck AG (Darmstadt, Germany).
Drugs
Tolbutamide was purchased from Sigma (St. Louis, MO, U.S.A.) and dissolved in NaHCO3/NaOH (ratio 4.5 : 0.5) and glibenclamide was purchased from Aldrich (Zwijndrecht, The Netherlands) and dissolved in NaHCO3. Pancreatic K-ATP channels can be inhibited by nanomolar concentrations of sulphonylurea drugs and therapeutic unbound concentrations are in this concentration range (Balant, 1981). Instead, epithelial K-ATP channels seem to be less sensitive and need micromolar concentrations of sulphonylurea drugs to be inhibited (Ashcroft & Ashcroft, 1990; Quast, 1996). Therefore, tolbutamide was added to the perfusate at a concentration of 200 μM and glibenclamide at an equipotent concentration of 10 μM.
Experimental procedure
The procedures for isolation and perfusion of the rat kidney were approved by the local Ethics Committee on animal experimentation and are described in detail elsewhere (Cox et al., 1990). Briefly, male Wistar-Hanover rats (200–300 g) were anaesthetized with pentobarbitone intraperitoneally (6 mg–100 g) after which the right kidney was exposed and the right renal artery and ureter were canulated without interruption of flow. The kidney was excised and placed in a perfusion chamber with a constant temperature (37.5°C) and the renal artery canula was connected to a circulating perfusate volume of 400 ml for 30 min. After this stabilization period the kidney was connected to a perfusate volume of 240 ml and the experimental procedure was started (0–10 min: control values, 10–40 min: stabilization with sulphonylurea drug or solvent, 40–70 min: hypoxia, 70–100 min: reoxygenation, 100–110 min: removal of sulphonylurea drug or solvent, 110–140 min: end values). During the stabilization period perfusate flow was set to values resulting in a perfusate pressure of 90 mmHg recorded just proximal to the kidney. After starting the experimental period no adjustments in perfusate flow were made. The perfusion fluid was oxygenated with a 95% O2/5% CO2 mixture during both the stabilization period and experimental period. Perfusate was pregassed in a reservoir and saturated with 95% O2/5% CO2 by use of a membrane oxygenator. For hypoxic perfusions gassing was changed to 95% N2/5% CO2 for 30 min. All studies were performed with a cell-free Krebs-Ringer-Henseleit based perfusate, containing 2.5% Pluronic F-108 as an oncotic agent and 20 μg/ml cyanocobalamin for the continuous colorimetric determination of the GFR (Cox et al., 1990). Urine samples were collected during the whole experimental period over 10 min intervals and analysed for glucose, sodium, potassium and lactate dehydrogenase (LDH). Tolbutamide (200 μM), glibenclamide (10 μM) or their respective solvents were added to the perfusion medium 30 min before the onset of the hypoxic perfusion to make sure no changes in perfusion pressure (RPP), perfusate flow (RPF) or GFR were recorded upon addition of the drug. A few experiments were also done with 100 μM glibenclamide, but this concentration turned out to be harmful, as assessed by an increase in morphological as well as functional damage compared to the hypoxic kidneys. Drugs were removed after 30 min reoxygenation by changing the reservoir with new drug-free perfusate and at the same time disconnecting the closed circulation for 10 min and discarding the venous effluent (which was subsequently substituted by drug-free perfusate to the reservoir to keep the same total amount of perfusate circulating).
Functional analysis
During the experimental period RPP, RPF, GFR, urine flow, urine pH and water reabsorption (R % H2O) were recorded continuously. Overall tubular functioning was assessed by calculation of fractional sodium excretion (FE % Na), while proximal tubular reabsorptive capacity was assessed by calculating the fractional excretion of glucose (FE % glucose), both at 10 min intervals. In order to study the effect of tolbutamide and glibenclamide on hypoxic perfused kidneys values of the last three sampling intervals (110–120, 120–130 and 130–140 min values) for GFR, FE % Na, FE % glucose and R % H2O were averaged and compared with the corresponding untreated hypoxic end-values. The solvents for tolbutamide or glibenclamide had no effect on recorded and measured parameters and these experiments were therefore grouped as hypoxia. Tubular epithelial cell damage was characterized by measurement of the total amount of LDH excreted in the urine during the experiment.
Morphological analysis
Morphological analysis was performed on normoxic and hypoxic perfused kidneys in order to make sure the method used to induce hypoxic damage would result in a proximal damage as in clinical and experimental ischaemia.
For light microscopic assessment kidney fragments were fixed in Bouin' solution, dehydrated, and embedded in paraplast, and 2 μm sections were stained with haematoxylin and eosin, Periodic Acid Schiff, and Silver methenamine (Assmann et al., 1983). For electron microscopy, small pieces of cortex were fixed in 2.5% glutaraldehyde dissolved in 0.1 M sodium cacodylate buffer, pH 7.4, for 4 h at 4°C, and washed in the same buffer. The tissue fragments were both fixed in cacodylate-buffered 1% OsO4 for 2 h, dehydrated, and embedded in Epon 812. Ultrathin sections were cut on an ultratome (LKB Instruments, Bromma, Sweden) and stained with 4% uranyl acetate for 45 min and with lead citrate for 2 min at room temperature. The sections were examined in an electron microscope (Jeol 1200 EX, Japan) (Assmann et al., 1983). Evaluation of light and electron microscopic preparations was done in a blinded fashion by an experienced renal pathologist.
Chemical analyses
Sodium and potassium were determined by flame photometry. Glucose was determined with a commercial glucose kit (GLUCO-QUANT, Boehringer, Manheim, Germany). Lactate dehydrogenase was determined routinely at the Clinical Chemistry Laboratory of the University Hospital Nijmegen.
Statistics
Data are expressed as means±standard error of the mean (s.e.mean). Student' t-test for unpaired data was used to detect a difference between single comparisons–between the mean end-values for untreated hypoxic perfusions versus normoxic perfusions. The statistical significance of the protective effect of tolbutamide and glibenclamide was assessed by ANOVA followed by a Student t-test with Bonferroni correction for multiple comparisons.
Results
Light microscopic assessment of kidneys perfused under normoxic conditions showed severe damage of the medullary thick ascending limb (mTAL), as reported previously for cell-free perfused isolated kidneys (Brezis et al., 1984). Hypoxic perfused kidneys showed no or only minor additional damage of mTAL, but did show an additional damage to proximal tubules. This proximal damage was light microscopically characterized by focal effacement and thinning of the brush border membrane in a heterogeneous manner (data not shown). Some proximal tubules seemed completely intact while others shed their brush borders entirely into the luminal cavity or even disrupted from the basement membrane.
More detailed assessment of proximal tubules from hypoxic kidneys by electron microscopy showed severe loss of the apical brush border, blebbing of apical membranes, vacuolization and swelling of mitochondria while normoxic kidneys showed only varying degrees of vacuolization (Figure 1A, B). As with light microscopic assessment of kidneys, a heterogeneous picture emerged from analysing different areas of the kidneys. Complete cellular destruction was seen as well as entirely intact proximal tubular cells. The degree and extent of proximal tubular damage of tolbutamide treated kidneys resembled that of hypoxic untreated kidneys, although more intact brush border fragments were seen (Figure 1C). It is technically difficult to quantify brush border preservation, but these results support the observed functional improvement described below.
Figure 1.
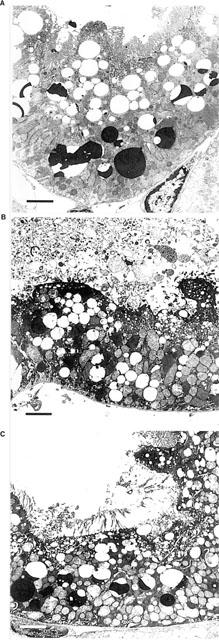
Electron microscopy images (3500×of proximal cells from normoxic perfused kidney (A) showing only vacuolization, and hypoxic perfused kidney (B) showing severe loss of brush border, apical blebbing, mitochondrial swelling and vacuolization. Tolbutamide treated hypoxic kidneys (C) showed a pattern similar to untreated hypoxic kidneys, although more intact brush border membranes were observed. The bar represents 3 μM.
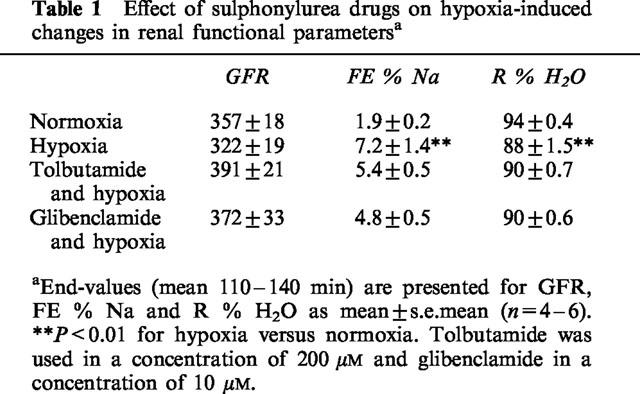
Upon starting the hypoxic perfusion of the isolated kidneys, a sharp increase was seen in GFR, RPP and diuresis (Figure 2). Diuresis increased over more than seven times basal values, which was accompanied by severe natriuresis. GFR values at the end of the experimental period did not significantly differ between normoxic and hypoxic perfused kidneys (Table 1), indicating full restoration of haemodynamic and glomerular functions. However, sodium and water reabsorption did not recover completely in hypoxic perfused kidneys as compared to normoxic perfused kidneys (Table 1), indicating sustained functional tubular damage due to the hypoxic period. Addition of tolbutamide or glibenclamide to the perfusate did not influence perfusate flow or perfusate pressure and both drugs had no significant effect on GFR, FE % Na and R % H2O, as compared to untreated hypoxic kidneys (Table 1).
Figure 2.
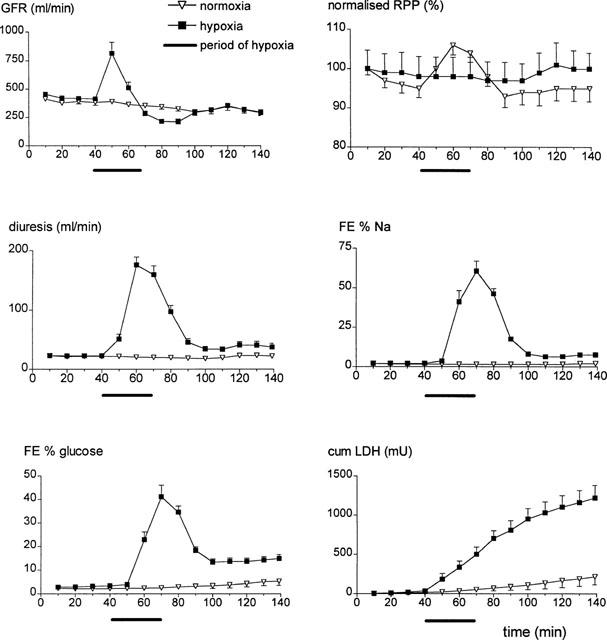
Mean values and s.e.mean for GFR, normalized RPP, diuresis, FE % Na, FE % glucose and cumulative urinary LDH excretion for normoxic (n=5) and hypoxic (n=9) perfusions plotted against time. The hypoxic period started at 40 min and ended at 70 min.
Table 1.
Effect of sulphonylurea drugs on hypoxia-induced changes in renal functional parametersa
Proximal reabsorptive capacity was assessed by measurement of fractional glucose excretion (FE % glucose). Hypoxic perfusion resulted in a sharp decline in proximal reabsorptive capacity, which did not completely return to baseline upon reoxygenation (Figure 2). Therefore, end-values for FE % glucose following hypoxic perfusions were significantly different from normoxic end-values (Figure 3). Both tolbutamide and glibenclamide reduced fractional glucose excretion in hypoxic perfused kidneys significantly, compared to untreated hypoxic perfused kidneys (Figure 3).
Figure 3.
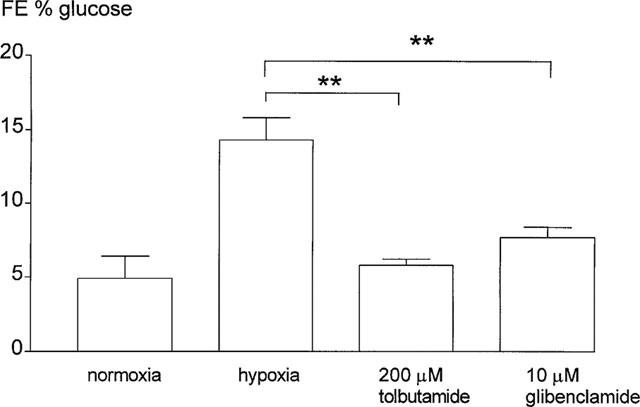
End-values (mean 110–140 min) for fractional excretion of glucose (FE % glucose) for normoxic and hypoxic perfused kidneys and for hypoxic kidneys treated with tolbutamide or glibenclamide. Data are expressed as mean±s.e.mean (n=4–6), for tolbutamide or glibenclamide treated kidneys versus hypoxia alone (**P<0.01).
This diminished reabsorptive capacity of the proximal tubule in hypoxic perfused kidneys was accompanied by a concurrent increase in urinary LDH excretion which returned to baseline levels after the hypoxic period (leading to flattening of the cumulative excretion curve of Figure 2). This resulted in a higher cumulative amount of LDH excreted in the urine as compared to normoxic perfusions (Figure 4). While 200 μM tolbutamide reduced the total amount of LDH excreted in the urine significantly the reduction in urinary LDH excretion seen with 10 μM glibenclamide failed to reach the level of 5% (β=0.35) significance (Figure 4).
Figure 4.
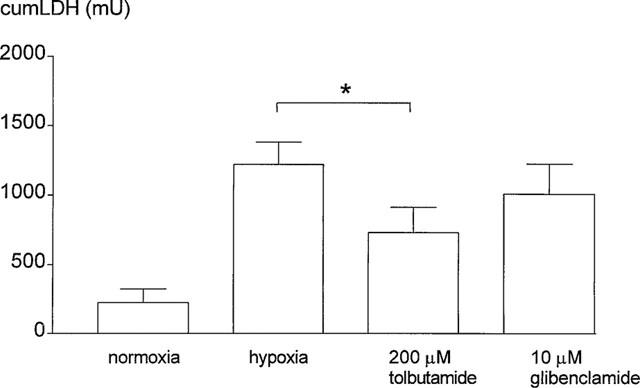
Cumulative urinary excretion of LDH (mU) for normoxic and hypoxic perfused kidneys and for hypoxic kidneys treated with tolbutamide or glibenclamide. Data are expressed as mean±s.e.mean (n=4–6), for tolbutamide or glibenclamide treated kidneys versus hypoxia alone (*P<0.05).
In order to further improve the observed protection with 10 μM glibenclamide we increased the applied concentration to 100 μM. This concentration is often used in experimental studies to inhibit epithelial K-ATP channels on isolated cells (Ashcroft & Ashcroft, 1990; Quast, 1996). However, in the isolated perfused kidney instead of protection, aggravation of hypoxic damage was observed as indicated by the reduced end-values for GFR (167±42 μl min−1, n=4, P<0.05) and increased end-values for FE % Na (17.5±2.0, n=4, P<0.01). Furthermore, this higher concentration of glibenclamide increased fractional glucose excretion (21.7±1.0, n=4, P<0.01) and urinary LDH excretion (2394±266 mU, n=4, P<0.01) indicating tubular damage.
Discussion
We showed a protective effect of the sulphonylureas, tolbutamide and glibenclamide, on morphological and functional parameters in hypoxic perfused rat kidney as reported previously for glibenclamide in isolated proximal tubules.
Proximal tubules are the most injured sites in clinical and experimental renal ischaemia. Isolated kidneys perfused with a medium devoid of an oxygen carrier develop a characteristic damage to the medullary thick ascending limb of Henle (Alcom et al., 1981; Brezis et al., 1984). However, Shanley et al. (1986a) showed an additional injury to proximal tubules in isolated cell-free perfused rat kidneys when medium was gassed with 95% N2/5% CO2 for 45 min. The results of the present study are in agreement with these findings. Hypoxic perfusions were damaging to proximal tubules, according to a reduced glucose reabsorption associated with an increase in LDH excretion. At the ultrastructural level this damage showed the characteristic features of that seen in clinical and experimental renal ischaemia (Shanley et al., 1986a; Brady et al., 1998; Spencer et al., 1991; Venkatachalam et al., 1978 Glaumann & Trump, 1975; Mergner et al., 1976). A similar heterogeneously distributed damage has been observed before in experimental studies by other investigators (Shanley et al., 1986a,1986b; Brezis et al., 1985). A profound morphological analysis of the injury to proximal tubules in the study of Shanley et al. (1986a) revealed a great variability in tubule responses to hypoxia with even complete protection to injury in peri-arterial zones, apparently due to gradients of oxygenation.
Normalization of GFR to baseline levels towards the end of the experiment indicates that the observed decrease in end-values for sodium, glucose and water reabsorption are due to a diminished tubular reabsorptive capacity, rather than an increased tubular load due to impaired filtration.
ATP-regulated potassium channels at the basolateral membrane of proximal tubules have been identified in rabbit, frog (Rana) and salamander (Ambystoma) using whole-cell and single channel patch clamp techniques (Tsuchiya et al., 1992; Robson & Hunter, 1997; Mauerer et al., 1998a). Channel activity was inhibited at micromolar concentrations of sulphonylurea drugs. Interestingly, Mauerer et al. (1998b) found a much more potent inhibition in cell-attached patches compared to the more often used inside-out patches. Out of six cell-attached recordings 10 μM glibenclamide exerted an 83±2% inhibition in three patches and 100 nM glibenclamide exerted a 70±3% inhibition in three other patches (Mauerer et al., 1998b). These findings suggest an augmentation of sulphonylurea potency by a cytosolic component, which may be an advantage of isolated cell and whole organ experiments. It could also provide an explanation for the relatively low concentrations needed in this study to reduce hypoxic damage in comparison with inhibitory concentrations for K-ATP channels in isolated membrane patches. Although these channels have not yet been described in rat proximal tubules, their existence seems likely, considering their pivotal role in recycling of potassium in concert with Na/K-ATPase activity (Tsuchiya et al., 1992). Whole organ experiments usually permit only limited conclusions as to the precise site of action of the applied drug. However, the most likely target for tolbutamide and glibenclamide would be proximal tubular basolateral K-ATP channels, although other targets, especially chloride channels, cannot be excluded (Rabe et al., 1995; Sheppard & Welsh, 1992; Reeves, 1997; Waters et al., 1997).
Tolbutamide and glibenclamide may also act at nephron segments other than proximal tubules, since K-ATP channels have also been described at the luminal site of medullary thick ascending limbs and cortical collecting ducts were they contribute to potassium recycling and secretion (Quast, 1996). It seems likely that the recently cloned subfamily of renal outer medulla potassium channels (ROMK) encodes for these luminal K-ATP channels (Ho et al., 1993). Expression in Xenopus oocytes of ROMK protein alone gave rise to a current, which was poorly inhibited by glibenclamide (Ki>mM), while co-expression with the ABC-superfamily member CFTR resulted in much higher affinities (Ki≈1 μM) for this drug (McNicholas et al., 1996). However, this higher susceptibility of ROMK to glibenclamide inhibition was lost under phosphorylating conditions (i.e. upon addition of PKA and MgATP), suggesting limited relevance of this observation under physiological conditions (McNicholas et al., 1996). Nevertheless, it seems likely that tolbutamide and glibenclamide acted directly on proximal tubules since they protected glucose reabsorption and at the same time reduced LDH excretion. In summary, this study describes for the first time, a protective effect of the K-ATP channel inhibitors tolbutamide and glibenclamide on hypoxic renal damage in a whole-organ experiment. The most likely target for both sulphonylureas would be the basolateral K-ATP channel of proximal tubules, but further studies are required to elucidate the exact site of action.
Acknowledgments
Dr K.J. Assmann from the Department of Pathology is gratefully acknowledged for his evaluation of light microscopic and electron microscopic preparations.
Abbreviations
- CFTR
cystic fibrosis transmembrane conductance regulator
- cumLDH
cumulative urinary LDH
- FE % glucose
fractional excretion of glucose
- FE % Na
fractional excretion of sodium
- IPK
isolated perfused kidney
- K-ATP
ATP regulated potassium channels
- Kir
inward rectifying potassium channel
- LDH
lactate dehydrogenase
- mTAL
medullary thick ascending limb of Henle' loop
- R % H2O
reabsorption of water
- ROMK
renal outer medulla potassium channel
- SUR
sulphonylurea receptor
References
- ALCORN D., EMSLIE K.R., ROSS B.D., RYAN G.B., TANGE J.D. Selective distal nephron damage during isolated kidney perfusion. Kidney Int. 1981;19:638–647. doi: 10.1038/ki.1981.63. [DOI] [PubMed] [Google Scholar]
- ASHCROFT S.J., ASHCROFT F.M. Properties and functions of ATP-sensitive K-channels. Cell. Signal. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- ASSMANN K.J., TANGELDER M.M., LANGE W.P., TADEMA T.M., KOENE R.A. Membranous glomerulonephritis in the mouse. Kidney Int. 1983;24:303–312. doi: 10.1038/ki.1983.159. [DOI] [PubMed] [Google Scholar]
- BABENKO A.P., AGUILAR BRYAN L., BRYAN J. A view of SUR/KIR6.X, KATP channels. Annu. Rev. Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- BALANT L. Clinical pharmacokinetics of sulphonylurea hypoglycaemic drugs. Clin. Pharmacokinet. 1981;6:215–241. doi: 10.2165/00003088-198106030-00003. [DOI] [PubMed] [Google Scholar]
- BEESLEY A.H., QURESHI I.Z., GIESBERTS A.N., PARKER A.J., WHITE S.J. Expression of sulphonylurea receptor protein in mouse kidney. Pflügers Arch. 1999;438:1–7. doi: 10.1007/s004240050872. [DOI] [PubMed] [Google Scholar]
- BRADY H.R., BRENNER B.M., LIEBERTHAL W. Acute Renal Failure. The Kidney. 1998. pp. 1201–1252.
- BREZIS M., ROSEN S., SILVA P., EPSTEIN F.H. Selective vulnerability of the medullary thick ascending limb to anoxia in the isolated perfused rat kidney. J. Clin. Invest. 1984;73:182–190. doi: 10.1172/JCI111189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREZIS M., SHANLEY P., SILVA P., SPOKES K., LEAR S., EPSTEIN F.H., ROSEN S. Disparate mechanisms for hypoxic cell injury in different nephron segments. Studies in the isolated perfused rat kidney. J. Clin. Invest. 1985;76:1796–1806. doi: 10.1172/JCI112171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKE T.J., SCHRIER R.W.Cell Death Molecular Biology of Membrane Transport Disorders 1996New York: Plenum Press; 485–505.Schultz , S., G pp [Google Scholar]
- COX P.G., MOONS M.M., SLEGERS J.F., RUSSEL F.G., VAN GINNEKEN C.A. The isolated perfused rat kidney as a tool in the investigation of renal handling and effects of nonsteroidal anti-inflammatory drugs. J. Pharmacol. Meth. 1990;24:89–103. doi: 10.1016/0160-5402(90)90020-l. [DOI] [PubMed] [Google Scholar]
- GLAUMANN B., TRUMP B.F. Studies on the pathogenesis of ischemic cell injury. III. Morphological changes of the proximal pars recta tubules (P3) of the rat kidney made ischemic in vivo. Virchows Arch. B. Cell Pathol. 1975;19:303–323. [PubMed] [Google Scholar]
- HEARSE D.J. Activation of ATP-sensitive potassium channels: a novel pharmacological approach to myocardial protection? [see comments] Cardiovasc. Res. 1995;30:1–17. [PubMed] [Google Scholar]
- HEURTEAUX C., BERTAINA V., WIDMANN C., LAZDUNSKI M. K+ channel openers prevent global ischemia-induced expression of c-fos, c-jun, heat shock protein, and amyloid beta-protein precursor genes and neuronal death in rat hippocampus. Proc. Natl. Acad. Sci. U. S. A. 1993;90:9431–9435. doi: 10.1073/pnas.90.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO K., NICHOLS C.G., LEDERER W.J., LYTTON J., VASSILEV P.M., KANAZIRSKA M.V., HEBERT S.C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- LIEBERTHAL W., RENNKE H.G., SANDOCK K.M., VALERI C.R., LEVINSKY N.G. Ischemia in the isolated erythrocyte-perfused rat kidney. Protective effect of hypothermia. Ren. Physiol. Biochem. 1988;11:60–69. doi: 10.1159/000173150. [DOI] [PubMed] [Google Scholar]
- MASON J., BECK F., DORGE A., RICK R., THURAU K. Intracellular electrolyte composition following renal ischemia. Kidney Int. 1981;20:61–70. doi: 10.1038/ki.1981.105. [DOI] [PubMed] [Google Scholar]
- MAUERER U.R., BOULPAEP E.L., SEGAL A.S. Regulation of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule. J. Gen. Physiol. 1998a;111:161–180. doi: 10.1085/jgp.111.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUERER U.R., BOULPAEP E.L., SEGAL A.S. Properties of an Inwardly Rectifying ATP-sensitive K+ Channel in the Basolateral Membrane of Renal Proximal Tubule. J. Gen. Physiol. 1998b;111:139–160. doi: 10.1085/jgp.111.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNICHOLAS C.M., GUGGINO W.B., SCHWIEBERT E.M., HEBERT S.C., GIEBISCH G., EGAN M.E. Sensitivity of a renal K+ channel (ROMK2) to the inhibitory sulfonylurea compound glibenclamide is enhanced by coexpression with the ATP-binding cassette transporter cystic fibrosis transmembrane regulator. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8083–8088. doi: 10.1073/pnas.93.15.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERGNER W.J., CHANG S.H., TRUMP B.F. Studies on the pathogenesis of ischemic cell injury. V. Morphologic changes of the pars convoluta (P1 and P2) of the proximal tubule of rat kidney made ischemic in vitro. Virchows Arch. B. Cell Pathol. 1976;21:211–228. [PubMed] [Google Scholar]
- MOLITORIS B.A. Ischemia-induced loss of epithelial polarity: potential role of the actin cytoskeleton [editorial] Am. J. Physiol. 1991;260:F769–F778. doi: 10.1152/ajprenal.1991.260.6.F769. [DOI] [PubMed] [Google Scholar]
- QUAST U. ATP-sensitive K+ channels in the kidney. Naunyn Schmiedebergs Arch. Pharmacol. 1996;354:213–225. doi: 10.1007/BF00171051. [DOI] [PubMed] [Google Scholar]
- RABE A., DISSER J., FROMTER E. Cl- channel inhibition by glibenclamide is not specific for the CFTR-type Cl- channel. Pflugers Arch. 1995;429:659–662. doi: 10.1007/BF00373986. [DOI] [PubMed] [Google Scholar]
- REEVES W.B. Effects of chloride channel blockers on hypoxic injury in rat proximal tubules. Kidney Int. 1997;51:1529–1534. doi: 10.1038/ki.1997.210. [DOI] [PubMed] [Google Scholar]
- REEVES W.B., SHAH S.V. Activation of potassium channels contributes to hypoxic injury in proximal tubules. J. Clin. Invest. 1994;94:2289–2294. doi: 10.1172/JCI117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBSON L., HUNTER M. Two K(+)-selective conductances in single proximal tubule cells isolated from frog kidney are regulated by ATP. J. Physiol. Lond. 1997;500:605–616. doi: 10.1113/jphysiol.1997.sp022046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANLEY P.F., BREZIS M., SPOKES K., SILVA P., EPSTEIN F.H., ROSEN S. Hypoxic injury in the proximal tubule of the isolated perfused rat kidney. Kidney Int. 1986a;29:1021–1032. doi: 10.1038/ki.1986.102. [DOI] [PubMed] [Google Scholar]
- SHANLEY P.F., BREZIS M., SPOKES K., SILVA P., EPSTEIN F.H., ROSEN S. Transport-dependent cell injury in the S3 segment of the proximal tubule. Kidney Int. 1986b;29:1033–1037. doi: 10.1038/ki.1986.103. [DOI] [PubMed] [Google Scholar]
- SHEPPARD D.N., WELSH M.J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J. Gen. Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITS P., BIJLSTRA P.J., RUSSEL F.G., LUTTERMAN J.A., THIEN T. Cardiovascular effects of sulphonylurea derivatives. Diabetes Res. Clin. Pract. 1996;31 Suppl:S55–S59. doi: 10.1016/0168-8227(96)01230-2. [DOI] [PubMed] [Google Scholar]
- SPENCER A.J., LEFURGEY A., INGRAM P., MANDEL L.J. Elemental microanalysis of organelles in proximal tubules. II. Effects of oxygen deprivation. J. Am. Soc. Nephrol. 1991;1:1321–1333. doi: 10.1681/ASN.V1121321. [DOI] [PubMed] [Google Scholar]
- TAKABA H., NAGAO T., YAO H., KITAZONO T., IBAYASHI S., FUJISHIMA M. An ATP-sensitive potassium channel activator reduces infarct volume in focal cerebral ischemia in rats. Am. J. Physiol. 1997;273:R583–R586. doi: 10.1152/ajpregu.1997.273.2.R583. [DOI] [PubMed] [Google Scholar]
- TANEMOTO M., VANOYE C.G., DONG K., WELCH R., ABE T., HEBERT S.C., XU J.Z. Rat homolog of sulfonylurea receptor 2B determines glibenclamide sensitivity of ROMK2 in Xenopus laevis oocyte. Am. J. Physiol. 2000;278:F659–F656. doi: 10.1152/ajprenal.2000.278.4.F659. [DOI] [PubMed] [Google Scholar]
- TSUCHIYA K., WANG W., GIEBISCH G., WELLING P.A. ATP is a coupling modulator of parallel Na,K-ATPase-K-channel activity in the renal proximal tubule. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6418–6422. doi: 10.1073/pnas.89.14.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENKATACHALAM M.A., BERNARD D.B., DONOHOE J.F., LEVINSKY N.G. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978;14:31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- WATERS S.L., MILLER G.W., ALEO M.D., SCHNELLMANN R.G. Neurosteroid inhibition of cell death. Am. J. Physiol. 1997;273:F869–F876. doi: 10.1152/ajprenal.1997.273.6.F869. [DOI] [PubMed] [Google Scholar]


