Abstract
The aim of this study was to elucidate the in vitro inhibitory potency of FK506 on production of the inflammatory cytokines, tumour necrosis factor (TNF)-α and interleukin (IL)-1β, with a view to assessing this immunosuppressive agent as a potential anti-rheumatic drug.
We employed an in vitro model which produces TNF-α and IL-1β through T cell activation. Human peripheral blood mononuclear cells (PBMC) were cultured with immobilized anti-CD3/CD28 monoclonal antibody in this model.
FK506 inhibited anti-CD3/CD28 induced TNF-α and IL-1β production at concentrations less than 1 ng ml−1. Flow cytometric analysis of intracellular TNF-α and IL-1β positive cells showed that FK506 potently suppresses inflammatory cytokine production from CD14+ monocytes as well as from T cells.
Cyclosporin A (CsA) and dexamethasone (DEX) also inhibited the anti-CD3/CD28 induced cytokine production, but were less potent than FK506. FK506 and CsA, but not DEX, specifically inhibited anti-CD3/CD28 induced inflammatory cytokine production without affecting the lipopolysaccaride (LPS) induced effect. Methotrexate (MTX) was completely inactive for suppressing cytokine production under either condition.
Anti-CD3/CD28 stimulated PBMC culture supernatants were found to enhance the expression of adhesion molecules in human vascular endothelial cells. FK506, CsA and DEX led to the suppression of adhesion molecule expression probably by inhibiting cytokine production from PBMC.
The inhibitory potency of agents on TNF-α and IL-1β production was compared with cytotoxicity and FK506 was not cytotoxic at concentrations several orders of magnitude greater than those required for cytokine inhibition.
These results strongly suggest that FK506 may be most effective to specifically prevent T cell activation mediated inflammatory cytokine production in a clinical setting.
Keywords: FK506, TNF-α, IL-1β, T cells, cytotoxicity, adhesion molecule, rheumatoid arthritis, cyclosporin A, dexamethasone, methotrexate
Introduction
FK506 is an immunosuppressive agent with an increasing number of clinical applications. Recently, FK506 has been examined as a treatment for rheumatoid arthritis (RA) and shown to be effective in RA patients failing methotrexate (MTX) (Furst et al., 1999). There is a substantial body of evidence supporting the hypothesis that T cells play a central role in initiating and perpetuating the chronic autoimmune response to RA (Panayi et al., 1992). Additionally, inflammatory cytokines such as TNF-α and IL-1β are reported to be responsible for the pathogenesis of RA (Arend & Dayer, 1995). Anti-cytokine therapy in clinical studies has also provided evidence for the involvement of these cytokines in RA (Elliott & Maini, 1995; Moreland et al., 1997). FK506 has been reported to inhibit T cell activation and T cell-derived cytokine production (Sawada et al., 1987; Tocci et al., 1989), however, the effect on inflammatory cytokine production has not been fully characterized. Specifically, the production of TNF-α and IL-1β by activated monocytes has been reported to be not affected by FK506 (Tocci et al., 1989; Andersson et al., 1992). Further insight into the effect of FK506 on T cell induced inflammatory cytokines may in part elucidate the mechanism of action of FK506 as a therapy for RA.
RA is an autoimmune dysfunction in which T cells play an important role in pathogenesis of the disease. Therefore, exogenous triggering agents such as LPS may not be appropriate stimulants to induce inflammatory cytokines in order to evaluate agents for treatment of RA, even though LPS has been used frequently as a stimulant of mononuclear cells (Segal et al., 1989; Finch-Arietta & Cochran, 1991; Bondeson & Sundler, 1995; Hartman et al., 1995; Joyce et al., 1997). It is possible that inflammatory cytokines are produced through activation of T cells and by subsequent interaction of the activated T cells and monocytes/macrophages in RA (Panayi et al., 1992).
T cells produce various cytokines including TNF-α. It has been reported that TNF-α and IL-1β are also produced from monocytes in PBMC during T cell activation (Landis et al., 1991; McAllister & Ellis, 1996; Sebbag et al., 1997). In order to estimate the efficacy of anti-rheumatic agents based on the effects on inflammatory cytokine production, we employed an in vitro model which produces TNF-α and IL-1β through T cell activation. Anti-CD3 monoclonal antibody and anti-CD28 monoclonal antibody (anti-CD3/CD28) were used as stimulants in the model. The effect of FK506 on TNF-α and IL-1β production in human PBMC was compared with the immunosuppressive anti-rheumatic drugs, cyclosporin A (CsA), dexamethasone (DEX) and MTX.
CsA is an immunosuppressive agent whose mode of action on the immune system is known to be very similar to FK506 but is 30–100 times less potent than FK506 (Ho et al., 1996). The major mode of action of this agent in RA has been assumed to be suppression of cytokine production from activated T cells (Barrera et al., 1996; Danning & Boumpas, 1998). Concerning inflammatory cytokines, it has been reported that CsA does not affect the production of TNF-α (Andersson et al., 1992) or IL-1β (Rordorf-Adam et al., 1989) from activated monocytes. Glucocorticoids are effective in suppressing the clinical spectra of many inflammatory diseases and have been used for the treatment of RA. Glucocorticoids affect many cellular and humoral mechanisms involved in the immune response (Barrera et al., 1996; Danning & Boumpas, 1998). Glucocorticoids, such as DEX, are known to decrease cytokine production from activated T cells (Schmidt et al., 1994; Van Wauwe et al., 1995) and monocytes/macrophages (Hartman et al., 1995; Joyce et al., 1997). MTX is a widely used anti-rheumatic drug, that sometimes leads to severe side effects such as bone marrow suppression (Segal et al., 1990). The mechanism of action of MTX has not been well defined, even though it has been shown to possess various anti-inflammatory effects. Due to the antifolate activity of MTX, it inhibits purine and pyrimidine synthesis and consequently impairs proliferation of immune cells (Baggott et al., 1993; Cronstein, 1996).
TNF-α and IL-1β participate in host defense, although overproduction of various inflammatory cytokines is associated with a wide range of medical disorders (Czuprynski et al., 1988; Marriott et al., 1997). Our contention is that the in vitro model of LPS-induced inflammatory cytokine production does not mimic monocyte activation in RA. In this report, we assess the effects of anti-rheumatic agents in an alternative, perhaps more relevant, in vitro system in which cytokine production is triggered by T cell activation.
Methods
Immunosuppressive agents
FK506, cyclosporin A (CsA) and Dexamethasone (DEX) were prepared in our research laboratories. Methotrexate (MTX) was purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). FK506, CsA and DEX were dissolved in ethanol. MTX was suspended and sonicated in ethanol. These reagents were diluted by culture medium.
PBMC preparation
Peripheral blood mononuclear cells (PBMC) were isolated from venous blood of healthy volunteers by centrifugation using Ficoll-Paque PLUS (Pharmacia Biotech, Uppsala, Sweden). PBMC were washed and resuspended in a culture medium of RPMI-1640 (Nikken Biomedical Laboratory, Kyoto, Japan) supplemented with 10% foetal bovine serum (FBS, HyClone Laboratories, Inc., Logan, UT, U.S.A.) and penicillin (50 IU ml−1)-streptomycin (50 μg ml−1) (ICN Biomedicals, Inc., Aurora, OH, U.S.A.).
Anti-CD3/CD28 induced cytokine production
A flat bottomed 24 well plate (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) was coated with 25 μg ml−1 of goat anti-mouse IgG Fc monoclonal antibody (Chemicon, Temecula, CA, U.S.A.). After the plate was washed with phosphate-buffered saline (PBS, Nikken Biomedical Laboratory), mouse anti-human CD3 monoclonal antibody (150 ng ml−1, Kyowa Hakko Kogyo Co., Ltd., Tokyo, Japan) and mouse anti-human CD28 monoclonal antibody (50 ng ml−1, Becton Dickinson, Franklin Lakes, NJ, U.S.A.) (anti-CD3/CD28) were immobilized to the plate. PBMC (3.0×106 cells ml−1well−1) were cultured in the anti-CD3/CD28 immobilized 24-well plate in 1 ml of culture medium containing various concentrations of agents for 24 h at 37°C. As a non-stimulated negative control, PBMC were cultured in goat anti-mouse IgG Fc monoclonal antibody-coated plate. After a 24 h culture at 37°C in 5% CO2 in humidified air, culture supernatants were harvested and stored at −80°C.
LPS-induced cytokine production
PBMC (2×106 cells ml−1, 0.5 ml well−1) were cultured in a flat bottomed 24 well plate in 0.5 ml of culture medium containing 2 μg ml−1 of LPS (Serotype 055:B4, Sigma Chemical Co., St Louis, MO, U.S.A.) and various concentrations of agents for 24 h at 37°C. After a 24 h culture at 37°C in 5% CO2 in humidified air, culture supernatants were harvested and stored at −80°C.
Determination of cytokines
TNF-α, IL-1β, IL-2, and IFN-γ in appropriately diluted culture supernatants were measured by cytokine ELISA kit (Amersham Pharmacia Biotech, Buckinghamshire, U.K.). ELISA plates were scanned in a Spectra MAX250 plate reader (WAKO, Osaka, Japan) using SOFTmax software (Molecular Devices Corp., Sunnyvale, CA, U.S.A.).
Flow cytometric analysis
Anti-CD3/CD28 antibody were immobilized in a flat bottomed 6 well plate (Sumitomo Bakelite Co., Ltd.) as described above. PBMC (3×106 cells well−1) were cultured in 5.1 ml of culture medium in the presence or absence of 10 ng ml−1 of FK506 at 37°C. After 12 h culture, GolgiStop solution (PharMingen, San Diego, CA, U.S.A.) was added to each well and incubated for another 6 h for storing cytokines in golgi (Sander et al., 1991). As a non-stimulated negative control, PBMC were cultured in the goat anti-mouse IgG Fc monoclonal antibody-coated plate. After the culture, PBMC were collected and resuspended in staining buffer containing 1% foetal calf serum (Dainippon Pharmaceutical Co. Ltd., Osaka, Japan) and 0.1% NaN3 in PBS. For staining cell surface marker, the PBMC were incubated with Cy-Chrome-labelled mouse anti-human CD4 monoclonal antibody (PharMingen), FITC-labelled mouse anti-human CD8 monoclonal antibody (PharMingen) or FITC-labelled mouse anti-human CD14 monoclonal antibody (PharMingen) for 20 min at 4°C. After staining, PBMC were washed in staining buffer and fixed with Cytofix/Cytoperm solution (PharMingen) for 20 min at 4°C. Then PBMC were washed and resuspended in Perm/Wash solution (PharMingen). For staining intracellular cytokines, the labelled PBMC were incubated with R-Phycoerythrin (PE)-labelled mouse anti-human TNF-α monoclonal antibody (PharMingen) or PE-labelled mouse anti-human IL-1β monoclonal antibody (Becton Dickinson) for 30 min at 4°C. After the double-staining, PBMC were washed and resuspended in Perm/Wash solution. PBMC were then analysed by FACScan (Beckton Dickinson) using LYSYS II software (Beckton Dickinson).
Cell ELISA of ICAM-1 and VCAM-1 on HUVEC
Human umbilical vein endothelial cells (HUVEC) were purchased from TOYOBO (Tokyo, Japan). HUVEC were maintained in growth medium (DMEM/F12 1 : 1 medium (Nikken Biomedical Laboratory), supplemented with 10% FBS (HyClone), penicillin (50 IU ml−1)-streptomycin (50 μg ml−1), endothelial cell growth supplement (50 μg ml−1, Collaborative Research Inc., Bedford, MA, U.S.A.) and Heparin (5 μg ml−1, Sigma) using a fibronectin-coated flask. Cell ELISA of ICAM-1 and VCAM-1 were performed according to the modified methods as described (Yamaguchi et al., 1997; Takami et al., 1998). Briefly, HUVEC (1×104 cells well−1) were seeded in a Fibronectin-coated 96-well microplate (Sumitomo Bakelite Co., Ltd.) and incubated for 4 h. The growth medium was then removed and 50 μl well−1 of appropriately diluted PBMC culture supernatants were added to the HUVEC monolayer. The culture was maintained for 16 h at 37°C. After the culture, the monolayer was fixed with 100 μl well−1 of 2% paraformaldehyde in PBS at room temperature (RT) for 15 min. After the fixation, HUVEC were incubated in 100 μl well−1 of 3% BSA-PBS at 37°C for 2 h. Then the cells were treated with 50 μl well−1 of mouse anti-human ICAM-1 monoclonal antibody (PharMingen) or mouse anti-human VCAM-1 monoclonal antibody (Genzyme Corporation, Cambridge, MA, U.S.A.) in 3% BSA-PBS. After 1 h incubation at 37°C, the cells were treated with 50 μl well−1 of peroxidase-F(ab′)2 rabbit anti-mouse IgG (H+L) (ZYMED Laboratories, Inc., San Francisco, CA, U.S.A.) at 37°C for 1 h. Then the cells were incubated in 0.1 ml well−1 of the substrate solution (0.4 mg ml−1 o-phenylendiamine, 0.003% H2O2 in 0.1 mol L−1 phosphate-citrate buffer) for 20 min at RT in a dark place. The reaction was stopped by adding 50 μl well−1 of 6.6% H2SO4, and spectrophotometric readings were made at 492 nm.
Cell proliferation assay
Saos-2 cells (human osteogenic carcinoma, American Type Culture Collection, Rockville, MD, U.S.A.) were maintained in Dulbecco' modified Eagle' medium (DMEM, Nikken Biomedical Laboratory), supplemented with 10% FBS (Intergen, Purchase, NY, U.S.A.) and penicillin (50 IU ml−1)-streptomycin (50 μg ml−1). The cells (1×104 cells well−1) were cultured in a 96-well flat bottomed microplate in the medium containing various concentrations of agents. After 72 h culture, determination of cell proliferation was undertaken using the Cell Proliferation Kit I (MTT) (Boehringer Mannheim GmbH, Mannheim, Germany).
Measurement of CFU-C
Haematopoietic progenitor cells (CFU-C) were assayed in semisolid cultures as described (Strømhaug et al., 1995). Briefly, bone marrow cells (1×105 cells) obtained from female C57BL/6 mice (Charles River Japan Inc., Kanagawa, Japan) in 1 ml of α-MEM supplemented with 0.88% methylcellulose, 20% FBS (Intergen), 10 ng ml−1 of recombinant mouse GM-CSF (Pepro Tech EC Ltd., London, U.K.), penicillin (50 IU ml−1)-streptomycin (50 μg ml−1) and various concentrations of agents were plated in a 6 well plate (35 mm diameter, IWAKI GLASS Co., Ltd., Chiba, Japan). After 8 days culture at 37°C, colony numbers were counted using a dissecting microscope.
Statistical analysis
Results are presented as mean±s.e.mean. Statistical analysis was performed using Dunnett' multiple comparison test following randomized block designed analysis of variance.
Results
The effect of FK506 on TNF-α production from anti-CD3/CD28 stimulated PBMC
To investigate the effect of FK506 on inflammatory cytokine production, we employed an in vitro model producing cytokines through T cell activation in human PBMC. FK506 has been reported to inhibit TNF-α production from activated T cells (Tocci et al., 1989; Dumont et al., 1998). TNF-α is not exclusive to T cells and is also produced from monocytes through interaction with activated T cells (Sebbag et al., 1997). We first examined the effect of FK506 on TNF-α production in PBMC by stimulating T cells with anti-CD3 antibody in conjugation with anti-CD28 antibody (anti-CD3/CD28). Cross-linking of a costimulatory molecule such as CD28 is known to amplify the TCR-coupled signal (Dumont et al., 1998). As shown in Figure 1A, TNF-α producing cells in CD4+ and CD8+ T cells and CD14+ monocytes were analysed by flow cytometry. Intracellular TNF-α positive cells were increased in CD4+ and CD8+ T cells and CD14+ monocytes by stimulation of anti-CD3/CD28. FK506 was found to decrease TNF-α positive cells in the CD4+ cells (22.8 to 6.7%), CD8+ cells (16.1 to 5.1%) and CD14+ cells (21.3 to 2.5%). These results indicate that FK506 suppresses T cell activation induced TNF-α production from both T cells and monocytes.
Figure 1.
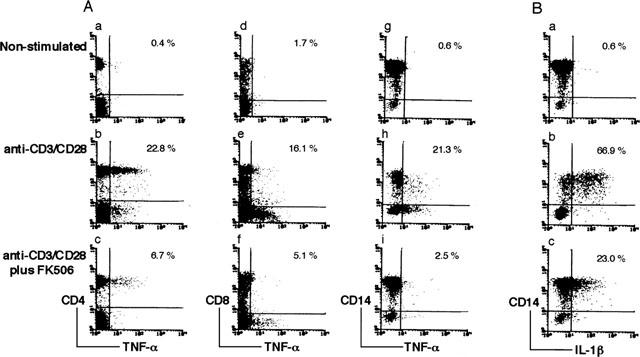
Flow cytometric analysis of intracellular TNF-α and IL-1β positive cells in T cells and monocytes. PBMC were cultured for 18 h in the absence (A-a,d,g, B-a) or presence (A-b,c,e,f,h,i, B-b,c) of anti-CD3/CD28. The effect of FK506 was examined by culturing PBMC with anti-CD3/CD28 and 10 ng ml−1 of FK506 (A-c,f,i, B-c). Six hours before the cell harvest, Golgi stop solution was added to the culture. Cells were stained with Cy-chrome-labelled anti-CD4 antibody (A-a,b,c) or FITC-labelled anti-CD8 antibody (A-d,e,f) or FITC-labelled CD14 antibody (A-g,h,i, B-a,b,c). After fixation, cells were stained with PE-labelled monoclonal antibodies against TNF-α (A) or IL-1β (B) and analysed by two-colour flow cytometry. The percentage of cytokine positive cells in CD4, CD8 and CD14 positive cells are shown in the upper right corner. Data are representative of three experiments.
Effects of FK506 on IL-1β production from anti-CD3/CD28 stimulated PBMC
IL-1β is produced from monocytes by stimulating PBMC with T cell mitogen (Landis et al., 1991; McAllister & Ellis, 1996). As it was confirmed that FK506 decreased TNF-α production from monocytes as well as T cells, we further investigated whether FK506 suppresses IL-1β production in monocytes during T cell activation. Flow cytometric analysis of intracellular IL-1β positive cells in Figure 1B indicates that CD14+ monocytes produce IL-1β by stimulation with anti-CD3/CD28. IL-1β positive cells in CD14+ monocytes were found to decrease from 66.9 to 23.0% in the presence of 10 ng ml−1 of FK506. The result indicates that FK506 blocks T cell activation mediated IL-1β production from monocytes.
Effects of immunosuppressive agents on anti-CD3/CD28 induced inflammatory cytokine production
The inhibitory potency of FK506 on anti-CD3/CD28 induced TNF-α and IL-1β production was compared with that of other immunosuppressive agents, CsA, DEX and MTX. As shown in Figure 2, FK506 potently inhibited both TNF-α and IL-1β production in a concentration dependent manner. CsA also inhibited production of these cytokines, but was approximately 100 times less potent than FK506. DEX also suppressed the cytokine production but was one order of magnitude less potent than FK506. MTX was completely inactive at concentrations up to 1000 ng ml−1.
Figure 2.
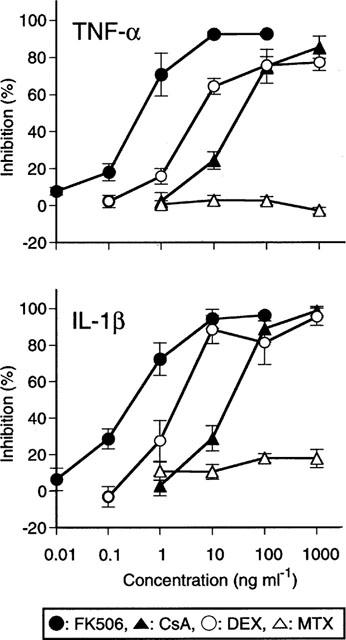
Effects of agents on the production of TNF-α and IL-1β by PBMC stimulated with anti-CD3/CD28. PBMC were stimulated with anti-CD3/CD28 in the presence of various concentrations of FK506, CsA, DEX and MTX. Results are expressed as the percentage inhibition of cytokine production relative to drug-free control. The baseline (100% inhibition) was represented by the unstimulated culture. The data are the mean±s.e.mean of five individuals. The control levels (mean±s.e.mean) were as follows; TNF-α : 7.6±1.4 ng ml−1, IL-1β : 0.23±0.05 ng ml−1.
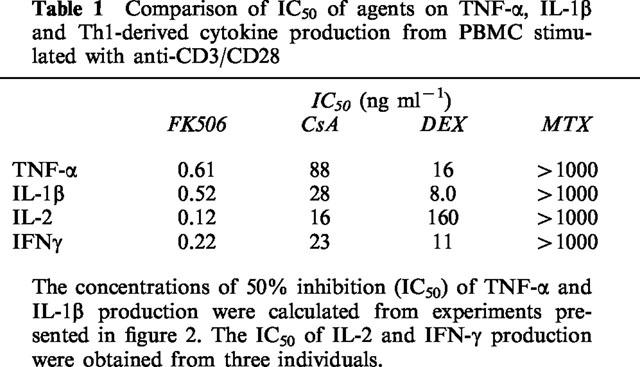
It is generally believed that FK506 exerts its therapeutic effects in organ transplantation principally by suppressing T cell activation and subsequent production of T cell derived cytokines such as IL-2 and IFN-γ (Sawada et al., 1987). The inhibitory potency of FK506 on the production of TNF-α and IL-1β was compared with that of FK506 on IL-2 and IFN-γ production as shown in Table 1. Inhibitory concentrations (IC50) of FK506 on TNF-α, IL-1β and the T cell derived cytokine production were not significantly different. The IC50 of FK506 for the production of each cytokine was <1.0 ng ml−1. The IC50s for CsA on inflammatory and T cell derived cytokine production were not significantly different from each other but were substantially higher than those obtained with FK506. These results indicate that the inhibitory potency of FK506 and CsA on T cell activation induced TNF-α and IL-1β production from monocytes was comparable to that for T cell exclusive cytokines.
Table 1.
Comparison of IC50 of agents on TNF-α, IL-1β and Th1-derived cytokine production from PBMC stimulated with anti-CD3/CD28
Plasma concentrations resulting from FK506 (Pou et al., 1998), CsA (Faulds et al., 1993) and DEX (Weijtens et al., 1998) clinical doses, are known to be above the IC50 of each agent for TNF-α and IL-1β production. On the other hand, the peak plasma concentration of MTX (Sinnett et al., 1989) at the dose used for RA is far below 1000 ng ml−1. Therefore, it is most likely that FK506, CsA and DEX, but not MTX are capable of inhibiting T cell activation mediated TNF-α and IL-1β production in a clinical setting.
Effects of immunosuppressive agents on LPS induced inflammatory cytokine production
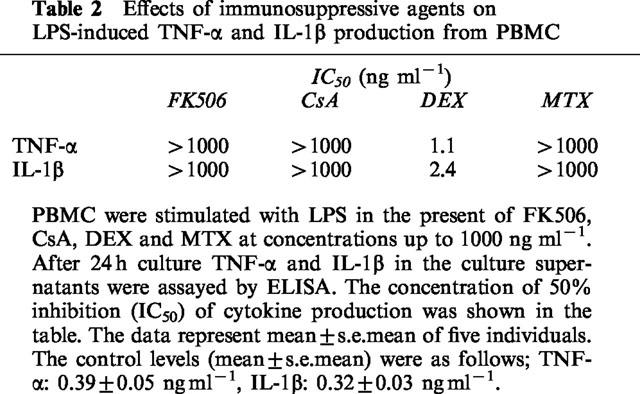
Inflammatory cytokine production from mononuclear cells has been investigated by stimulating cells with gram-negative bacterial component LPS in most studies. DEX has been reported to inhibit LPS-induced cytokine production (Hartman et al., 1995; Joyce et al., 1997). As shown in Table 2, the inhibitory potency of DEX on LPS-stimulated cytokine production was more potent than that on the anti-CD3/CD28 induced one. On the other hand, FK506, CsA and MTX did not show any inhibitory effect on LPS induced cytokine production. These results support the notion that FK506 and CsA, but not DEX, specifically suppress inflammatory cytokine production triggered by T cell activation without affecting the production induced by gram-negative bacterial cell wall components or LPS.
Table 2.
Effects of immunosuppressive agents on LPS-induced TNF-α and IL-1β production from PBMC
Anti-CD3/CD28 stimulated PBMC culture supernatants augmented the expression of ICAM-1 and VCAM-1 in HUVEC
Large amounts of TNF-α and IL-1β were produced by stimulation of PBMC with anti-CD3/CD28 as shown in Figure 2. Therefore we further investigated the effect of anti-CD3/CD28 stimulated PBMC culture supernatants on the expression of ICAM-1 and VCAM-1 in HUVEC. As shown in Figure 3, cultured HUVEC monolayers were treated with varying dilutions of anti-CD3/CD28 stimulated or non-stimulated PBMC culture supernatants. The stimulated PBMC culture supernatants were found to enhance cell surface expression of ICAM-1 and VCAM-1 compared to non-stimulated ones.
Figure 3.
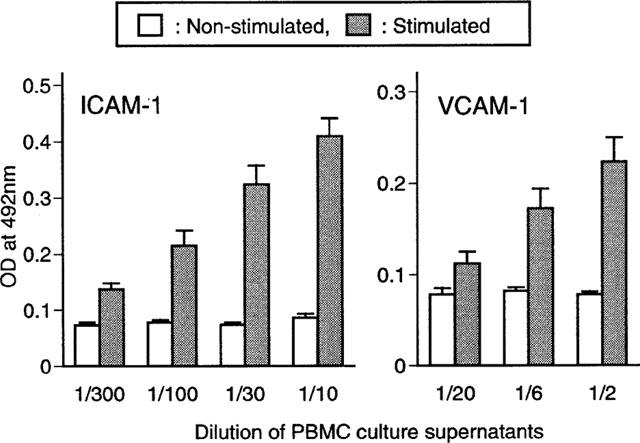
Effects of anti-CD3/CD28 stimulated PBMC culture supernatants on ICAM-1 and VCAM-1 expression in HUVEC. HUVEC were cultured with anti-CD3/CD28 unstimulated or stimulated PBMC culture supernatants. After 16 h culture, the expression of ICAM-1 and VCAM-1 on the HUVEC was determined by cell ELISA. The data represent mean±s.e.mean of culture supernatants of five individuals.
Effect of culture supernatants from anti-CD3/CD28 stimulated PBMC in the presence of agents on the expression of ICAM-1 and VCAM-1 in HUVEC
PBMC culture supernatants stimulated with anti-CD3/CD28 in the presence of various concentrations of FK506, CsA, DEX and MTX were added to the culture of HUVEC monolayers. The effects of the culture supernatants on the expression of the adhesion molecules were then investigated. As shown in Figure 4, expression of both ICAM-1 and VCAM-1 was inhibited when the HUVEC were incubated with supernatants from anti-CD3/CD28 activated PBMC which had been treated with FK506. The expression of adhesion molecules was not augmented, probably because the supernatants lack the stimulatory cytokines required to produce this effect. Additionally, CsA and DEX but not MTX decreased the enhancing activity of the PBMC culture supernatants to induce ICAM-1 and VCAM-1 in HUVEC. The inhibition of ICAM-1 and VCAM-1 expression was almost parallel to the suppression of TNF-α and IL-1β production by those agents. The level of expression of both adhesion molecules was not affected by the agents themselves (data not shown). Therefore it is most likely that FK506, CsA and DEX suppress T cell activation mediated adhesion molecule expression by inhibiting the production of adhesion molecule inducible cytokines including TNF-α and IL-1β.
Figure 4.
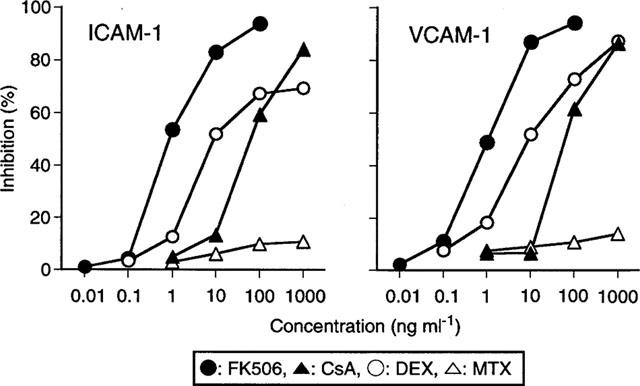
Effects of anti-CD3/CD28 stimulated PBMC culture supernatants in the presence of drugs on ICAM-1 and VCAM-1 expression of HUVEC. PBMC culture supernatants stimulated with anti-CD3/CD28 in the presence of various concentrations of FK506, CsA, DEX and MTX as shown in Figure 2 were added to HUVEC monolayers. After 16 h culture, the expression of ICAM-1 and VCAM-1 on the HUVEC was determined by Cell ELISA. The data are the mean of percentage inhibition of five individual PBMC culture supernatants.
Specificity of agents on the suppression of inflammatory cytokine production versus cell proliferation
The inhibitory potency of agents on anti-CD3/CD28 induced production of TNF-α and IL-1β was compared with their cytotoxicity. The proliferation of human osteogenic cell line, Saos-2, and colony formation of haematopoietic progenitor cells (CFU-C) in mouse bone marrow cells were employed to examine the cytotoxic effects of agents. CFU-C assay has been frequently employed to predict the toxic effects on bone marrow in vivo. FK506 and CsA (Kino et al., 1987) have already been shown to have a relatively weak effect on CFU-C. On the other hand, DEX (Golde et al., 1976) and MTX (Strømhaug et al., 1995) have been reported to suppress CFU-C at lower doses. For the purpose of comparing the effects on inflammatory cytokine production and haematopoietic cells more strictly, we examined the effects of agents on CFU-C. As shown in Table 3, the average IC50 of anti-CD3/CD28 induced TNF-α and IL-1β production was calculated from the results in Table 1 and compared with the IC50 against CFU-C. The relative specificity was presented as the ratio of CFU-C/TNF,IL-1.
Table 3.
Comparison of inhibitory potency of agents on inflammatory cytokine production and their cytotoxicity
FK506 showed no effect on the proliferation of Saos-2 and inhibited CFU-C at the highest dose of 5400 ng ml−1. It was notable that the ratio of CFU-C/TNF,IL-1 for FK506 was about 10,000. CsA inhibited CFU-C at a lower dose compared to FK506. The ratio of CFU-C/TNF,IL-1 was only 26. Therefore, FK506 is approximately 100 times more potent in suppressing T cell activation mediated inflammatory cytokine production as well as three times less potent at inhibiting CFU-C, translating to a 300 fold relative specificity over CsA, according to those parameters. DEX did not have any effect on the proliferation of Saos-2 but inhibited CFU-C at almost the same concentration as the inhibition of anti-CD3/CD28 induced TNF-α and IL-1β production. Consequently, CFU-C/TNF,IL-1 for DEX was found to be 0.92. MTX inhibited both the proliferation of Saos-2 and CFU-C at concentrations of 31 and 29 ng ml−1, respectively, although it had no effect on inflammatory cytokine production (CFU-C/TNF,IL-1 <0.029). Through strict comparison of in vitro efficacy and cytotoxicity, FK506 was found to be the most potent and least cytotoxic among the four agents in suppressing anti-CD3/CD28 induced inflammatory cytokine production.
Discussion
There are many reports that FK506 suppresses T cell activation and cytokine production from T cells (Sawada et al., 1987; Tocci et al., 1989; Dumont et al., 1998). However, in terms of production of inflammatory cytokines such as TNF-α and IL-1β, FK506 is reported not to inhibit production of these cytokines by direct stimulation of monocytes (Tocci et al., 1989; Andersson et al., 1992). FK506 is known to inhibit IL-1β production from human macrophage-like cell lines at high concentrations (Yoshimura et al., 1994), however, there are no reports showing a suppressive effect of FK506 on IL-1β production at clinically relevant concentrations. Recently it has been shown that inflammatory cytokines such as TNF-α and IL-1β play a central role in the pathogenesis of RA (Arend & Dayer, 1995; Elliott & Maini, 1995; Moreland et al., 1997). Therefore, it is useful to clarify the effects of potential therapies on inflammatory cytokine production in vitro.
In this study, we employed an in vitro system producing TNF-α and IL-1β through T cell activation in human PBMC since that T cells are thought to play an important role in the pathogenesis of RA. It is not clear that activation with anti-CD3/CD28 reflects on that which occurs in RA, however, this system may be more reflective of disease states than stimulation by LPS, which has been used broadly to assess cytokine inhibition in vitro. Agents that suppress anti-CD3/CD28 induced cytokine production may be widely applicable to prevent T cell activation mediated diseases, rather than RA specifically.
FK506 was found to inhibit both TNF-α and IL-1β production induced by the stimulation of anti-CD3/CD28 at lower concentrations, with IC50s comparable to those for Th1 derived cytokines such as IL-2 and IFN-γ. Furthermore, flow cytometric analysis demonstrated that FK506 also potently suppresses TNF-α and IL-1β production from monocytes. The signaling pathway for cytokine production from monocytes triggered by T cell activation has not been fully elucidated. However, it has been reported that IL-1β production from monocytes by activating T cells in PBMC is induced by direct cell contact between monocytes and activated T cells through ligation of CD40 and the CD40 ligand (Wagner et al., 1994; Suttles et al., 1999) or CD2 and lymphocyte function antigen-3 (LFA-3) (McAllister & Ellis, 1996). The interaction of T cells and monocytes is also required for induction of monocyte TNF-α during anti-CD3 induced T cell activation (Sebbag et al., 1997). Therefore, it is unlikely that the inhibition of production of both inflammatory cytokines by FK506 is mainly attributed to blockage of the induction of T cell-derived cytokines such as IL-2, which induces some monocyte-derived cytokines (Numerof et al., 1988).
The inhibitory potency of FK506 on inflammatory cytokine production was compared with the immunosuppressive anti-rheumatic drugs, CsA, DEX and MTX. The mechanism of action of CsA in RA has been poorly understood, although nearly two decades have passed since the first clinical application of this agent to RA. Its primary mode of action has been believed to be inhibition of the production of cytokines involved in regulation of T cell activation (Barrera et al., 1996; Ho et al., 1996; Danning & Boumpas, 1998). However, T cell-derived cytokines such as IL-2 are not currently considered to be the main cytokines involved in the pathogenesis of RA (Müller-Ladner, 1996). In this report, it was clearly shown that CsA is also capable of inhibiting anti-CD3/CD28 induced TNF-α and IL-1β production although the potency of inhibition of the agent was considerably less than FK506.
FK506 and CsA exert their effects after binding to intracellular proteins termed immunophilins; FK506 binding protein (FKBP) and cyclophilin, respectively. The drug-immunophilin complex inhibits calcineurin phosphatase, an enzyme involved in activation of the transcription factor NF-AT required for the expression of IL-2, IFN-γ and TNF-α genes in T cells (Ho et al., 1996). On the other hand, it is known that immunophilins and drug complexes have specific targets other than calcineurin phosphatase (Hutchinson et al., 1998). For instance, FK506 but not CsA increased nerve regeneration in rat sciatic nerve by a calcineurin-independent mechanism (Wang et al., 1997). In this study, both FK506 and CsA suppressed the production of TNF-α and IL-1β on the same order as the inhibitory concentration of T cell exclusive cytokines, IL-2 and IFN-γ. Therefore both agents likely suppress the anti-CD3/CD28 triggered inflammatory cytokine production from monocytes through the inhibition of calcineurin.
DEX suppresses cellular and humoral immunity and has already been reported to inhibit inflammatory cytokine production from monocytes by exogenous stimulants such as LPS (Hartman et al., 1995; Joyce et al., 1997). As shown in Tables 1 and 2, DEX inhibited anti-CD3/CD28 induced TNF-α and IL-1β production although the inhibition is slightly less potent than LPS induced production. Glucocorticoids bind to their receptors in the cytoplasm and inhibit the expression of multiple inflammatory genes through inhibition of transcription factors such as nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) (Barnes, 1998). Therefore, a signalling pathway including NF-κB or AP-1 may also be involved in T cell activation induced TNF-α and IL-1β production from monocytes.
Infiltration of mononuclear cells into synovia is involved in the process of chronic inflammation of RA. Lymphocyte function antigen-1 (LFA-1) and very late antigen-4 (VLA-4) mediate the adhesion and transmigration of mononuclear cells through activated endothelial cells by binding to their counter-receptors, ICAM-1 and VCAM-1, respectively. It is well established that ICAM-1 and VCAM-1 are upregulated by TNF-α and IL-1β (Lindsley et al., 1992; Cutolo et al., 1993). Anti-ICAM-1 monoclonal antibody is known to suppress the progression of RA in clinical studies (Kavanaugh et al., 1994) and adjuvant arthritis in rats (Iigo et al., 1991). Therefore, in this study, we investigated whether inhibition of inflammatory cytokine production was correlated with the suppression of the cytokine inducible adhesion molecule expression.
As shown in Figure 4, induction of ICAM-1 and VCAM-1 expression by anti-CD3/CD28 activated PBMC culture supernatants was decreased in FK506, CsA and DEX containing culture supernatants. The suppression of ICAM-1 and VCAM-1 expression was parallel to the inhibitory potency of inflammatory cytokine production by the four agents. The results suggest that FK506, CsA and DEX, but not MTX, may block T cell activation mediated adhesion molecule expression through the inhibition of cytokine production.
Ideally, drugs for the treatment of chronic inflammatory diseases should specifically suppress immune and inflammatory response without affecting other cellular functions including proliferation and differentiation. Therefore, the inhibitory potency of the agents on inflammatory cytokine production and the cytotoxicity of the drugs were compared. Antimetabolic drugs such as MTX sometimes lead to severe side effects such as bone marrow suppression (Segal et al., 1990; Baggott et al., 1993; Cronstein, 1996). MTX inhibited both the proliferation of Saos-2 and CFU-C at concentrations below 100 ng ml−1, although it has no effect on inflammatory cytokine production (CFU-C/TNF,IL-1 <0.029). Therefore, immunosuppression by MTX observed in clinical use may be attributed to its nonspecific suppression of cellular proliferation and function rather than the specific inhibition of cytokine production.
FK506, CsA and DEX showed almost no effects on the proliferation of Saos-2. However, the inhibitory effects on colony formation of haematopoietic progenitor cells (CFU-C) were significantly different between the three agents. FK506 exerted an effect on CFU-C only at high concentrations. The inhibitory effects of FK506 on anti-CD3/CD28 induced inflammatory cytokine production was extremely specific compared to the inhibition of CFU-C. The specificity shown as the ratio of CFU-C/TNF,IL-1 was close to 10,000. On the other hand, CsA suppressed CFU-C more potently than FK506, despite that CsA was about 100 times less potent than FK506 on inflammatory cytokine production. Consequently, the ratio of CFU-C/TNF,IL-1 of CsA was shown to be only 26, suggesting that CsA may exert a toxic effect on bone marrow at higher exposure. After the discovery of FK506, the difference between FK506 and CsA at the cellular level has focused on the potency of their immunosuppression. More importantly, the difference in specificity as presented here, is most striking from the point of view of clinical benefits. The effects of agents on the cellular function of immune or inflammatory cells have often been reported neglecting their cytotoxic nature. Strict comparison of in vitro efficacy and cytotoxicity as shown here is useful for comparing relative specificity between agents, although the in vitro analysis may not necessarily reflect on in vivo efficacy and side effects, particularly since different cell types from different species are compared.
DEX has been reported to inhibit proliferation and differentiation of bone marrow cells (Golde et al., 1976). The inhibition of CFU-C was almost the same as the dose for inhibition of anti-CD3/CD28 induced inflammatory cytokine production (CFU-C/TNF,IL-1=0.92). This result suggests that the inhibition of inflammatory cytokine production by DEX may be accompanied by bone marrow suppression. Furthermore, DEX inhibited LPS induced inflammatory cytokine production at the inhibitory concentration of the anti-CD3/CD28 induced one.
Anti-cytokine therapies have shown great promise for the treatment of RA (Moreland et al., 1997; Elliott & Maini, 1995). The LPS-induced cytokine production model has been frequently employed to study the potency of anti-rheumatic drugs in vitro (Segal et al., 1989; Finch-Arietts & Cochran, 1991; Bondeson & Sundler, 1995; Hartman et al., 1995; Joyce et al., 1997). Inflammatory cytokines are produced for host defence upon bacterial infection, although overproduction leads to various toxic effects in vivo (Czuprynski et al., 1988; Marriott et al., 1997). Therefore, agents that suppress LPS-induced inflammatory cytokine production in vitro are thought to be important for the treatment of infectious disease such as sepsis, but are not necessarily appropriate for applying to autoimmune diseases triggered by T cell activation.
We have tried to develop an in vitro system to evaluate agents for the treatment of RA. An agent which suppresses T cell activation mediated inflammatory cytokine production, and the subsequent inflammatory response such as adhesion molecule expression, without affecting cytokine production induced by bacterial infection may be an improvement over current anti-cytokine therapies, which may carry increased risks of compromising innate immunity. In this study, it was demonstrated that FK506 is the most potent and specific among the four agents for suppression of TNF-α and IL-1β production triggered by anti-CD3/CD28 in human PBMC. Taken together, the present study suggests that FK506 may potently suppress inflammatory cytokine production in RA, provided that these inflammatory cytokines are produced via T cell activation in this disease.
Acknowledgments
We thank Dr David Barrett, Medicinal Chemistry Research Laboratories, Fujisawa Pharmaceutical Co. Ltd., for critical reading of the manuscript.
Abbreviations
- anti-CD3/CD28
anti-CD3 monoclonal antibody and anti-CD28 monoclonal antibody
- CFU-C
haemopoietic progenitor cells
- CsA
Cyclosporin A
- DEX
dexamethasone
- FKBP
FK506 binding protein
- HUVEC
human umbilical vein endothelial cells
- IC50
50% inhibitory concentration
- ICAM-1
intercellular adhesion molecule-1
- IL
interleukin
- LPS
lipopolysaccaride
- MTX
Methotrexate
- PBMC
peripheral blood mononuclear cells
- RA
rheumatoid arthritis
- TNF
tumour necrosis factor
- VCAM-1
vascular cell adhesion molecule-1
References
- ANDERSSON J., NAGY S., GROTH C.-G., ANDERSSON U. Effects of FK506 and cyclosporin A on cytokine production studied in vitro at a single-cell level. Immunology. 1992;75:136–142. [PMC free article] [PubMed] [Google Scholar]
- AREND W.P., DAYER J.-M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor α in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- BAGGOTT J.E., MORGAN S.L., HA T.-S., ALARCÓN G.S., KOOPMAN W.J., KRUMDIECK C.L. Antifolates in rheumatoid arthritis: a hypothetical mechanism of action. Clin. Exp. Rheumatol. 1993;11 Suppl. 8:S101–S105. [PubMed] [Google Scholar]
- BARNES P.J. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- BARRERA P., BOERBOOMS A.M.T., VAN DE PUTTE L.B.A., VAN DE MEER J.W.M. Effects of antirheumatic agents on cytokines. Semin. Arthritis Rheu. 1996;25:234–253. doi: 10.1016/s0049-0172(96)80035-7. [DOI] [PubMed] [Google Scholar]
- BONDESON J., SUNDLER R. Auranofin inhibits the induction of interleukin 1β and tumor necrosis factor α mRNA in macrophages. Biochem. Pharmacol. 1995;50:1753–1759. doi: 10.1016/0006-2952(95)02030-6. [DOI] [PubMed] [Google Scholar]
- CRONSTEIN B.N. Molecular therapeutics. Methotrexate and its mechanism of action. Arthritis Rheum. 1996;39:1951–1960. doi: 10.1002/art.1780391203. [DOI] [PubMed] [Google Scholar]
- CUTOLO M., SULLI A., BARONE A., SERIOLO B., ACCARDO S. Macrophages, synovial tissue and rheumatoid arthritis. Clin. Exp. Rheumatol. 1993;11:331–339. [PubMed] [Google Scholar]
- CZUPRYNSKI C.J., BROWN J.F., YOUNG K.M., COOLEY A.J., KURTZ R.S. Effects of murine recombinant interleukin 1α on the host response to bacterial infection. J. Immunol. 1988;140:962–968. [PubMed] [Google Scholar]
- DANNING C.L., BOUMPAS D.T. Commonly used disease-modifying antirheumatic drugs in the treatment of inflammatory arthritis: An update on mechanisms of action. Clin. Exp. Rheumatol. 1998;16:595–604. [PubMed] [Google Scholar]
- DUMONT F.J., STARUCH M.J., FISCHER P., DASILVA C., CAMACHO R. Inhibition of T cell activation by pharmacologic disruption of the MEK1/ERK MAP kinase or calcineurin signaling pathways results in differential modulation of cytokine production. J. Immunol. 1998;160:2579–2589. [PubMed] [Google Scholar]
- ELLIOTT M.J., MAINI R.N. Anti-cytokine therapy in rheumatoid arthritis. Bailliere' Clinical Rheumatol. 1995;9:633–652. doi: 10.1016/s0950-3579(05)80306-1. [DOI] [PubMed] [Google Scholar]
- FAULDS D., GOA K.L., BENFIELD P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs. 1993;45:953–1040. doi: 10.2165/00003495-199345060-00007. [DOI] [PubMed] [Google Scholar]
- FINCH-ARIETTA M.B., COCHRAN F.R. Cytokine production in whole blood ex vivo. Agents Actions. 1991;34:49–52. doi: 10.1007/BF01993235. [DOI] [PubMed] [Google Scholar]
- FURST D.E., SHERRER Y., FLEISCHMANN R.M., BLOCK J., RUTSTEIN J., STAMLER D. Efficacy of FK506 in rheumatoid arthritis (RA): A 6 month, dose-ranging study in RA patients failing methotrexate (MTX) Arthritis Rheum. 1999;42:S271. [Google Scholar]
- GOLDE D.W., BERSCH N., QUAN S.G., CLINE M.J. Inhibition of murine granulopoiesis in vitro by dexamethasone. Am. J. Hematol. 1976;1:369–373. doi: 10.1002/ajh.2830010402. [DOI] [PubMed] [Google Scholar]
- HARTMAN D.A., OCHALSKI S.J., CARLSON R.P. The effects of antiinflammatory and antiallergic drugs on cytokine release after stimulation of human whole blood by lipopolysaccharide and zymosan A. Inflamm. Res. 1995;44:269–274. doi: 10.1007/BF02032567. [DOI] [PubMed] [Google Scholar]
- HO S., CLIPSTONE N., TIMMERMANN L., NORTHROP J., GRAEF I., FIORENTINO D., NOURSE J., CRABTREE G.R. The mechanism of action of cyclosporin A and FK506. Clinical Immunol. Immunopathol. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- HUTCHINSON I.V., BAGNALL W., BRYCE P., PUFONG B., GERAGHTY P., BROGAN I. Differences in the mode of action of cyclosporine and FK506. Transplant. Proc. 1998;30:959–960. doi: 10.1016/s0041-1345(98)00110-9. [DOI] [PubMed] [Google Scholar]
- IIGO Y., TAKASHI T., TAMATANI T., MIYASAKA M., HIGASHIDA T., YAGITA H., OKUMURA K., TSUKADA W. ICAM-1-dependent pathway is critically involved in the pathogenesis of adjuvant arthritis in rats. J. Immunol. 1991;147:4167–4171. [PubMed] [Google Scholar]
- JOYCE D.A., STEER J.H., ABRAHAM L.J. Glucocorticoid modulation of human monocyte/macrophage function: Control of TNF-α secretion. Inflamm. Res. 1997;46:447–451. doi: 10.1007/s000110050222. [DOI] [PubMed] [Google Scholar]
- KAVANAUGH A.F., DAVIS L.S., NICHOLS L.A., NORRIS S.H., ROTHLEIN R., SCHARSCHMIDT L.A., LIPSKY P.E. Treatment of refractory rheumatoid arthritis with a monoclonal antibody to intercellular adhesion molecule 1. Arthritis. Rheum. 1994;37:992–999. doi: 10.1002/art.1780370703. [DOI] [PubMed] [Google Scholar]
- KINO T., HATANAKA H., MIYATA S., INAMURA N., NISHIYAMA M., YAJIMA T., GOTO T., OKUHARA M., KOHSAKA M., AOKI H., OCHIAI T. FK506, a novel immunosupressant isolated from streptomyces. II Immunosuppressive effect of FK506 in vitro. J. Antibiot. (Tokyo). 1987;40:1256–1265. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- LANDIS R.C., FRIEDMAN M.L., FISHER R.I., ELLIS T.M. Induction of human monocyte IL-1 mRNA and secretion during anti-CD3 mitogenesis requires two distinct T cell-derived signals. J. Immunol. 1991;146:128–135. [PubMed] [Google Scholar]
- LINDSLEY H.B., SMITH D.D., DAVIS L.S., KOCH A.E., LIPSKY P.E. Regulation of the expression of adhesion molecules by human synoviocytes. Semin. Arthritis Rheu. 1992;21:330–334. doi: 10.1016/0049-0172(92)90026-a. [DOI] [PubMed] [Google Scholar]
- MARRIOTT J.B., WESTBY M., DALGLEISH A.G. Therapeutic potential of TNF-α inhibitors old and new. Drug Discovery Today. 1997;2:273–282. [Google Scholar]
- MCALLISTER P.T., ELLIS T.M. CD2 regulates T cell-dependent induction of monocyte IL-1β mRNA during anti-CD3 mitogenesis. Cell. Immunol. 1996;170:120–126. doi: 10.1006/cimm.1996.0141. [DOI] [PubMed] [Google Scholar]
- MORELAND L.W., HECK L.W., JR, KOOPMAN W.J. Biologic agents for treating rheumatoid arthritis. Concepts and progress. Arthritis Rheum. 1997;40:397–409. doi: 10.1002/art.1780400302. [DOI] [PubMed] [Google Scholar]
- MÜLLER-LADNER U. Molecular and cellular interactions in rheumatoid synovium. Curr. Opin. Rheumatol. 1996;8:210–220. doi: 10.1097/00002281-199605000-00008. [DOI] [PubMed] [Google Scholar]
- NUMEROF R.P., ARONSON F.R., MIER J.W. IL-2 stimulates the production of IL-1α and Il-1β by human peripheral blood mononuclear cells. J. Immunol. 1988;141:4250–4257. [PubMed] [Google Scholar]
- PANAYI G.S., LANCHBURY J.S., KINGSLEY G.H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992;35:729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- POU L., BRUNET M., BILBAO I., ANDREU H., ANDRES I., LOPEZ R., MARGARIT C., RIMOLA A., CORBELLA J. Therapeutic drug monitoring of tacrolimus in liver transplantation, phase III FK506 multicenter Spanish study group: a two-year follow-up. Ther. Drug Monit. 1998;20:602–606. doi: 10.1097/00007691-199812000-00003. [DOI] [PubMed] [Google Scholar]
- RORDORF-ADAM C., LAZDINS J., WOODS-COOK K., ALTERI E., HENN R., GEIGER T., FEIGE U., TOWBIN H., ERARD F. An assay for the detection of interleukin-1 synthesis inhibitors; effects of antirheumatic drugs. Drugs Exp. Clin. Res. 1989;15:355–362. [PubMed] [Google Scholar]
- SANDER B., ANDERSSON J., ANDERSSON U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol. Rev. 1991;119:65–92. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- SAWADA S., SUZUKI G., KAWASE Y., TAKAKU F. Novel immunosuppressive agent, FK506. In vitro effects on the cloned T cell activation. J. Immunol. 1987;139:1797–1803. [PubMed] [Google Scholar]
- SCHMIDT J., FLEIßNER S., HEIMAN-WEITSCHAT I., LINDSTAEDT R., POMBERG B., WERNER U., SZELENYI I. Effect of corticosteroids, cyclosporin A and methotrexate on cytokine release from monocytes and T-cell subsets. Immunopharmacol. 1994;27:173–179. doi: 10.1016/0162-3109(94)90013-2. [DOI] [PubMed] [Google Scholar]
- SEBBAG M., PARRY S.L., BRENNAN F.M., FELDMANN M. Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor-α, but not interleukin-10: possible relevance to pathophysiology of rheumatoid arthritis. Eur. J. Immunol. 1997;27:624–632. doi: 10.1002/eji.1830270308. [DOI] [PubMed] [Google Scholar]
- SEGAL R., MOZES E., YARON M., TARTAKOVSKY B. The effects of methotrexate on the production and activity of interleukin-1. Arthritis. Rheum. 1989;32:370–377. doi: 10.1002/anr.1780320403. [DOI] [PubMed] [Google Scholar]
- SEGAL R., YARON M., TARTAKOVSKY B. Methotrexate: Mechanism of action in rheumatoid arthritis. Semin. Arthritis Rheu. 1990;20:190–199. doi: 10.1016/0049-0172(90)90060-s. [DOI] [PubMed] [Google Scholar]
- SINNETT M.J., GROFF G.D., RADDATZ D.A., FRANCK W.A., BERTINO J.S., JR Methotrexate pharmacokinetics in patients with rheumatoid arthritis. J. Rheumatol. 1989;16:745–748. [PubMed] [Google Scholar]
- STRØMHAUG A., WARREN D.J., SLØRDAL L. Effect of methotrexate on murine bone marrow cells in vitro: Evidence of a reversible antiproliferative action. Exp. Hematol. 1995;23:439–443. [PubMed] [Google Scholar]
- SUTTLES J., MILHORN D.M., MILLER R.W., POE J.C., WAHL L.M., STOUT R.D. CD40 signaling of monocyte inflammatory cytokine synthesis through an ERK1/2-dependent pathway. J. Biol. Chem. 1999;274:5835–5842. doi: 10.1074/jbc.274.9.5835. [DOI] [PubMed] [Google Scholar]
- TAKAMI S., YAMASHITA S., KIHARA S., ISHIGAMI M., TAKEMURA K., KUME N., KITA T., MATSUZAWA Y. Lipoprotein(a) enhances the expression of intercellular adhesion molecule-1 in cultured human umbilical vein endothelial cells. Circulation. 1998;97:721–728. doi: 10.1161/01.cir.97.8.721. [DOI] [PubMed] [Google Scholar]
- TOCCI M.J., MATKOVICH D.A., COLLIER K.A., KWOK P., DUMONT F., LIN S., DEGUDICIBUS S., SIEKIERKA J.J., CHIN J., HUTCHINSON N.I. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J. Immunol. 1989;143:718–726. [PubMed] [Google Scholar]
- VAN WAUWE J., AERTS F., WALTER H., DE BOER M. Cytokine production by phytohematoagglutinin-stimulated human blood cells: Effects of corticosteroids, T cell immunosuppressants and phosphodiesterase IV inhibitors. Inflamm. Res. 1995;44:400–405. doi: 10.1007/BF01797868. [DOI] [PubMed] [Google Scholar]
- WAGNER D.W., JR, STOUT R.D., SUTTLES J. Role of the CD40-CD40 ligand interaction in CD4+ T cell contact-dependent activation of monocyte interleukin-1 synthesis. Eur. J. Immunol. 1994;24:3148–3154. doi: 10.1002/eji.1830241235. [DOI] [PubMed] [Google Scholar]
- WANG M.-S., ZELENY-POOLEY M., GOLD B.G. Comparative dose-dependence study of FK506 and cyclosporin A on the rate of axonal regeneration in the rat sciatic nerve. J. Pharmacol. Exp. Therapeutics. 1997;282:1084–1093. [PubMed] [Google Scholar]
- WEIJTENS O., SCHOEMAKER R.C., COHEN A.F., ROMIJN F.P.H.T.M., LENTJES E.G.W.M., VAN ROOIJ J., VAN MEURS J.C. Dexamethasone concentration in vitreous and serum after oral administration. Am. J. Ophthalmol. 1998;125:673–679. doi: 10.1016/s0002-9394(98)00003-8. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI M., SUWA H., MIYASAKA M., KUMADA K. Selective inhibition of vascular cell adhesion molecule-1 expression by verapamil in human vascular endothelial cells. Transplantation. 1997;63:759–764. doi: 10.1097/00007890-199703150-00024. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA N., OHMOTO Y., YASUI H., OHSAKA Y., BONG J.M., KOBAYASHI Y., OKA T. The direct effect of FK506 and rapamycin on interleukin 1 (β) and immunoglobulin production in vitro. Transplantation. 1994;57:1815–1818. [PubMed] [Google Scholar]


