Abstract
In the rat gastric fundus, non-adrenergic, non-cholinergic (NANC) relaxations are mediated by nitric oxide (NO), vasoactive intestinal polypeptide (VIP), and a third, as yet unidentified, neurotransmitter.
The possible involvement of adenosine 5′-triphosphate (ATP) in the NANC relaxations was examined using pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (PPADS), apamin and desensitization to α,β-methylene ATP. NANC responses were studied in the absence and presence of NG-nitro-L-arginine methyl ester (NAME; 100 μM) and α-chymotrypsin (1 u ml−1), to inhibit responses to NO and VIP, respectively.
PPADS (100 μM), apamin (1 μM) and desensitization to α,β-methylene ATP (10 μM, three additions) all significantly (P<0.05) reduced NANC relaxations to electrical field stimulation (0.5–4 Hz, 30 s trains) in longitudinal strips of rat gastric fundus and almost abolished the residual relaxation remaining in the presence of NAME and α-chymotrypsin.
PPADS had no effect on responses to the NO-donor, sodium nitroprusside (SNP), or VIP. Apamin slightly reduced relaxations to SNP, but did not affect those to VIP, whereas desensitization to α,β-methylene ATP markedly reduced responses to both SNP and VIP.
The effects of PPADS and apamin in this study provide strong evidence that the third inhibitory NANC neurotransmitter in the rat gastric fundus is ATP.
Keywords: Apamin, ATP, NANC nerves, PPADS, rat gastric fundus
Introduction
There is compelling evidence that nitric oxide (NO) and vasoactive intestinal polypeptide (VIP) are inhibitory non-adrenergic non-cholinergic (NANC) neurotransmitters in the rat gastric fundus (Li & Rand, 1990; D'Amato et al., 1992; Boeckxstaens et al., 1992). However, in the presence of blockade of both NO and VIP a residual relaxation remains, suggesting the involvement of a third inhibitory neurotransmitter in this tissue (Li & Rand, 1990; D'Amato et al., 1992; Boeckxstaens et al., 1992; Jenkinson & Reid, 1995).
In 1970, Burnstock and co-workers first proposed that the purine adenosine 5′-triphosphate (ATP) may act as an inhibitory NANC neurotransmitter in the gut (Burnstock et al., 1970). A number of lines of evidence support such a role in the rat gastric fundus: ATP both relaxes and then contracts the rat gastric fundus, probably acting on P2X- and P2Y-purinoceptor subtypes, respectively (Lefebvre & Burnstock, 1990; Matharu & Hollingsworth, 1992); ATP release has been demonstrated during electrical field stimulation (EFS) of the rat gastric fundus (Belai et al., 1991); and desensitization of rat gastric fundus to the P2X-purinoceptor agonist, α,β-methylene ATP has been reported to partially reduce relaxant responses to both ATP and EFS (Belai et al., 1991). In contrast, Lefebvre (1986) found that desensitization of the rat gastric fundus to ATP itself had no effect on EFS-induced relaxations. Furthermore, the bee venom apamin, a blocker of small conductance calcium-dependent potassium channels (Banks et al., 1979; Blatz & Magleby, 1986), reduces the relaxant response to ATP without affecting relaxations to EFS in this tissue (De Beurme & Lefebvre, 1989; Lefebvre et al., 1991). Due to these conflicting lines of evidence, the involvement of ATP in the inhibitory NANC innervation of the rat gastric fundus remains unresolved.
The aim of the present study was to investigate further the possible role of ATP as the third inhibitory neurotransmitter in the rat gastric fundus. The effects of the P2-purinoceptor antagonist pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (PPADS), apamin and desensitization to α,β-methylene ATP on NANC responses to EFS have been examined in the absence and presence of blockade or responses to NO and VIP.
Methods
Tissue preparation
Male Sprague-Dawley rats (320–580 g) were stunned and killed by decapitation, the stomach was immediately removed, and two longitudinal strips of the full wall of the stomach were prepared from the ventral part of the fundus as previously described (Jenkinson & Reid, 1995). Each fundus strip was mounted in a longitudinal orientation in a 6-ml water-jacketed organ bath under a resting tension of 1 g in physiological salt solution (PSS) of the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1.03, MgSO4 0.45, NaHCO3 25.0, D-(+)-glucose 11.1, disodium edetate 0.067, and ascorbic acid 0.14. The PSS was maintained at 37°C, and gassed with 5% CO2 in O2. Intramural nerves were electrically stimulated using a Grass S88 or S11 stimulator via two platinum wire electrodes, one placed on either side of the strip, with square wave pulses of 1-ms duration and supramaximal voltage (17 V cm−1). The PSS contained atropine (3 μM) and guanethidine (5 μM) throughout experiments to block cholinergic and nonadrenergic responses to EFS, respectively. Changes in tissue length were measured using a Ugo Basile isotonic transducer and recorded using a MacLab data acquisition system.
Experimental protocol
Each fundus strip was allowed to equilibrate for at least 30 min before serotonin (10 μM) was added to produce a sustained increase in tone of 10.1±0.1 mm (n=46). After a further 30-min equilibration period, a control response curve was obtained to either ATP (1–30 μM), EFS (0.5–4 Hz, 30-s train), sodium nitroprusside (SNP; 3 nM–30 μM) or VIP (0.1–100 nM). Relaxant responses to ATP (see Figure 1) and EFS (Figure 2) were obtained at 5-min intervals in random order, whereas relaxations to SNP and VIP were obtained in a cumulative manner. In some experiments responses to EFS (0.5–4 Hz, 30-s train) were obtained in physiological salt solution containing the NO synthase inhibitor NG-nitro-L-arginine methyl ester (NAME: 100 μM) and the peptidase α-chymotrypsin (1 u ml−1), to inhibit responses to NO and VIP, respectively. We have previously shown that α-chymotrypsin abolishes relaxations to VIP, and markedly reduces the duration of NANC relaxations, in this tissue (Jenkinson & Reid, 1995).
Figure 1.
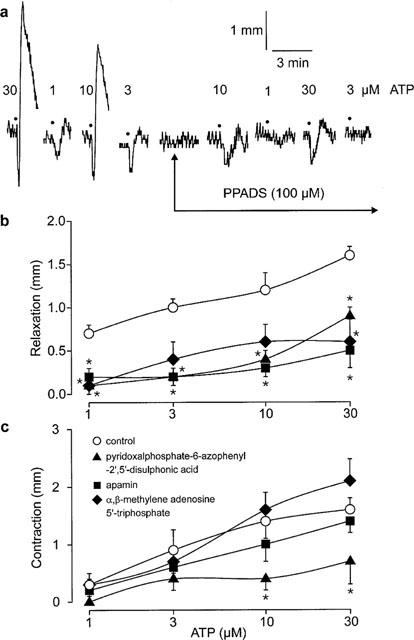
(a) Original trace showing the effect of pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (100 μM) on responses to adenosine 5′-triphosphate (ATP; •; 1–30 μM) in longitudinal strips of rat gastric fundus. Magnitude of (b) relaxant responses and (c) contractile responses to ATP (1–30 μM) in longitudinal strips of rat gastric fundus in the absence (control) and presence of pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (PPADS; 100 μM), apamin (1 μM) or desensitization to α,β-methylene adenosine 5′-triphosphate (10 μM, three additions). Values are means±s.e.mean for 4–5 experiments. *Significant difference from control (P<0.05, two-way MANOVA followed by Student-Newman-Keuls test).
Figure 2.
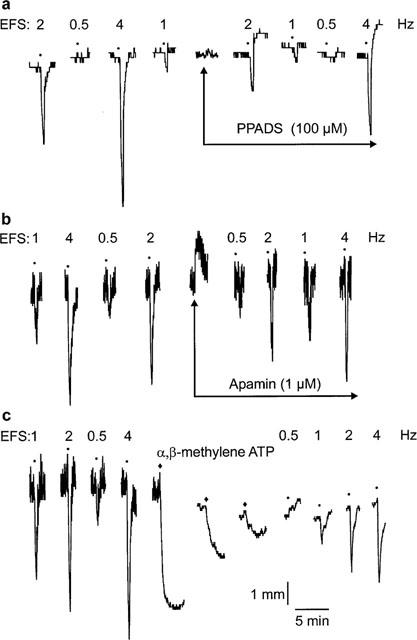
Original traces showing the effect of (a) pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (PPADS; 100 μM), (b) apamin (1 μM) and (c) desensitization to α,β-methylene adenosine 5′-triphosphate (α,β-methylene ATP; ♦; 10 μM, three additions) on responses to non-adrenergic, non-cholinergic electrical field stimulation (EFS; •; 0.5–4 Hz) in longitudinal strips of rat gastric fundus.
A second set of responses was obtained in the absence (time-control experiments) or presence of PPADS (100 μM) or apamin (1 μM), or following desensitization to α,β-methylene ATP (10 μM, three additions). Tissues were exposed to PPADS or apamin for at least 20 min before further responses were elicited. To desensitize tissue P2X-purinoceptors, α,β-methylene ATP (10 μM) was added repeatedly (three times), with tissue tone allowed to stabilize following each addition. The time period between α,β-methylene ATP additions varied from 15–35 min.
The magnitude of relaxations induced by SNP and VIP varied markedly between experimental groups. Therefore, responses to SNP and VIP obtained from the second curve have been expressed as a percentage of the initial maximum response obtained in the same tissue to 10 μM SNP or 100 nM VIP, respectively.
Analysis of results
Data are expressed as means±s.e.mean and n indicated the number of animals tested. Differences between means were assessed by unpaired Student' t-test, or by one-way analysis of variance (ANOVA) or multiple analysis of variance (MANOVA) followed by Student-Newman-Keuls test. Analyses were performed using the statistical software package Sigma Stat 1.0 (Jandel Scientific, U.S.A.). Probability values less than 0.05 (P<0.05) were taken to indicate statistical significance.
Drugs and drug solutions
The following drugs were used in the study: adenosine 5′-triphosphate disodium salt (ATP; Sigma, U.S.A.), apamin (Sigma, U.S.A.), atropine sulphate (Sigma, U.S.A.), α-chymotrypsin (bovine pancreas, Sigma, U.S.A.), guanethidine sulphate (Ciba-Geigy, Australia), 5-hydroxytryptamine creatine sulphate (serotonin; Sigma, U.S.A.) α,β-methylene adenosine 5′-triphosphate lithium salt (α,β-methylene ATP; Sigma, U.S.A.), NG-nitro-L-arginine methyl ester (NAME; Sigma, U.S.A.) pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (PPADS ; RBI, U.S.A.), sodium nitroprusside (SNP; Sigma, U.S.A.), tetrodotoxin (Sigma, U.S.A.), vasoactive intestinal polypeptide (VIP; human; Auspep, Australia). α-Chymotrypsin was dissolved in distilled water on the day of the experiment to give a stock solution of 100 u ml−1. All other drugs were dissolved in distilled water to give stock solutions of 10 mM, or 0.1 mM for VIP, and dilutions were made in PSS.
Results
Direct effects of PPADS, apamin and α,β-methylene ATP
Addition of PPADS (100 μM) to precontracted strips of rat gastric fundus had no effect on tissue tone (Figure 2a). Apamin (1 μM) produced transient contractile responses (Figure 2b) but had no sustained effect on tissue tone. The first addition of α,β-methylene ATP (10 μM) to precontracted tissues produced large, prolonged relaxant responses (Figure 2c). The second and third additions produced relaxations that were greatly reduced when compared to previous relaxations (Figure 2c). When tissue tone had stabilized following desensitization to α,β-methylene ATP (10 μM, three additions), the tone of gastric fundus strips was significantly reduced (Figure 2c; data not shown; P<0.05, paired t-test).
Responses to ATP
Addition of ATP (1–30 μM) to precontracted strips of rat gastric fundus produced biphasic responses: rapid, concentration-dependent relaxations were followed by concentration-dependent contractions of similar size (Figure 1). Neither relaxations nor contractions to ATP differed significantly between the first and the second response curves in time-control experiments (data not shown; P>0.05, two-way MANOVA).
Relaxant responses to ATP were significantly reduced by exposure to PPADS (100 μM) or apamin (1 μM) or by desensitization to α,β-methylene ATP (10 μM, three additions) (Figure 1b; P<0.05, two-way MANOVA). Contractile responses to ATP were significantly reduced by PPADS, but were not affected by either apamin or desensitization to α,β-methylene ATP (Figure 1c; P>0.05, two-way MANOVA).
Responses to NANC nerve stimulation
EFS (0.5–4 Hz, 30-s train) produced rapid, frequency-dependent relaxations (Figures 2 and 3) that remained consistent in time-control experiments. Relaxant responses to EFS were significantly reduced (P<0.05, two-way MANOVA) following exposure to PPADS or apamin, or following desensitization to α,β-methylene ATP (Figures 2 and 3a).
Figure 3.
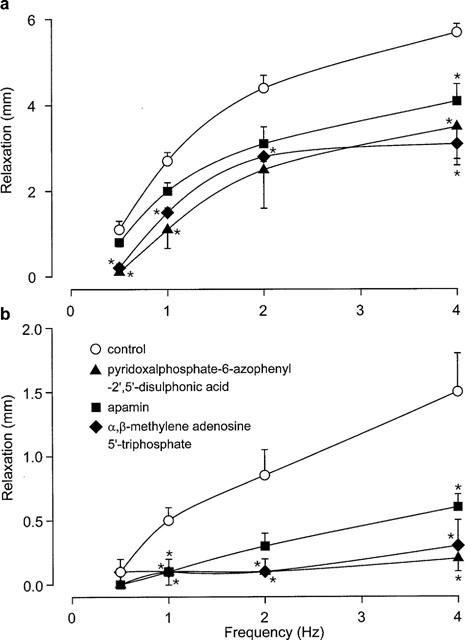
Magnitude of relaxant responses to non-adrenergic, non-cholinergic nerve stimulation (0.5–4 Hz) in longitudinal strips of rat gastric fundus in the absence (a) and presence (b) of NG-nitro-L-arginine methyl ester (100 μM) and α-chymotrypsin (1 u ml−1). Responses were obtained before (control) and after exposure to pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (100 μM) or apamin (1 μM) or desensitization to α,β-methylene adenosine 5′-triphosphate (10 μM, three additions). Values are means±s.e.mean for four experiments. *Significant difference from control (P<0.05, two-way MANOVA followed by Student-Newman-Keuls test).
Following incubation with NAME (100 μM) and α-chymotrypsin (1 u ml−1), the magnitude of relaxations to EFS was greatly reduced (Figure 3b; P<0.05, two-way ANOVA). The residual EFS-induced relaxations in the presence of NAME and α-chymotrypsin was almost completely abolished by PPADS or desensitization to α,β-methylene ATP, and was greatly reduced by apamin (Figure 3b; P<0.05, two-way MANOVA). In time-control experiments, relaxations to EFS did not differ between the first and second response curves in the presence of NAME and α-chymotrypsin (data not shown; P>0.05, two-way MANOVA).
Relaxations to SNP
The NO-donor, SNP (10 nM–10 μM) produced well maintained, concentration-dependent relaxations that remained consistent in time-control experiments. The magnitude of relaxations to SNP was not affected by PPADS (Figure 4a; P>0.05, two-way MANOVA), but was significantly reduced by exposure to apamin or by desensitization to α,β-methylene ATP (Figure 4a; P<0.05, two-way MANOVA).
Figure 4.
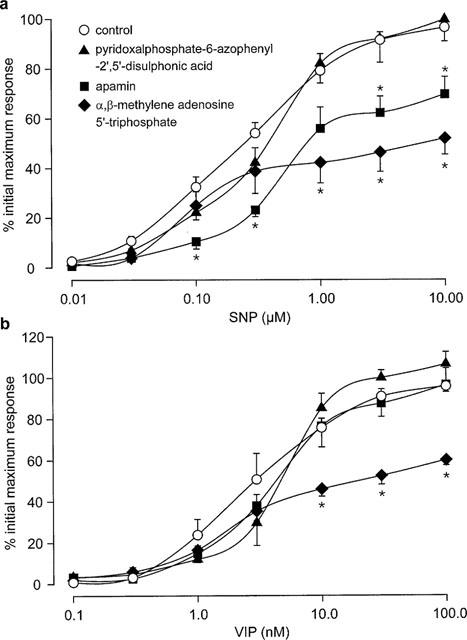
Magnitude of relaxant responses to (a) sodium nitroprisside (SNP; 10 nM–10 μM) and (b) vasoactive intestinal polypeptide (VIP; 0.1–100 nM) in longitudinal strips of rat gastric fundus in the absence (control) and presence of pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (100 μM), apamin (1 μM) or desensitization to α,β-methylene adenosine 5′-triphosphate (10 μM, three additions). Values are means±s.e.mean for 4–5 experiments. *Significant difference from control (P<0.05, two-way MANOVA followed by Student-Newman-Keuls test).
Relaxations to VIP
VIP (0.1–100 nM) produced slowly developing, concentration-dependent relaxations that did not alter over the time course of time-control experiments. Relaxant responses to VIP were not affected by exposure to PPADS or apamin (Figure 4b; P>0.05, two-way MANOVA), but were significantly reduced by desensitization to α,β-methylene ATP (Figure 4b; P<0.05, two-way MANOVA).
Discussion
The results of the present study provide the strongest evidence to date for an ATP-mediated component of inhibitory NANC responses in the rat gastric fundus. Previous studies examining this question have been hampered by a lack of effective purinoceptor antagonists and a lack of agents capable of blocking relaxations to NO and VIP, the primary inhibitory NANC neurotransmitters in this tissue (Li & Rand, 1990; D'Amato et al., 1992; Boeckxstaens et al., 1992). Consequently, results have been inconclusive. In the present study, we have used the potent and selective P2-purinoceptor antagonist, PPADS, to inhibit ATP-mediated responses. In addition, responses to neuronal NO and VIP were blocked by the NO synthase inhibitor, NAME, and the peptidase, α-chymotrypsin, respectively. PPADS greatly reduced both relaxations and contractions to exogenous ATP without affecting relaxations to either SNP or VIP, demonstrating its selectivity for purinoceptors in this tissue. In addition, PPADS markedly inhibited NANC relaxations and almost abolished the residual NANC relaxation remaining in the presence of blockade of relaxations to NO and VIP. The effects of PPADS strongly suggest that ATP is the third inhibitory neurotransmitter in the rat gastric fundus.
The peptide bee venom apamin greatly reduced relaxations to ATP in accord with previous findings in the rat gastric fundus (De Beurme & Lefebvre, 1989; Lefebvre et al., 1991). Apamin also produced a small reduction in the magnitude of NANC relaxations. Earlier studies (De Beurme & Lefebvre, 1989; Lefebvre et al., 1991), reported that apamin did not influence NANC relaxations in this tissue, however they examined its effects on EFS at 5 Hz for 5 min, and on a cumulative frequency-response curve to EFS (0.125–8 Hz); thus the small inhibitory effect of apamin observed in the present study may not be evident on relaxations produced by the high frequencies and prolonged periods of EFS used previously. Apamin reduced relaxations to SNP in the present study, and has previously been shown to reduce inhibitory junction potentials induced by the NO-donor S-nitrosocysteine in the rat gastric fundus (Kitamura et al., 1993), suggesting that the effects of apamin on NANC responses in the present study may be due to inhibition of relaxations to neuronally released NO. However, in the presence of NAME and α-chymotrypsin, apamin produced a marked reduction in the residual NANC relaxations, such that the responses were almost completely abolished. Moreover, apamin did not affect responses to VIP in either the present or previous studies (De Beurme & Lefebvre, 1989), and has been reported not to affect relaxations to isoprenaline in the guinea-pig gastric fundus, or to papaverine in the guinea-pig antrum (Costa et al., 1986). The effects of apamin in the present study suggest that the third NANC inhibitory neurotransmitter in the rat gastric fundus produces relaxations via activation of the apamin-sensitive calcium-dependent potassium channel, and support the suggestion that the transmitter is ATP.
Desensitization of rat gastric fundus strips to the stable P2X-purinoceptor agonist α,β-methylene ATP reduced relaxations to ATP and markedly reduced NANC relaxations. Both findings are in agreement with previous studies using α,β-methylene ATP in this tissue (Lefebvre & Burnstock, 1990; Belai et al., 1991; Matharu & Hollingsworth, 1992). In contrast, Lefebvre (1986) found that desensitization to ATP itself abolished relaxations to ATP without significantly affecting those to NANC nerve stimulation. However, the results are hard to interpret as the NANC relaxations were reduced by approximately 25%, but both tissue tone and NANC relaxations were markedly reduced in time control experiments (Lefebvre, 1986). In this study, desensitization to α,β-methylene ATP partially reduced relaxant responses to VIP and the NO-donor SNP and so its effect on NANC relaxations may be due to impairment of responses to neuronally released VIP and/or NO. However, in the presence blockade of responses to NO and VIP, α,β-methylene ATP-desensitization almost completely abolished NANC relaxations in fundus strips. This findings further supports a role for ATP in inhibitory NANC responses in this tissue, however it cannot be excluded that desensitization to α,β-methylene ATP has a non-selective inhibitory effect on NANC relaxations in the rat gastric fundus.
In summary, PPADS, apamin and desensitization to α,β-methylene ATP greatly reduced relaxant responses to ATP and NANC nerve stimulation in the rat gastric fundus. Furthermore, in the presence of blockade of responses to NO and VIP, the residual relaxation to NANC nerve stimulation was almost completely abolished by all three agents. However, desensitization to α,β-methylene ATP markedly reduced relaxations to SNP and VIP, and so is not suitable for examining ATP-mediated responses in this tissue. The effects of PPADS and apamin in this study provide strong evidence that the third inhibitory NANC neurotransmitter in the rat gastric fundus is ATP.
Acknowledgments
This work was supported by a Program Grant from the National Health and Medical Research Council of Australia.
Abbreviations
- ANOVA
analysis of variance
- ATP
adenosine 5′-triphosphate
- EFS
electrical field stimulation
- MANOVA
multiple analysis of variance
- NAME
NG-nitro-L-arginine methyl ester
- NANC
non-adrenergic non-cholinergic
- NO
nitric oxide
- PPADS
pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid
- PSS
physiological salt solution
- SNP
sodium nitroprusside
- VIP
vasoactive intestinal polypeptide
References
- BANKS B.E.C., BROWN C., BUGGESS G.M., BURNSTOCK G., CLARET M., COCKS T.M., JENKINSON D.H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979;282:415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- BELAI A., LEFEBVRE R.A., BURNSTOCK G. Motor activity and neurotransmitter release in the rat gastric fundus of streptozotocin-diabetic rats. Eur. J. Pharmacol. 1991;194:225–234. doi: 10.1016/0014-2999(91)90109-4. [DOI] [PubMed] [Google Scholar]
- BLATZ A.L., MAGLEBY K.L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986;323:718. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., DEMAN J.G., BULT H., HERMAN A.G., VAN MAERCKE Y.M. Evidence for a different release of nitric oxide and vasoactive intestinal polypeptide by nonadrenergic noncholingeric nerves in the rat gastric fundus. Arch. Int. Pharmacodyn. 1992;318:107–115. [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., SATCHELL D., SMYTHE A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic nerves in the gut. Br. J. Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTA M., FURNESS J.B., HUMPHREYS C.M.S. Apamin distinguishes two types of relaxation mediated by enteric nerves in the guinea-pig gastrointestinal tract. Naunyn-Schmiedberg' Arch. Pharmacol. 1986;332:79–88. doi: 10.1007/BF00633202. [DOI] [PubMed] [Google Scholar]
- D'AMATO M., CURRÒ D., MONTUSCHI P. Evidence for dual components in the non-adrenergic non-cholinergic relaxation in the rat gastric fundus: role of endogenous nitric oxide and vasoactive intestinal polypeptide. J. Auton. Nerve. Syst. 1992;37:175–186. doi: 10.1016/0165-1838(92)90039-j. [DOI] [PubMed] [Google Scholar]
- DE BEURME F.A., LEFEBVRE R.A. Influence of apamin on relaxations induced by ATP, VIP and NANC neurone stimulation in the rat and cat gastric fundus. Arch. Int. Pharmacodyn. 1989;298:296–297. [Google Scholar]
- JENKINSON K.M., REID J.J. Effect of diabetes on relaxations to non-adrenergic, non-cholinergic nerve stimulation in longitudinal muscle of the rat gastric fundus. Br. J. Pharmacol. 1995;116:1551–1556. doi: 10.1111/j.1476-5381.1995.tb16372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITAMURA K., LIAN Q., CARL A., KURIYAMA H. S-Nitrosocysteine, but not sodium nitroprisside, produces apamin-sensitive hyperpolarization in rat gastric fundus. Br. J. Pharmacol. 1993;109:415–423. doi: 10.1111/j.1476-5381.1993.tb13585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Study of the possible neurotransmitters of the non-adrenergic non-cholinergic innervation of the rat gastric fundus. Arch. Int. Pharmacodyn. 1986;280 Suppl.:110–136. [PubMed] [Google Scholar]
- LEFEBVRE R.A., BURNSTOCK G. Effect of adenosine triphosphate and related purines in the rat gastric fundus. Arch. Int. Pharmacodyn. 1990;303:199–215. [PubMed] [Google Scholar]
- LEFEBVRE R.A., DE BEURME F.A., SAS S. Effect of apamin on the responses to VIP, ATP and NANC neurone stimulation in the rat and cat gastric fundus. J. Auton. Pharmacol. 1991;11:73–83. doi: 10.1111/j.1474-8673.1991.tb00246.x. [DOI] [PubMed] [Google Scholar]
- LI C.C., RAND M.J. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur. J. Pharmacol. 1990;191:303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- MATHARU M.S., HOLLINGSWORTH M. Purinoceptors mediating relaxation and spasm in the rat gastric fundus. Br. J. Pharmacol. 1992;106:395–403. doi: 10.1111/j.1476-5381.1992.tb14346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


