Abstract
We report here the use of rat high-light social interaction to model the temporal anxiolytic/antidepressant effects of SSRIs seen in the clinic. Compared to vehicle controls, 21, but not 14, days of paroxetine treatment (3 mg kg−1, p.o., daily) produced a marked increase in rat social interaction (Vehicle=71.3±7.3 s; Paroxetine=116.7±14.7 s; P<0.01) with no concurrent effect on locomotor activity, consistent with anxiolysis.
To assess whether concurrent 5-HT1A receptor blockade reduces the time to onset of anxiolysis seen with paroxetine alone (21 days), rats were implanted with osmotic minipumps to continuously infuse the 5-HT1A receptor antagonist WAY100635 (1 mg kg−1 day−1, s.c., 7 days) alone or in combination with paroxetine (3 mg kg−1, p.o., daily, 7 days), prior to anxiety testing. Paroxetine (Veh/Par=61.9±7.9 s) or WAY100635 (WAY/Veh=71.6±4.7 s) alone, had no effect on social interaction time compared to vehicle treated controls (Veh/Veh=76.4±4.9 s), whilst coadministration of WAY100635 with paroxetine, produced a marked elevation in social interaction (WAY/Par=149.3±16.8 s; P<0.01) relative to all other groups with no concurrent change in locomotor activity.
In contrast, acute administration of WAY100635 (0.03 mg kg−1, s.c.) with paroxetine (3 mg kg−1, p.o.) did not alter any behavioural measure, suggesting that the anxiolysis induced by the combination after 7 days is attributable to a CNS adaptive response.
This data demonstrates that coadministration of a 5-HT1A receptor antagonist with paroxetine markedly reduces the latency to anxiolysis, in the rat.
This study supports the use of the rat social interaction test to further delineate adaptive changes in the CNS responsible for the anxiolytic/antidepressant action of SSRIs seen in humans.
Keywords: Serotonin, 5-hydroxytryptamine, 5-HT, anxiety, paroxetine, SSRI, 5-HT1A, WAY100635, anxiolysis
Introduction
The selective serotonin reuptake inhibitor (SSRI) paroxetine (Thomas et al., 1987) is a widely prescribed efficacious antidepressant and anxiolytic in humans (Bascara, 1989; Rickels et al., 1989; Cohn & Wilcox, 1992; Dunbar et al., 1991; Feighner & Boyer, 1992; Kiev, 1992). Similarly to all other SSRIs and pharmacologically different classes of antidepressants, these compounds are only active after chronic and not acute administration. However, recent clinical trials (Artigas et al., 1994; Blier & Bergeron, 1995) have supported the hypothesis that coadministration of SSRIs with the mixed β-adrenoceptor/5-HT1A receptor antagonist, pindolol, can hasten the onset to therapeutic effect compared to SSRI administration alone. It has been hypothesized, using preclinical rodent neurochemistry, that this accelerated onset of action is attributable to the blockade by pindolol of somatodendritic 5-HT1A autoreceptors in the midline raphé nuclei and the rapid elevation of extracellular forebrain 5-hydroxytryptamine (5-HT) which ensues (Artigas, 1993). The specific mediation of 5-HT1A autoreceptors in this process has been further verified by the demonstration that coadministration of the highly selective 5-HT1A receptor antagonist, WAY100635 (Forster et al., 1995; Fletcher et al., 1996) with an SSRI, markedly increased extracellular forebrain 5-HT (Gartside et al., 1995). However, whilst there is a convincing preclinical rationale for the rapid elevation of 5-HT in the rodent brain after the administration of a 5-HT1A receptor antagonist with an SSRI, there are few reports demonstrating that this combination of compounds can produce an enhanced antidepressant-like profile in rats. Indeed, whilst a number of behavioural paradigms demonstrate face and pharmacological validity for depression (e.g. the forced swim test), they do not model the temporal effects of SSRIs seen in the clinic.
Interestingly, Lightowler et al. (1994) have previously demonstrated that chronic (21 days), but not acute, paroxetine is anxiolytic in the rat high-light unfamiliar social interaction test test (File & Hyde, 1978; 1979), suggesting that it models the temporal effects of SSRIs seen in humans. This procedure has been previously shown to be sensitive to clinically active anxiolytic agents such as benzodiazepines (File, 1980) after acute administration. By using this behavioural paradigm, we have determined if coadministration of the highly selective 5-HT1A receptor antagonist, WAY100635 (Forster et al., 1995; Fletcher et al., 1996), with the SSRI paroxetine, can reduce the time to onset of anxiolysis seen with paroxetine alone.
Methods
All in vivo studies were conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986 and conformed to SmithKline Beecham ethical standards.
Assessment of 8-OH-DPAT-induced hyperlocomotion in rats acutely or chronically administered WAY100635
Animals
Male Sprague Dawley rats (250–350 g, Charles River) were group housed (4–6 per cage) for at least 5 days prior to behavioural testing and were allowed free access to food and water. Animals unit lights were switched on/off at 0600/1800 h.
Assessment of locomotor activity
Immediately after the final drug administration (Vehicle or 8-OH-DPAT), behavioural assessment was conducted in automated locomotor activity cages (57×16.6×25.3 cm) made of black perspex with a clear perspex lid and sawdust-covered floor under red light for 30 min. During this time, locomotion was recorded by means of alternately breaking two photocell beams traversing opposite ends of the box, 3.9 cm above floor level.
Assessment of 8-OH-DPAT-induced hyperlocomotion in rats acutely administered WAY100635
Rats were placed in a room adjacent to the experimental room on the day prior to behavioural assessment. Animals were allocated to a treatment group to receive a single pretreatment of either vehicle (water) or WAY100635 (0.003–0.1 mg kg−1, s.c., flank, 20 min pretest). Vehicle pretreated rats then received either vehicle (0.9% w v−1 NaCl) or 8-OH-DPAT (0.1 mg kg−1, s.c., neck); WAY100635 treated rats all received 8-OH-DPAT (0.1 mg kg−1).
Assessment of 8-OH-DPAT-induced hyperlocomotion in rats chronically administered WAY100635
Rats were individually implanted with a single osmotic minipump (Alzet Model 2001) under 2% isoflurane anaesthesia on day 1 to allow administration of vehicle (0.9% w v−1 NaCl, pH 5 with 0.5 M HCl) or WAY100635 (1 mg kg−1 day−1, pH 5 with 5 M NaOH) for 7 days. Immediately after surgery rats were group housed rats (four per cage) and placed in a room adjacent to the experimental room. On the seventh day after surgery both vehicle and WAY100635-treated rats were allocated to a treatment group to receive a single injection of vehicle (saline) or 8-OH-DPAT (0.1 mg kg−1, s.c.).
Statistics
Data was captured as total transits (locomotor activity) and presented as mean±s.e.mean for each treatment group.
The effects of acute 8-OH-DPAT vs WAY100635 were analysed by one-way ANOVA followed by Duncan' New Multiple Range post hoc analysis after identification of overall significance. The effect of chronic WAY100635 vs 8-OH-DPAT was assessed by two-way ANOVA; treatment 1 (WAY100635)×treatment 2 (8-OH-DPAT). Significant effects were followed by Newman-Keuls post hoc analysis.
Social interaction studies
Animals
Male Sprague Dawley rats (250–350 g, Charles River) were housed singly for a minimum of 5 days prior to behavioural testing and were allowed free access to food and water. Animal unit lights were switched on/off at 0600/1800 h.
Social interaction test
Social interaction studies were performed as previously described (Kennett et al., 1994; 1997). Rats were allocated to a test pair on the basis of weight (±10 g) and both received the same treatment. The extent of social interaction was determined in test arenas made of white perspex (54 (width)×36 (depth)×29 (height) cm), with a solid floor (divided into 24 squares 9×9 cm) and a transparent front to allow observation of rat interaction by a camera mounted above, and to the front of, the arena. Lighting was provided by a 60 W white light bulb mounted directly above the arena. Behaviour of pairs of rats was videotaped by remote VCR in a room adjacent to that in which behavioural testing was conducted in a 15 min test session. All behaviour was performed between 0900 and 1700 h sequentially in blocked behavioural groups. Duration of social interaction (Interaction time), defined as grooming, following, sniffing, boxing, climbing under and over another rat; and locomotion (zone transitions; number of squares traversed on the base of the arena) was scored for each rat using a microcomputer by an observer blind to experimental treatment.
Chronic social interaction studies
The effects of 21 and 14 days (3 mg kg−1, p.o., daily), and 7 days (10 mg kg−1, p.o., daily) dosing of paroxetine vs vehicle control, were assessed 1 h after the final dose. Separate groups of animals were used for each experiment.
To allow the effect of paroxetine (3 mg kg−1, p.o., daily) and WAY100635 coadministration on rat social behaviour to be assessed after 7 days administration, rats were individually implanted with a single osmotic minipump (Alzet Model 2001) under 2% isoflurane anaesthesia on day 1 to allow administration of vehicle (0.9% w v−1 NaCl, pH 5 with 0.5 M HCl) or WAY100635 (1 mg kg−1 day−1, pH 5 with 5 M NaOH) for 7 days. Immediately after surgery rats were singly housed and placed in a room adjacent to the experimental room. On the evening of day 1, rats were administered either vehicle or paroxetine (3 mg kg−1, p.o.) at 1700 h. On days 2–6 rats received, either vehicle or paroxetine (3 mg kg−1, p.o.) at 1200 h. On day 7 (test day) vehicle or paroxetine were administered 1 h prior to the assessment of rat social interaction.
Acute social interaction studies
The effects of a single dose of paroxetine (3 mg kg−1 p.o.) or vehicle, 60 min pretest, in combination with either WAY100635 (0.03 mg kg−1, s.c.) or vehicle (distilled water), 20 min pretest, were assessed on rat social interaction.
Statistics
Data was captured as total interaction time (seconds) and number of zone transitions (social interaction studies) and presented as mean±s.e.mean for each treatment group.
The effects of a single dose of paroxetine vs vehicle treated controls was assessed by unpaired t-test. For all antagonist studies, the effects of WAY100635 vs paroxetine were analysed by two-way analysis of variance (ANOVA); treatment 1 (WAY100635)×treatment 2 (paroxetine). Significant effects were followed by Newman-Keuls post hoc analysis.
Drugs and dosing
Paroxetine (SmithKline Beecham, SB) was administered orally in 1% methyl cellulose with water (vehicle) using an injection volume of 2 ml kg−1. Control animals received vehicle only. For acute studies WAY100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide) oxalate, synthesized by the Department of Medicinal Chemistry, SB, was administered sub-cutaneously in distilled water using an injection volume of 1 ml kg−1. (±)-8-hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT HBr; Semat) was administered subcutaneously in 0.9% w v−1 NaCl to give a constant injection volume of 1 ml kg−1. For chronic studies, WAY100635 was administered by osmotic minipump (Alzet model 2001) to administer 1 mg kg−1 day−1. WAY100635 was dissolved in 0.9% w v−1 NaCl after heating, and pH adjusted to 5.5 with 5 M NaOH. Vehicle controls received distilled water via osmotic minipump, pH adjusted to 5.5 with 0.5 M HCl.
Results
The effect of acute WAY100635 on 8-OH-DPAT-induced hyperlocomotion
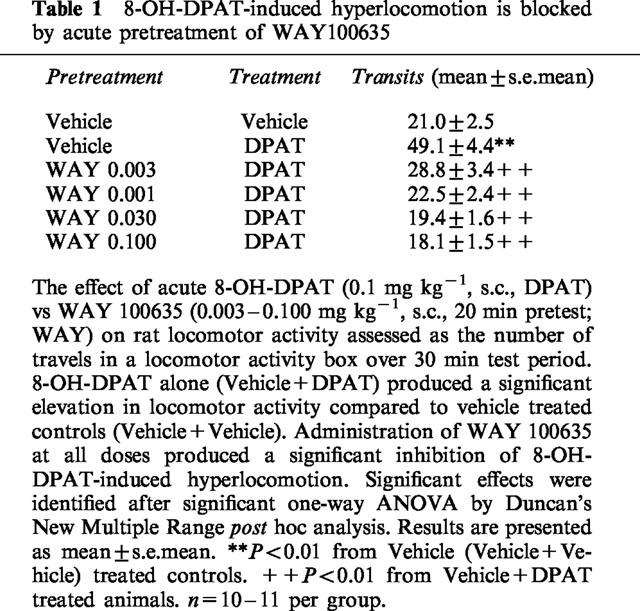
Administration of 8-OH-DPAT (0.1 mg kg−1, s.c.) immediately prior to the assessment of rat locomotor activity, produced the expected increase in locomotor activity relative to Vehicle (Veh/Veh) treated controls (F5.58=17.214; P<0.001). Pretreatment of rats with WAY100635 (0.003–0.100 mg kg−1, s.c., 20 min pretest) significantly and dose-dependantly inhibited 8-OH-DPAT-induced hyperlocomotor responses at all doses tested (Table 1).
Table 1.
8-OH-DPAT-induced hyperlocomotion is blocked by acute pretreatment of WAY100635
The effect of sub-chronic (7 day) administration of WAY100635 on 8-OH-DPAT-induced hyperlocomotion
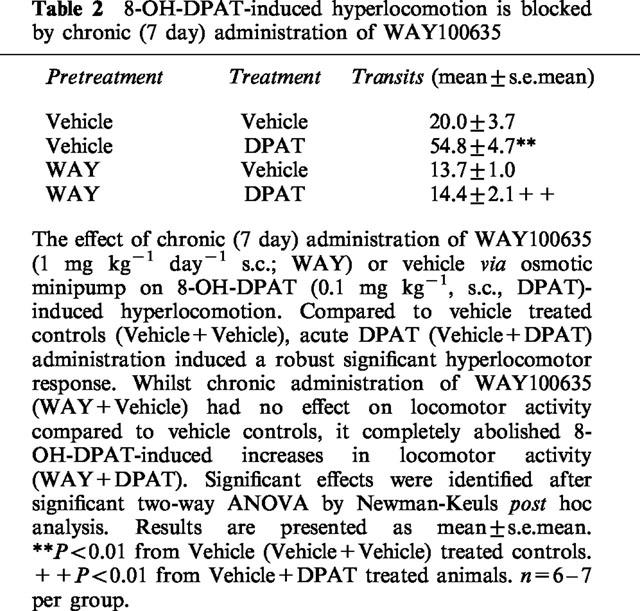
Administration of an acute dose of 8-OH-DPAT (0.1 mg kg−1, s.c.) to rats chronically administered vehicle via subcutaneous osmotic minipump, produced a robust increase (F1.22=52.830; P<0.001) in locomotor activity compared to vehicle controls (Veh/Veh, Table 2), similar in magnitude to that seen in rats treated acutely with vehicle prior to 8-OH-DPAT (see Table 1). Furthermore, baseline vehicle responses (approximately 20 travels in a 30 min test period) were similar between the two studies. Sub-chronic (7 day) administration of WAY100635 (1 mg kg−1 day−1, s.c.), at a dose and duration employed for the subchronic paroxetine/WAY100635 combination social interaction studies, had no effect on locomotor activity compared to vehicle controls (Veh/Veh) when administered alone (WAY/Veh), but produced a complete blockade of 8-OH-DPAT-induced hyperlocomotion at the same dose (F1.22=24.631; P<0.001; Table 2), thereby verifying that 5-HT1A receptors were blocked with this dose of compound in the 7 day social interaction study (see below).
Table 2.
8-OH-DPAT-induced hyperlocomotion is blocked by chronic (7 day) administration of WAY100635
The effect of chronic (21 and 14 days) paroxetine on rat social interaction behaviour
Administration of paroxetine (3 mg kg−1, p.o., daily) alone for 21 days produced a significant (P<0.01) increase in interaction time (Figure 1a) with no significant concurrent increase in locomotor activity (Figure 1b) compared to vehicle-treated controls, consistent with previous findings using an identical assay system (Lightowler et al., 1994). In contrast, administration of paroxetine (3 mg kg−1, p.o., daily) alone for 14 days had no effect on either interaction time (Figure 2a) or locomotor activity (Figure 2b).
Figure 1.
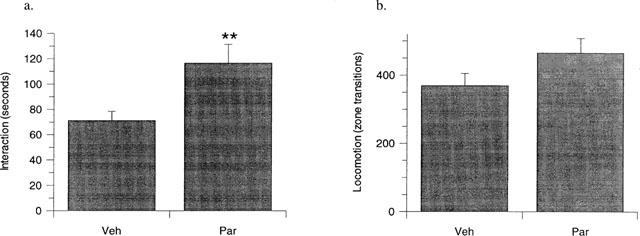
The effect of 21 days paroxetine (3 mg kg−1 day−1, p.o., Par) compared to vehicle treated controls (Veh) on rat behaviour in the high-light social interaction test. A significant increase in interaction time (a) was evident in rats treated with paroxetine, compared to vehicle treated controls, whilst no concurrent change was identified in locomotor activity (b) between the two treatment groups. Results are presented as mean±s.e.mean **P<0.01 from vehicle treated controls, unpaired t-test. n=12 (Veh), n=9 (Par).
Figure 2.
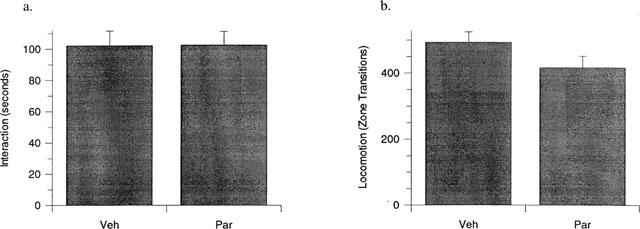
The effect of 14 days paroxetine (3 mg kg−1 day−1, p.o., Par) compared to vehicle treated controls (Veh) on rat behaviour in the high-light social interaction test. No significant change in interaction time (a) or locomotor activity (b) was evident in rats treated with paroxetine compared to vehicle treated controls. Results are presented as mean±s.e.mean. No significant effect by unpaired t-test. n=12 per group.
The effect of coadministration of sub-chronic (7 day) paroxetine and the 5-HT1A receptor antagonist WAY100635 on rat social interaction behaviour
The identification that the onset of paroxetine-induced anxiolysis occurred between days 14 and 21 led us to assess if coadministration of WAY100635 with paroxetine, could produce a more rapid anxiolytic response than that seen with the SSRI alone. Administration of paroxetine (3 mg kg−1, p.o. daily; Veh/Par) or WAY100635 (1 mg kg−1 day−1, s.c.; WAY/Veh) alone for 7 days had no effect on social interaction levels compared to vehicle-treated controls (Veh/Veh; Figure 3a). However, in contrast, in the same study coadministration of WAY100635 with paroxetine, produced a pronounced anxiolytic effect (F1,42=20.24, P<0.001) compared to all other treatment groups. Furthermore, this was not confounded by concurrent increases in locomotor activity in the same animals (Figure 3b).
Figure 3.
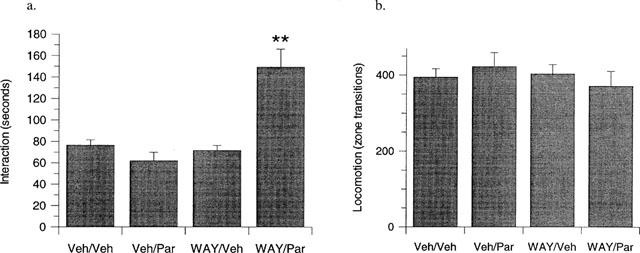
The effect of 7 days paroxetine (3 mg kg−1 day−1, p.o., Veh/Par) or WAY100635 (1 mg kg−1 day−1, s.c., WAY/Veh) alone, or in combination (WAY/Par) compared to vehicle treated controls (Veh/Veh) on rat behaviour in the high-light social interaction test. (a) Neither paroxetine nor WAY100635 had an effect on interaction time when administered alone, but produced a significant elevation in interaction time compared to vehicle treated controls (Veh/Veh) when administered in combination (WAY/Par). (b) No effect on locomotor activity was seen in any treatment group relative to vehicle treated controls. Results are presented as mean±s.e.mean. **P<0.01 from vehicle-treated controls after two-way ANOVA. n=10–12 per group.
The effect of sub-chronic (7 day) high dose paroxetine (10 mg kg−1) on rat social interaction behaviour
Treatment of rats with a high dose (10 mg kg−1, p.o.) of paroxetine daily, revealed no significant effects on either interaction or locomotor activity (Figure 4).
Figure 4.
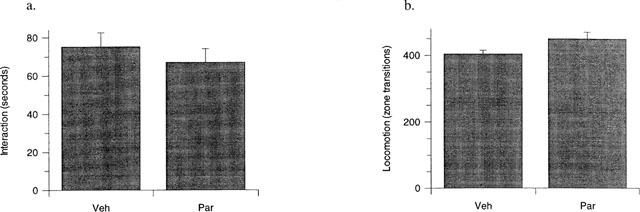
The effect of 7 days paroxetine (10 mg kg−1 day−1, p.o., Par) compared to vehicle treated controls (Veh) on rat behaviour in the high-light social interaction test. No significant change in interaction time (a) or locomotor activity (b) was evident in rats treated with paroxetine compared to vehicle treated controls. Results are presented as mean±s.e.mean. No significant effect by unpaired t-test. n=12 per group.
The effect of coadministration of acute paroxetine and the 5-HT1A receptor antagonist WAY100635 on rat social interaction behaviour
In contrast to the observed anxiolytic effects of coadministered WAY100635 and paroxetine after 7 days (see above), a single acute dose of these compounds either alone or in combination, had no effect on either rat social interaction (Figure 5a) or locomotor activity (Figure 5b).
Figure 5.
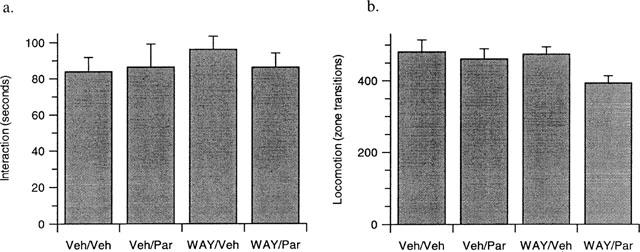
The effect of acute paroxetine (3 mg kg−1, p.o., Veh/Par) or WAY100635 (0.03 mg kg−1, s.c., WAY/Veh) alone, or in combination (WAY/Par) compared to vehicle treated controls (Veh/Veh)) on rat behaviour in the high light social interaction test. No significant effects were evident on either (a) interaction or (b) locomotion in any treatment group after two-way ANOVA. Results are presented as mean±s.e.mean. n=10 per group.
Discussion
The use of SSRIs to treat a number of anxiety disorders categorized in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV, 1994), including obsessive-compulsive and panic disorders, suggests that the actions of these compounds may be modelled in preclinical behavioural tests of anxiety. Indeed, a number of preclinical paradigms of anxiety have been used to model the delayed onset of therapeutic effect of this class of compounds seen in the clinic (Griebel et al., 1994; 1995; Berton et al., 1999; To & Bagdy, 1999; To et al., 1999). However, whilst SSRIs are proven highly efficacious anxiolytic/antidepressants in the clinic, it is accepted that SSRIs typically take weeks to reveal their clinical effect, thereby creating a need to identify novel agents with similar, or greater, efficacy and exhibiting a faster onset of action (Montgomery, 1999). To address this clinical need, it has been proposed that modulation of prescribed SSRIs by coadminstration of the 5-HT1A receptor antagonist (±)-pindolol, can reduce the latency to onset in clinical effect seen with the SSRI alone (Artigas, 1993). Although the majority of published studies confirm these findings, some negative reports have also been made (Berman et al., 1997; 1999) refuting this hypothesis. However, substantial preclinical neurochemical data support the proposal that concurrent blockade of somatodendritic 5-HT1A autoreceptors and the 5-HT transporter, will rapidly elevate 5-HT in the rat forebrain. Furthermore, many of these latter (preclinical) studies have been performed using agents possessing a pharmacologically cleaner profile at the 5-HT1A receptor (e.g. WAY100635) than pindolol. However, the results of a clinical trial performed with such a compound in combination with an SSRI, have yet to be reported.
Despite the convincing mechanistic evidence for a rapid elevation of 5-HT in the rodent CNS after concurrent 5-HT1A receptor and 5-HT reuptake blockade, there have been few reports equating this to a faster onset of behavioural effect relevant to the anxiolytic/antidepressant actions of SSRIs. Therefore, using a rat model of anxiety, the high-light unfamiliar social interaction test, the current study has attempted to address whether co-administration of the selective 5-HT1A receptor antagonist (WAY100635) can decrease the latency to anxiolytic-like effect seen with paroxetine alone after chronic (21 day) administration.
Confirming the effects seen by Lightowler et al. (1994), using identical social interaction test conditions, chronic 21 day administration of paroxetine (3 mg kg−1, p.o., daily) produced a robust increase in interaction time with no concomitant increase in locomotor activity, indicative of an anxiolytic-like effect. Verification that this behavioural paradigm models the delayed action of paroxetine seen in the clinic, was confirmed by the absence of anxiolysis after 7 or 14 days administration of the same dose of paroxetine. In order to assess whether coadministration of a 5-HT1A receptor antagonist could reduce the time taken to onset of anxiolysis seen with an SSRI alone, rats were implanted with osmotic minipumps to allow a constant infusion of WAY100635 (1 mg kg−1 day−1, s.c.) and dosed with paroxetine (3 mg kg−1, p.o., daily) for 7 days prior to testing in the social interaction paradigm. Blockade of 5-HT1A receptors by this dosing regime of WAY100635, was verified by the abolition of 8-OH-DPAT-induced hyperlocomotion (Tricklebank et al., 1984) in animals on the seventh day of antagonist administration. Consistent with a faster onset of anxiolysis after coadministration of WAY100635 and paroxetine, a marked increase in interaction time was seen in rats treated with this combination for 7 days, compared to animals treated with vehicle or either compound alone. Indeed, the magnitude of this response to the combination treatment was more marked than that of paroxetine alone, even when administered at a greater than 3 fold higher dose of 10 mg kg−1.
This finding suggests that using the high-light social interaction test to model anxiety, coadministration of a 5-HT1A receptor antagonist with paroxetine can reduce the latency to onset of paroxetine-induced anxiolysis by at least two-thirds. Upon identification of this effect, a single administration of WAY100635 (0.03 mg kg−1, s.c. also demonstrated to completely block 8-OH-DPAT-induced hyperlocomotion after a single acute administration) with paroxetine (3 mg kg−1, p.o.) was assessed on rat anxiety to determine if anxiolysis was evident after acute blockade of 5-HT1A receptors and 5-HT reuptake sites. In contrast to the 7 day coadministration, the acute combination of WAY100635 with paroxetine failed to produce any effect on rat anxiety measures.
Despite the consistent identification that combination of a 5-HT1A receptor antagonist with SSRIs produce robust increases in rat forebrain 5-HT, the data presented herein provides the first behavioural evidence, using an endpoint relevant to the clinical actions of an SSRI (anxiolysis), that coadministration of a 5-HT1A receptor antagonist with paroxetine, may produce a rapid anxiolytic effect not seen upon administration of an SSRI alone. However, other preclinical behavioural data also suggests that coadministration of 5-HT1A receptor antagonists with SSRIs may produce a more rapid onset to the behavioural effects seen with SSRIs alone. For example, using a similar drug administration strategy, Mitchell & Redfern (1997) demonstrated that WAY100635 (0.1 mg kg−1 day−1) hastened the delayed switch from a decrease to an increase in rat aggression seen with both fluoxetine (0.34 mg kg−1 day−1) and paroxetine (0.33 mg kg−1 day−1) after acute and chronic administration respectively. Despite these findings only a few other reports have attempted to address the behavioural effects of combining 5-HT1A receptor antagonists with SSRIs (for example see Cryan et al., 1999).
Whilst the above data provides in vivo proof of concept for the hypothesis that concurrent 5-HT1A receptor and 5-HT reuptake blockade can produce a rapid onset of anxiolysis in the rat, further investigation into the time between day 1 and day 7 that onset of anxiolysis occurs is needed. Indeed, it is possible that concurrent blockade of both pharmacological sites could result in an anxiolytic action at a time prior to the 7 day point chosen for the current studies. However, the previous identification that acute paroxetine with WAY100635 dis-inhibits dorsal raphé cell firing to elevate fronto-cortical 5-HT (Gartside et al., 1995), together with the absence of an anxiolytic-like effect in the current studies after acute administration, is consistent with the hypothesis that anxiolysis identified at the 7 day time point with the combination is attributable to an adaptive CNS change. Therefore, this dosing regime and behavioural model may provide a means by which the precise mechanism of anxiolytic/antidepressant action of SSRIs seen in humans, may be delineated in the rat. Interestingly, previous studies performed in SB labs (Kennett et al., 1994), suggest that 21 days administration of paroxetine, at a dose higher than that used in the current studies, can produce a desensitization to the hypolocomotor effects of the 5-HT2C receptor agonist mCPP. The blunted responses to mCPP seen in this study after chronic SSRIs are consistent with similar effects seen in humans on chronic antidepressant treatment (Zohar et al., 1988; Hollander et al., 1991; Quested et al., 1997). However, whilst assessment of 5-HT2C responsivity in identically treated animals awaits further evaluation, it is possible that adaptive changes in a number of other post-synaptic 5-HT receptors may also be responsible for the anxiolysis seen after chronic administration of paroxetine. The data presented herein suggests that the rapid anxiolysis seen when combining WAY100635 with paroxetine, is not attributable to post-synaptic 5-HT1A receptor activation (as WAY100635 blocks both pre- and post-synaptic 5-HT1A receptor sites). Moreover, whilst the adaptive CNS changes responsible for anxiolysis after 7 or 21 days of paroxetine in the presence and absence of WAY100635 respectively, may be common to both treatment groups, the above treatment regime and model will allow the potential differing mechanisms of these treatments to be explored.
Acknowledgments
We gratefully acknowledge the technical and surgical assistance provided by Karen Davis, Jackie College, Louise Chamberlain and Susan Barber.
Abbreviations
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders
- 8-OH-DPAT
(±)-8-hydroxy-2-dipropylaminotetralin hydrobromide
- SSRI
selective serotonin reuptake inhibitor
- WAY100635
(N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide oxalate
References
- ARTIGAS F. 5-HT and antidepressants: new views from microdialysis studies. Trends Pharmacol Sci. 1993;14:262. doi: 10.1016/0165-6147(93)90125-4. [DOI] [PubMed] [Google Scholar]
- ARTIGAS F., PEREZ V., ALVAREZ E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch. Gen. Psychiatry. 1994;51:248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]
- BASCARA L. A double blind study to compare the effectiveness and tolerability of paroxetine and amitriptyline in depressed patients. Acta Psychiatr. Scand. 1989;80 Suppl. 350:141–142. doi: 10.1111/j.1600-0447.1989.tb07196.x. [DOI] [PubMed] [Google Scholar]
- BERMAN R.M., ANAND A., CAPPIELLO A., MILLER H.L., HU X.S., OREN D.A., CHARNEY D.S. The use of pindolol with fluoxetine in the treatment of major depression: final results from a double-blind, placebo-controlled trial. Biol. Psychiatry. 1999;45:1170–1177. doi: 10.1016/s0006-3223(98)00383-7. [DOI] [PubMed] [Google Scholar]
- BERMAN R.M., DARNELL A.M., MILLER H.L., ANAND A., CHARNEY D.S. Effect of pindolol in hastening response to fluoxetine in the treatment of major depression: a double-blind, placebo-controlled trial. Am. J. Psychiatry. 1997;154:37–43. doi: 10.1176/ajp.154.1.37. [DOI] [PubMed] [Google Scholar]
- BERTON O., DURAND M., AGUERRE S., MORMEDE P., CHAOULOFF F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience. 1999;92:327–341. doi: 10.1016/s0306-4522(98)00742-8. [DOI] [PubMed] [Google Scholar]
- BLIER P., BERGERON R. Effectiveness of pindolol with selected antidepressant drugs in the treatment of major depression. J. Clin. Psychopharmacol. 1995;15:217–222. doi: 10.1097/00004714-199506000-00011. [DOI] [PubMed] [Google Scholar]
- COHN J.B., WILCOX C.S. Paroxetine in major depression: a double blind trial with imipramine and placebo. J. Clin. Psychiatry. 1992;53:52–56. [PubMed] [Google Scholar]
- CRYAN J.F., MCGRATH C., LEONARD B.E., NORMAN T.R. Onset of the effects of the 5-HT1A antagonist, WAY-100635, alone, and in combination with paroxetine, on olfactory bulbectomy and 8-OH-DPAT-induced changes in the rat. Pharmacol. Biochem. Behav. 1999;63:333–338. doi: 10.1016/s0091-3057(98)00245-7. [DOI] [PubMed] [Google Scholar]
- DUNBAR G.C., COHN J.B., FABRE L.F., FEIGHNER J.P., FIEVE R.R., MENDELS J., SHRIVASTAVA R.K. A comparison of paroxetine, imipramine and placebo in depressed out-patients. Br. J. Pharmacol. 1991;159:394–398. doi: 10.1192/bjp.159.3.394. [DOI] [PubMed] [Google Scholar]
- FEIGHNER J.P., BOYER W.F. Paroxetine in the treatment of depression: A comparison with imipramine and placebo. J. Clin. Psychiatry. 1992;53:44–47. [PubMed] [Google Scholar]
- FILE S.E. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J. Neurosci. Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- FILE S.E., HYDE J.R. Can social interaction be used to measure anxiety. Br. J. Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILE S.E., HYDE J.R. A test of anxiety that distinguishes between the actions of benzodiazepines and those of other minor tranquilisers and of stimulants. Pharmacol. Biochem. Behav. 1979;11:65–69. doi: 10.1016/0091-3057(79)90298-3. [DOI] [PubMed] [Google Scholar]
- FLETCHER A., FORSTER E.A., BILL D.J., BROWN G., CLIFFE I.A., HARTLEY J.E., JONES D.E., MCLENACHAN A., STANHOPE K.J., CRITCHLEY D.J., CHILDS K.J., MIDDLEFELL V.C., LANFUMEY L., CORRADETTI R., LAPORTE A.M., GOZLAN H., HAMON M., DOURISH C.T. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav. Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- FORSTER E.A., CLIFFE I.A., BILL D.J., DOVER G.M., JONES D., REILLY Y., FLETCHER A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur. J. Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- GARTSIDE S.E., UMBERS V., HAJOS M., SHARP T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br. J. Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIEBEL G., BLANCHARD D.C., AGNES R.S., BLANCHARD R.J. Differential modulation of antipredator defensive behavior in Swiss-Webster mice following acute or chronic administration of imipramine and fluoxetine. Psychopharmacology. 1995;120:57–66. doi: 10.1007/BF02246145. [DOI] [PubMed] [Google Scholar]
- GRIEBEL G., MOREAU J.L., JENCK F., MISSLIN R., MARTIN J.R. Acute and chronic treatment with 5-HT reuptake inhibitors differentially modulate emotional responses in anxiety models in rodents. Psychopharmacology. 1994;113:463–470. doi: 10.1007/BF02245224. [DOI] [PubMed] [Google Scholar]
- HOLLANDER E., DECARIA C., GULLY R., NITESCU A., SUCKOW R.F., GORMAN J.M., KLEIN D.F., LIEBOWITZ M.R. Effects of chronic fluoxetine treatment on behavioral and neuroendocrine responses to metachlorophenylpiperazine in obsessive-compulsive disorder. Psychiatry Res. 1991;36:1–17. doi: 10.1016/0165-1781(91)90113-4. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A., WOOD M.D., BRIGHT F., TRAIL B., RILEY G., HOLLAND V., AVENELL K.Y., STEAN T., UPTON N., BROMIDGE S., FORBES I.T., BROWN A.M., MIDDLEMISS D.N., BLACKBURN T.P. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A., WOOD M.D., GLEN A., GREWAL S., FORBES I., GADRE A., BLACKBURN T.P. In vivo properties of SB 200646A, a 5-HT2C/2B receptor antagonist. Br. J. Pharmacol. 1994;111:797–802. doi: 10.1111/j.1476-5381.1994.tb14808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIEV A. A double-blind, placebo-controlled study of paroxetine in depressed outpatients. J. Clin. Psychiatr. 1992;53:27–29. [PubMed] [Google Scholar]
- LIGHTOWLER S., KENNETT G.A., WILLIAMSON I.J., BLACKBURN T.P., TULLOCH I.F. Anxiolytic-like effect of paroxetine in a rat social interaction test. Pharmacol. Biochem. Behav. 1994;49:281–285. doi: 10.1016/0091-3057(94)90422-7. [DOI] [PubMed] [Google Scholar]
- MITCHELL P.J., REDFERN P.H. Potentiation of the time-dependent, antidepressant-induced changes in the agonistic behaviour of resident rats by the 5-HT1A receptor antagonist, WAY-100635. Behav. Pharmacol. 1997;8:585–606. doi: 10.1097/00008877-199711000-00016. [DOI] [PubMed] [Google Scholar]
- MONTGOMERY S.A.New developments in the treatment of depression J. Clin. Psychiatry 199960Suppl. 1410–15.discussion 31–35 [PubMed] [Google Scholar]
- QUESTED D.J., SARGENT P.A., COWEN P.J. SSRI treatment decreases prolactin and hyperthermic responses to mCPP. Psychopharmacology. 1997;133:305–308. doi: 10.1007/s002130050406. [DOI] [PubMed] [Google Scholar]
- RICKELS K., AMSTERDAM J., CLARY C., FOX I., SCHWEIZER E., WEISE C. A plecebo-controlled, double-blind, clinical trial of paroxetine in depressed outpatients. Acta Psychiatr. Scand. 1989;80 Suppl. 350:117–123. doi: 10.1111/j.1600-0447.1989.tb07188.x. [DOI] [PubMed] [Google Scholar]
- THOMAS D.R., NELSON D.R., JOHNSON A.M. Biochemical effects of the antidepressant paroxetine, a specific 5-hydroxytryptamine uptake inhibitor. Psychopharmacology. 1987;93:193–200. doi: 10.1007/BF00179933. [DOI] [PubMed] [Google Scholar]
- TO C.T., ANHEUER Z.E., BAGDY G. Effects of acute and chronic fluoxetine treatment of CRH-induced anxiety. Neuroreport. 1999;10:553–555. doi: 10.1097/00001756-199902250-00020. [DOI] [PubMed] [Google Scholar]
- TO C.T., BAGDY G. Anxiogenic effect of central CCK administration is attenuated by chronic fluoxetine or ipsapirone treatment. Neuropharmacology. 1999;38:279–282. doi: 10.1016/s0028-3908(98)00176-2. [DOI] [PubMed] [Google Scholar]
- TRICKLEBANK M.D., FORLER C., FOZARD J.R. The involvement of subtypes of the 5-HT1 receptor and of catecholaminergic systems in the behavioural response to 8-hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur. J. Pharmacol. 1984;106:271–282. doi: 10.1016/0014-2999(84)90714-3. [DOI] [PubMed] [Google Scholar]
- ZOHAR J., INSEL T.R., ZOHAR-KADOUCH R.C., HILL J.L., MURPHY D.L. Serotonergic responsivity in obsessive-compulsive disorder. Effects of chronic clomipramine treatment. Arch. Gen. Psychiatry. 1988;45:167–172. doi: 10.1001/archpsyc.1988.01800260081011. [DOI] [PubMed] [Google Scholar]


