Abstract
We investigated the ability of the cannabinoid agonists CP55,940 (CB1/CB2) and anandamide (endogenous cannabinoid) to modulate electrical field stimulation (EFS)-induced acetylcholine (ACh) release from parasympathetic nerve terminals innervating guinea-pig trachea. We assessed whether modulation of transmitter release translated to an impact on functional responses by investigating the effect of these agents on contractile responses evoked by EFS and ACh. Furthermore, we evaluated the ability of these compounds to elicit bronchodilation in pre-contracted guinea-pig tracheal strips.
CP55,940 and anandamide significantly inhibited EFS-evoked ACh release (maximal inhibition of 35.1±2.9% and 33.4±6.4% at 1 μM, P<0.05, respectively). The CB1 receptor antagonist SR 141716A (1 μM), had no effect on ACh release and failed to reverse the inhibitory effect of CP55,940 (1 μM).
Paradoxically, CP55,940 had no significant effect on EFS-evoked cholinergic contractile responses. Furthermore, CP55,940 did not relax pre-contracted tracheal strips or affect contractile responses to exogenous ACh. This lack of activity on smooth muscle tone is consistent with the fact that no detectable specific binding of [3H] CP55,940 was found in tracheal homogenates.
These data suggest that cannabinoid agonists inhibit ACh release from cholinergic nerve terminals via activation of CB2 receptors but that this inhibitory action does not impact on functional responses such as cholinergic contraction.
Keywords: Cannabinoid, trachea, neurotransmission, acetylcholine release, contraction, airways
Introduction
It is now clear that the biological activity of marijuana and related cannabinoids occurs through activation of two different subtypes of specific receptors, CB1 and CB2, which have been cloned and pharmacologically characterized (Matsuda et al., 1990; Munro et al., 1993; Felder et al., 1995; Howlett, 1995; Pertwee, 1997). These two receptors form a distinct class within the family of G protein-coupled receptors and share 40–50% amino acid sequence homology (Matsuda, 1997). CB1 receptors are widely distributed in mammalian tissues, with the highest density in the central nervous system (Herkenham et al., 1991; Galiegue et al., 1995). In contrast, the CB2 receptors, which are not expressed in the brain, are particularly abundant in immune tissues, and in some other peripheral tissues such as spleen, tonsils and nerve terminals (Howlet & Fleming, 1984; Galiegue et al., 1995; Griffin et al., 1997). Both receptors are negatively coupled to adenylyl cyclase and positively coupled to mitogen-activated protein kinase through a PTX-sensitive G-binding protein (Munro et al., 1993; Felder et al., 1995).
Although the neurobehavioural effects of cannabinoids have been extensively investigated, little is known about the pharmacological properties of these agents on the respiratory tract. Inhalation of Δ9-tetrahydrocannabinol, the major active component of marijuana, induces bronchodilation and protects against bronchoconstriction evoked by cholinergic agonists in the airways of asthmatic patients (Graham, 1986; Williams et al., 1976; Tashkin et al., 1977; Hartley et al., 1978) but it is not clear whether these effects are centrally mediated. The recent development of synthetic agonists with an affinity for cannabinoid receptors greater than that of Δ9-tetrahydrocannabinol has allowed further investigation of the cellular mechanisms underlying this bronchoprotective effect.
It has been reported that activation of pre-junctional cannabinoid receptors modulates neurotransmitter release from both central nervous system and peripheral nerves. This is probably due to a modulation of ion channels such as calcium and potassium channels located on the synaptic membrane. CP55,940 and WIN 55212-2, two potent non-selective cannabinoid agonists, inhibit acetylcholine (ACh) release in hippocampal slices from rat brain (Gifford et al., 1996, 1997). Cannabinoids also inhibit ACh release from guinea-pig myenteric plexus and noradrenaline release from peripheral sympathetic nerves (Pertwee et al., 1996; Ishac et al., 1996).
In order to clarify whether cannabinoids exert a neuromodulatory role also in the respiratory system we determined the effect of cannabinoid receptor agonists on cholinergic neurotransmission in guinea-pig airways. Quantitative measurement of [3H]-ACh overflow from post-ganglionic nerve endings provides a reliable direct method to demonstrate the occurrence of a pre-junctional modulation of cholinergic neurotransmission (Belvisi et al., 1996; Spicuzza et al., 1998). Therefore, we assessed the effects of CP55,940 (CB1/CB2 non-selective agonist) and anandamide (endogenous cannabinoid ligand) on electrical field stimulation (EFS)-evoked [3H]-ACh release from parasympathetic nerves innervating guinea-pig trachea. Furthermore, we assessed whether these agents had any effect on EFS-induced contractile responses to assess whether any effects seen on transmitter release translated to an impact on a functional response. In view of the observed bronchoprotective role of inhaled marijuana (Δ9-tetrahydrocannabinol) in asthmatics, we investigated the anti-spasmogenic of these compounds and their ability to elicit bronchodilation in guinea-pig tracheal strips.
Methods
Preparation of guinea-pig trachea
Male Dunkin-Hartley guinea-pigs (Harlan-Olac) (300–500 g) were killed by cervical dislocation and the tracheal tissue was prepared as described previously (Belvisi et al., 1996). The lungs, with trachea and bronchi attached, were rapidly removed and placed in oxygenating Krebs-Henseleit solution (KHS) of the following composition (in mM): NaCl 118, KCl 5.9, MgSO4 1.2, CaCl2 2.5, NaH2PO4 1.2, NaHCO3 25.5 and glucose 5.6. The trachea was dissected away from the lungs and main bronchi and opened longitudinally by cutting through the cartilage; the epithelium was subsequently removed, minimizing damage to the smooth muscle. Indomethacin (10 μM) was present throughout to prevent the formation of endogenous prostaglandins which have been demonstrated previously to affect cholinergic neurotransmission and ACh release per se (Wessler et al., 1994; Belvisi et al., 1996).
Measurement of ACh release from parasympathetic nerves
The release of ACh from cholinergic nerves was measured as described previously (Patel et al., 1995). Briefly, eight strips of smooth muscle with the cartilage and epithelium removed were studied in parallel. Each tissue was connected top and bottom with silver wire and mounted in a jacketed chamber. Tissues were superfused (Watson-Marlow model 503S; Smith and Nephew, Falmouth, U.K.) at a rate of 1 ml min−1 throughout the experiment with oxygenated KHS (pH 7.4) maintained at 37°C. The tissues were allowed to equilibrate for 30 min during which time they were continuously superfused with KHS solution. EFS (40V, 0.5 ms pulse width, 4 Hz) was applied continuously for the last 10 min delivered via the silver wire electrodes. Tissues were then placed into vials containing 1.5 ml of oxygenated KHS supplemented with [3H]-choline (67 nM; specific radioactivity: 2.78 TBq/mmol) and EFS was applied (40V, 0.5 ms pulse width, 4 Hz) for 45 min in order to facilitate uptake of [3H]-choline into cholinergic nerve terminals. At the end of this period, tissues were superfused with KHS containing hemicholinium-3 (10 μM) to prevent the re-uptake of unlabelled choline into the nerves. Preparations were washed for 2 h before the beginning of the experiment to achieve a stable baseline of tritium release. During this period the superfusate collected was discarded. It has been shown previously that most of the tritium outflow evoked by EFS of epithelium-containing trachea is [3H]-phosphorylcholine in addition to [3H]-ACh, whereas EFS of epithelium-denuded tracheal preparations does not elicit significant release of [3H]-phosphorylcholine (Wessler et al., 1990). Therefore, in the studies described herein epithelium-free tissue preparations were used (Ward et al., 1993; Patel et al., 1995).
EFS (40 V, 0.5 ms pulse width, 4 Hz for 1 min) was applied to each tissue and 1 ml samples were collected every minute for 3 min before, 1 min during and 3 min after stimulation and at 5 min intervals outside these times. Drugs were added to the KHS superfusing each tissue after one control EFS as detailed in the text and figure legends. A test EFS was then applied 15 min and 30 min after addition of the drugs as indicated and their effects evaluated. When the effect of an antagonist was evaluated, it (or vehicle) was added to the superfusing KHS solution 30 min before the third stimulation and in the presence of the agonist. At the end of the experiment, tissues were solubilized in 1 ml soluene tissue solubilizer (Canberra Packard, Pangbourne, U.K.). All samples of superfusate were aliquoted to 1 ml. After addition of 4 ml scintillant (Pico-Fluor® 40; Canberra Packard) to these samples and to the solubilized tissues, they were assayed for radioactivity by a liquid scintillation counter (Model 1900EA; Packard Instrument Co. Inc., Meriden, CT, U.S.A.). After determination of radioactivity, the fractional release of 3H from each preparation was calculated as a rate coefficient (min−1) of each collection period at the mid point time, as previously described (Patel et al., 1995). The increase in 3H overflow evoked by EFS was expressed as a percentage increase in the rate coefficient during the EFS period over the average for the preceding 3 min control period.
Measurement of contractile responses evoked by EFS and exogenous ACh and bronchodilator activity in guinea-pig trachea
Eight transverse segments of trachea, containing 3–4 cartilaginous rings, were prepared and suspended between platinum wire electrodes in 10 ml organ baths containing KHS supplemented with indomethacin (10 μM) at 37°C which was continually gassed with 95% O2/5% CO2 mixture. The tissues were allowed to equilibrate for 1 h with frequent washing under a resting tension of 1 g which was optimal for determining changes in tension.
Isometric contractile responses were measured with force-displacement transducers (model FT-03; Grass Instruments, Quincy, MA, U.S.A.) connected to a polygraph (Model 7D; Grass Instruments, Quincy, MA, U.S.A.). EFS was delivered by two platinum field electrodes inserted into parallel (10 mm apart) with the tissue suspended between them. A stimulator (model D345; Digitimer Ltd., Welwyn Garden City, Hertfordshire, U.K.) provided biphasic square wave pulses of supramaximal voltage of 40 V at source of 0.5 ms duration at a frequency of 4 Hz. EFS was applied for 15 s and after three stable responses where obtained the agonist was added in to the bath. Contractile responses were expressed as absolute changes in tension. The effect of the drug was expressed as percentage inhibition.
The effect of cannabinoid agonists on exogenous ACh-induced contraction was also evaluated. Three stable contractions to ACh (1 μM) were obtained before the agonists were added in to the bath (10 min incubation). The effect of the drug was expressed as percentage change in the ACh-evoked contractile response.
The ability of cannabinoids to evoke bronchodilation was assessed as follows. After an equilibration period tissue responses were standardized by evoking two contractile responses to a supramaximal concentration of acetylcholine (ACh, 10−2 M). Cumulative relaxation concentration response curves to anandamide and CP55,940 and their vehicles (0.1% ethanol for anandamide and 0.1% DMSO for CP55,940) were obtained in tissues where induced tone was provided by carbachol (1 μM). The bronchodilator activity of anandamide and CP55,940 was compared with the β-adrenoceptor agonist isoprenaline, a standard bronchodilator. The maximum relaxation of each tissue was determined by assessing the bronchodilator activity of the non-specific phosphodiesterase inhibitor papaverine (0.1 mM).
Radioligand binding studies
Membrane preparation
All membrane preparation procedures were performed at 4°C. Guinea-pig trachea and male Sprague-Dawley rat cerebellum, spleen and brain were dissected and finely minced with scissors and homogenized in 10–20 volumes (w v−1) of ice-cold 0.35 M sucrose–50 mM Tris-HCl with an Ultra-Turax homogenizer (10–15 bursts). After centrifugation at 800×g for 10 min at 4°C, the pellets were suspended, homogenized and resedimented at the same speed. The supernatants from these two stages were pooled, diluted with 50 volumes of ice-cold 50 mM Tris buffer (pH 7.4). The membranes were pelted by a centrifugation at 40,000×g for 20 min. The resulting pellets were resuspended in an appropriate volume of buffer. The membrane suspension was then stored as aliquots at −80°C. Protein concentration was determined using a Bio-Rad protein assay kit according to the manufacturer' instructions.
Binding assays
All binding experiments were performed in 1 ml of buffer consisting of 50 mM Tris-HCl, 2.5 mM EDTA and 5 mM MgCl2 (pH 7.4) containing 25–200 μg protein ml−1. Binding experiments were performed with 0.8 nM of the non-selective cannabinoid receptor agonist [3H]-CP55,940 (specific activity 165 Ci mmol). Non-specific binding was defined as the binding in the presence of 1 μM cold CP55,940. Incubations were performed at 25°C for 90 min and terminated by rapid vacuum filtration over 0.2% polyethyleneimine pre-treated Whatman GF/C glass fibre filters using a Brandel cell harvester. The filters were washed twice with 4 ml of ice-cold Tris buffer and placed in vials with 4 ml of scintillation cocktail (Filtron X, National Diagnostics, Manville, NJ, U.S.A.) and counted in a liquid scintillation counter (Packard 2200 CA model, Meriden, CT, U.S.A.).
Drugs chemicals and analytical reagents
The following drugs were obtained from the Sigma Chemical Company (Poole, Dorset, U.K.): indomethacin, ACh chloride, methacholine, hemicholinium-3, (±) isoprenaline hydrochloride, anandamide and DMSO. Methyl-[3H]-choline chloride (37 Ci mmol−1) was purchased from Amersham International (Amersham, Buckinghamshire, U.K.). [3H]-CP55,940 (specific activity 165 Ci mmol−1 was purchased from NEN (DuPont, New England Nuclear, Stevenage, Herts, U.K.). The CB1/CB2 receptor agonist CP55,940 and the CB1 receptor antagonist SR 141716A were synthesized by the Medicinal Chemistry department Rhône-Poulenc Rorer (Vitry, France).
All drugs were made up daily and dissolved in distilled water except the following: isoprenaline (all dilutions were made up in 10 mg ml−1 ascorbic acid); indomethacin (made up at 1 mg ml−1 in phosphate buffer (in mM): KH2PO4 20, Na2HPO4 120, pH 7.8). Stock solutions of anandamide were dissolved in ethanol and stock solutions of CP55,940 and SR 141716A were dissolved in DMSO at 10−2 M and stored at −20°C. The solvent in the assay never exceeded 0.1% (v v−1).
Statistical analysis
Data are expressed as mean±standard error of the mean (s.e.mean) of n independent observations. Contractile and relaxant responses are expressed as absolute changes in mg tension before and after drug additions and then normalized as a percentage change. In all experiments each tissue acted as its own control and results obtained before and after drug treatment were compared by Wilcoxon' rank order test for paired data. Log EC50 values (PD2 values i.e. the concentration of drug required to elicit 50% of the maximal response) were calculated by using non-linear iterative regression with the ‘PRISM' curve fitting programme (Graphpad Software, San Diego, CA, U.S.A.). The null hypothesis was rejected when P<0.05.
Results
Effect of cannabinoid agonists on [3H]-ACh release from guinea-pig trachea
The non selective cannabinoid agonist CP55,940 inhibited EFS-evoked [3H]-ACh release (at 1 μM, 35.1±2.9% inhibition, n=10, P<0.05 and at 10 μM, 26.8±8.5% inhibition, n=10, P<0.05) (Figures 1 and 2). Anandamide, an endogenous cannabinoid ligand inhibited [3H]-ACh release (at 1 μM, 33.4±6.4% inhibition, n=10, P<0.05 and at 10 μM, 22.1±5.8%, n=10, P<0.05) to a similar extent (Figure 2). Furthermore, there was no significant change in tritium efflux evoked by EFS in vehicle-treated tissues (Figure 2).
Figure 1.
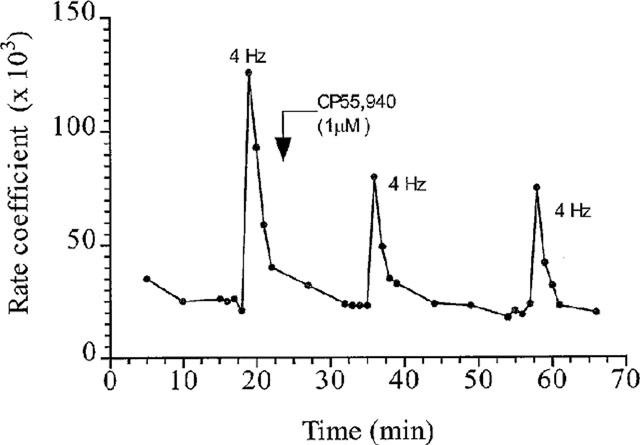
Inhibition by CP55,940 (1 μM) of EFS (40 V, 0.5 ms pulse width, 4 Hz for 1 min)-induced ACh release from an individual tracheal strip. The results are expressed as the rate coefficient which is a measure of the fractional 3H-release plotted against time (min).
Figure 2.
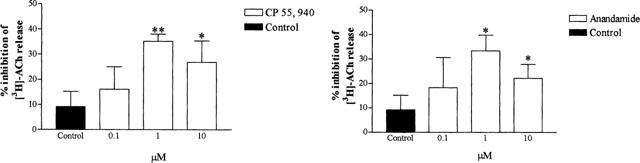
Effect of CP 55, 940 and anandamide on EFS (40 V, 0.5 ms pulse width, 4 Hz for 1 min)-induced [3H]-ACh release from guinea-pig tracheal strips. Control denotes the effect of the respective vehicles (0.1% DMSO and 0.1% ethanol which were the solvents for the highest concentrations used of CP55,940 and anandamide, respectively) on EFS-induced [3H]-ACh release. Each column shows the percentage change in the response after drug administration compared to the first control stimulation, and represents the mean±s.e.mean of 10 independent observations. * P<0.05, ** P<0.01 compared with control values preceding drug administration.
The selective CB1 receptor antagonist, SR141716A (1 μM), failed to reverse the inhibitory effect of CP55,940 (1 μM) (34.5±5.5% inhibition, in the absence and 38.6±9% inhibition, in the presence of the antagonist, n=6; P<0.001) and anandamide (1 μM) (33±3.5% inhibition in the absence, and 29.6±5.5% inhibition, in the presence of the antagonist, n=6, P<0.01) on ACh release (Figure 3).
Figure 3.

Effect of the selective CB1 receptor antagonist SR 141716 (1 μM) on the inhibitory effect of CP55,940 (1 μM) and anandamide (1 μM) on EFS (40 V, 0.5 ms pulse width, 4 Hz for 1 min)-induced [3H]-ACh release from guinea-pig trachea. Each column shows the percentage change in the response after drug administration compared to the first control stimulation, and represents the mean±s.e.mean of six independent observations. * P<0.05 compared with control values preceding drug administration.
Effect of cannabinoid agonists on contractile responses evoked by EFS and exogenous ACh and on carbachol-induced contraction in guinea-pig trachea
In order to establish whether cannabinoids exert a functional modulation of smooth muscle contractility we investigated the effect of CP55,940 on cholinergic contractile responses to EFS (40 V, 4 Hz, 0.5 ms pulse width for 15 s every 4 min) and ACh-induced contraction in epithelium-denuded trachea. CP55,940 (1 μM), added 10 min before the test and used at a concentration which was highly effective at inhibiting ACh release, had no significant effect on EFS-evoked contraction (before CP55,940, 419±50 mg compared to 374±62 mg after the addition of CP55,940, n=6, NS) and failed to impact on the contractile response to exogenous ACh (1 μM) (before CP55,940, 583±110 mg compared to 587±99 mg after the addition of CP55,940; n=6; NS).
In tissues contracted with carbachol (1 μM), isoprenaline, as expected, produced a clear concentration-related relaxation response (pD2 values of 7.599±0.09 and 7.074±0.11 in the presence and absence of indomethacin, respectively). However neither anandamide (1 nM–10 μM) nor CP55,940 (1 nM–10 μM) induced relaxation either in the presence or in the absence of indomethacin (10 μM).
Receptor binding studies
We have also investigated the cannabinoid receptor protein expression using ligand binding experiments. We have used rat brain and cerebellum membranes as a source of the central cannabinoid CB1 receptor, and rat spleen as an abundant source of the peripheral cannabinoid CB2 receptors (Munro et al., 1993). To investigate the expression of both receptors, we have used the non-selective cannabinoid receptor agonist [3H]-CP55,940. This ligand has been shown to bind both receptors with very high affinity in the nanomolar range (Devane et al., 1988; Bouaboula et al., 1993; Hillard et al., 1999). The specific binding of [3H]-CP55,940 to rat brain homogenates was linear over the protein concentrations investigated and represented over 60% of total binding at the concentration of the radioligand used (0.8 nM). Specific [3H]-CP55,940 binding was linear up to a protein concentration of 100 μg/assay. This protein concentration was chosen for further investigation of [3H]-CP55,940 binding in guinea-pig tracheal membranes. Using the same assay conditions, there was no detectable specific binding in tracheal homogenates (Figure 4). Parallel experiments performed in brain, cerebellum, and spleen did show specific [3H]-CP55,940 binding (Figure 4). These data suggest that guinea-pig tracheal membranes, unlike spleen, cerebellum and brain, do not express appreciable if not any [3H]-CP55,940 binding sites.
Figure 4.
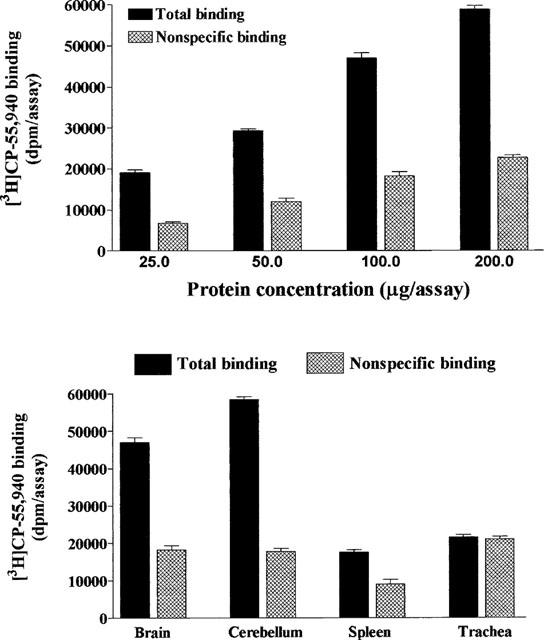
Binding of the non-selective cannabinoid receptor agonist [3H]-CP55,940 to rat brain homogenates (top panel) and guinea-pig trachea and rat tissue membranes (bottom panel). Values are the mean±s.e.mean of three experiments performed in duplicate in separate membrane preparations.
Discussion
The major finding of this study was that both CP55,940 and anandamide inhibit ACh release from post-ganglionic cholinergic nerves in guinea-pig trachea suggesting the presence of pre-junctional cannabinoid receptors. It has been previously suggested that cyclic AMP may be intimately involved in regulating the biosynthesis, storage and exocytosis of ACh (Wilson et al., 1974). In guinea-pig trachea we have shown that cyclic AMP-elevating agents, such as β-adrenoceptor agonists, facilitate ACh release from cholinergic nerves, whereas activation of pre-junctional receptors negatively coupled to adenylyl cyclase, such as the EP3 prostanoid receptor, inhibit ACh release (Belvisi et al., 1996; Spicuzza et al., 1998). Therefore, our finding that activation of pre-junctional cannabinoid receptors, which are known to inhibit the intracellular accumulation of cyclic AMP, decreases ACh release is consistent with these observations.
The potent and selective CB1 receptor antagonist SR141716A (Rinaldi-Carmona et al., 1994; 1995; 1996), at a concentration 1000 fold greater than its Ki value at CB1 receptors, failed to reverse the inhibitory effect of cannabinoid agonists on [3H]-ACh release. This result suggests that the effects of the cannabinoid agonist are mediated through activation of a cannabinoid receptor(s) insensitive to SR141716A, a possible candidate being the pre-junctional CB2 receptor. So far the neuromodulatory role of cannabinoids has been attributed to activation of either CB1 and/or CB2 receptors. Activation of pre-junctional CB1 receptors decreases [3H]-ACh output and EFS-induced contractions in the guinea-pig myenteric plexus longitudinal muscle preparation (Pertwee et al., 1996) and noradrenaline release from sympathetic nerves in rat atria and vas deferens (Ishac et al., 1996). In mouse vas deferens activation of pre-junctional CB2-like receptors accounts for the inhibitory effect of cannabinoids on EFS-evoked contraction (Griffin et al., 1997). Furthermore, CP55,940 has also been shown to inhibit electrically evoked [14C]-ACh release from hippocampal slices. This effect is likely to be mediated by pre-junctional CB1 and CB2 receptors (Gifford et al., 1997). However, in this study, due to the lack of a commercially available selective CB2 receptor agonist or antagonists, we cannot further substantiate the involvement of CB2 receptors in our system.
The most intriguing finding in this study was that CP55,940, used at the same concentration that significantly inhibited [3H]-ACh output, failed to impact on EFS-evoked smooth muscle contraction. Generally, agents that inhibit EFS-induced [3H]-ACh release (e.g. endomorphins, opioids) from parasympathetic nerves innervating guinea-pig trachea, to a similar extent as cannabinoids, also inhibit contractile responses evoked by EFS in these tissues (Belvisi et al., 1993; Patel et al., 1999). There are several possible explanations for this discrepancy between the effect of cannabinoids on transmitter release compared with their effects on functional contractile responses evoked by EFS. Firstly, [3H]-ACh evoked by EFS may be from a non neuronal source. However, this is unlikely since we have previously shown that EFS-evoked [3H]-ACh is neuronal in origin as tetrodotoxin, a selective neuronal conductance blocker, completely inhibits this response in guinea-pig and human airways (Ward et al., 1993). Moreover, in epithelium-denuded preparations addition of exogenous ACh does not increase [3H]-ACh release indicating that the contraction of the tissue itself does not cause a non-neuronal release of labelled ACh (Ward et al., 1993). Secondly, the possibility exists that this paradox could equally reflect a modulatory action of cannabinoids on ACh from nerves that do not innervate airway smooth muscle, but synapse with other effector cells such as submucosal glands and/or tracheobronchial blood vessels. Another reason for this discrepancy could be that despite the inhibition of neurotransmission, cannabinoids act predominantly at a post-junctional level to modulate airway smooth muscle tone thereby masking the more subtle effect on transmitter output. However, CP55,940 failed to modulate EFS- and ACh-induced contraction and to relax airways precontracted with carbachol. It seems clear that, at least in guinea-pig airways, cannabinoids lack a bronchodilator activity in vitro confirming a previous study showing that anandamide has minimal direct effect on bronchomotor tone in guinea-pigs in vivo (Stengel et al., 1998). However, specific airway conductance of healthy and asthmatic subjects has been shown to increase (Tashkin et al., 1973) and reversal of methacholine and exercise-induced bronchospasm in asthmatics (Tashkin et al., 1977) have been demonstrated after inhaled marijuana. It appears that a direct effect of cannabinoids on airway smooth muscle tone is unlikely and that the mechanisms involved in bronchodilation in vivo are unknown and may possibly be due to a central effect of cannabinoids.
The inability of cannabinoids to directly modulate airway smooth muscle tone is consistent with the fact that no detectable specific binding of [3H]-CP55,940 was found in tracheal homogenates. Although Rice et al. (1997), have shown a modest expression of CB1 receptor mRNA in freshly isolated rat lung and alveolar Type II cells, the lung was devoid of any [3H]-CP55,940 specific binding (Lynn & Herkenham, 1994). In fact, specific binding was found to be restricted to components of the immune system (spleen, lymph nodes and Peyer' patches) and confined to B lymphocyte-enriched areas (Lynn & Herkenham, 1994). Our functional experiments point to a role for CB2 receptors in the modulation of ACh release from cholinergic nerves. However, these ligand binding experiments performed in tracheal homogenates did not support the presence of either CB1 or CB2 receptors in this preparation in agreement with data obtained in rat lung. One potential explanation is that the CB1 and CB2 receptor protein expression may be below the level of detection. Alternatively, CB1 receptors may be distributed in discrete regions within the airways such as parasympathetic nerve endings.
In conclusion we have shown that in guinea-pig trachea, cannabinoid agonists significantly inhibit ACh release from cholinergic nerve endings, and this might be due to activation of either a CB2 receptor or to another inhibitory pre-junctional receptor which is insensitive to SR141716A. This study confirms that cannabinoids play a neuromodulatory role in the peripheral nervous system and provides the first evidence that such modulation occurs in the airways. However, this relatively small inhibitory action does not impact on functional responses such as cholinergic contraction.
Acknowledgments
Priya Venkatesan was supported by a ROPA award from the Medical Research Council (U.K.). Deborah Clarke was funded both by the Medial Research Council (U.K.) and Aventis Pharma on an Industrial/Academic Medical Research Council Studentship.
Abbreviations
- ACh
Acetylcholine
- CB
Cannabinoid
- EFS
electrical field stimulation
- KHS
Krebs-Henseleit solution
- s.e.
standard error
References
- BELVISI M.G., PATEL H.J., TAKAHASHI T., BARNES P.J., GIEMBYCZ M.A. Paradoxical facilitation of acetylcholine release from parasympathetic nerves innervating guinea-pig trachea by isoprenaline. Br. J. Pharmacol. 1996;117:1413–1420. doi: 10.1111/j.1476-5381.1996.tb15300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELVISI M.G., WARD J.K., PATEL H.J., TADJKARIMI S., YACOUB M.H., BARNES P.J. μ-Opioids inhibit electrically-evoked ACh release in human and guinea-pig trachea. Am. Rev. Respir. Dis. 1993;147:A02. [Google Scholar]
- BOUABOULA M., RINALDI M., CARAYON P., CARILLON C., DELPECH B., SHIRE D., LE FUR G., CASELLAS P. Cannabinoid-receptor expression in human leucocytes. Eur. J. Biochem. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., DYSARZ F.A., JOHNSON M.R., MELVIN L.S., HOWLETT A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., MANSOURI J., MACKIE K., BLOND O., LAI Y., MA A.L, MITCHELL R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- GALIEGUE S., MARY S., MARCHAND J., DUSSOSSOY D., CARRIERE D., CARAYON P., BOUABOULA M., SHIRE D., LE FUR G., CASELLAS P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- GIFFORD A.N., ASHBY C.R.J. Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid antagonist, SR 141716A. J. Pharmacol. Exp. Ther. 1996;277:431–436. [PubMed] [Google Scholar]
- GIFFORD A.N., SAMIIAN L., GATLEY S.J., ASHBY C.R., Jr Examination of the effect of the cannabinoid receptor agonist, CP 55,940, on electrically evoked transmitter release from rat brain slices. Eur. J. Phamacol. 1997;324:187–192. doi: 10.1016/s0014-2999(97)00082-4. [DOI] [PubMed] [Google Scholar]
- GRAHAM J.D.P.The bronchodilator action of cannabinoids Cannabinoids as Therapeutic Agents 1986CRC Press, Boca Raton, FL; 147–158.Mechoulam, R. (Ed.) [Google Scholar]
- GRIFFIN G., FERNANDO R.S., ROSS R.A., MCKAY N.G., ASHFORD M.L.J., SHIRE D., HUFMAN J.W., YU S., LAINTON J.A.H., PERTWEE R.G. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur. J. Pharmacol. 1997;339:53–61. doi: 10.1016/s0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- HARTLEY J.P., NOGRADY S.G., SEATON A. Bronchodilator effect of delta1-tetrahydrocannabinol. Br. J. Clin. Pharmacol. 1978;5:523–525. doi: 10.1111/j.1365-2125.1978.tb01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERKENHAM M., LYNN A.B., JOHNSON M.R., MELVIN L.S., DE COSTA B.R., RICE K.C. Characterisation and localisation of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–567. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLARD C.J.S., MANNA M.J., GREENBERG R., DICAMELLI R.A., ROSS L.A., STEVENSON V., MURPHY R.G., PERTWEE R.G., CAMPBELL W.B. Synthesis and Characterization of Potent and Selective Agonists of the Neuronal Cannabinoid Receptor (CB1)1. J. Pharmacol. Exp. Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- HOWLETT A.C. Pharmacology of cannabinoid receptors. Ann. Rev. Pharmacol. Toxicol. 1995;35:607–635. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C., FLEMMING R.M. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol. Pharmacol. 1984;26:532–538. [PubMed] [Google Scholar]
- ISHAC E.J., JIANG L., LAKE K.D., VARGA K., ABOOD M.E., KUNOS G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYNN A.B., HERKENHAM M. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding sites in peripheral tissues of the rat: implications for receptor-mediated immune modulation by cannabinoids. J. Pharmacol. Exp. Ther. 1994;268:1612–1623. [PubMed] [Google Scholar]
- MATSUDA L.A. Molecular aspects of cannabinoid receptors. Crit. Rev. Neurobiol. 1997;11:143–166. doi: 10.1615/critrevneurobiol.v11.i2-3.30. [DOI] [PubMed] [Google Scholar]
- MATSUDA L.A., LOLAIT S.J., BROWNSTEIN M.J., YOUNG A.C., BONNER T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- MUNRO S., THOMAS K.L., ABU-SHAAR M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- PATEL H.J., BARNES P.J., TAKAHASHI T., TADJKARIMI S., YACOUB M.H., BELVISI M.G. Evidence for pre-junctional muscarinic autoreceptors in human and guinea-pig trachea. Am. J. Respir. Crit. Care Med. 1995;152:872–878. doi: 10.1164/ajrccm.152.3.7663798. [DOI] [PubMed] [Google Scholar]
- PATEL H.J., VENKATESAN P., HALFPENNY J., YACOUB M.H., FOX A., BARNES P.J., BELVISI M.G. Modulation of acetylcholine release from parasympathetic nerves innervating guinea-pig and human trachea by endomorphin-1 and 2. Eur. J. Pharmacol. 1999;374:21–24. doi: 10.1016/s0014-2999(99)00308-8. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., NASH J.E., COUTTS A.A. Further evidence for the presence of cannabinoid CB1 receptors in guinea-pig small intestine. Br J Pharmacol. 1996;118:2199–2205. doi: 10.1111/j.1476-5381.1996.tb15663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICE W., SHANNON J.M., BURTON F., FIEDELDEY D. Expression of a brain-type cannabinoid receptor (CB1) in alveolar Type II cells in the lung: regulation by hydrocortisone. Eur. J. Pharmacol. 1997;327:227–232. doi: 10.1016/s0014-2999(97)89665-3. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HÉAULME M., ALONSO R., SHIRE D., CONGY C., SOUBRIÉ P., BRELIÈRE J-C, LE FUR G. Biochemical and pharmacological characterisation of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HÉAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NÉLIAT G., CAPUT D., FERRARA P., SOUBRIÉ P., BRELIÈRE J.-C., LE FUR G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., CALANDRA B., SHIRE D., BOUABOULA M., OUSTRIC D., BARTH F., CASELLAS P., FERRARA P., LE FUR G. SR141716A, Characterization of two cloned human CB1 cannabinoid receptor isoforms. J. Biol. Chem. 1996;278:871–878. [PubMed] [Google Scholar]
- SPICUZZA L., GIEMBYCZ M.A., BARNES P.J., BELVISI M.G. Prostaglandin E2 suppression of acetylcholine release from parasympathetic nerves innervating guinea-pig trachea by interacting with prostanoid receptors of the EP3-subtype. Br. J. Pharmacol. 1998;123:1246–1252. doi: 10.1038/sj.bjp.0701720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENGEL P.W., RIPPY M.K., COCKERHAM S.L., DEVANE W.A., SILBAUGH S.A. Pulmonary actions of anandamide, an endogenous cannabinoid receptor agonist, in guinea-pigs. Eur. J. Pharmacol. 1998;355:57–66. doi: 10.1016/s0014-2999(98)00472-5. [DOI] [PubMed] [Google Scholar]
- TASHKIN D.P., REISS S., SHAPIRO B.J., CALVARESE B., OLSEN J.L., LODGE J.W. Bronchial effects of aerosolized delta 9-tetrahydrocannabinol in healthy and asthmatic subjects. Am. Rev. Resp. Dis. 1977;115:57–65. doi: 10.1164/arrd.1977.115.1.57. [DOI] [PubMed] [Google Scholar]
- TASHKIN D.P., SHAPIRO B.J., FRANK I.M. Acute pulmonary physiologic effect of smoked marijuana and oral Δ9-tetrahydrocannabinol in healthy young men. N. Eng. J. Med. 1973;289:336–341. doi: 10.1056/NEJM197308162890702. [DOI] [PubMed] [Google Scholar]
- WARD J.K., BELVISI M.G., FOX A.J., TADJKARIMI S., YACOUB M.H., BARNES P.J. Modulation of cholinergic neural bronchoconstriction by endogenous nitric oxide and vasoactive intestinal peptide in human airways in vitro. J. Clin. Invest. 1993;92:736–743. doi: 10.1172/JCI116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESSLER I., HELLWIG D., RACKÉ K. Epithelium-derived inhibition of [3H] acetylcholine release from the isolated trachea. Naunyn-Schmied. Arch. Pharmacol. 1990;342:387–393. doi: 10.1007/BF00169454. [DOI] [PubMed] [Google Scholar]
- WESSLER I., REINHEIMER T., BRUNN G., ANDERSON G.P., MACLAGAN J., RACKÉ K. β-Adrenoceptors mediate inhibition of [3H]-acetylcholine release from the isolated rat and guinea-pig trachea by isoprenaline. Br. J. Pharmacol. 1994;113:1221–1230. doi: 10.1111/j.1476-5381.1994.tb17128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS S.J., HARTLEY J.P., GRAHAM J.D. Bronchodilator effect of delta1-tetrahydrocannabinol administered by aerosol of asthmatic patients. Thorax. 1976;31:720–723. doi: 10.1136/thx.31.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON D.F. The effects of dibutyryl cyclic adenosine 3,5-monophosphate, theophylline and aminophylline on neuromuscular transmission in the rat. J. Pharmacol. Exp. Ther. 1974;188:447–452. [PubMed] [Google Scholar]


