Abstract
Halofantrine is a widely used antimalarial agent which has been associated with prolongation of the ‘QT interval' of the electrocardiogram (ECG), torsades de pointes and sudden death. Whilst QT prolongation is consistent with halofantrine-induced increases in cardiac ventricular action potential duration, the cellular mechanism for these observations has not been previously reported.
The delayed rectifier potassium channel, IKr, is a primary site of action of drugs causing QT prolongation and is encoded by the human-ether-a-go-go-related gene (HERG). We examined the effects of halofantrine on HERG potassium channels stably expressed in Chinese hamster ovary (CHO-K1) cells.
Halofantrine blocked HERG tail currents elicited on repolarization to −60 mV from +30 mV with an IC50 of 196.9 nM. The therapeutic plasma concentration range for halofantrine is 1.67–2.98 μM.
Channel inhibition by halofantrine exhibited time-, voltage- and use-dependence. Halofantrine did not alter the time course of channel activation or deactivation, but inactivation was accelerated and there was a 20 mV hyperpolarizing shift in the mid-activation potential of steady-state inactivation. Block was enhanced by pulses that render channels inactivated, and channel blockade increased with increasing duration of depolarizing pulses.
We conclude that HERG channel inhibition by halofantrine is the likely underlying cellular mechanism for QT prolongation. Our data suggest preferential binding of halofantrine to the open and inactivated channel states.
Keywords: Halofantrine, human-ether-a-go-go-related gene (HERG), QT prolongation, cardiac arrhythmia, Chinese hamster ovary (CHO-K1) cells
Introduction
Between 300 and 500 million people in the world are infected with malaria, and 1.5–2.7 million people die from it every year (Pellegrini & Ruff, 1999). Halofantrine is a 9-phenanthrenemethanol antimalarial agent which is particularly effective against drug-resistant falciparum and vivax malaria. It has been in wide clinical use since 1984 and clinical trials have involved at least 3 million patients in more than 30 countries (Kano et al., 1995). In 1993, Nosten (Nosten et al., 1993), reported cardiac side effects associated with halofantrine treatment, (including one sudden death), in a group of patients on the Thai-Burmese border. Since then there have been several reports of prolongation of the ‘QT interval' of the electrocardiogram (ECG), torsades de pointes and fatal cardiac arrests in patients taking halofantrine (Castot et al., 1993; Monlun et al., 1993; WHO, 1993; Monlun et al., 1995).
One mechanism by which drugs can prolong the QT interval is through blockade of one or more repolarizing outward potassium channel currents in ventricular myocytes (Rampe et al., 1998). Of these, the delayed-rectifier, IK, is a common site of action of drugs causing QT lengthening and torsades de pointes (Roden, 1993; Thomas,1994; Woosley, 1996). There are two types of IK, rapidly activating, ‘IKr', and slowly activating, ‘Iks' (Sanguinetti & Jurkiewicz, 1990). Drugs that cause torsades de pointes appear to selectively block IKr over IKs ( e.g. dofetilide (Carmeliet, 1992; Williams & Beatch, 1997); quinidine (Balser et al., 1991); E4031 and sotalol (Sanguinetti & Jurkiewicz, 1990; Wettwer et al., 1992); terfenadine (Ming & Nordin, 1995; Berul & Morad, 1995).
Recently, the gene encoding the major subunit of the IKr channel has been identified as the human ether-a-go-go-related gene, HERG, and it is strongly expressed in the heart (Sanguinetti et al., 1995; Curran et al., 1995). When transfected into heterologous cell lines, HERG expresses a potassium channel with properties similar to native IKr (Sanguinetti et al., 1995; Trudeau et al., 1995). The profile of HERG and native IKr currents during the ventricular action potential have been recorded in action potential clamp experiments (Zhou et al., 1998; Hancox et al., 1998). These studies show that both HERG and native IKr currents develop progressively during the action potential plateau to reach a maximum amplitude during phases 2 and 3 (plateau and terminal repolarization phase) of the action potential. The recorded profiles provide direct demonstrations of the crucial role of HERG/IKr in ventricular action potential repolarization, and suggest similar profiles between cloned and native channels.
The finding that HERG encodes IKr is of particular clinical relevance as mutations in HERG cause a major form of the congenital long QT syndrome (Curran et al., 1995) and blockade of HERG by drugs is the likely mechanism underlying the acquired long QT syndrome. Indeed recent studies from several groups including our own have shown potent inhibition of transfected HERG channels by Class III (QT prolonging) antiarrhythmic drugs such as dofetilide (Snyders & Chaudhary, 1996; Ficker et al., 1998) and amiodarone (Kiehn et al., 1999), as well as many non-cardiac drugs known to cause QT prolongation such as haloperidol (Suessbrich et al., 1997), thoridazine (Drolet et al., 1999), sertindole (Rampe et al., 1998), terfenadine, astemizole (Suessbrich et al., 1996), cisapride (Mohammad et al., 1997; Rampe et al., 1997; Walker et al., 1999a) and ketoconazole (Dumaine et al., 1998).
The cellular mechanism underlying QT prolongation and torsades de pointes associated with halofantrine has not been previously reported. We therefore investigated the effects of halofantrine on HERG channels stably expressed in Chinese hamster ovary (CHO-K1) cells.
Methods
These have previously been reported by us in detail, including a full biophysical characterization of the HERG channel (Walker et al., 1999b).
Molecular biology
The CHO-K1 cells (American Type Culture Collection, Bethesda, MD, U.S.A.) used in the following experiments were maintained in Dulbecco's modified Eagle's medium-F12 (DMEM-F12, Gibco, BRL, Gaithersburg, MD, U.S.A.), supplemented with 5% foetal calf serum. Eukaryotic expression of HERG was performed by directionally cloning the coding region of the HERG gene (gift from Dr G. Robertson, Department of Physiology, University of Wisconsin Medical School, Madison WI, U.S.A.) into the expression vector pRc/CMV (Invitrogen, San Diego, CA, U.S.A.), which also carries the G418 resistance gene. This construct was then transfected into CHO-K1 cells. Cell monolayers in 35 mm2 dishes were transfected using 9 μl Lipofectamine Reagent (Gibco, BRL) and 1 μg DNA. Stably transfected cells were then selected with 1000 μg ml−1 G418 (Boehringer, Mannheim). These were subcloned to isolate individual cell clones which expressed substantial HERG-related potassium current. Individual subclones were maintained long-term in tissue culture and used for the patch clamping experiments to be described below.
Electrophysiology
Currents were recorded at room temperature (20–22°C), using the whole-cell patch-clamp technique. CHO-K1 cells plated on coverslips were placed at the bottom of a 2 ml perfusion chamber mounted on the stage of an inverted phase-contrast microscope (Nikon Diaphot, Nikon Corporation, Tokyo, Japan). Electrodes were positioned using a micromanipulator (Narishige WB 90, Tokyo, Japan). A silver/silver-chloride reference electrode was placed directly in the perfusion chamber. Membrane potentials were adjusted by −15 mV to correct for the junction potential between high K+ pipette and external bath solution (calculated using commercial software, JpCalc, Barry, 1994). Cells were patched using micropipettes fabricated from thin-walled borosilicate glass (Vitrex Microhematocrit Tubes, Modulohm 1/S, Denmark) with a vertical pipette puller (Model 720, David Kopf Instruments, CA, U.S.A.). Tip diameters varied between 0.9 and 1.5 μm (pipette resistance =5.9+2.9 MΩ, n=24). Currents were amplified and filtered at 2 kHz with a 4-pole Bessel filter (−3dB point) using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA, U.S.A.). Currents were sampled at 0.25 kHz for the prolonged depolarization (40-s) protocol and 1 kHz for all other protocols. Stimulation protocols and data acquisition were carried out using a microcomputer (IBM Pentium), running commercial software and hardware (pClamp6.0/ Digidata 1200, Axon Instruments Inc. and Scientific Solutions Inc., Foster City, CA, U.S.A.) Whole-cell capacitance was determined from capacitative transient decay in current recordings following voltage steps of ±10 mV from the holding potential. The median CHO-K1 cell capacitance was 47.7 pF (range 11.2–209 pF). At least 80% series resistance compensation was achieved in all reported experiments (series resistance 13.3±6.4 MΩ before compensation). Leak subtraction was performed in some experiments by applying three hyperpolarizing pre-pulses before the test pulses (P/3 subtraction protocol).
Solutions and drugs
The intracellular pipette solution contains (mM): K gluconate 120, KCl 20, MgATP 1.5, EGTA 5, N-2-hydroxylethylpiperazine-N1-2-ethanesulphonic acid (HEPES) 10, adjusted to pH 7.3. The superfusion solution contains (mM): NaCl 130, KCl 4.8, MgCl2 1.2, NaH2PO4 1.2, HEPES 10, glucose 12.5, CaCl2 1.0, adjusted to pH 7.4. Halofantrine was made as stock solution dissolved in absolute ethanol (maximum final ethanol concentration =0.1% v v−1) and stored at −4°C. In preliminary experiments, we confirmed that ethanol at 0.1% v v−1 had no effect on the parameters under study.
Statistics
Current analyses were performed using the Clampfit module of pClamp software. Data are expressed as mean±s.e.mean for n experiments. Statistical analyses were performed using Prism 2.0 (Graphpad Software, San Diego, CA, U.S.A). Unpaired t-tests were used for comparisons of two groups and repeated measures ANOVA with post-hoc comparison of means using Dunnett's test for multiple group comparisons. A P value <0.05 was considered significant. The amplitude of the activating current was calculated as the difference between the initial current recorded just after the step depolarization and the maximum reached at the end of the step. Similarly, the amplitude of the tail current was recorded as the difference between the peak and steady state current after repolarization to −60 mV. The voltage-dependence of current activation was determined by fitting the values of the normalized tail currents to a Boltzmann function:
where I represents the relative tail current, V1/2, the voltage at which the current was half activated, Vt, the test potential and k, the slope factor. The relationship between drug concentration and current blockade was determined by fitting values to a Hill equation after normalization of post-drug current to control current:
where I represents the relative tail current, IC50 the concentration required for 50% channel blockade, D the drug concentration and n the Hill coefficient.
Results
CHO-K1 cells transfected with HERG possess a potassium channel with activation and rectification properties very similar to endogenous IKr and also to HERG-transfected cells previously described (e.g. Human embryonic kidney 293, Snyders & Chaudhary, 1996; Xenopus Oocytes, Suessbrich et al., 1997, CHO-K1, Walker et al.,1999b). When activated by step depolarizations, these channels yielded activating outward currents which decreased in amplitude at potentials positive to 0 mV due to rapid C-type inactivation (Smith et al., 1996; Spector et al., 1996). The steady state current-voltage (I-V), curve has a bell-shaped waveform which peaked at 0 mV. On repolarization, large tail currents were elicited as a result of fast relief from inactivation combined with slow deactivation (Sanguinetti et al., 1995). The tail current I-V curve has a typical sigmoid shape, that is, tail currents increased with voltage and then reached plateau for membrane potentials positive to +10 mV.
Halofantrine applied to the superfusate blocked both HERG activating and tail currents (Figure 1a,b). Analysis of peak tail currents, elicited on repolarization to −60 mV after 3.9-s prepulses from −80 mV to +30 mV, revealed concentration-dependent inhibition by halofantrine with an IC50 value of 196.9 nM (95% CI 151.8–245.8 nM) and Hill coefficient −0.96±0.06 (Figure 1c. n=5). The blocking effects of halofantrine was very slow to washout and only slightly reversible (21.1±6.3% recovery after 20 min, n=7).
Figure 1.
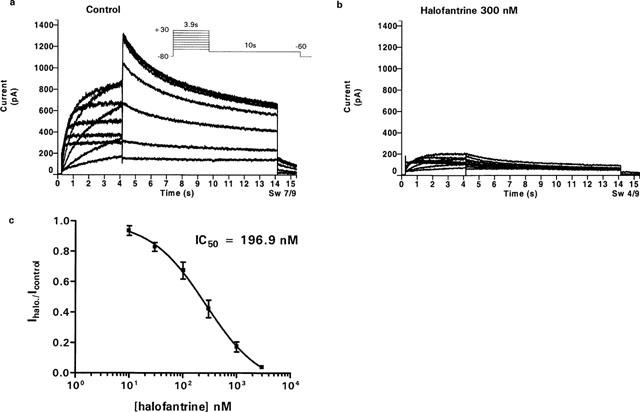
HERG channel blockade by halofantrine. Currents were elicited before, (a), and after 300 nM halofantrine, (b), using the potocol shown. Depolarizing steps 3.9-s in duration were applied from a holding potential of −80 mV, to test potentials from −50 to +30 mV in 10-mV increments. Tail currents were generated by repolarization to −60 mV for 10 s. (c) HERG tail currents recorded after repolarization to −60 mV from +30 mV in the presence of varying concentrations of halofantrine were normalized to the control amplitude (Ihalo./ Icontrol) and plotted against drug concentration to yield the dose response. A Hill equation fit yielded an IC50 of 196.9 nM (95% CI: 151.8–245.8 nM) and Hill slope of −0.96±0.06 (n=5).
Onset of block
Cells were held at −80 mV to keep channels in the closed state during wash-in of 300 nM halofantrine. The solution flow rate was 1 ml min−1 ensuring complete exchange of the bath within 30 s. After 3 min without stimulation, the first 40-s depolarization to 0 mV yielded only a small reduction in activating current (<12%, n=5). However, blockade progressively increased with subsequent pulses until steady state was reached after about 6 min (Figure 2).
Figure 2.
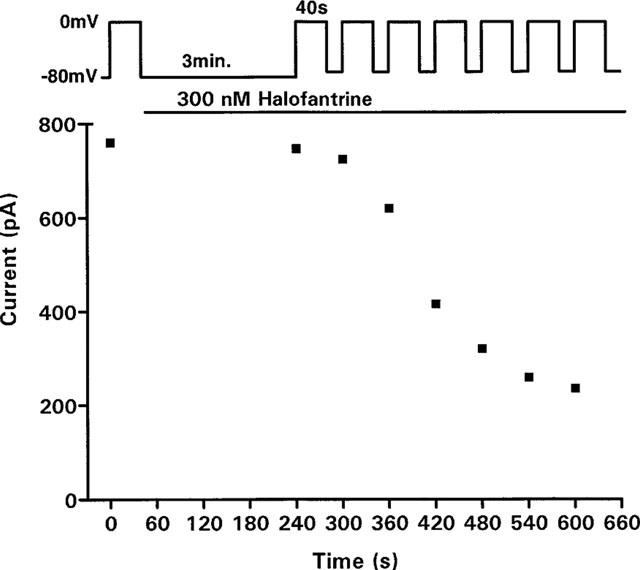
Pulse-dependent development of block with 300 nM halofantrine. During wash-in of halofantrine, the cell was held at −80 mV for 3 min without stimulation. A series of steps (40 s at 0 mV, 20 s at −60 mV) were then applied at 60-s intervals.
Voltage dependence
We studied the voltage-dependence of block by halofantrine by analysing the effects of the drug on the HERG current-voltage relationship. We first used a standard protocol in which activating currents were elicited by 3.9-s depolarizing pulses from a holding potential of −80 mV to potentials between −50 mV and +30 mV. Tail currents were elicited by repolarization to −60 mV (Figure 3a, top panel). Voltage steps were delivered at an interpulse interval of 20 s. Individual peak tail currents were normalized to the maximal control amplitude and fitted with a Boltzmann function (Figure 3a, middle panel). The voltage required for half-maximal activation (V1/2) was shifted from −17.5±0.4 mV under control conditions to −23.4±2.5 mV after 300 nM halofantrine (P<0.05, n=5). The reduction in tail current by 300 nM halofantrine increased significantly from 24±13% at −30 mV to 71±6% at +30 mV (P<0.05, n=5; Figure 3a, bottom panel). Halofantrine therefore exerted a voltage-dependent block on HERG. The degree of blockade was more pronounced with stronger depolarization from −30 mV to +30 mV as channels were rendered inactivated at positive potentials. This suggests interaction of halofantrine with the inactivated state. If so, removal of inactivation might eliminate the voltage-dependence of channel blockade. To investigate this, we used a protocol which allowed us to plot an instantaneous I-V relationship (Figure 3b, top panel). Cells were first depolarized to +30 mV for 500 ms to inactivate the channels. Following this, a short 40-ms repolarizing step to −80 mV removed inactivation without allowing sufficient time for deactivation (Smith et al., 1996). After this brief repolarizing step, a series of 1-s test pulses were delivered to potentials between −100 mV and +40 mV. Currents recorded at the test pulses were fitted by single exponential functions and the peak amplitude obtained was plotted against test potentials to yield instantaneous I-V curves. With inactivation removed, the I-V relationship was linear (Figure 3b, middle panel) and HERG channel blockade was significantly less (Figure 3b, bottom) when compared with the standard I-V protocol (Figure 3a, bottom panel). For example, at +20 mV, 300 nM halofantrine reduced HERG currents by 68.1±6.1% with the ‘standard' protocol but only 34.8±8.9% with the ‘instantaneous' protocol (P<0.05, n=5).
Figure 3.
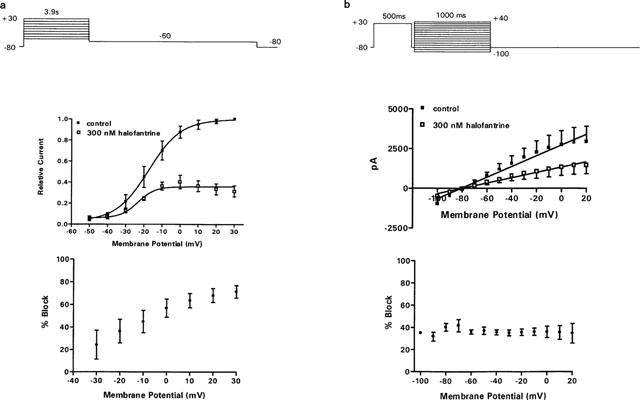
Voltage-dependent block of HERG by halofantrine. (a) Top: Standard current-voltage (I-V) protocol. Relative I-V relationships for peak tail currents in controls and halofantrine 300 nM were fitted with a Boltzmann function. V1/2 shifted from −17.5±0.4 mV in controls to −23.4±2.5 mV after halofantrine, P<0.05, n=5). Bottom: Fraction of control tail currents blocked at every given potential increased from 24±13% at −30 mV to 71±6% at +30 mV (P<0.05, n=5). (b) Top: Instantaneous I-V protocol yielding linear I-V relationship. Bottom: Fraction of control current blocked was significantly less compared with (a).
Use dependence
To analyse use-dependent block, HERG channels were activated by 500-ms depolarizing steps from −80 mV to +30 mV at frequencies of 0.1 and 0.5 Hz in the presence of 300 nM halofantrine. Immediately after each step, tail current amplitudes were measured following repolarization to −60 mV. Figure 4 indicates that the time course by which HERG channel blockade occurs is dependent upon the stimulation frequency (After 150 s, 16.6±3.5% block at 0.1 Hz versus 56.4±11.8% block at 0.5 Hz, P<0.05, n=6). Therefore, block by halofantrine exhibits positive use-dependence.
Figure 4.
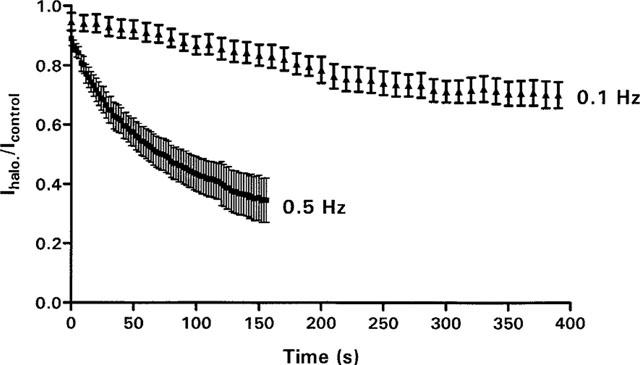
Use-dependent block of HERG by 300 nM halofantrine. Tail currents were recorded at −60 mV after a 500-ms pre-pulse from −80 mV to +30 mV at 0.1 Hz and 0.5 Hz. Before the first depolarization, cells were superfused for 2 min to allow complete exchange of the bath.
Effect of halofantrine during prolonged depolarization
Figure 5a shows the effect of halofantrine on HERG current amplitude during prolonged depolarizing steps. Halofantrine, 300 nM was applied for 3 min to cells held at −80 mV without stimulation. Current was then activated by 40-s depolarizations to +10 mV. The first pulse after this equilibration period showed no effect on the initial time course of current activation and the initial peak current was only reduced slightly (10±1.9% block, n=5). However, there is a time-dependent decline in current during the depolarizing step. A single exponential fit to this decaying current yielded a time constant of 16.85±2.07 s (n=5). Figure 5b shows the time course of the ‘difference' current obtained by subtraction of the activating currents (control–halofantrine). A single exponential fit to this ‘difference' current yielded a time constant of 14.42±2.31 s (n=5).
Figure 5.
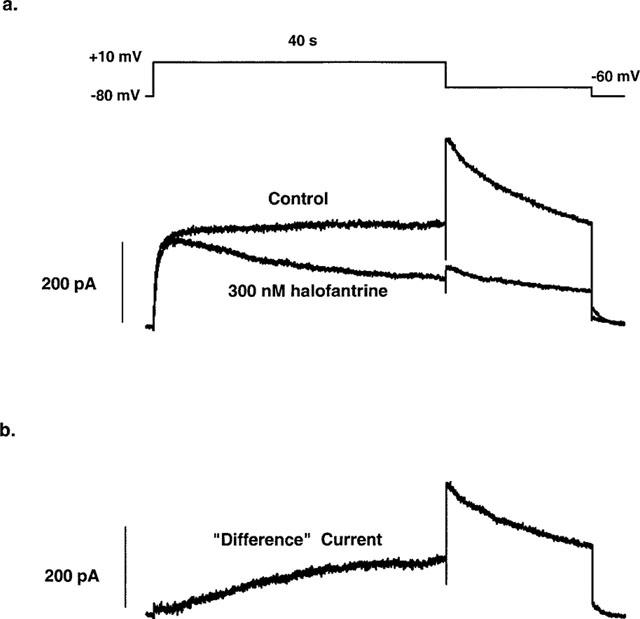
Effect of halofantrine on HERG current during prolonged depolarization. (a) Representative current traces (control and after 300 nM halofantrine) generated by a 40-s depolarizing step to +10 mV. (b) ‘Difference' current obtained by subtracting the current after halofantrine from the control current.
Development of channel blockade
To further examine the time course of onset of the time-dependent block on HERG, we used an envelope of tails protocol. Peak tail current amplitude was measured after a series of consecutive depolarizing steps to +30 mV, the duration of which was progressively increased in 80-ms increments, followed by a 5-s return to −60 mV to maximize the tail current (Figure 6). This protocol removed inactivation and allowed us to analyse the effects of halofantrine on the fully-activated current. Figure 6a shows that under control conditions, with depolarizing steps ⩾520 ms, peak tail currents reached steady state, that is, HERG channels were maximally activated. 300 nM halofantrine caused a small reduction (18±0.03%, n=7) in peak tail current after the first (shortest) depolarizing pulse duration of 40 ms (Figure 6c). Although HERG channel activation was maximal after depolarizations ⩾520 ms in duration, HERG blockade by 300 nM halofantrine continued to increase progressively with longer depolarizing pulse durations (20±7.6% block after a 120-ms step versus 59.5±4.5% after a 1880-ms step (P<0.05, n=7, Figure 6c). Therefore the fraction of open channels does not exclusively determine the degree of blockade. To measure the onset of the block, currents were normalized to their maximal amplitude and fitted with a sum of two exponentials (Figure 6d). With 300 nM halofantrine, we obtained values of 164.6±13.4 ms and 455.8±56.1 ms. The control currents could be well fitted with a single exponential function with a time constant of 140.6±3.6 ms.
Figure 6.
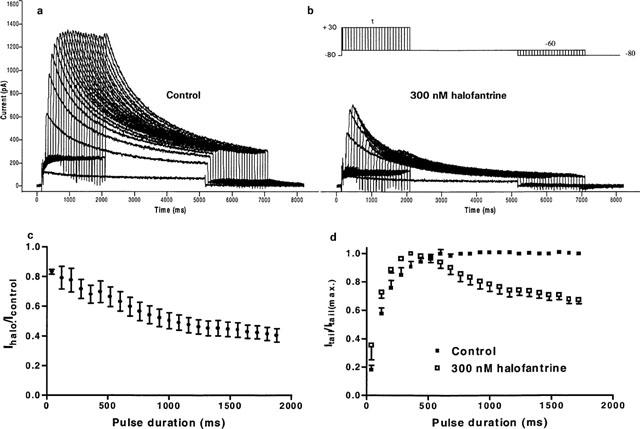
Envelope of tails protocol for HERG channels. Representative current traces from a cell under control conditions (a) and 300 nM halofantrine (b) using the protocol as shown. (c) Relative tail current (Ihalo/Icontrol) plotted as a function of depolarizing pulse duration (n=7). (d) Peak tail currents were normalized to their respective maximal values (n=7). The decaying phase of the normalized tail current plot shows the onset of block developing after channel opening without contamination from fast inactivation.
Effect on kinetics
The use-, voltage- and time-dependence of halofantrine block of HERG channel suggest that the drug binds to the inactivated and/or open states (Rampe et al., 1997; Toyama et al., 1997; Suessbrich et al., 1997). Therefore we studied the effects of 300 nM halofantrine on the time course of activation, deactivation and inactivation. Halofantrine 300 nM did not significantly alter the time course of channel activation or deactivation at the potentials studied (P=ns, n=4–8, Figure 7). We next studied channel inactivation using a ‘dual-pulse protocol'. Currents were recorded after a 500-ms depolarization to +30 mV from a holding potential of -80 mV, followed by a 40-ms hyperpolarizing step to −80 mV to relieve inactivation without allowing sufficient time for deactivation, then a second depolarization to potentials between −100 mV and +40 mV (Figure 8b). The time course for the onset of inactivation was determined by fitting a single exponential function to the tail current elicited after the second depolarizing step. In the presence of halofantrine 300 nM, there was a significant acceleration in the time course for onset of inactivation at most potentials. At −30 mV, τinactivation was 13.6±1.2 ms under control conditions versus 10.4±0.3 ms with 300 nM halofantrine (P<0.05, n=7, Figure 8c). Recovery from inactivation was determined by fitting a single exponential to the initial ‘hook' preceding slower deactivation of tail currents elicited by stepping to potentials between −120 mV and 0 mV following a 500-ms depolarization to +30 mV (Figure 8a). After halofantrine there was no significant change in the time constant of recovery from inactivation (Figure 8c). We next looked for changes in the steady state inactivation of the channels. We used a double pulse protocol with varying interpulse potential amplitude, first to inactivate the channels and then to remove inactivation (Figure 8d). The maximal amplitude of the current during the second pulse to +30 mV represents the number of channels inactivated during the interpulse . To avoid any contribution from the capacitance of the cells, current traces were fitted in a region where the capacitative artifact had completely resolved. The capacitative transient was fully resolved within 3 ms from the beginning of the pulse at all tested potentials. We then plotted the amplitude of the peak current against the interpulse potential to obtain the steady state inactivation (Figure 8e). The raw data were fitted to a Boltzmann distribution and used to calculate the asymptotic value at the plateau. Peak currents were then normalized to this asymptotic value and the data in Figure 8f plotted. Averaged mid-activation potential (V1/2) was shifted 20 mV in the hyperpolarizing direction by 300 nM halofantrine (V1/2=−62±0.4 mV in controls versus V1/2=−82±0.7 mV with 300 nM halofantrine, P<0.05, n=8.). The slope factor was essentially unchanged (−19±0.4 mV for controls versus −21±0.7 mV for halofantrine, P=ns, n=8).
Figure 7.
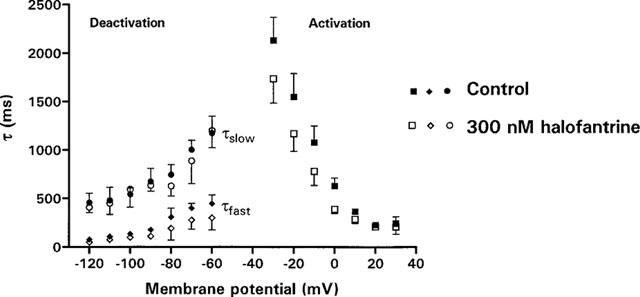
Effect of halofantrine on activation and deactivation time constants. Single exponential functions were fitted to activating currents generated as in Figure 1 to yield activation time constants. Time constants of deactivation were obtained by fitting a double exponential function to the deactivating tails currents as in Figure 1. τfast, τslow: fast and slow components of deactivation respectively.
Figure 8.
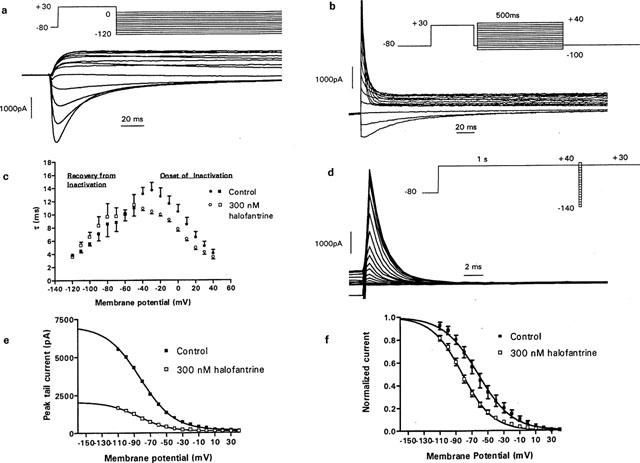
Effect of halofantrine on HERG channel inactivation. (a) Time course of recovery from inactivation was obtained by a single exponential fit to the initial ‘hook' elicited by the steps to between −120 mV and 0 mV using the protocol shown. (b) Onset of inactivation was measured by fitting a single exponential function to the tail current elicited after the second depolarization to potentials between −100 mV and +40 mV using the ‘dual-pulse' protocol as shown. (c) Time constants for recovery and onset of inactivation plotted against membrane potential under control conditions and 300 nM halofantrine (n=7). (d) Steady state inactivation was studied using a double-pulse protocol with varying interpulse repolarization levels as shown. (e) Peak current amplitude obtained from fits to current traces in panel (d) under control conditions and 300 nM halofantrine were plotted against membrane potential. A Boltzmann distribution fit to the data was used to calculate the asymptotic value. (f) Data from eight cells were normalized to the asymptotic value of their respective Boltzmann fit. A Boltzmann distribution fitted to the normalized currents gave averaged mid-activation potentials of −62±0.4 mV for control and −82±0.7 mV for 300 nM halofantrine.
Discussion
This is the first report of direct blockade of the HERG channel by halofantrine, an antimalarial agent in wide clinical use which has been well documented to cause QT prolongation, torsades de pointes and fatal cardiac arrests. We found that halofantrine caused potent inhibition of HERG currents with an IC50 value of 196.9 nM (95% CI 151.8–245.8 nM). The potency of halofantrine blockade is similar to the methanesulphonanilides which cause block of this channel at nanomolar concentrations (dofetilide, Snyders & Chaudhary, 1996; E4031, Walker et al., 1999b). Several other non-cardiac drugs which prolong QT have also been shown to block HERG or native IKr currents. (e.g. macrolide antibiotics (Daleau et al., 1995; West et al., 1998), haloperidol (Suessbrich et al., 1997), thoridazine (Drolet et al., 1999), sertindole (Rampe et al., 1998), terfenadine, astemizole (Suessbrich et al., 1996), cisapride (Mohammad et al., 1997; Rampe et al., 1997; Walker et al., 1999a), ketoconazole (Dumaine et al., 1998) and terodiline (Jones et al., 1998).
Therapeutic plasma halofantrine concentration in patients receiving halofantrine treatment is 1.67 to 2.98 μM (Veenendaal et al., 1991; Karbwang et al., 1991). QT interval has been shown to be significantly correlated with plasma level of the parent drug, halofantrine, but not with its major metabolite, N-desbutyl-halofantrine (Touze et al., 1996). In the report by Nosten et al. (1993), a high dose regimen of halofantrine (72 mg kg−1), induced consistent dose-dependent QT prolongation in all 61 patients treated. Our finding of a concentration-dependent block of HERG channels by halofantrine therefore correlates well with this observation.
In two cases of serious cardiotoxicity in which plasma concentration was measured at the time of death or syncope, it was approximately 1.6 μM in both patients (Nosten et al., 1993). Thus, halofantrine-induced cardiotoxicity can occur at therapeutic concentrations of the drug. The IC50 value for halofantrine block of HERG currents is an order of magnitude lower than this (196.9 nM) but halofantrine is very highly bound in whole blood (83% to serum proteins, 17% to erythrocytes; Cenni et al., 1995). It is also possible of course, that the potency of blockade of native human IKr by halofantrine may be different from that of HERG currents expressed in heterologous cell lines.
Mechanism of blockade
Channel inhibition by halofantrine exhibited time-, voltage- and use-dependence, suggesting that it preferentially binds to open and/or inactivated states of HERG channels (Rampe et al., 1997; Toyama et al., 1997; Suessbrich et al., 1997). We observed strong evidence for binding of halofantrine to the inactivated state. Thus, block was enhanced by protocols which render more channels inactivated and weakened by removal of inactivation. Halofantrine increased the rate of inactivation and caused a marked hyperpolarizing shift in the V1/2 of steady-state inactivation. This hyperpolarizing shift reflects a reduction in channel availability at a given potential, and effectively produces enhanced inward rectification (Smith et al., 1996).
Our data do not exclude the possibility that halofantrine also interacts with the open-channel state. Open-channel blockers often cause apparent acceleration of the activation time constant and/or deceleration of the deactivation time constant due to reopening of channels caused by drug unbinding (Yang et al., 1995; Wang et al., 1999). Although halofantrine did not alter activation or deactivation time constants in our studies, this is not conclusive evidence against open-state blockade, because drug-induced modification of deactivation kinetics depends critically on the relative rates of channel deactivation and drug/channel interaction. For example, dofetilide blocks HERG channels in the open state but does not change the deactivation time course because it is a slow-onset/slow-offset open-channel blocker (Snyders & Chaudhary, 1996). Our results with halofantrine suggest that binding and unbinding of halofantrine to the open-channel state occurs slowly for the following reasons: firstly, the envelope of tails test demonstrated that HERG channel blockade by halofantrine increased progressively with increasing depolarizing pulse duration even after steady state activation had been reached, and secondly, the rate of HERG channel deactivation was not affected by halofantrine, consistent with slow unbinding before channel closing.
A small amount of HERG channel blockade by halofantrine was observed on the first depolarizing pulse after drug wash-in at the holding potential. This block, therefore, does not require previous channel activation, which would suggest either an initial component of rapid open-channel block or some small contribution of closed channel block. Since taken collectively, our data do not appear to support rapid open-channel binding, this minor degree of block initially probably reflects a small level of binding to the closed channel state.
In conclusion, we have found that the widely used antimalarial agent, halofantrine, blocks HERG potassium channels. This blockade is the likely underlying cellular mechanism for QT prolongation and torsades de pointes seen during therapy with this drug. Our data suggest that blockade of HERG by halofantrine is predominantly due to high affinity binding to the open and inactivated channel states, with only a small contribution from lower affinity binding to closed channels.
Acknowledgments
This work was supported by research grants from the National Health and Medical Research Council of Australia, National Heart Foundation of Australia, St. Vincent's Clinic and The Clive and Vera Ramaciotti Foundation.
Abbreviations
- CHO-K1
Chinese hamster ovary
- HEPES
N-2-hydroxylethylpiperazine-N1-2-ethanesulphonic acid
- HERG
human ether-a-go-go-related-gene
- IC50
drug concentration producing 50% channel blockade
References
- BALSER J.R., BENNETT P.B., HONDEGHEM L.M, RODEN D.M. Suppression of time-dependent outward current in guinea pig ventricular myocytes. Actions of quinidine and amiodarone. Circ. Res. 1991;69:519–529. doi: 10.1161/01.res.69.2.519. [DOI] [PubMed] [Google Scholar]
- BARRY P.H. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilyer measurements and for correcting junction potential measurements. J. Neuroscience Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- BERUL C.I., MORAD M. Regulation of potassium channels by non-sedating antihistamines. Circulation. 1995;91:2220–2225. doi: 10.1161/01.cir.91.8.2220. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J. Pharmacol. Exp. Ther. 1992;262:809–817. [PubMed] [Google Scholar]
- CASTOT A., RAPOPORT P., LE COZ P. Prolonged QT interval with halofantrine. Lancet. 1993;341:1541. [PubMed] [Google Scholar]
- CENNI B., MEYER J., BRANDT R., BETSCHART B. The antimalarial drug halofantrine is bound mainly to low and high density lipoproteins in human serum. Br. J. Clin Pharmacol. 1995;39:519–526. doi: 10.1111/j.1365-2125.1995.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN M.E., SPLAWSKI I., TIMOTHY K.W., VINCENT G.M., GREEN E.D., KEATING M.T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- DALEAU P., LESSARD E., GROLEAU M.F., TURGEON J. Erythromycin blocks the rapid component of the delayed rectifier potassium current and lengthens repolarization of guinea pig ventricular myocytes. Circulation. 1995;91:3010–3016. doi: 10.1161/01.cir.91.12.3010. [DOI] [PubMed] [Google Scholar]
- DROLET B., VINCENT F., RAIL J., CHAHINE M., DESCHENES D., NADEAU S., KHALIFA M., HAMELIN B.A., TURGEON J. Thioridazine Lengthens Repolarization of Cardiac Ventricular Myocytes by Blockinh the Delayed Rectifier Potassium Current. JPET. 1999;288:1261–1268. [PubMed] [Google Scholar]
- DUMAINE R., ROY M-L., BROWN A.M. Blockade of HERG and Kv1.5 by ketoconazole. J. Pharmacol. Exp. Ther. 1998;286:727–735. [PubMed] [Google Scholar]
- FICKER E., JAROLIMEK W., KIEHN J., BAUMANN A., BROWN A.M. Molecular determinants of dofetilide block of HERG K+ channels. Circ. Res. 1998;82:386–395. doi: 10.1161/01.res.82.3.386. [DOI] [PubMed] [Google Scholar]
- HANCOX J.C., LEVI A.J., WITCHEL H.J. Time course and voltage dependence of expressed HERG current compared with native ‘rapid' delayed rectifier K current during the cardiac ventricular action potential. Pflügers Arch.- Eur. J. Physiol. 1998;436:843–853. doi: 10.1007/s004240050713. [DOI] [PubMed] [Google Scholar]
- JONES S.E., OGURA T., SHUBA L.M., MCDONALD T.F. Inhibition of the rapid component of the delayed-rectifier K+ current by therapeutic concentrations of the antispasmodic agent terodiline. Br. J. Pharmacol. 1998;125:1138–1143. doi: 10.1038/sj.bjp.0702173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANO S., HAYASHI A., KANDA T., SUZUKI M. Prolongation of the QT interval observed in a Japanese patient with vivax malaria following treatment with halofantrine. Kansenshogaku Zasshi. 1995;69:1408–1412. doi: 10.11150/kansenshogakuzasshi1970.69.1408. [DOI] [PubMed] [Google Scholar]
- KARBWANG J., MILTON K.A., NA BANGCHANG K., WARD S.A. , EDWARDS G., BUNNAG D. Pharmacokinetics of halofantrine in Thai patients with acute uncomplicated falciparum malaria. Br. J. Clin. Pharmacol. 1991;31:484–487. doi: 10.1111/j.1365-2125.1991.tb05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIEHN J., THOMAS D., KARLE C.A., SCHOLS W., KUBLER W. Inhibitory effects of the class III antiarrhythmic drug amiodarone on cloned HERG potassium channels. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:212–219. doi: 10.1007/pl00005344. [DOI] [PubMed] [Google Scholar]
- MING Z., NORDIN C. Terfenadine blocks time-dependent Ca2+, Na+, and K+ channels in guinea pig ventricular myocytes. J. Cardiovasc. Pharmacol. 1995;26:761–769. doi: 10.1097/00005344-199511000-00013. [DOI] [PubMed] [Google Scholar]
- MOHAMMAD S., ZHOU Z., GONG Q., JANUARY C.T. Blockade of the HERG human cardiac K+ channel by the gastrointestinal prokinetic agent cisapride. Am. J. Physiol. 1997;273:H2534–H2538. doi: 10.1152/ajpheart.1997.273.5.H2534. [DOI] [PubMed] [Google Scholar]
- MONLUN E., LE METAYER P., SZWANDT S., NEAU D., LONGY-BOURSIER M., HORTON J., LE BRAS M. Cardiac complications of halofantrine: a prospective study of 20 patients. Trans. R. Soc. Trop. Med. Hyg. 1995;89:430–433. doi: 10.1016/0035-9203(95)90041-1. [DOI] [PubMed] [Google Scholar]
- MONLUN E., PILLET O., COCHARD J.F., FAVAREL GARRIGUES J.C., LE BRAS M. Prolonged QT interval with halofantrine. Lancet. 1993;341:1541–1542. [PubMed] [Google Scholar]
- NOSTEN F., TER KUILE F.O., LUXEMBERGER C., WOODROW C., KYLE D.E., CHONGSUPHAJAISIDDHI T., WHITE N.J. Cardiac effects of antimalarial treatment with halofantrine. Lancet. 1993;341:1054–1056. doi: 10.1016/0140-6736(93)92412-m. [DOI] [PubMed] [Google Scholar]
- PELLEGRINI M., RUFF T.A. Malaria. The latest advice for travellers. Aust. Fam. Physician. 1999;28:683–688. [PubMed] [Google Scholar]
- RAMPE D., MURAWSKY M.K., GRAU J., LEWIS E.W. The antipsychotic agent sertindole is a high affinity antagonist of the human cardiac potassioum channel HERG. J. Pharmacol. Exp. Ther. 1998;286:788–793. [PubMed] [Google Scholar]
- RAMPE D., ROY M.L. , DENNIS A., BROWN A. A mechanism for the proarrhythmic effects of cisapride (Propulsid): high affinity blockade of the human cardiac potassium channel HERG. FEBS Lett. 1997;417:28–32. doi: 10.1016/s0014-5793(97)01249-0. [DOI] [PubMed] [Google Scholar]
- RODEN D.M. Current status of class III antiarrhythmic drug therapy. Am. J. Cardiol. 1993;72:44B–49B. doi: 10.1016/0002-9149(93)90040-j. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JIANG C., CURRAN M.E., KEATING M.T. A mechanistic link between inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P.L., BAUKROWITZ T., YELLEN G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- SNYDERS D.J., CHAUDHARY A. High affinity open channel block by dofetilide of HERG expressed in a human cell line. Mol. Pharmacol. 1996;49:949–955. [PubMed] [Google Scholar]
- SPECTOR P.S., CURRAN M.E., ZOU A., KEATING M.T., SANGUINETTI M.C. Fast inactivation causes rectification of the IKr channel. J. Gen. Physiol. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUESSBRICH H., SCHONHERR R., HEINEMANN S.H., ATTALI B., LANG F., BUSCH AE. The inhibitory effect of the antipsychotic drug haloperidol on HERG potassium channels expressed in Xenopus oocytes. Br. J. Pharmacol. 1997;120:968–974. doi: 10.1038/sj.bjp.0700989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUESSBRICH H., WALGEGGER S., LANG F., BUSCH A.E. Blockade of HERG channels expressed in Xenopus oocytes by the histamine receptor antagonists terfenadine and astemizole. FEBS Lett. 1996;385:77–80. doi: 10.1016/0014-5793(96)00355-9. [DOI] [PubMed] [Google Scholar]
- THOMAS S. H. Drugs, QT interval abnormalities and ventricular arrhythmias. Adverse Drug React. Toxicol. Rev. 1994;13:77–102. [PubMed] [Google Scholar]
- TOUZE J.E., BERNARD J., KEUNDJIAN A., IMBERT P., VIGUIER A., CHAUDET H., DOURY J.C. Electrocardiographic changes and halofantrine plasma level during acute falciparum malaria. Am. J. Trop. Med. Hyg. 1996;54:225–228. doi: 10.4269/ajtmh.1996.54.225. [DOI] [PubMed] [Google Scholar]
- TOYAMA J., KAMIYA K., CHENG J., LEE J.K., SUZUKI R., KODAMA I. Vesnarinone prolongs action potential duration without reverse frequency dependence in rabbit ventricular muscle by blocking the delayed rectifier K+ current. Circulation. 1997;96:3696–3703. doi: 10.1161/01.cir.96.10.3696. [DOI] [PubMed] [Google Scholar]
- TRUDEAU M.C., WARMKE J.W., GANETZKY B., ROBERTSON G.A. HERG, an inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- VEENENDAAL J.R., PARKINSON A.D., KERE N., RIECKMANN K.H., EDSTEIN M.D. Pharmacokinetics of halofantrine and n-desbutylhalofantrine in patients with falsiparum malaria following a multiple dose regimen of halofantrine. Eur. J. Clin. Pharmacol. 1991;41:161–164. doi: 10.1007/BF00265910. [DOI] [PubMed] [Google Scholar]
- WALKER B.D., SINGLETON C.B., BURSILL J.A., WYSE K.R., VALENZUELA S.M., QIU M.R., BREIT S.N., CAMPBELL T.J. Inhibition of the human ether-a-go-go-related gene (HERG) potassium channel by cisapride: affinity for open and inactivated states. Br. J. Pharmacol. 1999a;128:444–450. doi: 10.1038/sj.bjp.0702774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER B.D., VALENZUELA S.M., SINGLETON C.B., TIE H., BURSILL J.A., WYSE K.R., QIU M.R., BREIT S.N., CAMPBELL T.J. Inhibition of HERG channels stably expressed in a mammalian cell line by the antianginal agent perhexiline maleate. Br. J. Pharmacol. 1999b;127:243–251. doi: 10.1038/sj.bjp.0702502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H.-Z., SHI H., LIAO S-J., WANG Z. Inactivation gating determines nicotine blockade of human HERG channels. Am. J. Physiol. 1999;277:H1081–H1088. doi: 10.1152/ajpheart.1999.277.3.H1081. [DOI] [PubMed] [Google Scholar]
- WEST P.D., MARTIN D.K., BURSILL J.A., WYSE K.R., CAMPBELL T.J. Comparative Study of the Effects of Erythromycin and Roxithromycin on Action Potential Duration and Potassium Currents in Canine Purkinje Fibers and Rabbit Myocardium. J. Cardiovasc. Pharmacol. Ther. 1998;3:29–36. doi: 10.1177/107424849800300104. [DOI] [PubMed] [Google Scholar]
- WETTWER E., GRUNDKE M., RAVENS U. Differential effects of the new class III antiarrhythmic agents alomkalant, E-4031 and d-sotalol, and of quinidine on delayed rectifier currents in guinea pig ventricular myocytes. Cardiovasc. Res. 1992;26:1145–1152. doi: 10.1093/cvr/26.11.1145. [DOI] [PubMed] [Google Scholar]
- WHO Halofantrine: revised data sheet. WHO Drug Inf. 1993;7:66–67. [Google Scholar]
- WILLIAMS B.A., BEATCH G.N. Magnesium shifts voltage dependence of activation of delayed rectifier IK in guinea pig ventricular myocytes. Am. J. Physiol. 1997;272:H1292–H1301. doi: 10.1152/ajpheart.1997.272.3.H1292. [DOI] [PubMed] [Google Scholar]
- WOOSLEY R.L. Cardiac actions of antihistamines. Annu. Rev. Pharmacol. Toxic. 1996;36:233–252. doi: 10.1146/annurev.pa.36.040196.001313. [DOI] [PubMed] [Google Scholar]
- YANG T., PRAKASH C., RODEN D.M., SNYDERS D.J. Mechanism of block of a human cardiac potassium channel by terfenadine racemate and enantiomers. Br. J. Pharmacol. 1995;115:267–274. doi: 10.1111/j.1476-5381.1995.tb15873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU Z., GONG Q., YE B., FAN Z., MAKIELSKI J.C., ROBERTSON G.A., JANUARY C.T. Properties of HERG Channels Stably Expressed in HEK 293 Cells Studied at Physiological Temperature. Biophys. J. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


