Abstract
I2-imidazoline receptors are mainly expressed on glial cells in the rat brain. This study was designed to test the effect of treatment with the I2-imidazoline selective receptor ligand LSL 60101 [2-(2-benzofuranyl)imidazole] on the morphology of astrocytes in the neonate and adult rat brain, and to explore the putative neuroprotective effects of this glial response.
Short-term (3 days) or chronic (7–10 days) treatment with LSL 60101 (1 mg kg−1, i.p. every 12 h) enhanced the area covered by astroglial cells in sections of facial motor nucleus from neonate rats processed for glial fibrillary acidic protein (GFAP) immunostaining. Facial motoneurons surrounded by positive glial cell processes were frequently observed in sections of LSL 60101-treated rats. A similar glial response was observed in the parietal cortex of adult rats after chronic (10 days) treatment with LSL 60101 (10 mg kg−1, i.p. every 12 h).
Western-blot detection of the specific astroglial glutamate transporter GLT-1, indicated increased immunoreactivity after LSL 60101 treatment in the pons of neonate and in the parietoccipital cortex of adult rats.
In the facial motor nucleus of neonate rats, the glial response after LSL 60101 treatment was associated to a redistribution of the immunofluorescence of the basic fibroblast growth factor (FGF-2) from the perinuclear area of motoneurons to cover most of their cytoplasm, suggesting a translocation of this mitogenic and neurotrophic factor towards secretion pathways.
The neuroprotective potential of the above effects of LSL 60101 treatment was tested after neonatal axotomy of facial motor nucleus. Treatment with LSL 60101 (1 mg kg−1, i.p. every 12 h from day 0 to day 10 after birth) significantly reduced (38%) motoneuron death rate 7 days after facial nerve axotomy performed on day 3 after birth.
It is concluded that treatment with the I2-imidazoline selective receptor ligand LSL 60101 provokes morphological/biochemical changes in astroglia that are neuroprotective after neonatal axotomy.
Keywords: I2-imidazoline receptors, glial fibrillary acidic protein, GLT-1, basic fibroblast growth factor, neuronal death, neuroprotection, motoneurons
Introduction
Several studies have demonstrated that imidazol(ine)/guanidine compounds elicit central and peripheral effects through the interaction with non-adrenoceptor sites, the so-called imidazoline receptors (Bousquet, 1995; French, 1995; Regunathan & Reis, 1996; Molderings, 1997), which have been classified into two main types: I1- and I2-imidazoline receptors (Michel & Insel, 1989; Ernsberger, 1992). The I2-imidazoline receptors are widely distributed and in the brain are expressed on neurons, but mainly on glial cells (Regunathan et al., 1993; Olmos et al., 1994; Ruggiero et al., 1998; Sastre & García-Sevilla, 1993). The amine agmatine (decarboxylated arginine) has been proposed as an endogenous agonist at imidazoline receptors (Li et al., 1994) and to act as a neurotransmitter or neuromodulator for some members of this receptor family (Li et al., 1994; Regunathan & Reis, 1996; Reis & Regunathan, 1998; Otake et al., 1998). Astrocytes are also a site of synthesis and storage of agmatine (Regunathan et al., 1995).
The imidazoline drug idazoxan is neuroprotective in in vivo models of necrotic neuronal cell death, i.e. administration of this drug leads to reductions in hippocampal cell loss after global ischaemia (Gustafson et al., 1989; 1990) and in the infarction areas after focal ischaemia (Maiese et al., 1992). In addition, agmatine can exert neuroprotection in both in vitro and in vivo rodent models of neurotoxic ischaemia brain injuries (Gilad et al., 1996). Recently, various selective I2-imidazoline receptor ligands have been shown to induce neuroprotective effects on chronic opiate exposure, thus suggesting a neuroprotective role of the glial I2-imidazoline receptor (Boronat et al., 1998).
Death of motoneurons can be induced by axotomy when the motor axon is transected soon after birth (Schmalbruch, 1984). In this sense, the rodent periferal nerve transection is a well characterized model of neuronal cell death (Crews & Wigston, 1990; Sendtner et al, 1990; 1992; Pollin et al., 1991; Snider et al., 1992). The interruption of an axonally transported neurotrophic factor, delivered by target tissues, and the reduction in the ability of neurons to handle a calcium load, evoked by excitatory neurotransmitters like glutamate, have been proposed as the mechanisms involved in neuronal death after axotomy (Houenou et al., 1994). Thus, glutamate excitoxicity plays a key role in the motoneuron death that occurs following nerve injury during early postnatal development (Mentis et al., 1993; Greensmith et al., 1994; Casanovas et al., 1996). It has been demonstrated, both in vitro and in vivo, that neurons show greater resistance to the excitotoxic effects of glutamate when contacting with astrocytes (Mattson & Rychlik, 1990; Delaney et al., 1996; Beaman-Hall et al, 1998). In this sense, chronic treatment with the imidazole compound LSL 60101 [2-(2-benzofuranyl)imidazole)] (Figure 1), an agonist at I2-imidazoline receptors (Sánchez-Blázquez et al., 2000) displaying very low affinity for α2-adrenoceptors (Ki ratio for α2/I2-receptors=286) (Alemany et al., 1995), increases the immunoreactivity of glial fibrillary acidic protein (GFAP) (Alemany et al., 1995), the main constituent of intermediate filaments of astrocytes. These results suggested a functional involvement of glial I2-imidazoline receptors in inducing reactive astrocytosis (Olmos et al., 1994; Alemany et al., 1995). The main aim of this study was to characterize morphologically the glial response associated with the chronic treatment with I2-imidazoline ligands and to assess whether or not this effect on glial cells could be related to the neuroprotective effects of these drugs. Thus, the present work was designed (1) to study the effect of LSL 60101 treatment on the immunocytochemical distribution of GFAP in sections of facial motor nucleus from neonate rats and in the parietal cortex of adult rats; (2) to assess if these drug effects on astroglial cells are associated with changes in the expression of the glial glutamate transporter GLT-1 and that of basic fibroblast growth factor (FGF-2 or bFGF), a trophic factor that exhibits a diverse range of mitogenic and neurotrophic activities (Bikfalvi et al., 1997); and (3) to assess if the effects of LSL 60101 on the facial motor nucleus are neuroprotective during motoneuron death following neonatal axotomy of the rat facial nerve.
Figure 1.
Chemical structure of LSL 60101 [2-(2-benzofuranyl)imidazole].
Methods
Animals and treatments
Neonate (5–7 g) and adult male (250–300 g) Sprague-Dawley rats were used. In all animal manipulations, the local government specific rules and regulations to minimize animal suffering were taken into account (Generalitat de Catalunya DOGC 2073, 1995). Animals were administered i.p. every 12 h either with 0.9% pyrogen-free saline vehicle or with LSL 60101. Neonates received from day 0 to day 10 after birth (P0–P10) LSL 60101 at 1 mg kg−1 dissolved in saline solution. Adult rats were also treated i.p. at 10 mg kg−1 for 10 days. This dose of LSL 60101 has been shown to increase significantly GFAP immunoreactivity in the adult rat brain as assessed by Western blot techniques (Alemany et al., 1995).
Immunocytochemistry for GFAP and FGF-2
The presence of astrocytes was evidenced in sections processed for GFAP immunocytochemistry from the brainstem of neonate rats, and from the parietal cortex of adult rats after drug treatment. Animals were anaesthetized by hypothermia (neonates) or by chloral hydrate (450 mg kg−1) (adults) and killed by intracardiac perfusion with 0.9% saline solution followed by 4% paraformaldehyde in 0.1 M phosphate buffer pH 7.4. Then, the brainstem (neonates) or the whole brain (adults) were immersed in a fresh solution of fixative for a further 12 h at 4°C. Samples were then washed in phosphate buffered saline (PBS) and cryoprotected with 30% sucrose in PBS. Transversal cryosections (16 μm thick) were cut through the facial motor nucleus from neonate rats or at ca. −1.3 to −3.6 mm from the bregma in adult rats according to the atlas of Paxinos & Watson, 1986. Sections were mounted on silane-coated slides, air dried and treated in a microwave oven with 10 mM citrate buffer pH 6.0 for 5 min at 750 w. Sections were then permeabilized with 0.1% Triton X-100 in PBS for 30 min. Endogenous peroxidase was blocked by incubating the sections with 0.3% hydrogen peroxide for 30 min and non-specific binding was blocked by incubating the sections with 3% normal goat serum in PBS. Incubation with the primary polyclonal antibody to GFAP (DAKO A/S, Denmark) diluted 1:200 was done overnight at 4°C. After several washes in PBS, the sections were incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories, CA, U.S.A.) diluted 1:100 for 30 min and then with freshly prepared avidin–biotin–peroxidase complex (Vector) for 60 min. Peroxidase activity was visualized using 3,3′-diaminobenzidine solution (Sigma Chemical Co., U.S.A.) with nickel end-product amplification (Shu et al., 1988).
In sections of the facial motor nucleus from neonate rats, the optical weight of GFAP immunoreactivity around motoneurons was measured using PC-image 2.2 (Newcastle Technopole, U.K.). Measures are referred to as the percentage of GFAP-immunoreactive area respect to the total visual field.
In adult rats a more detailed morphometric analysis was performed in order to evaluate the effects of LSL 60101 treatment on the number of immunoreactive astrocytes and on the surface density of GFAP-immunoreactive profiles, as previously described for other treatments (Tranque et al., 1987). The parietal cortex (areas 1 and 2) was chosen for the study. Immunostained sections were observed through a microscope provided with a camera lucida attachment. For each brain and selected area, the number of immunoreactive cells was recorded with the aid of a test square grid delimiting 10,000 μm2 in the section. Only cells showing their nucleus in the same plane of focus in the section were recorded. Results are expressed as number of cells mm−2. In addition, a quantitative evaluation of the surface density of GFAP-immunoreactive cell bodies and cell processes (SvGFAP) was performed by using a stereologic grid, according to the point-counting method of Weibel (1979) in which the ratio of the surface immunoreactive profiles to the volume of a given structure (surface density, Sv) is calculated by the following formula: Sv=2I/L, where I is the number of points at which the immunoreactive profiles (cell bodies and cell processes) cross the test grid lines and L is the test line length in the tissue. The test grid used is based on the C16 grid of Weibel (1979) and has 10×10 lines of a total length of 2000 μm (L=2 mm). Magnification was calculated with a calibrated slide (100 lines mm−1, Reichert). For each animal, four sections and 30,000 μm2 per section were evaluated. All immunoreactive profiles, whether heavily or less heavily labelled, were considered for quantification. No significant variations in the volume of the parietal cortex, estimated according to the Cavalieri principle (Uylings et al., 1986) using consecutive serial sections were detected among the experimental groups studied. All the material was quantified without previous knowledge of the experimental group from which sections were obtained.
The expression of FGF-2 was studied in sections of brainstem of neonate rats after LSL 60101 treatment by using fluorescent procedures. Sections were permeabilized with 0.1% Triton X-100 in PBS for 30 min and non-specific binding was blocked by incubating with 3% normal goat serum. They were then processed for immunofluorescence histochemistry using a primary polyclonal antibody against FGF-2 (Santa Cruz Biotechnology Inc., CA, U.S.A.) diluted 1:50 in PBS and finally were incubated with fluorescein-conjugated anti-rabbit IgG (Vector) diluted 1:100. After mounting the sections on Vectashield (Vector) for reducing photobleaching, they were examined in a Zeiss LSM 310 confocal microscope (Zeiss, Oberkochen, Germany) using the 488 nm argon ion excitation source and appropriate selective barrier filters.
Omission of the primary antibody was used as a negative control in the immunocytochemical detection of GFAP and FGF-2; i.e. immunoreactivity was absent under these conditions.
Axotomized rats (see below) were not included in this study because the lesion itself could interfere with the evaluation of the effects of LSL 60101 treatment on GFAP and FGF-2 immunoreactivities. Axotomy itself induces a well-known microglial (Graeber et al., 1988) and astroglial (Casanovas et al., 1996) reaction in the facial motor nucleus and also this lesion modulates FGF-2 mRNA and protein expression (Huber et al., 1997).
Immunoblot detection and quantitation of the glial glutamate transporter GLT-1
The effect of chronic LSL 60101 treatment on the immuno-reactivity of the glial glutamate transporter GLT-1 was assessed in neonate and adult rats. Neonate and adult rats were treated with LSL 60101 as described before. Neonate rats were anaesthetized by hypothermia and their brainstems rapidly removed on ice. Adult rats were decapitated and their parietooccipital cortex were removed on ice.
The pons of neonate rats or 30–50 mg of brain parietooccipital cortex of adult rats were homogenized (30 s) with an Ultraturrax homogenizer in 33 volumes of (in mM): Tris HCl buffer 50, pH 7.5 containing MgCl2 2, EDTA 1, phenylmethylsulphonylfluoride (PMSF) 1, iodoacetamide 5, 10 μg ml−1 of trypsin-chymotrypsin inhibitor and 1 μg ml−1 of each leupeptin and aprotinin. The samples were centrifuged at 4°C and 800×g for 10 min. The resulting pellet was discarded, and the supernatant was centrifuged at 40,000×g for 10 min. The final pellet was resuspended in 500 μl of 40 mM Tris-HCl, 4% sodium dodecyl sulphate, pH 6.8 and incubated at 75°C for 5 min. Thereafter, electrophoresis loading buffer (62.5 mM Tris-HCl, 3% SDS, 20% glycerol, 0.1% 2-mercaptoethanol, 0.005% bromophenol blue, pH 6.8) was added to a final protein content of 0.1 mg ml−1 for samples from neonate rats or 0.02 mg ml−1 for samples from adult rats, as determined by the bycinchoninic acid method (Smith et al., 1985). The samples were then boiled and submitted to sodium dodecyl sulphate-polyacrilamide gel electrophoresis (SDS–PAGE) in a 10% Laemmli gel (1.5 mm thickness). Proteins were transferred to 0.45 micron nitrocellulose membranes (immunoblotting, Western blotting), and blocked at room temperature for 1 h with PBS containing 5% nonfat dry milk, 0.5% bovine serum albumin and 0.2% Tween 20 (blocking solution). The primary antibody, rabbit anti-rat GLT-1 (Calbiochem-Novabiochem Ltd., U.K.) was then added in fresh blocking solution (1:4000 dilution) and incubated for 14–16 h at 4°C. After two quick rinses with PBS, membranes were washed, for 10 min at room temperature, three times with PBS. The secondary antibody, horseradish peroxidase-linked sheep anti-mouse IgG (Amersham International plc. U.K.), was then added in fresh blocking solution (1:5000 dilution), and incubated for 2 h at room temperature. Immunoreactivity was detected with the Enhanced Chemiluminescence (ECL) Western Blot Detection system (Amersham) followed by exposure to Hyperfilm ECL film (Amersham). Omission of the primary antibody was used as a negative control; i.e. immunoreactivity was absent under this condition. Films were scanned in the image analyser Bio Image (Millipore, Ann Arbor MI, U.S.A.).
After scanning, standard curves were constructed using samples from saline-treated rats. In these curves, the total protein loaded in at least five wells (0.5 μg–2.5 μg for samples of pons from neonate rats and 0.1 μg–0.5 μg for samples of cerebral cortex from adult rats) was plotted against the integrated optical density (IOD). A linear relationship (correlation coefficient; r=0.98–0.99) between the amount of protein loaded in the gel and the IOD was found all over the range of protein content used. For the quantitation of GLT-1 protein immunoreactivity, samples from saline-treated and drug-treated rats were loaded in the same gel as well as the standard curve and, for every sample, a theoretical amount of protein loaded in the gel (Pt) was obtained by intrapolation of its IOD into the standard curve. The percentage of GLT-1 immunoreactivity of a given sample respect to the standard (saline-treated) samples was calculated as (Pt/Pr) × 100; where Pr is the real amount of protein loaded in the gel well. This quantitation procedure was repeated at least five times for each sample in different gels. The mean intra- and inter-assay coefficients of variation were 4 and 7%, respectively.
Neonatal axotomy and motoneuron counting
Other groups of neonate rats were also treated with saline vehicle or with LSL 60101 (1 mg kg−1) from day 0 to day 10 after birth, as described before. After 3 days of treatment (P3), rats were anaesthetized by hypothermia and subjected to unilateral facial nerve transection distal to its exit from the stylomastoid foramen, as previously described (Casanovas et al., 1996). In order to prevent spontaneous reinnervation, a ligature was applied to the proximal nerve stump and the maximum available length of the distal part of the nerve was removed. Neonates were left to recover for 6 h in individual cages after surgery before returning them to their dams. Treatment with saline vehicle or with LSL 60101 was maintained for 7 days after nerve transection. On day 10 after birth (P10), animals were deeply anaesthetized as described above and killed by intracardiac perfusion with 25 ml of physiological saline solution followed by 4% paraformaldehyde in 0.1 M phosphate buffer pH 7.4. Facial motoneurons were counted from serial Nissl stained paraffin sections (9 μm) of rat brainstems. Large motoneurons, with clearly defined nucleolus, were easily distinguishable from non neuronal cells and were counted from every five sections (average of 18 sections counted) with the aid of a camera lucida.
The effect of treatment with LSL 60101 on motoneuron death after axotomy was not studied in adult rats because motoneurons become progressively resistant to death during their developmental maturation; therefore, the highest rates of motoneuron death after axotomy are obtained when the motor axon is transected soon after birth (see Schmalbruch, 1984).
Statistics
Data were analysed by the Student's unpaired 2-tailed t-test. A P value less than 0.05 was taken as a significant difference between the groups. The experimental data are presented throughout as mean±s.e.mean.
Drugs and reagents
LSL 60101 [2-(2-benzofuranyl)imidazole] HCl was synthesized by Dr F. Pla at Ipsen Pharma S.A., Barcelona (Spain). Other reagents were obtained from Sigma Chemical Co., U.S.A.
Results
Effects of LSL 60101 on astroglia
The effect of short-term (3 days) or chronic (7–10 days) treatment with LSL 60101 on the distribution of GFAP-immunoreactivity was studied in sections of brainstem from neonate rats containing the facial motor nucleus. GFAP-immunoreactive astrocytes showing a typical stellate morphology with several processes radiating from the cell body were observed in the facial motor nucleus of neonate rats (P3–P10) (Figure 2A). The GFAP-immunoreactive area significantly increased with age in the facial motor nucleus (Figure 3). Administration of LSL 60101 (1 mg kg−1, i.p. every 12 h) to newborn rats (P0) enhanced the area covered by astroglial cells in the facial motor nucleus, as denoted by the increment in GFAP-immunoreactive area after 3 (P3), 7 (P7) and 10 days (P10) of treatment (Figures 2B and 3). Hypertrophic astrocytes were frequently observed in sections containing the facial motor nucleus of rats treated with LSL 60101 in comparison with that of saline-treated rats (Figure 2A,B). In rats treated with LSL 60101 some perykaria of facial motoneurons appeared completely surrounded by glial processes (Figure 2B, inset).
Figure 2.

Immunocytochemistry of rat facial motor nucleus for GFAP performed on day 3 after birth in rats treated with saline vehicle (A) or LSL 60101 [2-(2-benzofuranyl)imidazole] (1 mg kg−1, i.p. every 12 h) from day 0 (B). Inset: Detail of a motoneuron of the facial motor nucleus surrounded by positive astroglial cells processes. Scale bars=25 μm.
Figure 3.
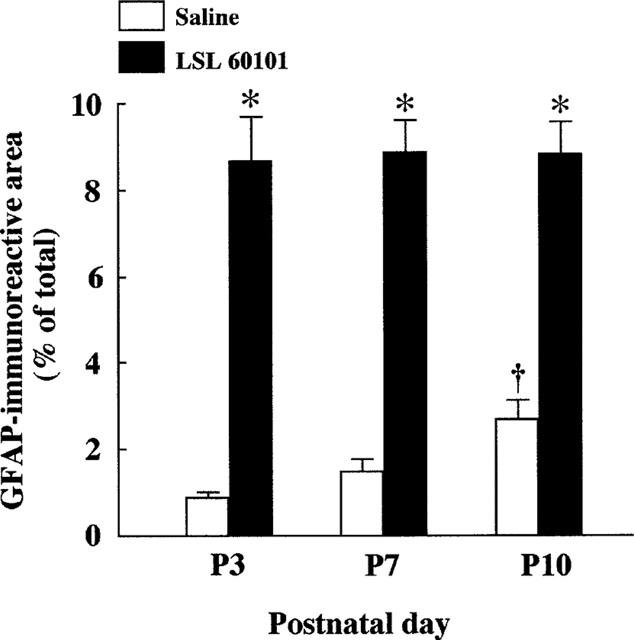
Percentage of area covered by GFAP-immunoreactive cell bodies and cell processes in the rat facial motor nucleus from rats treated either with saline vehicle or LSL 60101 [2-(2-benzofuranyl) imidazole] (1 mg kg−1, i.p. every 12 h) from day 0 to days 3 (P3), seven (P7) or 10 (P10) after birth. Columns represent mean±s.e.mean of four animals for each treatment. *P<0.001 respect to the corresponding saline-treated rats and †P<0.05 respect to P3.
Next, the question was addressed as to whether the chronic treatment with LSL 60101 could also affect astroglial cells in the adult rat brain. The parietal cortex (areas 1 and 2) was chosen for this study. Morphometric analysis of sections processed for GFAP-immunoreactivity revealed a significant increase in the mean number of immunoreactive cells (Table 1) after chronic (10 days) LSL 60101 (10 mg kg−1, i.p. every 12 h) treatment in comparison with saline-treated rats (Table 1 and Figure 4). Examination of the sections revealed the presence of hypertrophic astrocytes (Figure 4B, inset), as previously found in the facial motor nucleus after LSL 60101 treatment. Together, these effects of chronic LSL 60101 treatment resulted in increased surface density of GFAP (SvGFAP) immunoreactive profiles in the parietal cortices of drug-treated respect to saline-treated rats (Table 1 and Figure 4).
Table 1.
Effect of chronic treatment with LSL 60101 on the number and surface density of GFAP-immunoreactive cells in the parietal cortex of adult rats
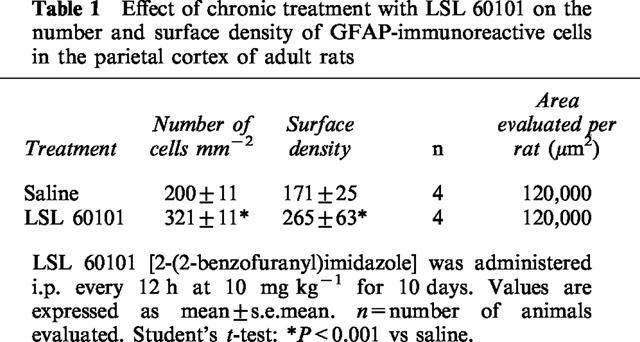
Figure 4.
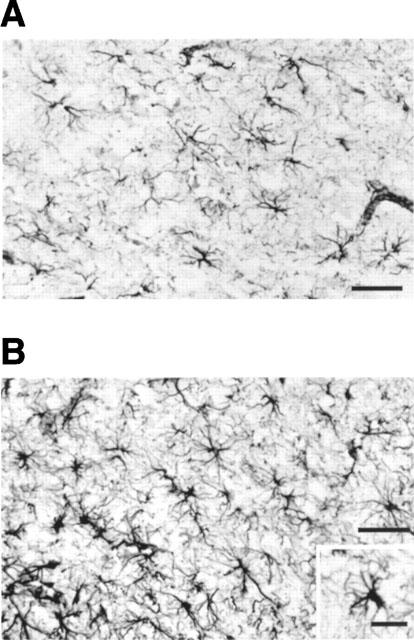
Immunocytochemical localization of GFAP in the parietal cortex of adult rats treated with saline vehicle (A) or with LSL 60101 [2-(2-benzofuranyl)imidazole] (10 mg kg−1, i.p., every 12 h for 10 days) (B). Inset: Detail of one astrocyte showing hypertrophic morphology. Scale bars=35 μm.
The effect of chronic LSL 60101 on astroglial cells was further assessed by determining the expression levels of the astroglial specific glutamate transporter protein GLT-1 (Rothstein et al., 1994). A typical broad GLT-1 band (∼73 kDa) was detected by Western-blot techniques in membrane preparations from the pons of 10-day-old neonate rats and from the parietoocciptal cortex of adult rats (Figure 5). Chronic treatment i.p. every 12 h for 10 days to neonate rats (P0–P10) with LSL 60101 at 1 mg kg−1 or to adult rats at 10 mg kg−1, significantly increased GLT-1 immunoreactivity (Table 2 and Figure 5).
Figure 5.
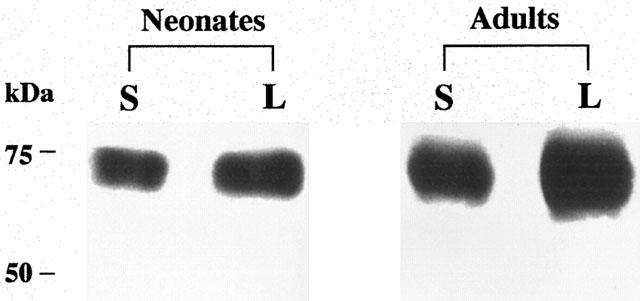
Representative immunoreactive bands using antisera against GLT-1 proteins (Mr=73 kDa) in the pons of neonate rats and in the parietoccipital cortex of adult rats after saline (S) or chronic treatment with LSL 60101 [2-(2-benzofuranyl)imidazole] (L). The drug was administered i.p. ever 12 h at 1 mg kg−1 from P0 to P10 to neonate rats and at 10 mg kg−1 to adult rats for 10 days. Samples were subjected to SDS–PAGE, transferred to nitrocellulose membranes (immunoblotting), incubated with the specific primary and secondary antibodies and visualized by the ECL method. The apparent molecular mass of GLT-1 protein was determined by calibrating the blots with prestained molecular weight markers as shown on the left-hand side. All samples were run in the same gel. The amount of total protein loaded per well was as follows (in μg): Neonates: 1.94 (S); 2.22 (L) and adults: 0.37 (S); 0.44 (L). See Table 2 for changes in mean percentage values of GLT-1 immunoreactivity after chronic treatment with LSL 60101 and other details.
Table 2.
Effect of chronic treatment with LSL 60101 on glial glutamate transporter GLT-1 immunoreactivity in the pons of neonate rats and in the parietooccipital cortex of adult rats
Effects of LSL 60101 on FGF-2 immunofluorescence
Differentiation and proliferation of astroglia have been found to be affected by a variety of growth factors, among them, the basic fibroblast growth factor (FGF-2) is well known as a mediator in the transition of astrocytes from a quiescent to a reactive state (Perraud et al., 1988; Liu & Chen, 1994). Thus the potential effects of LSL 60101 treatment on FGF-2 abundance was studied on sections of brainstem from neonate rats containing the facial motor nucleus.
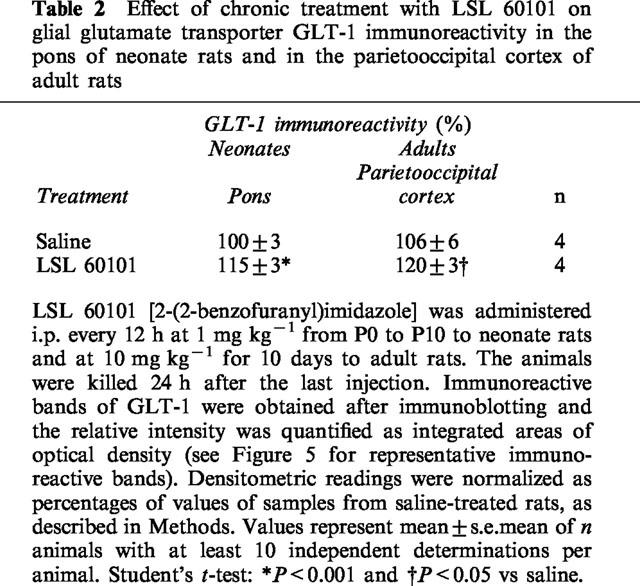
FGF-2 immunofluorescence was mainly observed within the cytoplasm of motoneurons of the facial nerve nucleus (Figure 6). In saline-treated rats, strong FGF-2 immunofluorescence was observed around the perinuclear area and a weaker staining in the rest of cytoplasm, suggesting a possible association with the cisterns of endoplasmic reticulum, as previously described in hippocampal neurons (Gomez-Pinilla et al., 1992) (Figure 6A). Treatment of newborn rats (P0) with LSL 60101 (1 mg kg−1, every 12 h) for 3 days enhanced the immunofluorescence of FGF-2 in the cytoplasm of motoneurons and changed its distribution pattern from the perinuclear area to cover most of their cytoplasm (Figure 6B). Treatments with LSL 60101 for longer periods (P0–P7 or P0–P10) also resulted in increased levels of FGF-2 immunofluorescence (not shown).
Figure 6.
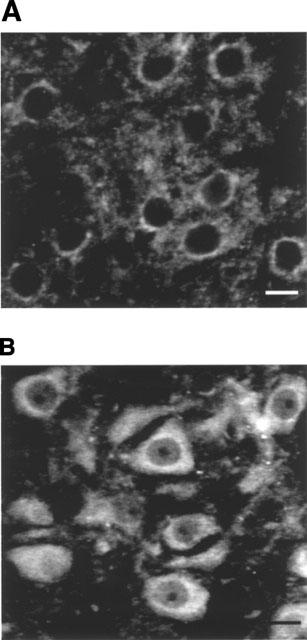
Basic fibroblast growth factor (FGF-2) immunofluorescence in sections containing the facial motor nucleus from rats on day 3 after birth and treated either with saline vehicle (A) or with LSL 60101 [2-(2-benzofuranyl)imidazole] (1 mg kg−1, i.p. every 12 h) from day 0 (B). Note the translocation of FGF-2 immunofluorescence from the perinuclear area in saline-treated rats to cover most of the cytoplasm after LSL 60101 treatment. Scale bars=100 μm.
Effects of LSL 60101 on motoneuron death
The above morphological/biochemical changes in the facial motor nucleus after LSL 60101 treatment suggested a potential neuroprotective role for this drug. This question was addressed by testing the effects of LSL 60101 treatment after neonatal axotomy of facial nerve, a model of apoptotic neuronal cell death (Casanovas et al., 1996).
Facial nerve transection performed on 3-day-old (P3) rats induced an extensive rate of motoneuron death in the facial motor nucleus as assessed 7 days later (P10). Motoneurons count showed that, at this time, 72% of the neurons were lost in saline-treated rats (Table 3, Figures 7 and 8). Another group of rats was treated with LSL 60101 (1 mg kg−1, i.p. every 12 h) 3 days before the axotomy (P0–P3) in accordance with the hypothesis that increased FGF-2 levels and reactive astrocytes surrounding motoneurons, present at the moment of the injury (see above results), may rescue motoneurons from cell death. This drug treatment was maintained up to 7 days after nerve transection (P10). LSL 60101 treatment significantly reduced the percentage of motoneuron loss evaluated on P10 (Table 3; Figures 7 and 8); thus, motoneuron death rate was reduced by 38% (P<0.01) respect to saline-treated rats.
Table 3.
Prevention by LSL 60101 of motoneuron cell death in the rat facial nucleus after neonatal axotomy
Figure 7.
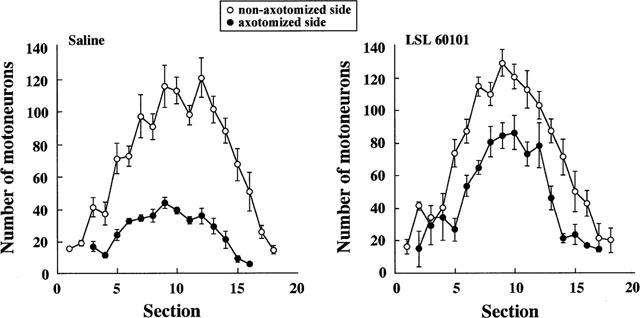
Number of motoneurons along serially sectioned rat facial motor nucleus after unilateral facial nerve transection performed on day 3 after birth and evaluated at day 10. Animals were treated either with saline vehicle (n=5) or with LSL 60101 [2-(2-benzofuranyl)imidazole] (1 mg kg−1, i.p. every 12 h) (n=7) from day 0. Mean±s.e.mean are shown.
Figure 8.
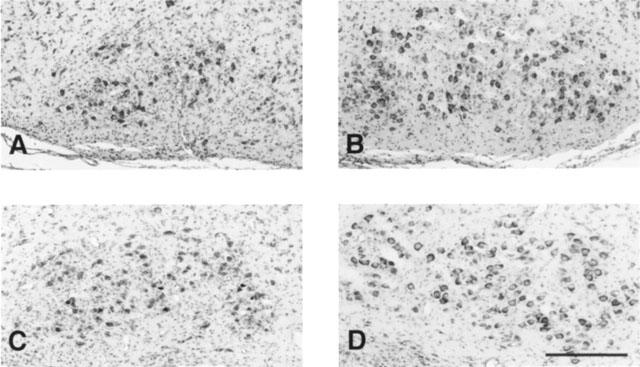
Representative Nissl-stained sections showing stained motoneurons of the facial motor nucleus after unilateral facial nerve transection performed on day 3 after birth and evaluated at day 10. Photomicrographs A and C correspond to the axotomized nucleus and B and D to the non-axotomized nucleus. Neonatal rats were treated either with saline vehicle (A, B) or with LSL 60101 [2-(2-benzofuranyl)imidazole] (1 mg kg−1, i.p. every 12 h) from day 0 (C,D). Scale bars=300 μm. See Table 3 and Figure 7 for other details.
Discussion
Previous in vitro and in vivo studies have implicated I2-imidazoline receptors on the regulation of the expression of GFAP. Thus, exposure of rat cortical astrocytes in culture to the I2-imidazoline ligand idazoxan, resulted in a marked concentration-dependent increase in mRNA levels for GFAP (Regunathan et al., 1993), and chronic treatment with various I2-ligands including LSL 60101, resulted in a marked increase (45%) of GFAP immunoreactivity determined by Western blotting in the cerebral cortex of adult rats (Olmos et al., 1994; Alemany et al., 1995). Because this effect was not achieved after chronic treatment with other structurally related imidazoline drugs displaying low affinity for I2-imidazoline receptors, the increment on GFAP-immunoreactivity appeared to be related to the stimulation of these receptors and not due to nonspecific toxic effects (Olmos et al., 1994). Moreover, exposure for 24 h of cultured cerebellar granule cells to concentrations up to 500 micromolar of LSL 60101 did not affect mitochondrial function (Olmos et al., 1999); thus indicating the lack of toxicity of this compound. In the present study, LSL 60101 treatment was associated in the adult and neonate rat brains with astrocytic hypertrophia, resulting in increased GFAP immunoreactivity. Although formal cell count in the cerebral cortex of adult LSL 60101-treated rats revealed increased number of astrocytes as compared with the saline-treated group (Table 1), it cannot be completely discarded that some grey matter astrocytes become GFAP-positive after LSL 60101 treatment or that because their cytoplasmic hypertrophy they are better recognized by the anti-GFAP antibody (see also Norton et al., 1992; Norenberg, 1996).
Overexpression of GFAP has been regarded for years as the most common indicator of the reactive astrocytosis that occurs in response to the tissue injury (O'Callaghan & Jensen, 1992; O'Callaghan, 1994), and still there is controversy as to whether this response is beneficial or deleterious to repair. In this sense, both axotomy and I2-imidazoline drug treatment, induce marked gliosis and increased expression of GFAP (Ginsberg et al., 1995; 1996; Olmos et al., 1994; Alemany et al., 1995; present results), however, axotomy is not associated with increased levels of the glial glutamate transporter GLT-1, and rather decreased expression of this protein and other specific glial glutamate transporters is found (Ginsberg et al., 1995; 1996). Also in this sense, GFAP up-regulation after I2-imidazoline drug treatment is associated to a parallel increase in the density of glial I2-imidazoline receptors (Olmos et al., 1994); however, increases in GFAP in the rat hippocampus following global forebrain ischaemia are not associated with changes in I2-imidazoline receptors density (Conway et al., 1998). These findings are in line with previous studies indicating that although increased GFAP expression is a common feature of reactive astrocytes, they may differ in the expression of other proteins with quite different activities in the context of tissue response against injury and, in this way, some reactive astrocytes may promote, while others may inhibit the regenerative process (Malhotra & Shnitka, 1994; Norenberg, 1996; Ridet et al., 1997).
Although the mechanisms by which resting astrocytes become reactive are not fully understood, several growth factors are clearly implicated. Among them, the FGF-2, a heparin-binding multifunctional growth factor has been shown to promote morphological changes in cultured astrocytes leading to morphological maturation and increased expression of GFAP (Perraud et al., 1988). The FGF-2 also displays mitogenic activities for astrocytes (Pettmann et al., 1985) and oligodendrocytes (Eccleston & Silberberg, 1985). In the rat facial motor nucleus, the FGF-2 is expressed mainly in motoneurons of the neonate (Weise et al., 1993) and adult brain (Gomez-Pinilla et al., 1992). In the present study, short-term (3 days) treatment of neonate rats with LSL 60101 increased the expression of FGF-2 in motoneurons of the facial motor nucleus, as denoted by the increment in its specific immunofluorescence. This effect was associated with a redistribution from the perinuclear area to cover most of the cytoplasm; this redistribution suggests a possible translocation of FGF-2 towards secretion pathways. Interestingly, such as translocation has also been described in normal to reactive astrocytes when these glial cells provide trophic support to injured neurons (Gomez-Pinilla et al., 1992). Thus, it is possible that after LSL 60101 treatment, neurons release higher amounts of FGF-2 which may have paracrine effects on neighbouring astroglial cells, leading to morphological changes, resulting in increased GFAP immunoreactivity. Alternatively, it cannot be discarded a direct effect of LSL 60101 on glial I2-imidazoline receptors stimulating GFAP expression (see Regunathan et al., 1993) and production of glial-derived growth factor(s) (Gomez-Pinilla et al., 1992; Oderfeld-Nowak et al., 1992; Yan et al., 1995) that, in turn, may act on motoneurons stimulating secretion of FGF-2.
In addition to its effects on glial cells, FGF-2 exhibits potent neurotrophic activities (Logan & Berry, 1993) and its administration to the proximal stump of transected peripheral nerves protects axotomized motoneurons (Grothe & Unisicker, 1992). It has been reported that a few days after axotomy there is a complete down-regulation of the FGF-2 protein in lesioned motoneurons and that about 2 weeks are needed for the reexpression of the FGF-2 protein (Grothe & Unsicker, 1992; Huber et al., 1997); thus suggesting a delay in the response of motoneurons to this peripheral nerve injury. Since in the present study axotomy was performed 3 days after LSL 60101 treatment, increased FGF-2 levels are already present at the moment in which axotomy occurs and this may contribute to the neuroprotective effects of LSL 60101 treatment after axotomy of the facial nerve. In this model of neural death, it seems that glutamate-mediated neurotoxicity is involved as the mechanism determining motoneuron death (Lowrie & Vrbova, 1992; Casanovas et al., 1996). In this sense, the FGF-2 has been demonstrated to protect against the excitotoxic effects of glutamate through distinct mechanisms including suppression of the expression of a 71 kDa N-methyl-D-aspartate (NMDA) receptor protein (Mattson et al., 1993), induction of the expression of the 28 kDa calcium-binding protein calbindin (Collazo et al., 1992), increase in antioxidant enzyme activities (Mattson et al., 1995) and down-regulation of the c-Jun transcription (Blottner & Herdegen, 1998). Interestingly, it has been demonstrated that some survival–promoting effects of FGF-2 are dependent on glial cell proliferation, i.e. the trophic effect of FGF-2 is totally blocked when glial cell growth is inhibited by various drugs (Engele & Bohn, 1991; Hou et al., 1997). Together, these results suggest that treatment with LSL 60101 could be related to translocation of neuronal FGF-2 towards secretion pathways leading to autocrine neuroprotective effects on facial motoneurons and to paracrine effects on surrounding glia; thus promoting transition of astrocytes from a quiescent to a reactive state. Reactive astrocytes after LSL 60101 treatment could also play a role in the regenerative process after axotomy by increased glutamate uptake through increased expression of the GLT-1 transporter (Table 2 and Figure 5), thus counteracting the reported down-regulation after axotomy of the glial GLT-1 transporter (Ginsberg et al., 1995; 1996). Also in this sense, it has been proposed that increased expression of a glial glutamate transporter may protect axotomized motoneurons of the facial nucleus against glutamate toxicity (Yamashita et al., 1996). Reactive astrocytes after LSL 6011 treatment could also provide neurotrophic support by maintenance of neuronal glutathione levels, a tripeptide that plays an important role in cell protection against glutamate-mediated oxidative stress (Sagara et al., 1993).
In conclusion, treatment with LSL 60101, a selective I2-imidazoline ligand, results in marked morphological changes in astrocytes of the neonate and adult rat brain. Since in our experimental paradigm axotomy was performed 3 days after LSL 60101 treatment, reactive astrocytes are already present, surrounding and providing trophic support to the motoneurons at the moment of injury (see also Mattson & Rychlik, 1990; Delaney et al., 1996; Beaman-Hall et al., 1998). Thus, the reduction in the onset time for the glial/neuronal response after axotomy, together with the increased levels of GLT-1 and FGF-2 may account for the neuroprotective effects of this drug after this peripheral injury.
Acknowledgments
This study was supported by DGICYT Grants PB94-0002 Mod C (J.A. García-Sevilla) and SAF 970083 (J.E. Esquerda). M.A. Boronat was supported by a fellowship from the Universitat de les Illes Balears. We are grateful to Ipsen Pharma S.A., Barcelona (Spain) for gifts of drugs and to Consell Insular de Mallorca Paeria of Lleida and Marató of TV3 for support. J.A. García-Sevilla is a member of the Institut d'Estudis Catalans, Barcelona (Spain).
Abbreviations
- FGF-2
basic fibroblast growth factor
- GFAP
glial fibrillary acidic protein
- IOD
integrated optical density
- LSL 60101
2-(2-benzofuranyl)imidazole
- P0–P10
days 0 to 10 after birth
- PBS
phosphate buffered saline
- SvGFAP
surface density (Sv) of GFAP-immunoreactive cell bodies and cell processes
References
- ALEMANY R., OLMOS G., ESCRIBÁ P.V., MENARGUES A., OBACH R., GARCÍA-SEVILLA J.A. LSL 60101, a selective ligand for I2 receptors, on glial fibrillary acidic protein concentration. Eur. J. Pharmacol. 1995;280:205–210. doi: 10.1016/0014-2999(95)00214-6. [DOI] [PubMed] [Google Scholar]
- BEAMAN-HALL C.M., LEAHY J.C., BENMANSOUR S., VALLANO M.L. Glia modulate NMDA-mediated signaling in primary cultures of cerebellar granule cells. J. Neurochem. 1998;71:1993–2005. doi: 10.1046/j.1471-4159.1998.71051993.x. [DOI] [PubMed] [Google Scholar]
- BIKFALVI A., KLEIN S., PINTUCCI G., RIFKIN D.B. Biological roles of fibroblast growth factor-2. Endocrine Revs. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- BLOTTNER D., HERDEGEN T. Neuroprotective fibroblast growth factor type-2 down-regulates the c-Jun transcription factor in axotomized sympathetic preganglionic neurons of adult rat. Neuroscience. 1998;82:283–292. doi: 10.1016/s0306-4522(97)00287-x. [DOI] [PubMed] [Google Scholar]
- BORONAT M.A., OLMOS G., GARCÍA-SEVILLA J.A. Attenuation of tolerance to opioid-induced antinociception and protection against morphine-induced decrease of neurofilament proteins by idazoxan and other I2-imidazoline ligands. Br. J. Pharmacol. 1998;125:175–185. doi: 10.1038/sj.bjp.0702031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUSQUET P. Imidazoline receptors: from basic concepts to recent developments. J. Cardiovasc. Pharmacol. 1995;26:S1–S6. [PubMed] [Google Scholar]
- CASANOVAS A., RIBERA J., HUKKANEN M., RIVEROS-MORENO V., ESQUERDA J.E. Prevention by lamotrigine, MK-801 and NΩ-nitro-L-arginine methyl ester of motoneuron cell death after neonatal axotomy. Neuroscience. 1996;71:313–325. doi: 10.1016/0306-4522(95)00461-0. [DOI] [PubMed] [Google Scholar]
- COLLAZO D., TAKAHASHI H., MCKAY R.D. Cellular targets and trophic functions of neurotrophin-3 in the developing rat hippocampus. Neuron. 1992;9:643–656. doi: 10.1016/0896-6273(92)90028-c. [DOI] [PubMed] [Google Scholar]
- CONWAY E.L., GUNDLACH A.L., CRAVEN J.A. Temporal changes in glial fibrillary acidic protein messenger RNA and [3H]PK11195 binding in relation to imidazoline-I2-receptor and α2-adrenoceptor binding in the hippocampus following transient global forebrain ischaemia in the rat. Neuroscience. 1998;82:805–817. doi: 10.1016/s0306-4522(97)00321-7. [DOI] [PubMed] [Google Scholar]
- CREWS L.L., WIGSTON D.J. The dependence of motoneurons on their target muscle during postnatal development of the mouse. J. Neurosci. 1990;10:1643–1653. doi: 10.1523/JNEUROSCI.10-05-01643.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELANEY C.L., BRENNER M., MESSING A. Conditional ablation of cerebellar astrocytes in postnatal transgenic mice. J. Neurosci. 1996;16:6908–6918. doi: 10.1523/JNEUROSCI.16-21-06908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLESTON P.A., SILBERBERG D.H. Fibroblast growth factor is a mitogen for oligodendrocytes in vitro. Dev. Brain Res. 1985;21:315–318. doi: 10.1016/0165-3806(85)90221-4. [DOI] [PubMed] [Google Scholar]
- ENGELE J., BOHN M.C. The neurotrophic effects of fibroblast growth factors on dopaminergic neurons in vitro are mediated by mesencephalic glia. J. Neurosci. 1991;11:3070–3078. doi: 10.1523/JNEUROSCI.11-10-03070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERNSBERGER P. Heterogeneity of imidazoline binding sites: proposed I1 and I2 subtypes. Fundam. Clin. Pharmacol. 1992;6 55s:P18. [Google Scholar]
- FRENCH N. α2-adrenoceptors and I2 sites in the mammalian central nervous system. Pharmacol. Ther. 1995;68:175–208. doi: 10.1016/0163-7258(95)02005-5. [DOI] [PubMed] [Google Scholar]
- GILAD G.M., SALAME K., RABEY J.M., GILAD V.H. Agmatine treatment is neuroprotective in rodent brain injury models Life Sci. 19965841–46.PL [DOI] [PubMed] [Google Scholar]
- GINSBERG S.D., MARTIN L.J., ROTHSTEIN J.D. Regional deafferentiation down-regulates subtypes of glutamate transporter proteins. J. Neurochem. 1995;65:2800–2803. doi: 10.1046/j.1471-4159.1995.65062800.x. [DOI] [PubMed] [Google Scholar]
- GINSBERG S.D., ROTHSTEIN J.D., PRICE L.D., MARTIN L.J. Fimbria-fornix transections selectively down-regulate subtypes of glutamate transporter and glutamate receptor proteins in septum and hippocampus. J. Neurochem. 1996;67:1208–1216. doi: 10.1046/j.1471-4159.1996.67031208.x. [DOI] [PubMed] [Google Scholar]
- GOMEZ-PINILLA F., LEE J.W., COTMAN C.W. Basic FGF in adult rat brain: cellular distribution and response to entorhinal lesion and fimbria-fornix transection. J. Neurosci. 1992;12:345–355. doi: 10.1523/JNEUROSCI.12-01-00345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAEBER M.B., STREIT W.J., KREUTZBERG G.W. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J. Neurosci. Res. 1988;21:18–24. doi: 10.1002/jnr.490210104. [DOI] [PubMed] [Google Scholar]
- GREENSMITH L., HASAN H.I., VRBOVA G. Nerve injury increases the susceptibility of motoneurons to N-methyl-D-aspartate-induced neurotoxicity in the developing rat. Neuroscience. 1994;58:727–733. doi: 10.1016/0306-4522(94)90450-2. [DOI] [PubMed] [Google Scholar]
- GROTHE C., UNSICKER K. Basic fibroblast growth factor in the hypoglossal system: specific retrograde transport, trophic and lesion-related responses. J. Neurosci. Res. 1992;32:317–328. doi: 10.1002/jnr.490320304. [DOI] [PubMed] [Google Scholar]
- GUSTAFSON I., WESTERBORG E., WIELOCH T.W. Protection against ischemia-induced neuronal damage by the α2-adrenoceptor antagonist idazoxan: Influence of time of administration and possible mechanisms of action. J. Cereb. Flow Metab. 1990;10:885–894. doi: 10.1038/jcbfm.1990.145. [DOI] [PubMed] [Google Scholar]
- GUSTAFSON I., YOSHITOYO M., WIELOCH T.W. Postischemic administration of idazoxan, an α2-adrenergic receptor antagonist, decreases neuronal damage in the rat brain. J. Cereb. Blood Flow Metab. 1989;9:171–174. doi: 10.1038/jcbfm.1989.25. [DOI] [PubMed] [Google Scholar]
- HOU J.-G., COHEN G., MYTILINEOU C. Basic fibroblast growth factor stimulation of glial cells protects dopamine neurons from 6-hydroxydopamine toxicity: involvement of the glutathione system. J. Neurochem. 1997;69:76–83. doi: 10.1046/j.1471-4159.1997.69010076.x. [DOI] [PubMed] [Google Scholar]
- HOUENOU L.J., LI L., LO A.C., YAN Q., OPPENHEIM R.W. Naturally occurring and axotomy-induced motoneuron death and its prevention by neurotrophic agents: a comparison between chick and mouse. Prog. Brain. Res. 1994;102:217–226. doi: 10.1016/S0079-6123(08)60542-7. [DOI] [PubMed] [Google Scholar]
- HUBER K., MEISINGER C., GROTHE C. Expression of fibroblast growth factor-2 in hypoglossal motoneurons is stimulated by peripheral nerve injury. J. Comp. Neurol. 1997;382:189–198. [PubMed] [Google Scholar]
- LI G., REGUNATHAN S., BARROW C.J., ESHRAGHI J., COOPER R., REIS D.J. Agmatine–an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- LIU H.M., CHEN H.H. Correlation between fibroblast growth factor expression and cell proliferation in experimental brain infarct: studied with proliferating cell nuclear antigen immunohistochemistry. J. Neuropathol Exp. Neurol. 1994;53:118–126. doi: 10.1097/00005072-199403000-00002. [DOI] [PubMed] [Google Scholar]
- LOGAN A., BERRY M. Transforming growth factor-b1 and basic fibroblast growth factor in the injured CNS. Trends Pharmacol. Sci. 1993;14:337–342. doi: 10.1016/0165-6147(93)90007-7. [DOI] [PubMed] [Google Scholar]
- LOWRIE M.B., VRBOVA G. Dependence of postnatal motoneurons on their targets: review and hypothesis. Trends Neurosci. 1992;15:80–84. doi: 10.1016/0166-2236(92)90014-y. [DOI] [PubMed] [Google Scholar]
- MAIESE K., PEK L., BERGER S.B., REIS D.J. Reduction in focal cerebral ischemia by agents acting at imidazole receptors. J. Cereb. Blood Flow Metab. 1992;12:53–63. doi: 10.1038/jcbfm.1992.7. [DOI] [PubMed] [Google Scholar]
- MALHOTRA S.K., SHNITKA T.K. Adaptive plasticity and diversity among reactive astrocytes in central nervous system lesions. Biomed. Lett. 1994;49:273–302. [Google Scholar]
- MATTSON M.P., RYCHLIK B. Glia protect hippocampal neurons against excitatory amino acid-induced degeneration: involvement of fibroblast growth factor. Int. J. Devl. Neurosci. 1990;8:399–415. doi: 10.1016/0736-5748(90)90073-b. [DOI] [PubMed] [Google Scholar]
- MATTSON M.P., KUMAR K.N., WANG H., CHENG B., MICHAELIS E.K. Basic FGF regulates the expression of a functional 71 kDa NMDA receptor protein that mediates calcium influx and neurotoxicity in hippocampal neurons. J. Neurosci. 1993;13:4575–4588. doi: 10.1523/JNEUROSCI.13-11-04575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTSON M.P., LOVELL M.A., FURUKAWA K., MARKESBERY W.R. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca+2 concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J. Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- MENTIS G.Z., GREENSMITH L., VRBOVA G. Motoneurons destined to die are rescued by blocking N-methyl-D-aspartate receptors by MK-801. Neuroscience. 1993;54:283–285. doi: 10.1016/0306-4522(93)90253-c. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., INSEL P.A. Are there multiple imidazoline binding sites. Trends Pharmacol. Sci. 1989;10:342–344. doi: 10.1016/0165-6147(89)90002-3. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J. Imidazoline receptors: basic knowledge, recent advances and future prospects for therapy and diagnosis. Drugs Fut. 1997;22:757–772. [Google Scholar]
- NORENBERG M.D. Reactive astrocytosis The Role of Glia in Neurotoxicity 1996New York: CRC Press; 93–107.eds. Aschner, M. & Kimelberg, H.K. pp [Google Scholar]
- NORTON W.T., AQUINO D.A., HOZUMI I., CHIU F.-C., BROSNAN C.F. Quantitative aspects of reactive gliosis: a review. Neurochem. Res. 1992;17:877–885. doi: 10.1007/BF00993263. [DOI] [PubMed] [Google Scholar]
- O'CALLAGHAN J.P. Biochemical analysis of glial fibrillary acidic protein as a quantitative approach to neurotoxicity assessment: advantages, disadvantages and application to the assessment of NMDA receptor antagonist-induced neurotoxicity. Psychopharmacol. Bull. 1994;30:549–554. [PubMed] [Google Scholar]
- O'CALLAGHAN J.P., JENSEN K.F. Enhanced expression of glial fibrillary acidic protein and the cupric silver degeneration reaction can be used as sensitive and early indicators of neurotoxicity. Neurotoxicology. 1992;13:113–122. [PubMed] [Google Scholar]
- ODERFELD-NOWAK B., BACIA A., GRADKOWSKA M., FUSCO M., VANTINI G., LEON A., ALOE L. In vivo activated brain astrocytes may produce and secrete nerve growth factor-like molecules. Neurochem. Int. 1992;21:455–461. doi: 10.1016/0197-0186(92)90197-y. [DOI] [PubMed] [Google Scholar]
- OLMOS G., ALEMANY R., ESCRIBÁ P.V., GARCÍA-SEVILLA J.A. The effects of chronic imidazoline drug treatment on glial fibrillary acidic protein concentrations in rat brain. Br. J. Pharmacol. 1994;111:997–1002. doi: 10.1111/j.1476-5381.1994.tb14842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLMOS G., DEGREGORIO-ROCASOLANO N., REGALADO M.P., GASULL T., BORONAT M.A., TRULLAS R., VILLARROEL A., LERMA J., GARCÍA-SEVILLA J.A. Protection by imidazol(ine) drugs and agmatine of glutamate-induced neurotoxicity in cultured cerebellar granule cells through blockade of NMDA receptor. Br. J. Pharmacol. 1999;127:1317–1326. doi: 10.1038/sj.bjp.0702679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTAKE K., RUGGIERO D.A., REGUNATHAN S., WANG H., MILNER T.A., REIS D.J. Regional localization of agmatine in the rat brain: an immunocytochemical study. Brain Res. 1998;787:1–14. doi: 10.1016/s0006-8993(97)01200-6. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- PERRAUD F., LABOURDETTE G., MIEHE M., LORET C., SENSENBRENNER M. Comparison of the morphological effects of acidic and basic fibroblast growth factors on rat astroblasts in culture. J. Neurosci. Res. 1988;20:1–11. doi: 10.1002/jnr.490200102. [DOI] [PubMed] [Google Scholar]
- PETTMANN B., LABOURDETTE G., WEIBEL M., SENSENBRENNER M. Brain-derived astroglial growth factors. Funct. Biol. Med. 1985;4:243–248. doi: 10.1016/0014-5793(85)80851-6. [DOI] [PubMed] [Google Scholar]
- POLLIN M.M., MCHANWELL S., SLATER C.R. The effect of age on motor neurone death following axotomy in mouse. Development. 1991;112:83–89. doi: 10.1242/dev.112.1.83. [DOI] [PubMed] [Google Scholar]
- REGUNATHAN S., REIS D.J. Imidazoline receptors and their endogenous ligands. Annu. Rev. Pharmacol. Toxicol. 1996;36:511–544. doi: 10.1146/annurev.pa.36.040196.002455. [DOI] [PubMed] [Google Scholar]
- REGUNATHAN S., FEINSTEIN D.L., REIS D.J. Expression of non-adrenergic imidazoline sites in rat cerebral cortical astrocytes. J. Neurosci. Res. 1993;34:681–688. doi: 10.1002/jnr.490340611. [DOI] [PubMed] [Google Scholar]
- REGUNATHAN S., FEINSTEIN D.L., RAASCH W., REIS D.J. Agmatine (decarboxylated arginine) is synthesized and stored in astrocytes. NeuroReport. 1995;6:1897–1900. doi: 10.1097/00001756-199510020-00018. [DOI] [PubMed] [Google Scholar]
- REIS D.J., REGUNATHAN S. Agmatine: a novel neurotransmitter. Adv. Pharmacol. 1998;42:645–649. doi: 10.1016/s1054-3589(08)60834-0. [DOI] [PubMed] [Google Scholar]
- RIDET J.L., MALHOTRA S.K., PRIVAT A., GAGE F.H. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN J.D., MARTIN L., LEVEY A.I., DYKES-HOBERG M., JIN L., WU D., NASH N., KUNCL R.W. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- RUGGIERO D.A., REGUNATHAN S., WANG H., MILNER T.A., REIS D.J. Immunocytochemical localization of an imidazoline receptor protein in the central nervous system. Brain Res. 1998;780:270–293. doi: 10.1016/s0006-8993(97)01203-1. [DOI] [PubMed] [Google Scholar]
- SAGARA J., MIURA K., BANNAI S. Maintenance of neuronal glutathione by glial cells. J. Neurochem. 1993;61:1672–1676. doi: 10.1111/j.1471-4159.1993.tb09802.x. [DOI] [PubMed] [Google Scholar]
- SÁNCHEZ-BLÁZQUEZ P., BORONAT M.A., OLMOS G., GARCÍA-SEVILLA J.A., GARZÓN J. Activation of I2-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br. J. Pharmacol. 2000;130:146–152. doi: 10.1038/sj.bjp.0703294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASTRE M., GARCÍA-SEVILLA J.A. Opposite age-dependent changes of α2A-adrenoceptors and non-adrenoceptor [3H]idazoxan binding sites (I2-imidazoline sites) in the human brain: strong correlation of I2 with MAO-B sites. J. Neurochem. 1993;61:881–889. doi: 10.1111/j.1471-4159.1993.tb03599.x. [DOI] [PubMed] [Google Scholar]
- SCHMALBRUCH H. Motoneuron death after sciatic nerve section in newborn rats. J. Comp. Neurol. 1984;224:252–258. doi: 10.1002/cne.902240206. [DOI] [PubMed] [Google Scholar]
- SENDTNER M., HOLTMANN B., KOLBECK R., THOENEN H., BARDE Y.A. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992;360:757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- SENDTNER M,. , KREURTZBERG G.W., THOENEN H. Ciliary neurotrophic factor prevents the degeneration of moto neurons after axotomy. Nature. 1990;345:440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- SHU S.Y., JU G., FAN L.Z. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci. Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- SMITH P.K., KROHN R.I., HERMANSON G.T., MALLIA A.K., GARTNER F.H., PROVENZANO M.D., FUJIMOTO E.K., GOEKE N.M., OLSON B.J., KLENK D.. Measurements of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- SNIDER W.D., ELLIOT J.L., YAN Q. Axotomy-induced neuronal death during development. J. Neurobiol. 1992;23:1231–1246. doi: 10.1002/neu.480230913. [DOI] [PubMed] [Google Scholar]
- TRANQUE P.A., SUAREZ I., OLMOS G., FERNANDEZ B., GARCÍA-SEGURA L.M. Estradiol-induced redistribution of glial fibrillary acidic protein immunoreactivity in the rat brain. Brain Res. 1987;406:348–351. doi: 10.1016/0006-8993(87)90805-5. [DOI] [PubMed] [Google Scholar]
- UYLINGS H.B.M., VAN EDEN C.G., HOFMAN M.A. Morphometry of size/volume variables and comparison of their bivariate relations in the nervous system under different conditions. J. Neurosci. Meth. 1986;18:19–37. doi: 10.1016/0165-0270(86)90111-1. [DOI] [PubMed] [Google Scholar]
- WEIBEL E.R. Practical Methods for Biological Morphometry. London: Academic Press; 1979. Stereological Methods; p. 1. [Google Scholar]
- WEISE B., JANET T., GROTHE C. Localization of bFGF and FGF-receptor in the developing nervous system of the embryonic and newborn rat. J. Neurosci. Res. 1993;34:442–453. doi: 10.1002/jnr.490340409. [DOI] [PubMed] [Google Scholar]
- YAMASHITA T., KOHMURA E., YUGUCHI T., SHIMADA S., TANAKA K., HAYAKAWA T., TOHYAMA M. Changes in glutamate/aspartate transporter (GLAST/GluT-1) mRNA expression following nerve transection. Brain Res. Mol. Brain Res. 1996;38:294–299. doi: 10.1016/0169-328x(96)00043-5. [DOI] [PubMed] [Google Scholar]
- YAN Q., MATHESON C., LOPEZ O.T. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]


