Abstract
The possible existence of a β3-adrenoceptor (β3-AR) in human near-term myometrium was investigated by in vitro functional and biochemical studies and analysis of mRNA expression.
SR 59119A and SR 59104A and CGP 12177 (two selective agonists and a partial agonist, respectively, of the β3-AR), salbutamol and terbutaline (β2-AR agonists) each produced a concentration-dependent relaxation of the myometrial spontaneous contractions. There were no differences in pD2 values for the relaxing potencies of terbutaline, salbutamol, CGP 12177 and SR 59119A. The rank order for their relaxing efficacies was SR 59119A>SR 59104A>terbutaline≈salbutamol≈CGP 12177 (Emax=52±7%, 42±12% and ≈ 30% respectively).
Propranolol, a β1- and β2-AR antagonist, and ICI 118551, a β2-AR antagonist (both at 0.1 μM), did not affect the SR 59119A-induced relaxation whereas SR 59230A, a selective β3-AR antagonist (1 μM), significantly reduced the maximal relaxing effect of SR 59119A.
SR 59119A and salbutamol induced a significant increase in cyclic AMP levels that was antagonized by SR 59230A but not by propranolol for SR 59119A, and by propranolol but not by SR 59230A for salbutamol.
The β3-AR mRNA was positively expressed in myometrium preparations in a reverse transcription polymerase chain assay.
The results presented provide the first evidence for the existence of the β3-AR subtype in human near-term myometrium and suggest that the effects of SR 59119A might be mediated through an increase in cyclic AMP level.
Keywords: Human myometrium, near-term, β3-adrenoceptor mRNA, β3-adrenoceptor, tocolysis, cyclic AMP, SR 59119A, RT–PCR
Introduction
The incidence of premature birth has risen over the past 15 years and remains relatively high in developed countries, despite preventative measures (Goldenberg & Rouse, 1998). Around 6% of all pregnancies end pre-term, and deaths of premature babies represent ∼85% of perinatal mortality. A reduction in the number of premature deliveries depends on the early and specialized management of pregnancy and parturition, including the development of new tocolytic agents (Jannet et al., 1997). β2-adrenoceptor agonists are widely used in the treatment of pre-term labour, but β2-adrenoceptors undergo desensitization after prolonged stimulation, with a decrease in efficacy (Berg et al., 1985). Although β-adrenoceptors (Lands et al., 1967a,1967b) were originally subclassified into β1- and β2-adrenoceptors, another subtype, the β3-subtype, has since been reported (Emorine et al., 1989; 1992; 1994). The β3-adrenoceptor shares 40–50% amino acid sequence identity with β1- and β2-adrenoceptor (Granneman et al., 1993) and lacks recognition sites for the cyclic AMP-dependent protein kinase and β-adrenoceptor kinase implicated in the desensitization of β2-adrenoceptor (Strosberg, 1993). β3-adrenoceptor has been shown to mediate lipolysis in white adipose tissue and thermogenesis in brown adipose tissue (Arch et al., 1984; Lonnqvist et al., 1993; Zaagsma & Nahorski, 1990), to inhibit the contractile activity of ileum and colon (Bardou et al., 1998; Bond & Clarke, 1988; Manara & Bianchetti, 1990). In dogs, β3-adrenoceptor produces sustained peripheral vasodilatation that is predominant in skin and fat (Berlan et al., 1994; Shen et al., 1994). In the heart, the existence of non β1-, non β2-adrenoceptors has been the subject of much debate. The heart β3-adrenoceptor has been described by Gauthier et al. (1996) to be negatively coupled to adenylate cyclase through stimulation of a Gi protein, or to be coupled to a nitric oxide synthase pathway through a Gi/o protein (Gauthier et al., 1998). A heart β4-adrenoceptor (Kaumann, 1989; Kaumann & Molenaar, 1997; Kaumann et al., 1998; Molenaar & Summers, 1987), that induce positive inotropic effects and that is positively coupled to adenylate cyclase through stimulation of a Gs protein has been described. The heart β4-adrenoceptor, as described by Kauman & Molenaar (1997) is more likely to be stimulated by atypical β-adrenoceptor agonists (like CGP 12177) than by β3-adrenoceptor agonists (BRL 37344, SR 58611A for example). Myometrium has always been described as expressing predominantly β2-adrenoceptor (Doggrell, 1995; Engstrom et al., 1999; Story et al., 1988; Sugrue et al., 1985) and the presence of a β3-adrenoceptor in human near-term myometrium has never been studied. The purpose of the present study was to investigate the presence of the β3-adrenoceptor and the effects of β3-adrenoceptor agonists on contractions and cyclic AMP production in human myometrium obtained from women undergoing caesarean delivery.
Methods
Human myometrial tissue preparation
Myometrial tissue samples were obtained from 35 women (mean age 31±4 years) with normal uncomplicated pregnancy near term (between the 38th and 40th weeks of gestation) but undergoing caesarean section. None of the women had been treated with β2-adrenoceptor agonists prior to caesarean delivery. Tissue samples were excised from the longitudinal layer in the uterine body, at the antiplacental site, and were immediately placed in pre-oxygenated Krebs solution at 4°C (composition, mM: NaCl, 118; KCl, 5.4; CaCl2, 2.5; KH2PO4 0.6, MgSO4, 1.2; NaHCO3, 25; glucose, 11.7) and transported to the laboratory. Tissues were dissected free from serosa and used fresh (functional studies) or quickly frozen at −80°C (biochemical and molecular studies). The use of human myometrial tissue for experiments was approved by the local ethical committees.
Functional study
Myometrial tissues were cut into 6–8 strips (8–10 mm long by 2–3 mm in cross section) from each muscle piece and were suspended isometrically under a resting tension of 2 g in a 10 ml organ bath containing Krebs solution (composition as above) at 37°C and continuously gassed with a mixture of 95% oxygen and 5% carbon dioxide (pH 7.40). After 1 h during which the myometrial strips were washed every 15 min and the resting tension readjusted to 2 g, the strips were allowed to equilibrate for a further 1 h until they showed regular spontaneous rhythmic contractile activity. One end of each strip was connected to a force-displacement transducer and tension changes were measured with Pioden strain gauges (UF1), amplified (EMKA, Paris, France), and recorded on a pen-writing oscillograph (Linseis, L65514, Munich, Germany). Once contractions became regular in amplitude, inhibitory cumulative concentration-response curves (from 0.1–30 μM) were determined for each compound studied: salbutamol and terbutaline (β2-adrenoceptor agonists), SR 59119A, SR 59104A (Bardou et al., 1998) and SR 58611A (all three being full β3-adrenoceptor agonists) and CGP 12177, usually described as a β1- and β2-adrenoceptor antagonist and as a partial β3-adrenoceptor agonist (Gauthier et al., 1996; Longhurst & Levendusky, 1999). Each concentration-response curve was constructed by adding the next concentration when contractions had reached a steady state (about 45 min). In additional experiments, concentration-response curves for SR 59119A and salbutamol were obtained after 45 min incubation with 1 μM of the β3-adrenoceptor antagonist SR59230A (De Ponti et al., 1996; Malinowska & Schlicker, 1997; Manara et al., 1996) or 0.1 μM of either propranolol (β1- and β2-adrenoceptor antagonist), ICI 118551 (a selective β2-adrenoceptor antagonist) or a combination of both SR 59230A and ICI 118551. Amplitude of contraction was recorded at steady state for all concentrations. Since 3–4 h was required for the construction of a cumulative concentration-response curve, only one complete curve was obtained in each strip. Each drug was tested on at least eight preparations, each one derived from a different patient, and time-matched control experiments were carried out using drug vehicles.
Biochemical study
Myometrial strips were equilibrated in Krebs solution (composition as above) continuously gassed with a mixture of 95% oxygen and 5% carbon dioxide at 37°C (pH 7.40) for 50 min, and then exposed to antagonists (0.1 μM propranolol or 1 μM SR 59230A), or their vehicle for 20 min, followed by addition of agonists (salbutamol or SR 59119A, each at 10 μM) for 5 min. In paired uterine strips, time-matched control experiments were carried out for a total incubation period of 75 min to determine the basal cyclic nucleotide content of unstimulated tissues. At the end of the incubation period, the tissues were processed as previously outlined by Buhimschi et al. (1995). The samples were immediately transferred into liquid nitrogen and homogenized in ice-cold 10% trichloroacetic acid. The homogenate was centrifuged at 10,000×g for 15 min at 4°C. The pH of the supernatant was neutralized by the addition of excess calcium carbonate followed by low-speed centrifugation. Aliquots of the supernatant were tested for cyclic AMP and cyclic GMP by enzyme immunoassay kits (RPN 225 and RPN 226, respectively; Amersham Pharmacia Biotech Ltd, Little Chalfont, U.K.) following the instructions of the manufacturer, without acetylation.
Analysis of functional and biochemical studies
In functional experiments the effect of each relaxant agent, including its maximal effect (Emax), was expressed as a percentage of the initial amplitude of spontaneous contractions. Drug potency is expressed as pD2 values (negative logarithm of the molar concentration of the drug to produce half of its maximal effect). pD2 values were calculated using Microsoft Excel 97. Data are expressed as mean±s.e.mean. Differences among groups were analysed by analysis of variance (ANOVA) followed by the Bonferoni-corrected t-test or by Student's t-test for paired or unpaired data, as appropriate.
In functional studies, the β3-adrenoceptor antagonist SR 59230A depressed the maximal response to SR 59119A. In order to evaluate the potency of this antagonist, we have calculated a pKB value and its s.e.mean by applying the following equation as previously described by Paquet et al. (1999).
in which slope is that of the double-reciprocal plot of equieffective concentrations of agonist (A) in the absence (1/A) or in the presence (1/A′) of the antagonist (B) and [B] represents the antagonist concentration.
Molecular study: β3-adrenoceptor transcript analysis by RT–PCR
Total RNA from human uterus tissue was prepared by using a single-step acid guanidium isothiocyanate-phenol-chloroform method as previously described by Chomczynski & Sacchi (1987). To avoid contamination with genomic DNA, total RNA was treated with DNase I (Gibco/BRL, Cergy Pontoise, France). Digestion was carried out at room temperature for 15 min and stopped by addition of 20 mM EDTA, pH 8.0, and incubated at 65°C for 10 min.
Isolated human myometrial polyA+ RNA was treated with Superscript II RNAse H− reverse transcriptase (Gibco/BRL, Cergy Pontoise, France) and oligo(dT)12–18 primers as described previously by Gauthier et al. (1996). cDNA synthesis was performed using 5 μg of total RNA incubated in a 20 μl reaction containing 50 mM Tris-HCl (pH 8.3), 3 mM DTT, 10 mM KCl, 0.5 mM dNTP, 40 u. RNAsin, 200 u superscript reverse transcriptase (Gibco/BRL, Cergy Pontoise, France) for 1 h at 37°C. A control without reverse transcription was performed to verify that amplification did not proceed from residual genomic cDNA.
PCR reactions were performed with 2 μl of reverse transcriptase products in 50 μl reaction containing 50 mM Tris-HCl (pH 9.2), 16 mM (NH4)2SO4, 1.75 mM MgCl2 10% DMSO, 0.3 mM each primer, and 3.5 μg of TAQ and PWO DNA polymerases (Boehringer Mannheim, Germany). The amplification sequence consisted of 30 cycles of 94°C for 0.5 min, 58°C for 1 min, 68°C for 2.5 min, preceded by a denaturing step at 95°C for 1 min and followed by treatment at 68°C for 10 min. To rule out the possibility of amplifying genomic DNA, PCR was carried out with no prior RT of the RNA in some experiments.
The amplicons were generated using specific primers derived from the cDNA sequence (sense 5′-CGC GTA GGG GCC GAC GC antisense 5′-CCT GGG CTG CGC TGG GCT). The expected length of the fragment was 650 bp with this sense and antisense combination. PCR products were separated by electrophoresis through 1% agarose ethinium bromide-stained gels.
Drugs and solutions
The drugs and chemicals used and their sources were: SR59119A (N-[(7-methoxy-1,2,3,4-tetrahydronaphthalen-(2R) >-2-yl)methyl]-(2R)-2-hydroxy-2-(3-chlorophenyl)ethanamine hydrochloride), SR 59104A (N-[(6-hydroxy-1,2,3,4-tetrahydronaphthalen - (2R) - 2 - yl)methyl] - (2R) - 2-hydroxy - 2 -(3-chlorophenyl)ethanamine hydrochloride), SR 58611A (ethyl{ (7S)-7-[(2R)-2-(3-chlorophenyl)-2-hydroxyethylamino]-5,6,7,8-tetrahydronaphthalen-2-yloxy}acetate hydrochloride) and SR 59230A (3 - (2 - ethylphenoxy) - 1 - [(1S)-1,2,3,4-tetrahydronaphthalen-1-ylamino]-(2S)-2-propanol oxalate) were gifts from Sanofi-Synthelabo Research Centre (Milan, Italy), (±)-CGP12177A ((±)-4-(3-t-butylamino)-2-hydroxypropoxy)-1,3-dihydro-2H-benzimidazol-2-one); salbutamol sulphate, terbutaline, ICI 118551 hydrochloride (erythro-(±)-1-(7-methylindan-4-yloxy)-3-isopropylaminobutan-2-ol hydrochloride) (Sigma, St Louis, MO, U.S.A.); theophylline sodium anisate (Delalande, Quétigny, France). Drugs were dissolved in distilled water, absolute ethanol or 20% polyethyleneglycol 300 as appropriate, and diluted in Krebs solution as required. Drug concentrations are given as final bath concentrations.
Results
Relaxation response to β-adrenoceptor agonists
The two β3-adrenoceptor agonists, SR 59119A (Figure 1) and to a lesser extent SR 59104, induced a dose-dependent inhibition of in vitro contractions of human near-term myometrium at concentrations ranging from 0.1–30 μM (Figure 2, Table 1). The maximum effect obtained at a concentration of 30 μM for SR 59119A and SR 59104A was 52±7% and 42±12% respectively, a value greater than that obtained for salbutamol and terbutaline that was 27±6% and 30±12% respectively, although significance was reached only for SR 59119A vs salbutamol. SR 58611A and the partial agonist CGP 12177A were only marginally effective but were as potent as all the other drugs tested in the present study (Figure 2 and Table 1). The pD2 values did not differ significantly.
Figure 1.
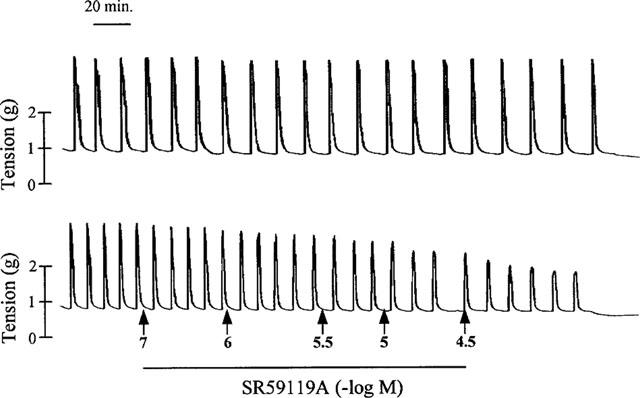
Representative recording of the effect of SR 59119A on spontaneous contraction of human near-term myometrium (lower trace) and time-matched control (upper trace).
Figure 2.
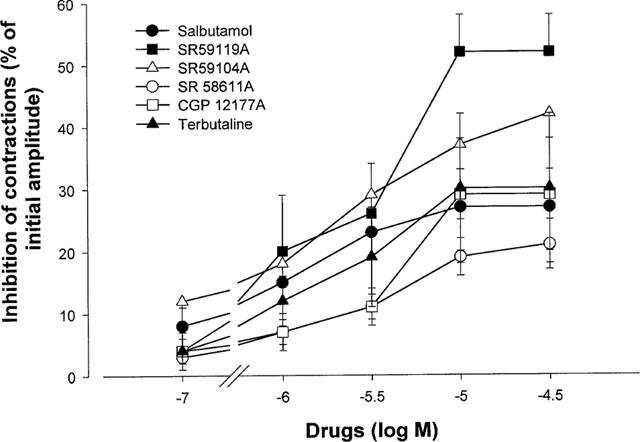
Effect of three full β3-adrenoceptor agonists SR 59119A, SR 59104A and SR 58611A, of a partial β3-adrenoceptor agonist CGP 12177, and of two β2-adrenoceptor agonists salbutamol and terbutaline, on spontaneous contraction of human near term contractions. Results are expressed as mean±s.e.mean. Inhibition of contractions is expressed as a percentage of initial amplitude of contractions.
Table 1.
Maximal effect (Emax) and potency (pD2) values for salbutamol, terbutaline and selective β3-adrenoceptor agonists in human pregnant myometrium near term
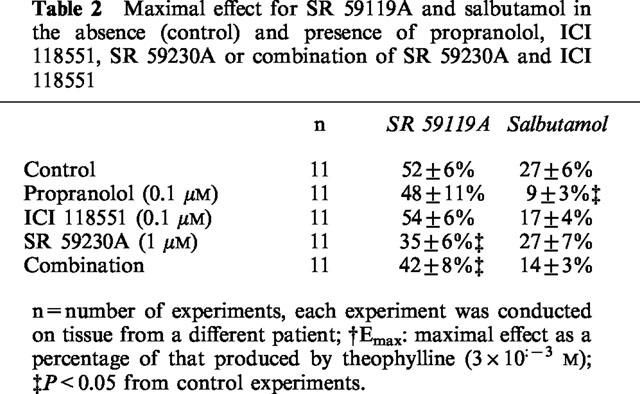
The dose-response curve of SR 59119A and salbutamol were then performed in the presence of 0.1 μM propranolol, a concentration that blocks 97 and 98% of β1- and β2-adrenoceptors respectively, but only 3% of β3-adrenoceptors (Roberts et al., 1995), 0.1 μM ICI 118551, a selective β2-adrenoceptor antagonist, 1 μM SR 59230A, a selective β3-adrenoceptor antagonist, and in the presence of a combination of ICI 118551 and SR 59230A. A 45-min pretreatment with each of the antagonists had no effect on the amplitude of contractions. However, the inhibition of contractions obtained in the presence of SR 59119A was not antagonized by either propranolol or ICI 118551 but was statistically reversed, in terms of efficacy, in the presence of SR 59230A (Emax 35±6% in SR 59230A experiments compared with 52±6% in control experiments, P<0.05, with a pKB value of 6.57±0.32), without further antagonism by the combination of the β2- and the β3-adrenoceptor antagonists ICI 118551 and SR 59230A (Figure 3A, Table 2). The pD2 value for SR 59119A was not significantly modified by SR 59230A. This might be partly explained because the maximal effect was decreased by the antagonists. The inhibitory effect on myometrial contractions of salbutamol was significantly reduced by propranolol (Emax=9±3% in propranolol experiments vs 27±6% in control experiments, pKB value of 8.22±0.24) but was not antagonized by SR 59230A. The antagonism produced by ICI 118551 failed to reach statistical significance but gave a pKB value of 7.44±0.35, and further antagonism was not observed with the combination of ICI 118551 and SR 59230 (Figure 3B and Table 2).
Figure 3.
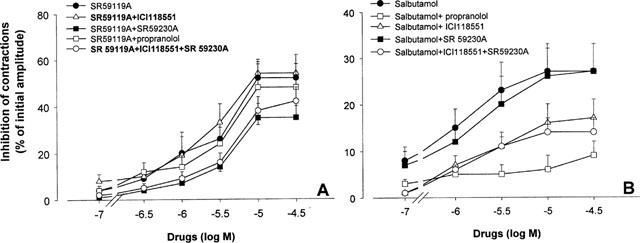
Effects of SR 59119A (A) or salbutamol (B), alone or after pretreatment with either propranolol (0.1 μM), ICI 118551 (0.1 μM), SR 59230A (1 μM) or combination of ICI 118551 (0.1 μM) and SR 59230A (1 μM) on spontaneous contraction of human near-term contractions. Results are expressed as mean±s.e.mean. Inhibition of contractions is expressed as a percentage of initial amplitude of contractions.
Table 2.
Maximal effect for SR 59119A and salbutamol in the absence (control) and presence of propranolol, ICI 118551, SR 59230A or combination of SR 59230A and ICI 118551
Cyclic AMP and cyclic GMP myometrial content after stimulation with salbutamol and SR 59119A.
Influence of propranolol and SR 59230A
SR 59119A and salbutamol (each at 10 μM) induced a significant increase in cyclic AMP level compared with the control (Figure 4). The trend towards a greater increase in cyclic AMP level after stimulation with SR 59119A compared with salbutamol failed to reach statistical significance. The effect of SR 59119A on cyclic AMP stimulation was not modified after pretreatment with propranolol (0.1 μM) whereas it was strongly antagonized (P<0.05) by SR 59230A (1 μM). In contrast, the effect of salbutamol was not antagonized by SR 59230A but was abolished after pretreatment with propranolol, P<0.05 (Figure 4).
Figure 4.
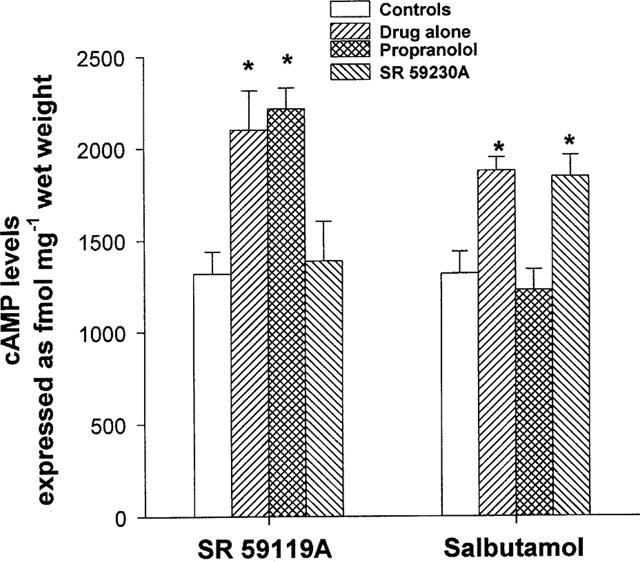
Effects of SR 59119A and salbutamol (both at 10 μM) on cyclic AMP production expressed as fmol mg−1 wet weight and influence of either propranolol (0.1 μM) or SR 59230A (1 μM). Results are expressed as mean±s.e.mean. Asterisks indicate a cyclic AMP level that was statistically different from baseline value (*P<0.05).
The cyclic GMP levels were about 20 fold lower than the corresponding cyclic AMP levels, and they were not increased either by SR 59119A or by salbutamol (data not shown).
Isolation of mRNA by RT–PCR
To determine whether the presence of a β3-adrenoceptor-mediated inhibition of human near-term myometrium contractions was associated with the expression of β3-adrenoceptor transcripts we used an RT–PCR assay. RNA was treated with DNase I to prevent contamination by genomic DNA.
As shown in Figure 5, β3-adrenoceptor mRNA expression was detected, as a 650 bp fragment, in human myometrium (lane 6, n=5). Hybridization to human β3-adrenoceptor cDNA confirmed the identity of the amplified products. No amplification was found in human bronchi (lanes 3 and 4, n=5). As a control for the assay, reverse transcribed β3-adrenoceptor mRNA was readily amplified by PCR in SK-N-MC cell line known to express the β3-adrenoceptor (Esbenshade et al., 1992).
Figure 5.
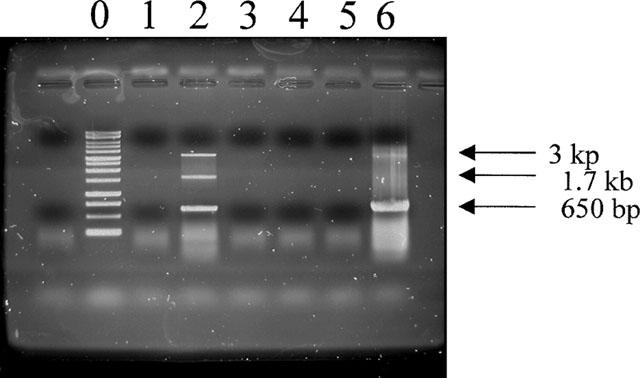
Agarose gel electrophoresis of RT–PCR products of β3-adrenoceptors using RNA extracted from human near-term myometrium. Lane 0, size marker, lines 1 and 2 cell line SK-N-MC used as positive control, lanes 3 and 4 human bronchi, lanes 5 and 6 human near-term myometrium. Lanes 1, 3 and 5 are without reverse transcription, lanes 2, 4 and 6 are with reverse transcription.
PCR products without prior RT of the RNA did not reveal any positive bands (Figure 5).
Discussion
Various studies have shown that β2-adrenoceptors exist in human gravid myometrium and that their stimulation produces an increase in cyclic AMP level and relaxation (Andersson et al., 1980; Berg et al., 1985). But treatment with β2-adrenoceptor agonists is often accompanied by a decrease in efficacy and of binding sites for β2-adrenoceptor agonists (Andersson et al., 1980; Berg et al., 1985; 1987). This is to our knowledge the first study that demonstrates the presence of functional β3-adrenoceptor in human near term myometrium. The functional and biochemical effects observed in the present study are very likely to be mediated through stimulation of the β3-adrenoceptor. Indeed, in a previous report we have shown that on spontaneous contractions of human colon, the inhibitory effect of SR 59119A, the most efficient agonist in this study, was insensitive to inhibition of 0.1 μM of propranolol (Bardou et al., 1998). The human colon is known to express β3-adrenoceptors (Bardou et al., 1998; Berkowitz et al., 1995; Krief et al., 1993). These results confirm the selectivity of this β3-adrenoceptor agonist. The pharmacological evidence for the presence of β3-adrenoceptor in human myometrium was also supported by the use of β-adrenoceptor antagonists. The inhibitory effects of SR 59119A on human myometrial contractions were antagonized by the selective β3-adrenoceptor antagonist SR 59230A but not by the β1- and β2-adrenoceptor antagonist propranolol or by the selective β2-adrenoceptor antagonist ICI 118551. The selectivity of the β3-adrenoceptor antagonist used in this study, SR 59230A, has been reported in previous studies (De Ponti et al., 1996; MacDonald & Watt, 1999; Malinowska & Schlicker, 1997; Manara et al., 1996). Thus SR 59230A antagonized the SR 58611A-induced relaxation of rat thoracic aorta (Trochu et al., 1999) and bound with high affinity to β3-adrenoceptor cloned in Chinese hamster ovary cells even if it has been described, in a single study, to be able to bind to both β1- and β2-adrenoceptor (Candelore et al., 1999). The relatively weak inhibitory effect of salbutamol found in the present study was not affected by SR 59230A but was antagonized by propranolol and, although not reaching statistical significance, also by ICI 118551. Similar results have been reported previously in human bronchi, which are known to express both β1- and β2-adrenoceptor but not β3-adrenoceptor (Mak et al., 1996), where SR 59230A failed to antagonize the in vitro relaxation induced either with isoprenaline or with salbutamol (Bardou et al., 1998).
The functional part of our study fulfils the four criteria defined by Kaumann & Molenaar (1996) for the β3-adrenoceptor: (1) the receptor has been selectively stimulated by β3-adrenoceptor-selective agonists; (2) the receptor has been stimulated by a non-conventional partial agonist; (3) the receptor has been resistant to blocking by antagonists possessing only high affinity for β1- and β2-adrenoceptor; (4) the receptor has been blocked by β3-adrenoceptor-selective antagonists.
In our study, SR 59119A and salbutamol were responsible for a significant increase in cyclic AMP level but failed to increase the cyclic GMP level. The stimulation of cyclic AMP production after exposure to SR 59119A was not modified by pre-treatment with propranolol but was virtually abolished by SR 59230A, providing confirmation of the β3-adrenoceptor-mediated nature of this stimulation. The relationship between cyclic AMP and smooth muscle relaxation has been well documented and shows that agents that increase the synthesis of cyclic AMP, e.g. β-adrenoceptor stimulants, as well as agents that inhibit the degradation of cyclic AMP, such as phosphodiesterase inhibitors, all decrease smooth muscle contraction (Bardou et al., 1999; Komas et al., 1991; Torphy et al., 1991). In our study, the functional inhibition of spontaneous contractions and the stimulation of cyclic AMP production induced by SR 59119A appears to be mediated through stimulation of β3-adrenoceptors. Therefore, this increase in cyclic AMP level is probably responsible for the inhibitory effect on contraction since it has previously been demonstrated that inhibitors of phosphodiesterase type 4 (such as rolipram) are very potent as in vitro inhibitors on human near-term myometrium spontaneous contractions (Bardou et al., 1999; Leroy et al., 1989). It has recently been shown that in human heart β3-adrenoceptor stimulation might be responsible for a negative inotropic effect mediated through stimulation of a nitric oxide synthase pathway and stimulation of cyclic GMP production (Gauthier et al., 1998). These conflicting results confirm the contrasting effects of β3-adrenoceptor agonists in various tissues.
In the functional study, SR 59119A was more efficient than salbutamol whereas both agonists induced similar increases in cyclic AMP production. An explanation of this discrepancy is that β2-adrenoceptor has been shown to undergo acute desensitization after exposure to β2-adrenoceptor agonists whereas β3-adrenoceptor does not (Nantel et al., 1995). In biochemical experiments samples were exposed for 5 min to agonists whereas the time needed to construct a full concentration-response curve in the functional study was about 3 h. Nantel et al. (1995) have shown that in CHW and L cells expressing either β2-adrenoceptor or β3-adrenoceptor, cyclic AMP content reached a maximum level during the first hour of exposure to isoprenaline and then decreased more rapidly in cells expressing the β2-adrenoceptor (Nantel et al., 1995). Moreover, this long-term desensitization does not occur in CHO cells transfected with the human cloned β3-adrenoceptor (Nantel et al., 1994). Furthermore, it has been shown that β3-adrenoceptor mRNA expression was increased by BRL 35135, a selective β3-adrenoceptor agonist (Emilsson et al., 1998).
Finally, pharmacological evidence for myometrial β3-adrenoceptor was strengthened by detection of β3-adrenoceptor transcripts in the human myometrium using a reverse transcription polymerase chain assay. As adipocytes are rare in myometrial tissue, it is unlikely that expression of β3-adrenoceptor is due to the presence of this type of cell.
Although the therapeutic potential of the findings reported in this study remains to be established, the use of selective β3-adrenoceptor agonist might be of interest in the pharmacological treatment of premature labour and in other conditions where inhibition of uterine contractility is indicated, since data from randomized trials indicate that tocolytic drugs, if used frequently usually fail to induce a strong and sustained uterine relaxation (Goldenberg & Rouse, 1998).
Acknowledgments
The authors are indebted to the surgical teams of the Services of Obstetrics and Gynaecology at the University Hospitals, Antoine Béclère Hospital (Clamart, France), CHU du Bocage Hospital (Dijon, France) and La Fe Hospital (Valencia, Spain) for making the myometrial tissue available to us. Special thanks go to Mrs Ruth Bardou for her careful reading of this manuscript.
Abbreviations
- AR
adrenoceptor
References
- ANDERSSON R.G., BERG G., JOHANSSON S.R., RYDEN G. Effects of non-selective and selective beta-adrenergic agonists on spontaneous contractions and cyclic AMP levels in myometrial strips from pregnant women. Gynecol. Obstet. Invest. 1980;11:268–293. [PubMed] [Google Scholar]
- ARCH J.R., AINSWORTH A.T., CAWTHORNE M.A., PIERCY V., SENNITT M.V., THODY V.E., WILSON C., WILSON S. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984;309:163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- BARDOU M., CORTIJO J., LOUSTALOT C., TAYLOR S., PERALES-MARIN A., MERCIER F.J., DUMAS M., DENEUX-THARAUX C., FRYDMAN R., MORCILLO E., ADVENIER C. Pharmacological and biochemical study on the effects of selective phosphodiesterase inhibitors on human term myometrium. Naunyn Schmiedebergs Arch. Pharmacol. 1999;360:457–463. doi: 10.1007/s002109900092. [DOI] [PubMed] [Google Scholar]
- BARDOU M., DOUSSET B., DENEUX-THARAUX C., SMADJA C., NALINE E., CHAPUT J.C., NAVEAU S., MANARA L., CROCI T., ADVENIER C. In vitro inhibition of human colonic motility with SR 59119A and SR 59104A: evidence of a beta3-adrenoceptor-mediated effect. Eur. J. Pharmacol. 1998;353:281–287. doi: 10.1016/s0014-2999(98)00419-1. [DOI] [PubMed] [Google Scholar]
- BERG G., ANDERSSON R.G., RYDEN G. Beta-adrenergic receptors in human myometrium during pregnancy: changes in the number of receptors after beta-mimetic treatment. Am. J. Obstet Gynecol. 1985;151:392–396. doi: 10.1016/0002-9378(85)90310-2. [DOI] [PubMed] [Google Scholar]
- BERG G., ANDERSSON R.G., RYDEN G. Effects of enprofylline, a new xanthine derivate, on human pregnant myometrium. Am. J. Obstet Gynecol. 1987;156:958–962. doi: 10.1016/0002-9378(87)90366-8. [DOI] [PubMed] [Google Scholar]
- BERKOWITZ D.E., NARDONE N.A., SMILEY R.M., PRICE D.T., KREUTTER D.K., FREMEAU R.T., SCHWINN D.A. Distribution of beta 3-adrenoceptor mRNA in human tissues. Eur. J. Pharmacol. 1995;289:223–228. doi: 10.1016/0922-4106(95)90098-5. [DOI] [PubMed] [Google Scholar]
- BERLAN M., GALITZKY J., BOUSQUET-MELOU A., LAFONTAN M., MONTASTRUC J.L. Beta-3 adrenoceptor-mediated increase in cutaneous blood flow in the dog. J. Pharmacol. Exp. Ther. 1994;268:1444–1451. [PubMed] [Google Scholar]
- BOND R.A., CLARKE D.E. Agonist and antagonist characterization of a putative adrenoceptor with distinct pharmacological properties from the alpha- and beta-subtypes. Br. J. Pharmacol. 1988;95:723–734. doi: 10.1111/j.1476-5381.1988.tb11698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUHIMSCHI I., YALLAMPALLI C., DONG Y.L., GARFIELD R.E. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. Am. J. Obstet. Gynecol. 1995;172:1577–1584. doi: 10.1016/0002-9378(95)90500-6. [DOI] [PubMed] [Google Scholar]
- CANDELORE M.R., DENG L., TOTA L., GUAN X.M., AMEND A., LIU Y., NEWBOLD R., CASCIERI M.A., WEBER A.E. Potent and selective human beta(3)-adrenergic receptor antagonists. J. Pharmacol. Exp. Ther. 1999;290:649–655. [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DE PONTI F., GIBELLI G., CROCI T., ARCIDIACO M., CREMA F., MANARA L. Functional evidence of atypical beta 3-adrenoceptors in the human colon using the beta 3-selective adrenoceptor antagonist, SR 59230A. Br. J. Pharmacol. 1996;117:1374–1376. doi: 10.1111/j.1476-5381.1996.tb15294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOGGRELL S.A. Comparison of the attenuating effects of four beta-adrenoceptor agonists on rat isolated uterus and aorta. Clin. Exp. Pharmacol. Physiol. 1995;22:670–674. doi: 10.1111/j.1440-1681.1995.tb02086.x. [DOI] [PubMed] [Google Scholar]
- EMILSSON V., SUMMERS R.J., HAMILTON S., LIU Y.L., CAWTHORNE M.A. The effects of the beta3-adrenoceptor agonist BRL 35135 on UCP isoform mRNA expression. Biochem. Biophys. Res. Commun. 1998;252:450–454. doi: 10.1006/bbrc.1998.9667. [DOI] [PubMed] [Google Scholar]
- EMORINE L.J., BLIN N., STROSBERG A.D. The human beta 3-adrenoceptor: the search for a physiological function. Trends Pharmacol. Sci. 1994;15:3–7. doi: 10.1016/0165-6147(94)90118-x. [DOI] [PubMed] [Google Scholar]
- EMORINE L.J., FEVE B., PAIRAULT J., BRIEND-SUTREN M.M., NAHMIAS C., MARULLO S., DELAVIER-KLUTCHKO C., STROSBERG A.D. The human beta 3-adrenergic receptor: relationship with atypical receptors. Am. J. Clin. Nutr. 1992;55:215S–218S. doi: 10.1093/ajcn/55.1.215s. [DOI] [PubMed] [Google Scholar]
- EMORINE L.J., MARULLO S., BRIEND-SUTREN M.M., PATEY G., TATE K., DELAVIER-KLUTCHKO C., STROSBERG A.D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989;245:1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- ENGSTROM T., BRATHOLM P., VILHARDT H., CHRISTENSEN N.J. Effect of oxytocin receptor and beta2-adrenoceptor blockade on myometrial oxytocin receptors in parturient rats. Biol. Reprod. 1999;60:322–329. doi: 10.1095/biolreprod60.2.322. [DOI] [PubMed] [Google Scholar]
- ESBENSHADE T.A., HAN C., THEROUX T.L., GRANNEMAN J.G., MINNEMAN K.P. Coexisting beta 1- and atypical beta-adrenergic receptors cause redundant increases in cyclic AMP in human neuroblastoma cells. Mol. Pharmacol. 1992;42:753–759. [PubMed] [Google Scholar]
- GAUTHIER C., LEBLAIS V., KOBZIK L., TROCHU J.N., KHANDOUDI N., BRIL A., BALLIGAND J.L., LE MAREC H. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J. Clin. Invest. 1998;102:1377–1384. doi: 10.1172/JCI2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUTHIER C., TAVERNIER G., CHARPENTIER F., LANGIN D., LE MAREC H. Functional beta3-adrenoceptor in the human heart. J. Clin. Invest. 1996;98:556–562. doi: 10.1172/JCI118823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDENBERG R.L., ROUSE D.J. Prevention of premature birth. N. Engl. J. Med. 1998;339:313–320. doi: 10.1056/NEJM199807303390506. [DOI] [PubMed] [Google Scholar]
- GRANNEMAN J.G., LAHNERS K.N., CHAUDHRY A. Characterization of the human beta 3-adrenergic receptor gene. Mol. Pharmacol. 1993;44:264–270. [PubMed] [Google Scholar]
- JANNET D., ABANKWA A., GUYARD B., CARBONNE B., MARPEAU L., MILLIEZ J. Nicardipine versus salbutamol in the treatment of premature labor. A prospective randomized study. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997;73:11–16. doi: 10.1016/s0301-2115(97)02701-2. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. Is there a third heart beta-adrenoceptor. Trends Pharmacol. Sci. 1989;10:316–320. doi: 10.1016/0165-6147(89)90065-5. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., MOLENAAR P. Differences between the third cardiac beta-adrenoceptor and the colonic beta 3-adrenoceptor in the rat. Br. J. Pharmacol. 1996;118:2085–2098. doi: 10.1111/j.1476-5381.1996.tb15648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., MOLENAAR P. Modulation of human cardiac function through 4 beta-adrenoceptor populations. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:667–681. doi: 10.1007/pl00004999. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., PREITNER F., SARSERO D., MOLENAAR P., REVELLI J.P., GIACOBINO J.P. (−)-CGP 12177 causes cardiostimulation and binds to cardiac putative beta 4-adrenoceptors in both wild-type and beta 3-adrenoceptor knockout mice. Mol. Pharmacol. 1998;53:670–675. doi: 10.1124/mol.53.4.670. [DOI] [PubMed] [Google Scholar]
- KOMAS N., LUGNIER C., STOCLET J.C. Endothelium-dependent and independent relaxation of the rat aorta by cyclic nucleotide phosphodiesterase inhibitors. Br. J. Pharmacol. 1991;104:495–503. doi: 10.1111/j.1476-5381.1991.tb12457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRIEF S., LONNQVIST F., RAIMBAULT S., BAUDE B., VAN SPRONSEN A., ARNER P., STROSBERG A.D., RICQUIER D., EMORINE L.J. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J. Clin. Invest. 1993;91:344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDS A.M., ARNOLD A., MCAULIFF J.P., LUDUENA F.P., BROWN T.G. , JR Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967a;214:597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- LANDS A.M., LUDUENA F.P., BUZZO H.J. Differentiation of receptors responsive to isoproterenol. Life Sci. 1967b;6:2241–2249. doi: 10.1016/0024-3205(67)90031-8. [DOI] [PubMed] [Google Scholar]
- LEROY M.J., CEDRIN I., BREUILLER M., GIOVAGRANDI Y., FERRE F. Correlation between selective inhibition of the cyclic nucleotide phosphodiesterases and the contractile activity in human pregnant myometrium near term. Biochem. Pharmacol. 1989;38:9–15. doi: 10.1016/0006-2952(89)90142-1. [DOI] [PubMed] [Google Scholar]
- LONGHURST P.A., LEVENDUSKY M. Pharmacological characterization of beta-adrenoceptors mediating relaxation of the rat urinary bladder in vitro. Br. J. Pharmacol. 1999;127:1744–1750. doi: 10.1038/sj.bjp.0702709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONNQVIST F., KRIEF S., STROSBERG A.D., NYBERG S., EMORINE L.J., ARNER P. Evidence for a functional beta 3-adrenoceptor in man. Br. J. Pharmacol. 1993;110:929–936. doi: 10.1111/j.1476-5381.1993.tb13902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD A., WATT K. Characterisation of the atypical beta-adrenoceptor in rabbit isolated jejunum using BRL 37344, cyanopindolol and SR 59230A. J. Auton. Pharmacol. 1999;19:91–95. doi: 10.1046/j.1365-2680.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- MAK J.C., NISHIKAWA M., HADDAD E.B., KWON O.J., HIRST S.J., TWORT C.H., BARNES P.J. Localisation and expression of beta-adrenoceptor subtype mRNAs in human lung. Eur. J. Pharmacol. 1996;302:215–221. doi: 10.1016/0014-2999(96)00104-5. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., SCHLICKER E. Further evidence for differences between cardiac atypical beta-adrenoceptors and brown adipose tissue beta3-adrenoceptors in the pithed rat. Br. J. Pharmacol. 1997;122:1307–1314. doi: 10.1038/sj.bjp.0701516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANARA L., BIANCHETTI A. Further heterogeneity of the beta-adrenoceptor. The phenylethanolaminotetralines: new selective agonists for atypical beta-adrenoceptors. Trends Pharmacol. Sci. 1990;11:229–230. doi: 10.1016/0165-6147(90)90247-6. [DOI] [PubMed] [Google Scholar]
- MANARA L., BADONE D., BARONI M., BOCCARDI G., CECCHI R., CROCI T., GIUDICE A., GUZZI U., LANDI M., LE FUR G. Functional identification of rat atypical beta-adrenoceptors by the first beta 3-selective antagonists, aryloxypropanolaminotetralins. Br. J. Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLENAAR P., SUMMERS R.J. Characterization of beta-1 and beta-2 adrenoceptors in guinea pig atrium: functional and receptor binding studies. J. Pharmacol. Exp. Ther. 1987;241:1041–1047. [PubMed] [Google Scholar]
- NANTEL F., BOUVIER M., STROSBERG A.D., MARULLO S. Functional effects of long-term activation on human beta 2- and beta 3-adrenoceptor signalling. Br. J. Pharmacol. 1995;114:1045–1051. doi: 10.1111/j.1476-5381.1995.tb13311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NANTEL F., MARULLO S., KRIEF S., STROSBERG A.D., BOUVIER M. Cell-specific down-regulation of the beta 3-adrenergic receptor. J. Biol. Chem. 1994;269:13148–13155. [PubMed] [Google Scholar]
- PAQUET J.L., LUCCARINI J.M., FOUCHET C., DEFRENE E., LOILLIER B., ROBERT C., BELICHARD P., CREMERS B., PRUNEAU D. Pharmacological characterization of the bradykinin B2 receptor: inter-species variability and dissociation between binding and functional responses. Br. J. Pharmacol. 1999;126:1083–1090. doi: 10.1038/sj.bjp.0702403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS S.J., RUSSELL F.D., MOLENAAR P., SUMMERS R.J. Characterization and localization of atypical beta-adrenoceptors in rat ileum. Br. J. Pharmacol. 1995;116:2549–2556. doi: 10.1111/j.1476-5381.1995.tb17206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN Y.T., ZHANG H., VATNER S.F. Peripheral vascular effects of beta-3 adrenergic receptor stimulation in conscious dogs. J. Pharmacol. Exp. Ther. 1994;268:466–473. [PubMed] [Google Scholar]
- STORY M.E., HALL S., ZICCONE S.P., PAULL J.D. Effects of adrenaline, isoprenaline and forskolin on pregnant human myometrial preparations. Clin. Exp. Pharmacol. Physiol. 1988;15:703–713. doi: 10.1111/j.1440-1681.1988.tb01130.x. [DOI] [PubMed] [Google Scholar]
- STROSBERG A.D. Structure, function, and regulation of adrenergic receptors. Protein Sci. 1993;2:1198–1209. doi: 10.1002/pro.5560020802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGRUE M.F., ARMSTRONG J.M., GAUTHERON P., MALLORGA P., VIADER M.P. A study on the ocular and extraocular pharmacology of metipranolol. Graefes. Arch. Clin. Exp. Ophthalmol. 1985;222:123–127. doi: 10.1007/BF02173535. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J., ZHOU H.L., BURMAN M., HUANG L.B. Role of cyclic nucleotide phosphodiesterase isozymes in intact canine trachealis. Mol. Pharmacol. 1991;39:376–384. [PubMed] [Google Scholar]
- TROCHU J.N., LEBLAIS V., RAUTUREAU Y., BEVERELLI F., LE MAREC H., BERDEAUX A., GAUTHIER C. Beta 3-adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracic aorta. Br. J. Pharmacol. 1999;128:69–76. doi: 10.1038/sj.bjp.0702797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAAGSMA J., NAHORSKI S.R. Is the adipocyte beta-adrenoceptor a prototype for the recently cloned atypical ‘beta 3-adrenoceptor'. Trends Pharmacol. Sci. 1990;11:3–7. doi: 10.1016/0165-6147(90)90032-4. [DOI] [PubMed] [Google Scholar]


