Abstract
To characterize the volume-sensitive, osmolyte permeable anion channels responsible for the osmodependent release of taurine from supraoptic nucleus (SON) astrocytes, we investigated the pharmacological properties of the [3H]-taurine efflux from acutely isolated SON.
Taurine release induced by hypotonic stimulus (250 mosmol l−1) was not antagonized by the taurine transporter blocker guanidinoethyl sulphonate, confirming the lack of implication of the transporter.
The osmodependent release of taurine was blocked by a variety of Cl− channel inhibitors with the order of potency: NPPB>niflumic acid>DPC>DIDS>ATP. On the other hand, release of taurine was only weakly affected by other compounds (dideoxyforskolin, 4-bromophenacyl bromide, mibefradil) known to block volume-activated anion channels in other cell preparations, and was completely insensitive to tamoxifen, a broad inhibitor of these channels.
Although the molecular identity of volume-sensitive anion channels is not firmly established, a few genes have been postulated as potential candidates to encode such channels. We checked the expression in the SON of three of them, ClC3, phospholemman and VDAC1, and found that the transcripts of these genes are found in SON neurons, but not in astrocytes. Similar observation was previously reported for ClC2.
In conclusion, the osmodependent taurine permeable channels of SON astrocytes display a particular pharmacological profile, suggesting the expression of a particular type or subtype of volume-sensitive anion channel, which is likely to be formed by yet unidentified proteins.
Keywords: Taurine; volume-sensitive anion channels; astrocytes; supraoptic nucleus; Cl− channel inhibitors, osmoregulation, neuroendocrine cells, hypothalamus
Introduction
The supraoptic nucleus (SON) is an osmosensitive hypothalamic structure involved in the regulation of the whole body fluid balance, mainly through the release of the antidiuretic hormone vasopressin (AVP) in the blood circulation. Osmosensitivity of SON neurons partly results from the expression of mechanoreceptors on their membrane (Bourque & Oliet, 1997). We also recently provided evidence for an involvement of glial cells in the osmoregulation of SON neuron activity (Hussy et al., 1997; Deleuze et al., 1998). SON astrocytes specifically accumulate high levels of the β amino acid taurine (Decavel & Hatton, 1995). Taurine is released from these cells upon hypo-osmotic stimulation (Deleuze et al., 1998) and likely acts on glycine receptors of SON neurons (Hussy et al., 1997). Activation of glycine receptors contributes to the inhibition of AVP neurons induced by a decrease in plasma osmotic pressure (Hussy et al., 1997).
The osmodependent release of taurine from cultured astrocytes and many other cell types is believed to occur through volume-sensitive anion channels termed VSOAC (for volume-sensitive organic osmolyte and anion channel; Strange et al., 1996; Kirk, 1997; Nilius et al., 1997a; Pasantes-Morales & Schousboe, 1997). This conclusion has been inferred from several observations. First, release of taurine does not involve the taurine transporter, since it is Na+ independent, temperature insensitive and not antagonized by the carrier blocker ouabain. Rather, taurine efflux is a diffusion process that depends on the taurine concentration gradient (Pasantes-Morales & Schousboe, 1997). Second, the osmodependent release of taurine and VSOAC show a similar pharmacological profile (Jackson & Strange, 1993; Sànchez-Olea et al., 1996; Manolopoulos et al., 1997). Last, ionic currents carried by taurine through VSOAC have been directly recorded from several cell types including astrocytes (Bandelari & Roy, 1992; Jackson & Strange, 1993; Roy, 1995). Although a few genes have been postulated to encode volume-sensitive anion channels, VSOAC have not yet been identified at the molecular level (see Strange 1998; Clapham, 1998). Nevertheless, these channels likely represent a family of membrane proteins, an idea that is based on the diversity of the pharmacological properties and of the activation or regulatory mechanisms observed in various cell preparations. Moreover, the osmodependent efflux of taurine has also been proposed to occur through volume-dependent anion channels other than VSOAC (Lambert & Hoffmann, 1994; Moorman et al., 1995; Stutzin et al., 1999).
In SON astrocytes, taurine release also appears to be mediated by volume-sensitive anion channels. Indeed, release is independent of external Ca2+ and Na+, which rules out the participation of a vesicular release or of the taurine transporter, and it is antagonized by the Cl− channel inhibitors DIDS and DPC (Deleuze et al., 1998). We recently showed that the osmosensitivity of this channel is uniquely regulated by tyrosine protein kinase, pointing to the possible expression of a particular channel type in these cells (Deleuze et al., 2000). The present study was designed to characterize more extensively the pharmacological properties of the osmodependent release of taurine from SON glial cells in order to compare these properties with those of other taurine-permeable channels studied in other cell types. As an attempt to identify the molecular correlate of the channel, we checked the cellular expression in the SON of the known genes that have been proposed to encode volume-sensitive anion channels.
Methods
Release of taurine
Measurements of [3H]-taurine release from SON acutely isolated from adult male Wistar rats (Depré, France) was performed according to the method described previously (Deleuze et al., 1998; 2000). Briefly, rats were killed by decapitation without anaesthesia, SON were rapidly dissected and carefully isolated in a cold (4°C) oxygenated Locke solution (in mM: NaCl, 132; KCl, 5; CaCl2, 2; MgCl2, 2; KH2PO4, 1.2: HEPES, 10; glucose, 10; pH 7.4; osmolarity, 300 mosmol l−1). They were then incubated for 40 min in a Locke medium supplemented with 500 nM [3H]-taurine (Amersham) at 35°C, rinsed three times, placed in perfusion chambers (250 μl, one SON per chamber) at 35°C, and perfused at a rate of 250 μl min−1 with oxygenated Locke solution. After 20 min rest, perfusate was collected every 2 min with a sample collector (Gilson FC204). [3H]-taurine release was estimated by scintillation counting. Iso-osmotic medium (300 mosmol l−1) was a Locke solution to which 50 mosmol NaCl was replaced with an equi-osmotic amount of sucrose. Hypo-osmotic medium (250 mosmol l−1) was the same solution without sucrose. Basal release of taurine in iso-osmotic medium was fitted with a monoexponential function, and the fit was used to normalize all data, in order to express release as per cent of basal release (Deleuze et al., 1998; 2000). Two chambers were systematically used as control, and the effects of the various drugs were always compared to the controls of the same experiment. All experiments were realized on at least two different preparations. Inhibition curves were fitted with the Hill equation Y=100/(1+(A/IC50)nH), where A is the antagonist concentration, IC50 the concentration of half maximal inhibition, and nH the Hill coefficient. Analysis was performed with Origin 5.0 software (Microcal Inc.). Data are given as means±s.e.mean.
Taurine, niflumic acid, 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS), tamoxifen, dideoxyforskolin (DDF), 4-bromophenacyl bromide (pBPB), and ATP-Na were from Sigma, N-phenylanthranylic acid (DPC) and 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) were from RBI, guanidinoethyl sulphonate (GES) was from Toronto Research Chemical, and mibefradil was a gift from E. Bourinet (CNRS-UPR 1142, Montpellier, France).
Primers for reverse transcription-polymerase chain reaction (RT–PCR) and probes for in situ hybridization
The primers for RT and PCR, and/or the probes for in situ hybridization (Table 1) were designed from the rat mRNA sequences of ClC1, ClC2, ClC3, ClC4, ClC5, ClC6, ClC7, VDAC1, and phospholemman using CPrimer 1.09 (G. Bristol and R. D. Andersen, University of California, Los Angeles, CA, U.S.A.) and Amplify 1.2 (W. Engels, University of Wisconsin, Madison, WI, U.S.A.) softwares. When gene structure is known, forward and reverse primers were chosen on different exons. Specificity of the primer sequences for their target cDNA, particularly the absence of cross-matching with the cDNAs of proteins of the same family, was carefully verified. The BLAST software (National Center for Biotechnology Information, Bethesda, MD, U.S.A.) set at its maximal sensitivity was also used to check that the primers and probes did not match significantly any other known RNA or DNA sequence. To improve the sensitivity of detection by in situ hybridization, two probes were simultaneously used for ClC3 and for VDAC1. Oligonucleotides were synthesized by Genosys.
Table 1.
Primers for RT–PCR, PCR conditions and restriction enzymes used, and probes for in situ hybridization
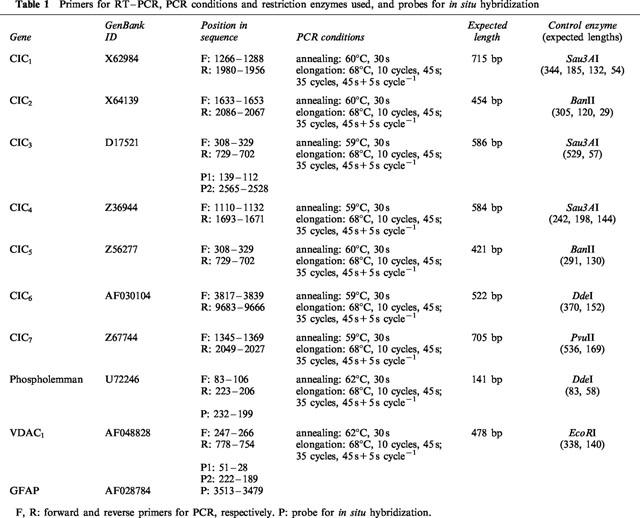
RT–PCR
RNAs were extracted from isolated SON (pooled from 10 adult male rats) using the RNeasyTM kit (Qiagen) and digested with DNase I (Life technologies) to avoid amplification of contaminating genomic DNA. RT–PCR was realized in a thermocycler PTC-100/16 (MJ-Research) using the TitanTM One Tube RT–PCR System (Roche) and RNasin (Promega Corp.) as a RNase inhibitor. PCR settings are indicated in Table 1. PCR products were run in a 2% agarose gel in TAE buffer (in mM: Tris-acetate 40, EDTA 1, pH 8) containing 0.5 μg ml−1 ethidium bromide, and bands were revealed under UV light. The length of the PCR products were as expected; the identity of the PCR bands was systematically checked by appropriate restriction enzymes (Table 1). Heat denaturation of the reverse transcriptase suppressed all PCR signals, confirming the absence of amplification of residual contaminating genomic DNA.
In situ hybridization
Brains from adult male rats were rapidly dissected, frozen at −40°C in isopentane and stored at −80°C. Coronal sections of hypothalamus (12 μm) were cut with a cryostat (Leica CM3000), thaw mounted on polylysin coated slides, fixed in 4% paraformaldehyde in PBS buffer (in mM: NaCl 130, Na2HPO4 7, NaH2PO4 3) for 20 min at room temperature, rinsed in PBS, dehydrated in increasing concentrations of ethanol (70 and 95%) and air dried. Oligonucleotide probes were labeled at their 3′ end with digoxygenin 11-dUTP using terminal transferase (Roche). Sections were incubated in a humid chamber overnight at 42°C with the primers (10 nM) in hybridization buffer (50% deionized formamide, Tris buffer 0.08 M pH 7.5, NaCl 0.6 M, EDTA 4 mM, Na2H2P2O7 0.05%, Na4P2O7 0.05%, N-lauroylsarcosine 0.2%, and 1×Denhardt solution [polyvinylpyrrolidone 0.02%, BSA 0.02%, Ficoll 0.02%]). They were rinsed at 45°C in decreasing concentrations (2×, 0.5×, and 0.1×, 30 min each) of saline-sodium citrate (in mM: NaCl 150, sodium citrate 15, pH 7), followed by a final 30 min wash in the buffer A (Tris 0.1 M pH 7.5, NaCl 1 M, MgCl2 2 mM) supplemented with 5% BSA and 0.1% N-lauroylsarcosine. Slides were then incubated 2–3 h with a sheep antidigoxygenin antibody coupled to alkaline phosphatase (Roche) diluted 1/500 in buffer A supplemented with 5% BSA, rinsed in three baths (10 min each) of buffer A, buffer B (Tris 0.1 M pH 9.5, NaCl 1 M, MgCl2 5 mM) and buffer C (Tris 0.1 M pH 9.5, NaCl 0.1 M, MgCl2 5 mM). Detection was performed by 2–12 h incubation with nitroblue tetrazolium and 5-bromo 4-chloro 3-indolylphosphate (Roche), and slides were viewed under a light microscope after mounting in Mowiol. Omission of probes or different molecules involved in digoxigenin detection returned the hybridization signals to background levels.
Results
Osmodependent release of taurine is a diffusional process
Reduction in osmolarity of perfusing medium from 300 to 250 mosmol l−1 induced a rapid and reversible increase in [3H]-taurine release (Figure 1). We showed previously that such release of taurine is exclusively of glial origin (Deleuze et al., 1998). The lack of involvement of the taurine transporter in the mechanism of release has been suggested by the independence on external Na+ (Deleuze et al., 1998). We confirmed this by studying the effect of the taurine transporter inhibitor guanidinoethyl sulphonate (GES). Application of GES (300 μM) increased basal release by 58±6% (n=8), indicative of a transporter-dependent uptake of taurine under control conditions. However, GES did not prevent the hypo-osmolarity-evoked release (Figure 1). In fact, release induced by the hypotonic stimulus peaked at 494±12% of the control basal release (n=8), so that the ratio of the evoked release/baseline was similar in the absence (319±17%) and the presence of GES (314±13%). The osmosensitive release of taurine is therefore independent of the functionality of the taurine transporter.
Figure 1.
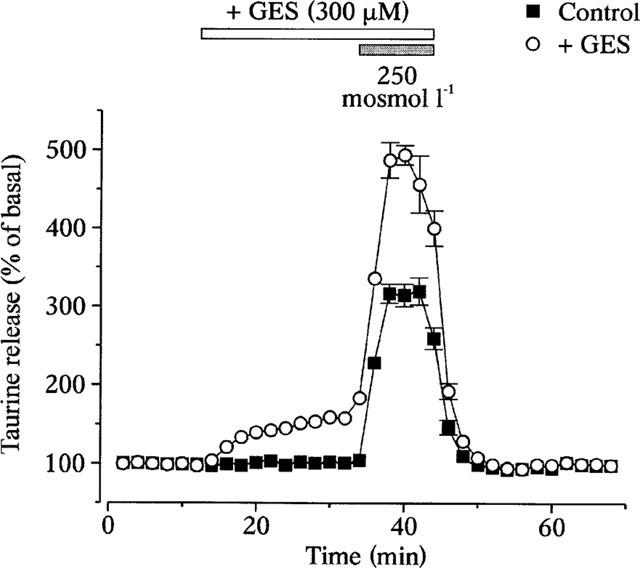
The osmodependent taurine efflux does not involve the taurine transporter. [3H]-taurine release from isolated SON in response to a 15% hypotonic stimulus. Application of the taurine transporter inhibitor GES increases both basal and evoked release, such that the ratio peak/basal stays constant (see text). Points are the mean of eight measurements. Standard errors are indicated when exceeding the size of the symbol.
Pharmacological properties of taurine release
To increase the basal release and minimize the possible interaction of the Cl− channel inhibitors with the taurine transporter, all experiments were carried out in the continued presence of 300 μM GES in the perfusion media. Both basal and hypo-osmolarity-evoked release were inhibited by a variety of Cl− channel blockers. Examples of such blockade by niflumic acid and NPPB are shown on Figure 2a. The degree of inhibition of hypotonicity-evoked release was obtained by measuring the amplitude of evoked release (peak–basal) in the presence of the drug, and by normalizing this amplitude to that of the control evoked release. Dose-response relationships for the antagonists gave an order of potency of NPPB>niflumic acid>DPC>DIDS>ATP (Figure 2b). Basal release was affected within similar windows of concentrations, although inhibition curves could not be constructed due to higher variability in the degree of blockade. Volume-dependent, taurine permeable Cl− channels in several cell types are also blocked by a variety of other compounds. These include dideoxyforskolin (DDF), the ‘Ca2+ channel' inhibitor mibefradil, the phospholipaseA2 inhibitor 4-bromophenacyl bromide (pBPB), as well as the anti-oestrogen tamoxifen (Strange et al., 1996; Kirk, 1997; Manolopoulos et al., 1997; Nilius et al., 1997a,1997b). We checked the sensitivity of SON glial release of taurine to these compounds, using them at concentrations shown to largely inhibit the channels in other preparations (Figure 3). Taurine release was only weakly decreased by DDF (100 μM), mibefradil (30 μM) and pBPB (50 μM), and completely insensitive to tamoxifen (30 μM). These latter compounds had no effect on basal release.
Figure 2.
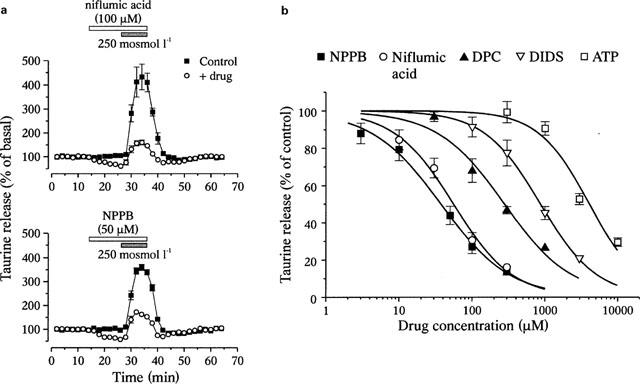
Blockade of osmodependent taurine release by Cl− channel inhibitors. (a) Examples of blockade of both basal and hypotonicity-evoked release of [3H]-taurine by niflumic acid (n=4) and NPPB (n=6). (b) Inhibition curves of the evoked release for the compounds indicated. Solid lines are fits with a Hill equation (see Methods) that gave IC50 of 38±3 μM (NPPB), 56±7 μM (niflumic acid), 280±55 μM (DPC), 869±22 μM (DIDS) and 4.09±0.74 mM (ATP). Hill coefficients are 0.9–1.2. Each point is the mean of 4–8 measurements.
Figure 3.
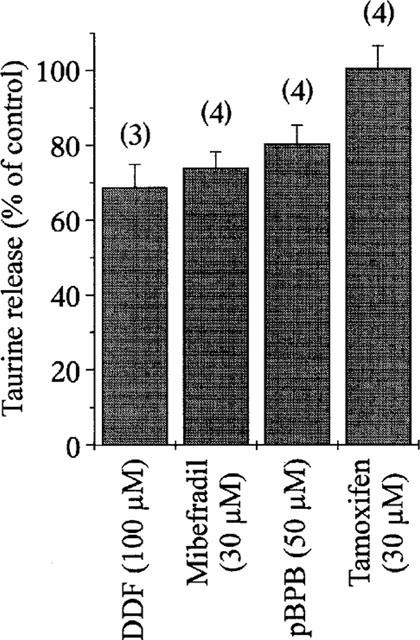
Weak sensitivity of taurine release to other anion channel blockers. Graph showing the peak of [3H]-taurine release evoked by a hypotonic stimulus in the presence of the compounds indicated. Release is expressed as per cent of control observed in the absence of the drug. Note the total lack of effect of tamoxifen. Number of observations is indicated above each bar.
Expression of the mRNA encoding potential volume-sensitive Cl− channels
As an attempt to identify the taurine-permeable channel of SON astrocytes, we first used RT–PCR to detect the expression of genes encoding known or putative anion channels. Primers were designed to amplify the mRNA of several members of the ClC family, ClC1–7 (Jentsch et al., 1999), as well as of the small protein phospholemman (Moorman et al., 1995) and the voltage-dependent anion channel VDAC1 (Dermietzel et al., 1994). All these messengers were detected in RNA extract from total SON (Figure 4). ClC1 encodes a volume-independent, voltage-activated Cl− channel mostly found in skeletal muscle, and ClC4 to ClC7 most likely encode Cl− channels of intracellular organelles (Jentsch et al., 1999). The other four genes, ClC2, ClC3, phospholemman and VDAC1 have been proposed to potentially encode volume-sensitive Cl− channels in various preparations (see Discussion). Among these, the ClC2 channel is unlikely to carry taurine efflux from SON astrocytes, because of its prominent neuronal distribution in the brain, including in the SON (Smith et al., 1995). To check whether ClC3, VDAC1, and phospholemman could correlate with the glial taurine-permeable channel, the cellular distribution of the expression of the genes encoding these proteins was investigated by in situ hybridization. Expression of ClC3, VDAC1 and phospholemman was detected exclusively in the neuronal part of the SON, in the large cell bodies of magnocellular neurons (Figure 5a–c). This distribution contrasted with that of the mRNA for the glial fibrillary acidic protein (GFAP, Figure 5d), a classical marker of astrocytes which was found in the somata of glial cells arranged on the ventral surface of the SON, the ventral glial lamina (Hatton, 1999). Therefore, SON glial cells either do not express these proteins or show a level of expression under the detection threshold.
Figure 4.
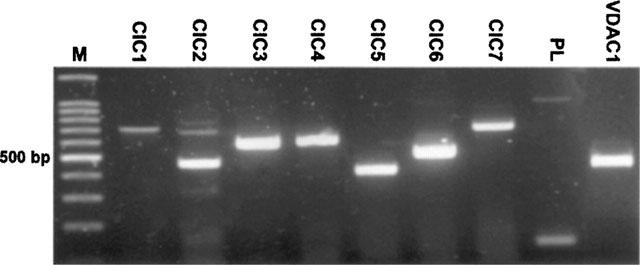
All ClCs, as well as phospholemman (PL) and VDAC1 genes are expressed in the SON. Electrophoresis gel showing the PCR products amplified from the nine mRNA encoding potential anion channels. Bands of expected size were amplified (see Table 1), and identity was checked by digestion with appropriate restriction enzymes. M, markers.
Figure 5.
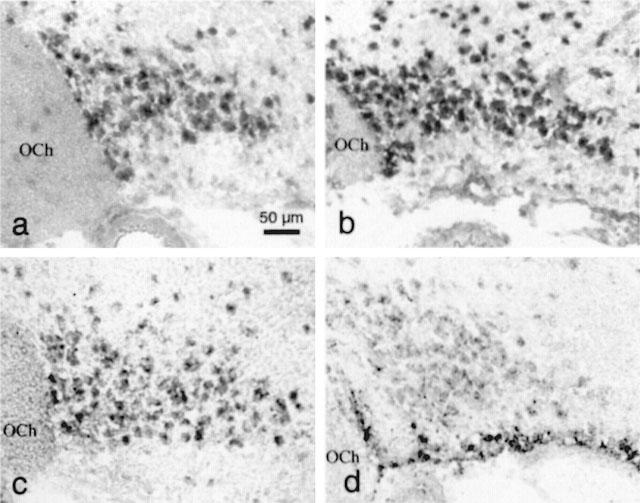
Neuronal localization of the expression of ClC3, phospholemman (PL) and VDAC1 in the SON. In situ hybridization showing the cellular expression of the three mRNA in the SON (a: CIC3; b: PL; c: VDAC1). Labelling is restricted to the large cell bodies located in the neuronal part of the nucleus. Note the absence of labelling in the ventral glia limitans where lie the somata of the astrocytes, as shown by the visualization of the expression of GFAP (d). OCh, optic chiasm.
Discussion
We here report the detailed pharmacological characterization of taurine release from SON glial cells. The fact that only glial cells accumulate and release taurine in this structure (Decavel & Hatton, 1995; Deleuze et al., 1998) allows study of the properties of the release from a homogeneous cell population in an in situ preparation. We showed that the release of taurine is not antagonized by the taurine transporter inhibitor GES, confirming the lack of implication of the transporter in the osmodependent efflux of taurine (Deleuze et al., 1998). Taurine then likely leaves the cells by a diffusional pathway, in agreement with the involvement of an ionic channel. The fact that GES increases basal release of taurine indicates the presence of a significant efflux under isotonic conditions, which is partly masked by the rapid re-uptake of taurine. We previously showed that a fraction of this basal release can be inhibited by hypertonic stimuli (Deleuze et al., 1998; 2000). Basal release would then consist of both an osmodependent and an osmo-independent component.
Taurine release, both basal and hypo-osmolarity-evoked, is inhibited by classical Cl− channel blockers. Sensitivity to the inhibitors could be reliably estimated only for the evoked release, possibly due the dual nature of basal release. NPPB blocks the osmodependent taurine efflux with a sensitivity similar to that found for VSOAC in other cell preparations (Sànchez-Olea et al., 1996; Manolopoulos et al., 1997; Okada, 1997). Niflumic acid was almost as potent as NPPB in inhibiting taurine efflux, and its IC50 is lower than that reported in many other cell types (Sànchez-Olea et al., 1996; Manolopoulos et al., 1997; Okada, 1997). Release is also antagonized by DIDS, albeit with a very low sensitivity. This could result from the voltage dependence of DIDS blockade (Gosling et al., 1995), since SON astrocytes are strongly hyperpolarized (Deleuze & Hussy, personal observation). Release is also inhibited by DPC, a blocker of several anion channels including some but not all VSOAC (Okada, 1997). Interestingly, taurine release and regulatory volume decrease in cultured cortical and cerebellar astrocytes has been reported to be largely insensitive to DPC (Sànchez-Olea et al., 1993; Pasantes-Morales et al., 1994). Further specificity in pharmacological properties is indicated by the weak sensitivity of SON taurine release to several compounds that have been shown to potently block VSOAC in other preparations (Strange et al., 1996, Pasantes-Morales & Schousboe, 1997; Nilius et al., 1997a,1997b). Among these, DDF, pBPB and mibefradil were found to have only a small inhibitory effect on taurine release from SON astrocytes. Lastly, tamoxifen, one of the most widely used blockers of VSOAC, completely failed to affect taurine release in the SON. Tamoxifen-insensitive VSOAC have also been described in a few cell types (Nilius et al., 1994; Leaney et al., 1997).
All these observations point to the specificity of the pharmacological profile of the taurine release from SON astrocytes. This could reflect the expression of a specific subtype of volume-sensitive, taurine permeable anion channel in these cells, either a VSOAC-like or a different type of anion channel. This would be in agreement with the peculiar regulation of SON taurine release by protein tyrosine kinase. Indeed, in several cell preparations including cultured astrocytes (Tilly et al., 1993; Crepel et al., 1998; Voets et al., 1998), swelling-induced opening of VSOAC has been shown to critically depend upon the activation of tyrosine kinase. However, in SON astrocytes, protein tyrosine kinase activation does not appear necessary for the osmodependent induction of taurine release, although the level of tyrosine phosphorylation modulates the osmosensitivity of taurine efflux (Deleuze et al., 2000). Alternatively, the differences seen in various cell preparations may result from specific properties of the activation and regulation of the channels rather than of the channels themselves. Solving whether or not this diversity in the properties of the osmodependent anion fluxes reflects the heterogeneity of volume-sensitive anion channels in mammalian cells will require the molecular identification of the channels.
So far, six genes have been postulated to encode volume-sensitive Cl− channels. Among these, the P-glycoprotein and pICln have later been shown to regulate the expression or the function of VSOAC, but not to be channels themselves (Strange, 1998; Clapham, 1998). Two members of the ClC chloride channel family, ClC2 and ClC3, have been reported to be activated by cell swelling (Gründer et al., 1992; Duan et al., 1997). However, ClC2 displays anionic selectivity, voltage dependence, single channel conductance, and pharmacological properties different from VSOAC (Bond et al., 1998; Okada et al., 1998; Jentsch et al., 1999). ClC3 appears as one of the best candidates for VSOAC, as its expression in heterologous systems leads to the presence in the cell membrane of volume-activated Cl− channels with properties strikingly similar to VSOAC (Duan et al., 1997; Strange, 1998), although taurine permeability has yet to be shown. However, its unusual regulation by protein kinase C, plus the fact that other groups have failed to reproduce the functional expression of this clone has questioned the general correlation between ClC3 and VSOAC (Strange, 1998; Jentsch et al., 1999). Moreover, ClC3 shows a very high sequence homology to ClC5, a known volume-insensitive Cl− channel expressed in the membrane of intracellular organelles (Jentsch et al., 1999). Further work is needed to clarify the role of ClC3. Other candidates for non-VSOAC channels carrying volume-sensitive taurine efflux include the voltage-dependent anion channel (VDAC) that belongs to the porin family (Kirk & Strange, 1998), originally identified in yeast mitochondria outer membrane (Colombini et al., 1996). The rat homologue VDAC1 has been shown to be expressed in the plasma membrane of astrocytes and neurons (Dermietzel et al., 1994; Moon et al., 1999), and to support large-conductance Cl− channels (Dermietzel et al., 1994) shown previously to be volume-sensitive (Jalonen, 1993). Interestingly, these channels are known to carry the diffusion of large anionic molecules (Kirk & Strange, 1998). Lastly, the small protein phospholemman cloned from the heart generates large conductance, highly taurine permeable anion channels when incorporated into lipid bilayers (Moorman et al., 1995). The volume sensitivity of these channels has yet to be demonstrated.
In the supraoptic nucleus, the transcripts encoding most members of the ClC family, including ClC2 and ClC3, plus VDAC1 and phospholemman can be amplified by RT–PCR. However, we showed by in situ hybridization that the three latter mRNA are highly expressed in neurons but are not detected in glial cells in this structure. A previous study has also reported the exclusive neuronal expression of ClC2, including in the SON (Smith et al., 1995). Although these results do not exclude low levels of expression of these mRNA in astrocytes, they strongly argue for the lack of correlation between these molecules and the taurine permeable channel of SON glial cells. Indeed, it is unlikely that the channel would be less expressed in the cell population most obviously concerned by taurine fluxes in the SON. Moreover, as neurons are devoid of taurine under isotonic conditions (Decavel & Hatton, 1995), activation of these channels by hypotonic stimuli should lead to massive entry of taurine in magnocellular neurons. Such taurine flux should influence global release of taurine from SON, which cannot be evidenced (Deleuze et al., 1998). The blockade of ClC3 by tamoxifen, protein kinase C and protein kinase A (Duan et al., 1997; Nagasaki et al., 2000) are also at variance with the properties of taurine release from SON astrocytes (Deleuze et al., 2000), further arguing against a correlation with this protein. The question subsists as to the physiological role of these Cl− channel candidates in SON neurons, keeping in mind that the level of mRNA might not be fully representative of the level of protein expression. Indeed, despite extensive work over the past two decades on the osmosensitivity of SON neurons, volume-activated anion channels have never been reported on these cells (Bourque & Oliet, 1997). If ClC2 has been postulated a role in the regulation of the Cl− equilibrium potential in neurons (Staley et al., 1996), this would have to be confirmed in the present preparation. The function of the three other membrane proteins is yet to be defined.
In conclusion, the specificity of the pharmacological profile of the taurine efflux pathway of SON astrocytes, together with its peculiar regulation by tyrosine kinase, may suggest the expression of a particular anion channel in these cells. None of the proteins postulated to underlie volume-sensitive anion channels is likely to be involved in the taurine efflux from SON glial cells. Until recently, only ClC3 had been convincingly associated with a VSOAC subtype (Duan et al., 1997). However, given the ongoing controversy about the true nature of ClC3 (see Jentsch et al., 1999), plus the fact that the ClC family does not appear to be able to account for the diversity of the VSOAC described so far, it seems reasonable to speculate that most, if not all, VSOAC have still to find their molecular match. We would favour the idea of a yet to be identified protein family of which the SON glial channel would be a particular subtype.
Acknowledgments
We thank M.G. Desarménien for critical reading of the manuscript, and E. Bourinet for kindly providing mibefradil. This work was supported in part by the Centre National d'Etudes Spatiales (CNES no. 98/7346/793).
Abbreviations
- AVP
vasopressin
- DDF
dideoxyforskolin
- DIDS
4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid
- DPC
N-phenylanthranylic acid
- GES
guanidinoethyl sulphonate
- GFAP
glial fibrillary acidic protein
- NPPB
5-nitro-2-(3-phenylpropylamino)benzoic acid
- pBPB
4-bromophenacyl bromide
- RT–PCR
reverse transcription-polymerase chain reaction
- SON
supraoptic nucleus
- VSOAC
volume-sensitive organic osmolyte and anion channel
References
- BANDELARI U., ROY G. Anion channels for amino acids in MDCK cells. Am J. Physiol. 1992;263:C1200–C1207. doi: 10.1152/ajpcell.1992.263.6.C1200. [DOI] [PubMed] [Google Scholar]
- BOND T.D., AMBIKAPATHY S., MOHAMMAD S., VALVERDE M.A. Osmosensitive Cl− currents and their relevance to regulatory volume decrease in human intestinal T84 cells: outwardly vs inwardly rectifying currents. J. Physiol. 1998;511:45–54. doi: 10.1111/j.1469-7793.1998.045bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOURQUE C.W., OLIET S.H.R. Osmoreceptors in the central nervous system. Annu. Rev. Physiol. 1997;59:601–619. doi: 10.1146/annurev.physiol.59.1.601. [DOI] [PubMed] [Google Scholar]
- CLAPHAM D.E. The list of potential volume-sensitive chloride currents continues to swell (and shrink) J. Gen. Physiol. 1998;111:623–624. doi: 10.1085/jgp.111.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLOMBINI M., BLACHY-DYSON E., FORTE M. VDAC, a channel in the outer mitochondrial membrane Ion channels 1996Plenum Publishing Corp.: New York, NY; 4, 169–202.ed. Narahashi, T. pp [DOI] [PubMed] [Google Scholar]
- CREPEL V., PANENKA W., KELLY M.E.M., MACVICAR B.A. Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. J. Neurosci. 1998;18:1196–1206. doi: 10.1523/JNEUROSCI.18-04-01196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECAVEL C., HATTON G.I. Taurine immunoreactivity in the rat supraoptic nucleus: prominent localization in glial cells. J. Comp. Neurol. 1995;354:13–26. doi: 10.1002/cne.903540103. [DOI] [PubMed] [Google Scholar]
- DELEUZE C., DUVOID A., HUSSY N. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J. Physiol. 1998;507:463–471. doi: 10.1111/j.1469-7793.1998.463bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELEUZE C., DUVOID A., MOOS F.C., HUSSY N. Tyrosine phosphorylation modulates the osmosensitivity of volume-dependent taurine efflux from glial cells in the rat supraoptic nucleus. J. Physiol. 2000;523:291–299. doi: 10.1111/j.1469-7793.2000.t01-2-00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERMIETZEL R., HWANG T.-K., BUETTNER R., HOFER A., DOTZLER E., KREMER M., DEUTZMANN R., THINNES F.P., FISHMAN G.I., SPRAY D.C., SIEMEN D. Cloning and in situ localization of a brain-derived porin that constitutes a large-conductance anion channel in astrocytic plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 1994;91:499–503. doi: 10.1073/pnas.91.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUAN D., WINTER C., COWLEY S., HUME J.R., HOROWITZ B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- GOSLING M., SMITH J.W., POYNER D.R. Characterization of a volume-sensitive chloride current in rat osteoblast-like (ROS 17/2.8) cells. J. Physiol. 1995;485:671–682. doi: 10.1113/jphysiol.1995.sp020761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRÜNDER S., THIEMANN A., PUSCH M., JENTSCH T.J. Regions involved in the opening of ClC-2 chloride channel by voltage and cell volume. Nature. 1992;360:759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- HATTON G.I. Astrological modulation of neurotransmitter/peptide release from the neurohypophysis: present status. J. Chem. Neuroanat. 1999;16:203–222. doi: 10.1016/s0891-0618(98)00067-2. [DOI] [PubMed] [Google Scholar]
- HUSSY N., DELEUZE C., PANTALONI A., DESARMÉNIEN M.G., MOOS F. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmo-regulation. J. Physiol. 1997;502:609–621. doi: 10.1111/j.1469-7793.1997.609bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON P.S., STRANGE K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am. J. Physiol. 1993;265:C1489–C1500. doi: 10.1152/ajpcell.1993.265.6.C1489. [DOI] [PubMed] [Google Scholar]
- JALONEN T. Single-channel characteristics of the large-conductance anion channel in rat cortical astrocytes in primary culture. Glia. 1993;9:227–237. doi: 10.1002/glia.440090308. [DOI] [PubMed] [Google Scholar]
- JENTSCH T.J., FRIEDRICH T., SCHRIEVER A., YAMADA H. The CLC chloride channel family. Pflügers Arch. 1999;437:783–795. doi: 10.1007/s004240050847. [DOI] [PubMed] [Google Scholar]
- KIRK K. Swelling-activated organic osmolyte channels. J. Memb. Biol. 1997;158:1–16. doi: 10.1007/s002329900239. [DOI] [PubMed] [Google Scholar]
- KIRK K., STRANGE K. Functional properties and physiological roles of organic solute channels. Annu. Rev. Physiol. 1998;60:719–739. doi: 10.1146/annurev.physiol.60.1.719. [DOI] [PubMed] [Google Scholar]
- LAMBERT I.H., HOFFMANN E.K. Cell swelling activates separate taurine and chloride channels in Ehrlich mouse ascites tumor cells. J. Memb. Biol. 1994;142:289–298. doi: 10.1007/BF00233436. [DOI] [PubMed] [Google Scholar]
- LEANEY J.L., MARSH S.J., BROWN D.A. A swelling-activated chloride current in rat sympathetic neurones. J. Physiol. 1997;501:555–564. doi: 10.1111/j.1469-7793.1997.555bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANOLOPOULOS V.G., VOETS T., DECLERCQ P.E., DROOGMANS G., NILIUS B. Swelling-activated efflux of taurine and other organic osmolytes in endothelial cells. Am. J. Physiol. 1997;273:C214–C222. doi: 10.1152/ajpcell.1997.273.1.C214. [DOI] [PubMed] [Google Scholar]
- MOON J.I., JUNG Y.W., KO B.H., DE PINTO V., JIN I., MOON I.S. Presence of a voltage-dependent anion channel 1 in the rat postsynaptic density fraction. Neuroreport. 1999;10:443–447. doi: 10.1097/00001756-199902250-00001. [DOI] [PubMed] [Google Scholar]
- MOORMAN J.R., ACKERMAN S.J., KOWDLEY G.C., GRIFFIN M.P., MOUNSEY J.P., CHEN Z., CALA S.E., O'BRIAN J.J., SZABO G., JONES L.R. Unitary currents through phospholemman channel molecules. Nature. 1995;377:737–740. doi: 10.1038/377737a0. [DOI] [PubMed] [Google Scholar]
- NAGASAKI M., YE L., DUAN D., HOROWITZ B., HUME J.R. Intracellular cyclic AMP inhibits native and recombinant volume-regulated chloride channels from mammalian heart. J. Physiol. 2000;523:705–717. doi: 10.1111/j.1469-7793.2000.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., EGGERMONT J., VOETS T., BUYSE G., MANOLOPOULOS V., DROOGMANS G. Properties of volume-regulated anion channels in mammalian cells. Prog. Biophys. Molec. Biol. 1997a;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., KAMOUCHI M., VIANA F., VOETS T., DROOGMANS G. Inhibition by mibefradil, a novel calcium channel antagonist, of Ca2+ - and volume-activated Cl− channels in macrovascular endothelial cells. Br. J. Pharmacol. 1997b;121:547–555. doi: 10.1038/sj.bjp.0701140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., SEHRER J., VIANA F., DE GREEF C., RAEYMAEKERS L., EGGERMONT J., DROOGMANS G. Volume-activated Cl− currents in different mammalian non-excitable cell types. Pflügers Arch. 1994;428:364–371. doi: 10.1007/BF00724520. [DOI] [PubMed] [Google Scholar]
- OKADA Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am. J. Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- OKADA Y., OIKI S., HAZAMA A., MORISHIMA S. Criteria for the molecular identification of the volume-sensitive outwardly rectifying Cl− channel. J. Gen. Physiol. 1998;112:365–367. doi: 10.1085/jgp.112.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASANTES-MORALES H., MURRAY R.A., LILJA L., MORÁN J. Regulatory volume decrease in cultured astrocytes. I. Potassium- and chloride-activated permeability. Am. J. Physiol. 1994;266:C165–C171. doi: 10.1152/ajpcell.1994.266.1.C165. [DOI] [PubMed] [Google Scholar]
- PASANTES-MORALES H., SCHOUSBOE A. Role of taurine in osmoregulation in brain cells: mechanisms and functional implications. Amino Acids. 1997;12:281–292. [Google Scholar]
- ROY G. Amino acid current through anion channels in cultured human glial cells. J. Memb. Biol. 1995;147:35–44. doi: 10.1007/BF00235396. [DOI] [PubMed] [Google Scholar]
- SÁNCHEZ-OLEA R., MORALES M., GARCÍA O., PASANTES-MORALES H. Cl channel blockers inhibit the volume-activated efflux of Cl and taurine in cultured neurons. Am. J. Physiol. 1996;270:C1703–C1708. doi: 10.1152/ajpcell.1996.270.6.C1703. [DOI] [PubMed] [Google Scholar]
- SÁNCHEZ-OLEA R., PEÑA C., MORÁN J., PASANTES-MORALES H. Inhibition of volume regulation and efflux of osmoregulatory amino acids by blockers of Cl− transport in cultured astrocytes. Neurosci. Lett. 1993;156:141–144. doi: 10.1016/0304-3940(93)90458-w. [DOI] [PubMed] [Google Scholar]
- SMITH R.L., CLAYTON G.H., WILCOX C.L., ESCUDERO K.W., STALEY K.J. Differential expression of an inwardly rectifying chloride conductance in rat brain neurons: a potential mechanism for cell-specific modulation of postsynaptic inhibition. J. Neurosci. 1995;15:4057–4067. doi: 10.1523/JNEUROSCI.15-05-04057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STALEY K., SMITH R., SCHAACK J., WILCOX C., JENTSCH T.J. Alteration of GABAA receptor function following gene transfer of the CLC-2 chloride channel. J. Neurosci. 1996;17:543–551. doi: 10.1016/s0896-6273(00)80186-5. [DOI] [PubMed] [Google Scholar]
- STRANGE K. Molecular identity of the outwardly rectifying, swelling-activated anion channel: time to reevaluate pICln. J. Gen. Physiol. 1998;111:617–622. doi: 10.1085/jgp.111.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE K., EMMA F., JACKSON P.S. Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- STUTZIN A., TORRES R., OPORTO M., PACHECO P., EGUIGUREN A.L., PABLO CID L., SEPÚLVEDA F.V. Separate taurine and chloride efflux pathways activated during regulatory volume decrease. Am. J. Physiol. 1999;277:C392–C402. doi: 10.1152/ajpcell.1999.277.3.C392. [DOI] [PubMed] [Google Scholar]
- TILLY B.C., VAN DEN BERGHE N., TERTOOLEN L.G.J., EDIXHOVEN M.J., DE JONGE H.R. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J. Biol. Chem. 1993;268:19919–19922. [PubMed] [Google Scholar]
- VOETS T., MANOLOPOULOS V., EGGERMONT J., ELLORY C., DROOGMANS G., NILIUS B. Regulation of a swelling-activated chloride current in bovine endothelium by protein ty-rosine phosphorylation and G proteins. J. Physiol. 1998;506:341–352. doi: 10.1111/j.1469-7793.1998.341bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


