Abstract
Striatal microdialysate levels of dopamine (DA) in conscious guinea-pigs were measured following acute (1 day) and chronic (21 days) treatment with deprenyl (2 and 0.25 mg kg−1 s.c., respectively) or clorgyline (4 and 1 mg kg−1 s.c., respectively), as well as by combination treatment using the same doses of the two inhibitors. These treatments caused selective inhibition of monoamine oxidase type B (MAO-B) or monoamine oxidase type A (MAO-A) respectively.
Neither acute nor chronic treatments with deprenyl or clorgyline increased basal or KCl-induced DA levels. Acute and chronic clorgyline treatments were accompanied by significant reductions in striatal microdialysate 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). On the other hand, both acute and chronic deprenyl treatments were accompanied by significant increases in microdialysate HVA with no effect on DOPAC levels.
Acute or chronic combined treatment with clorgyline and deprenyl increased tissue but not microdialysate DA levels. The combination treatment given chronically also reduced KCl-induced DA release but enhanced amphetamine-induced DA release.
Microdialysate DA levels increased to a smaller extent in guinea-pig than in rat following local striatal infusion of GBR-12909 (100 μM).
The difference between guinea-pigs and rats in the response to GBR-12909, could be the result of a lower dopaminergic innervation and/or density of DA transporter. This difference may explain why striatal microdialysate DA levels increased following chronic deprenyl treatment in the rat but not in the guinea-pig.
Keywords: Selegiline, clorgyline, MAO-A, MAO-B, dopamine, microdialysis, GBR-12909, amphetamine, tyrosine hydroxylase
Introduction
Administration of the selective monoamine oxidase type B (MAO-B) inhibitor drug, deprenyl (selegiline), to human parkinsonian patients causes a symptomatic antiparkinsonian response, indicative of a dopaminergic effect in the striatum (Myllyla et al., 1992; Olanow et al., 1996). However, the way in which subtype-selective MAO inhibition affects synaptic dopamine (DA) levels is not completely understood. Dopamine is a substrate for both monoamine oxidase type A (MAO-A) and MAO-B in about equal affinities (O'Carroll et al., 1983). Most microdialysis studies on the effect of selective MAO subtype inhibition on striatal microdialysate levels have been carried out in the rat, and have shown an increase in extracellular DA levels following selective MAO-A, but not MAO-B inhibition (Butcher et al., 1990; Colzi et al., 1990; Kato et al., 1986). Since most released DA is taken up back into the dopaminergic terminal by the high-affinity DA transporter (DAT), this is consistent with intraneuronal metabolism of DA by MAO-A, since this is the enzyme form localized in neurones of the dopaminergic system (Levitt et al., 1982).
In our previous study in the rat (Lamensdorf et al., 1996), we found that chronic (but not acute) administration of deprenyl as well as clorgyline, in doses selective for inhibition of MAO-B and -A respectively, resulted in an increased DA striatal microdialysate level. One possible explanation for this enhancing effect of deprenyl on DA release is that this substance increases catecholamine release by an intrinsic property of the molecule, not connected to inhibition of MAO-B (Knoll, 1987; Knoll et al., 1996). However, a second selective MAO-B inhibitor, rasagiline, which is free of the amphetamine-like properties of deprenyl, also caused an increase in striatal extracellular DA levels on chronic administration (Lamensdorf et al., 1996). Our explanation of the effect of chronic deprenyl and rasagiline to elevate striatal extracellular DA was that the chronic MAO-B inhibition leads to the build up of endogenous β-phenylethylamine (PEA), which acts both to inhibit DA reuptake and to cause its release from storage granules, as proposed by Paterson (Berry et al., 1994; Paterson et al., 1991).
Since the MAO-A/MAO-B ratio in the guinea-pig striatum is similar to that of the human striatum, i.e. about 80% MAO-B, the guinea-pig striatum is a better model for human striatal DA metabolism than the rat (Azzaro et al., 1985; Ross, 1987). Also, the ratio of homovanillic acid/3,4-dihydroxyphenylacetic acid (HVA/DOPAC) in guinea-pig striatum is similar to that in man, with about five times more HVA than DOPAC, but in the rat there is a greater amount of DOPAC than HVA (Wetherell et al., 1989). Accordingly, we decided to determine the effect of acute and chronic selective inhibition of MAO-A and MAO-B (or both together), on DA basal release, and on KCl-induced DA release, in the guinea-pig striatum. We also examined additional processes associated with synaptic DA levels, such as synthesis, neuronal uptake and amphetamine-induced release.
Methods
Animals and drug treatments
Male English Short Hair guinea-pigs (350–400 g) were used in all experiments. Animals were housed in temperature-controlled rooms, maintained on a 12 h light/dark cycle (lights on 0700 h, off 1900 h) and were given food and water ad libitum. Drugs were given by s.c. injection. All animals received a total of 21 daily injections with saline or drug. Control animals received daily saline injections for 21 days, acutely-treated animals received saline for 20 days followed by drug on day 21, and chronically-treated animals received drug for 21 days. Doses of deprenyl and clorgyline differed between acute and chronic administrations, so that a similar degree of enzyme inhibition was obtained with each type of treatment. The doses of clorgyline in acute and chronic treatments were 4 and 1 mg kg−1, and of deprenyl, 2 and 0.25 mg kg−1, respectively. All experimental protocols were approved by the Animal Experimentation Committee of the Technion, Israel Institute of Technology.
Microdialysis procedure
Animals were anaesthetized with pentobarbitone/chloral hydrate solution (12/60 mg kg−1 i.p.) on day 21 of drug or saline treatment. Microdialysis probes (4 mm active length, Lamensdorf et al., 1996) were placed in the left striatum using the coordinates: A −1.3, L 3.2, V −0.75 (with respect to bregma). The probes were perfused with artificial CSF (composition in mM: NaCl 150, CaCl2 1.7, KCl 3.0, MgCl2 0.9) at a rate of 2 μl min−1. Following recovery, the animals were maintained in glass-sided cages. Microdialysate samples were collected 24 h after operation into 0.5 ml polyethylene vials containing 10 μl of 0.1 M perchloric acid, over 20 min periods. After the dialysis experiment, animals were decapitated, and correct position of the microdialysis probe verified. The striatum was rapidly dissected out on ice, the left side taken for determination of MAO activity (see below) and the right side was homogenized in 600 μl 0.1 M perchloric acid. After centrifugation of the tissue homogenate, 100 μl of the supernatant layer were removed for assay by h.p.l.c. with electrochemical detection of DA, DOPAC and HVA.
Dialysate analysis
Samples of microdialysate or tissue homogenate (20 μl) were injected into the solvent stream of an h.p.l.c. apparatus equipped with a Microsorb column (packing 3 μm, 4.6 mm diameter, 12.5 cm long). The mobile phase was composed of NaH2PO4 13.6 g, heptane-1-sulphonic acid 200 mg, disodium ethylenediaminetetracetic acid 100 mg, methanol 15 ml, acetonitrile 10 ml per 1l h.p.l.c. grade deionized water, at a flow rate of 1.5 ml min−1. Compounds were detected with an ESA Coulochem model 5014 detector (Bedford, MA, U.S.A.). Column eluates were initially oxidized by an ESA guard cell (model 5020) at +300 mV. The first electrode of the model 5014 analytical cell was set at +60 mV and compounds of interest were measured at electrode 2 set at −250 mV.
Determination of MAO activity
MAO activity was measured in vitro using a modification (O'Carroll et al., 1983) of the method of Otsuka & Kobayashi (1964). Striatal tissue was homogenized in 0.32 M sucrose.
Tissue aliquots were diluted appropriately in phosphate buffer (0.1 M, pH 7.4) and preincubated for 1 h at 37°C. An additional 20 min (MAO-B) or 30 min (MAO-A) incubation was performed in the presence of the appropriate radiolabelled substrate (14C-PEA 40 μM for MAO-B or 14C-5-hydroxytryptamine 400 μM for MAO-A). The reaction was terminated by the addition of 2 M citric acid. Samples were centrifuged, and radiolabelled deaminated metabolites were extracted into toluene/ethyl acetate 1 : 1 v v−1. Labelled metabolites in the organic phase were determined by liquid scintillation counting following addition of 2,5-diphenyloxazole solution (2% w v−1).
Determination of tyrosine hydroxylase activity
Tyrosine hydroxylase activity in the striatum was measured ex-vivo using the method described by Hayashi et al. (1988). Accumulation of striatal 3,4-dihydroxyphenylalanine (DOPA) was measured by h.p.l.c. 30 min after NSD-1015 injection (100 mg kg−1 i.p.).
3H-Dopamine uptake
Determination of 3H-DA uptake was carried out as described by Fang & Yu (1994), following preparation of synaptosomes as described by Cooper & Carlson (1983). A 50 μl aliquot of the synaptosomal-rich suspension was added to 0.35 ml of Krebs'-Henseleit buffer containing GBR-12909 (10−3–10−12 M), and preincubated for 5 min at 37°C in a shaking water bath. The incubation was continued for an additional 5 min in the presence of 0.1 μM 3H-DA, and uptake terminated by addition of cold 0.32 M sucrose (1 ml) and filtration on GF/B filters. The filters were added to 4 ml Optiflor (Packard, Groningen, The Netherlands), and analysed for 3H by liquid scintillation counting.
Statistical analysis
Monoamine oxidase activity in striatum from drug-treated guinea-pigs was expressed as a percentage of activity in that of control animals. Statistical significance of the calculated activities was tested using one-way analysis of variance (ANOVA). Other data are expressed as mean values±s.e.mean. The significance of differences between treated and control animals' tissue and microdialysate amine and metabolite levels was determined by ANOVA followed by Dunnett's test. The effect of KCl-stimulated release was calculated by subtracting the average of four baseline collections from the levels obtained at 20, 40 and 60 min after infusion of the KCl bolus, and the significance of difference between control and treated animals at each time point was estimated using ANOVA followed by Dunnett's test. For comparison of microdialysate DA between rats and guinea-pigs, Students' t-test was used. The level of statistical significance was set at a probability value of 0.05.
Materials
Deprenyl hydrochloride and (±)-amphetamine sulphate were gifts of Teva (Jerusalem, Israel). 14C-5HT, 14C-PEA and 3H-DA were purchased from New-England-Nuclear Dupont (Boston, MA, U.S.A.). 3-Hydroxybenzylhydrazine (NSD-1015) and 1-[2-bis(4-fluorophenyl)methoxy]ethyl]-4-[3-phenylpropyl]piperazine dihydrochloride (GBR-12909) were purchased from Research Biochemicals International (Natick, MA, U.S.A.). All other reagents and chemicals were purchased from either Sigma (Natick, MA, U.S.A.) or Merck (Darmstadt, Germany) and were analytical or h.p.l.c. grade.
Results
Inhibition of striatal MAO activity by MAO inhibitor treatments
Both acute and chronic treatments with clorgyline or deprenyl resulted in selective inhibition of the appropriate enzyme subtype (Figure 1). Acute and chronic treatments with clorgyline resulted in some degree of inhibition of MAO-B which, however, was not statistically significant.
Figure 1.
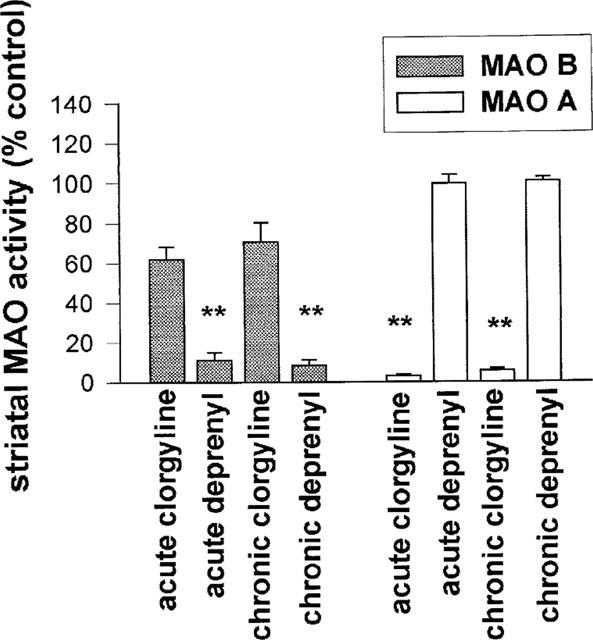
Effect of clorgyline and deprenyl on MAO enzyme activity in guinea-pig striatal tissue 30 h after last dose of inhibitor. Doses of MAO inhibitors used were: acute clorgyline 4 mg kg−1, chronic clorgyline 1 mg kg−1, acute deprenyl 2 mg kg−1, chronic deprenyl 0.25 mg kg−1 (acute treatment=single dose, chronic treatment=21 daily doses, s.c.). Results expressed as per cent enzyme activity in control animals (mean±s.e.mean for n=6–12 animals per group). **P<0.01 for difference from control animals.
Basal and KCl-stimulated microdialysate catecholamine levels
The acute or chronic administration of deprenyl or clorgyline, or a mixture of both, did not result in a significant change in basal striatal microdialysate concentration of DA by comparison with the control group (Figure 2a). Acute and chronic clorgyline treatment, as well as acute and chronic combination treatments, but neither acute nor chronic deprenyl treatments, were accompanied by significant reductions in striatal microdialysate DOPAC (Figure 2b). On the other hand, both acute and chronic deprenyl treatments were accompanied by significant increases in microdialysate HVA, whereas treatment with clorgyline, or combination of clorgyline and deprenyl, was accompanied by reductions in HVA (Figure 2c). Potassium-stimulated DA release was not significantly changed by acute or chronic treatment with clorgyline, or chronic deprenyl, but acute deprenyl caused a slight but significant (P<0.05) reduction. The acute combination treatment also had no effect on KCl-induced release, but chronic treatment with a combination of deprenyl and clorgyline produced a highly significant decrease (P<0.01) in KCl-induced DA release measured 40 min after the start of KCl infusion (Figure 3a,b).
Figure 2.
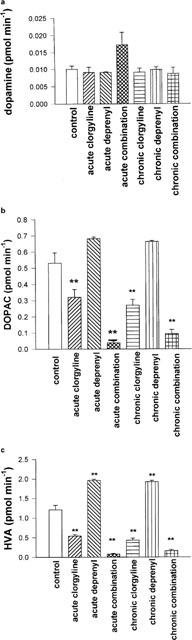
(a-c) Effect of acute and chronic treatment with MAO inhibitors on basal striatal microdialysate levels of DA (a), DOPAC (b) and HVA (c) in the guinea-pig. Results shown are mean±s.e.mean of four 20 min microdialysate collections from each guinea-pig (n=6–12 guinea-pigs in each group), commencing 24 h after last dose of MAO inhibitor. Doses of MAO inhibitors used are as shown in legend to Figure 1. Statistical analysis was performed using ANOVA and Dunnett's test. **=P<0.01 for comparison with control group.
Figure 3.
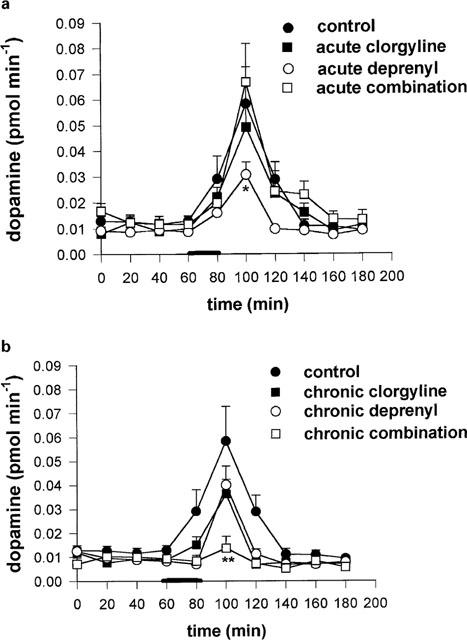
(a,b) Effect of acute (a) or chronic (b) MAO inhibition on KCl induced DA release in striatum of guinea-pigs. An artificial CSF containing 90 mM KCl was perfused for 20 min at period shown by horizontal bar. Doses of MAO inhibitors used are as shown in legend to Figure 1. Results shown are mean±s.e.mean for n=6–11 animals per group. Statistical analysis was performed at each time interval, on incremental effect above mean baseline value using ANOVA followed by Dunnett's test. *=P<0.05; **=P<0.01.
Steady-state tissue catecholamine levels
Administration of clorgyline alone, acutely as well as chronically, did not result in a significant change in striatal tissue content of DA. Acute deprenyl caused no change in tissue DA, but chronic deprenyl caused a small but significant (P<0.05) reduction. A combination of deprenyl and clorgyline in acute or chronic adminstration resulted in elevated striatal tissue content of DA (Figure 4a). Acute or chronic treatment with deprenyl produced no changes in tissue DOPAC or HVA, except for a small reduction in DOPAC levels following chronic deprenyl (P<0.05). Clorgyline, both acutely and chronically, caused significant reductions in tissue contents of both metabolites (Figure 4b). Combination treatment (acute and chronic) markedly suppressed tissue contents of DOPAC and HVA.
Figure 4.
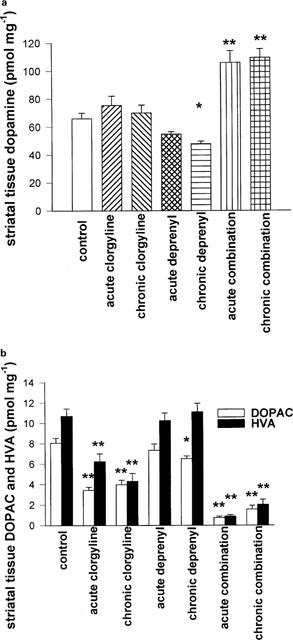
(a,b) Effect of different treatments with MAO inhibitors on striatal tissue content of DA (a), DOPAC and HVA (b) in the guinea-pig. Tissue levels were determined 24 h after last dose of MAO inhibitor. Doses of MAO inhibitors used are as shown in Figure 1. Results shown are mean±s.e.mean for n=6–12 animals per group. Statistical analysis was performed using ANOVA followed by Dunnett's test. *=P<0.05; **=P<0.01, for comparison with control group.
Striatal tyrosine hydroxylase activity
Chronic treatment with a combination of deprenyl and clorgyline resulted in a 60±3.2 (n=15; P<0.001) per cent reduction in DOPA striatal tissue content measured 30 min after NSD-1015 injection. However, no significant difference in DOPA striatal tissue content was detected as a result of other treatments with deprenyl or clorgyline (data not shown; n=6–9 animals per group).
Comparison of the effect of GBR-12909 on microdialysate DA and synaptosomal 3H-DA uptake in rats and guinea-pigs
Inclusion of GBR-12909 (100 μM) in the artificial CSF caused an increase in DA microdialysate levels both in guinea-pigs and rats. However, there was a notable difference in the extent of DA microdialysate elevation between the two species, 167±3.9% in guinea-pigs and 377±30.2% in rats, in comparison to their basal DA release (n=5 animals per group; P<0.0001 for comparison between rats and guinea-pigs; Figure 5).
Figure 5.
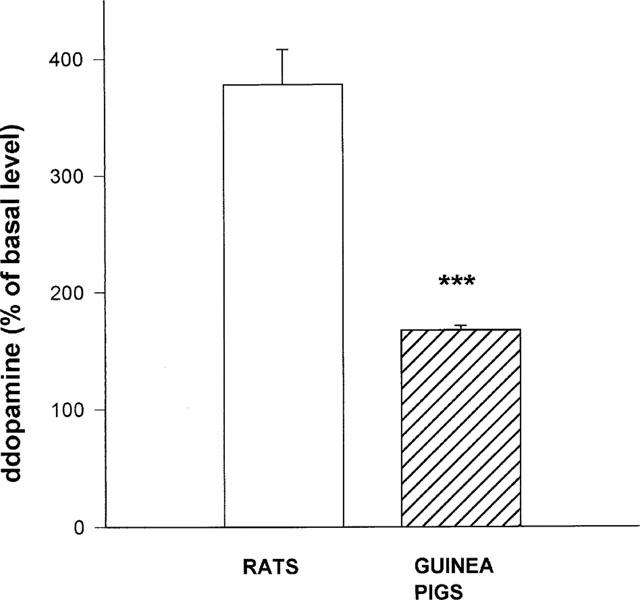
Striatal DA release in the rat and guinea-pig following administration of GBR-12909. An artificial CSF containing GBR-12909 (100 μM) was perfused for 40 min, then microdialysate samples were collected for an additional 20 min. Results are mean±s.e.mean of the number of animals in each group (5–6). ***=P<0.0001 for comparison between rats and guinea-pigs (Students' t-test).
The inhibition curves of GBR-12909 for 3H-DA uptake into synaptosomes from striatal tissue of guinea-pig and rat were identical for both species. The IC-50 values were equal to about 1 nM (data not shown; n=6 separate experiments for each species).
Effect of local amphetamine administration in guinea-pigs treated with deprenyl-clorgyline combination
Striatal DA release following perfusion with amphetamine (10 μM for 20 min) in the microdialysis cannula was significantly higher in guinea-pigs that were chronically treated with a combination of deprenyl and clorgyline, by comparison with control animals (1806±199 and 748±115% respectively, measured 20–40 min after amphetamine bolus; n=6 for each treatment, P<0.001).
Discussion
The present results showing the effects of acute and chronic treatments with deprenyl and clorgyline in the guinea-pig show a marked difference from the findings in our previous work in the rat (Lamensdorf et al., 1996). In the rat, chronic (but not acute) inhibition of either MAO-A or MAO-B, by treatment with clorgyline, deprenyl or another selective MAO-B inhibitor, rasagiline, was accompanied by significant elevations in basal striatal microdialysate DA concentration, as well as by increases in KCl-evoked DA release. In the case of the guinea-pig, neither basal nor KCl-induced microdialysate DA was elevated by inhibitors of MAO-A or MAO-B administered separately or together, in either acute or chronic administration.
We were not able to determine all potential metabolites of DA in the current study (e.g. conjugates, 3-methoxytyramine), due to the difficulty of processing the small amounts of microdialysis fluid, and the low concentrations of some of these metabolites. However, it was significant that HVA levels were increased by both acute and chronic deprenyl treatments. Since DOPAC levels were not reduced, and even showed a non-significant trend to increase, this indicates that DA release was enhanced by deprenyl, but without a significant increase in microdialysate DA concentration, or in KCl-evoked release. Tissue amine and metabolite levels to a large extent resembled the microdialysate data, since DOPAC and HVA tissue and microdialysate levels were reduced by clorgyline but not by deprenyl separately, as well as by the combination treatments.
Our findings differ from those of previous workers (Juorio et al., 1994) who found reductions in DOPAC and HVA concentrations in striatal microdialysate following acute deprenyl treatment in the guinea-pig. However, in the study of Juorio et al. (1994) a dose of 4 mg kg−1 deprenyl was administered 2 h before microdialysis, and microdialysate collections were commenced immediately after surgery for probe implantation. We studied tissue and microdialysate levels of DA and metabolities 24 h after MAO inhibitor administration and probe implantation, in order to avoid acute effects of anaesthetic and other pharmacological actions of the inhibitors. As described previously (Finberg et al., 1981; 1982) both deprenyl and clorgyline possess other pharmacological actions in addition to irreversible MAO inhibition. By conducting our microdialysis collections 24 h after drug administration, we were able to study the effect of MAO inhibition in isolation from pharmacological effects of the inhibitor molecules on amine uptake or release, since most of the administered drug and its metabolites would have left the animal's body by this time. Juorio et al. (1994) also showed that levels of endogenous PEA were not increased after single doses of clorgyline of up to 8 mg kg−1 s.c., reinforcing our data showing a lack of significant inhibition of MAO-B at 24 h after a single dose of 4 mg kg−1.
Our results on the effect of single-dose MAO inhibitor treatment on striatal tissue DA and metabolite levels are similar to those of Azzaro et al. (1985), but since our study was based on microdialysis determinations in animals treated chronically with the inhibitors, we present here also the tissue DA and metabolite levels following chronic as well as acute treatments. In our previous study in the rat, we proposed that the reason for increased microdialysate DA levels following MAO-B inhibition was an increase in endogenous PEA levels, leading to inhibition of DA reuptake, as a result of competition between DA and PEA at the DA transporter sites. According to this view, the increase in microdialysate DA following MAO-B inhibition is the result of a reduction in reuptake by the DAT. In the case of MAO-A inhibition in the rat, because of the neuronal location of MAO-A (Levitt et al., 1982), microdialysate DA levels may increase as a result of the increased axoplasmatic DA levels, leading to an increase in efflux and a reduction in net reuptake from the synaptic cleft. As is well known, the major factor controlling striatal microdialysate DA levels in the rat is the activity of the neuronal DAT (Smith & Justice, 1994). It occurred to us that in the guinea-pig, the influence of the transporter on microdialysate DA might be different from the rat. Accordingly, we designed an experiment to check this hypothesis, by comparing the effects of the DAT inhibitor, GBR-12909, on striatal microdialysate DA in rat and guinea-pig. Initially, we tested the ability of the inhibitor to reduce DA uptake in synaptosomes prepared from rat and guinea-pig striatal tissue, and found a similar inhibitory activity in both species. Subsequently, we tested the effect of locally-infused GBR-12909 to enhance microdialysate DA levels in rat and guinea-pig, and found a markedly lower effect of the inhibitor to increase DA levels in the guinea-pig. The reduced effect of GBR-12909 on striatal DA in the guinea-pig as compared with the rat could be the result of various factors. One possibility is an altered synaptic architecture, whereby an increased synaptic cleft width in guinea-pig striatal dopaminergic neurons would lead to a reduced effect of DAT inhibition on synaptic DA levels, as proposed by Kopin for sympathetic neurons of different tissues (Kopin et al., 1978). Another possibility is an altered distribution of DAT at the dopaminergic terminal in the two species, i.e., a lower concentration of DAT at the synaptic area in guinea-pig striatal DA neurons. Such a difference in DAT distribution between different brain areas of the rat was recently shown by Sesack et al. (1998) for prefrontal cortex and striatum.
Another puzzling observation was that KCl-induced augmentation of DA microdialysate levels was inhibited following combined MAO-A and MAO-B inhibition, although amphetamine-induced release was enhanced. This effect was accompanied by a decrease in DA synthesis rate, as shown by reduced levels of DOPA following administration of a decarboxylase inhibitor (NSD-1015), and by elevated tissue DA level. These data may be explained by the fact that KCl-induced release is mainly from the recently synthesized pool of DA (Fairbrother et al., 1990), which could have been depleted by the reduction in synthesis rate. Amphetamine releases from both vesicular and cytoplasmic pools, which are increased by MAO-inhibition. In addition, however, an effect of MAO inhibition to enhance inhibitory presynaptic or somatodendritic receptor tone could explain the reduction in KCl-induced release. An inhibitory effect on striatal DA release can be exerted both at the level of axonal prejunctional receptors on DA neurones as well as in the midbrain. It is interesting that in slices of rat midbrain, inhibition of both types of MAO was found to be effective in causing inhibition of DA neuronal activity via activation of D2/D3 receptors, probably by increased levels of endogenous DA (Mercuri et al., 1996). Such an effect could also be produced by the in vivo treatment with MAO-A and MAO-B inhibitors, and could result in a reduction in axonal DA release in the striatum.
Recently, Chen et al. (1999) showed that striatal extracellular DA levels were not enhanced in mice lacking the MAO-B gene. In the absence of data in control wild-type mice administered MAO-B inhibitors, however, we cannot presently conclude whether normal mice resemble guinea-pigs or rats in their response to drug-induced MAO-B inhibition, or whether the lack of increase in striatal DA in MAO-B knockout animals is because the striatal DA levels are suppressed because of some secondary change resulting from absence of the enzyme during foetal development.
In conclusion, control of striatal DA levels in the guinea-pig differs from that in the rat, in that neither MAO-A nor MAO-B inhibition given chronically significantly enhances extracellular fluid levels of the free amine in the guinea-pig striatum. Our study points to an increased neuronal release of DA following MAO-B inhibition in the guinea-pig. Confirmation of this finding necessitates collection of all potential metabolites of DA, including 3-methoxytyramine, and conjugates. Finally, the level of extracellular DA is controlled to a lesser extent by the activity of DAT in the guinea-pig than in the rat.
Acknowledgments
This study was in part requirement for the M.Sc. degree (Technion) of T. Ilani.
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- DOPA
3,4-dihydroxyphenylalanine
- DOPAC
3,4-dihydroxyphenylacetic acid
- HVA
homovanillic acid
- MAO-A
monoamine oxidase type A
- MAO-B
monoamine oxidase type B
- PEA
β-phenylethylamine
References
- AZZARO A.J., KING J., KOTZUK J., SCHOEPP D.D., FORST J., SCHOCHET S. Guinea-pig striatum as a model of human dopamine deamination: the role of monoamine oxidase isozyme ratio, localization, and affinity for substrate in synaptic dopamine metabolism. J. Neurochem. 1985;45:949–956. doi: 10.1111/j.1471-4159.1985.tb04086.x. [DOI] [PubMed] [Google Scholar]
- BERRY M.D., SCARR E., ZHU M.Y., PATERSON I.A., JUORIO A.V. The effects of administration of monoamine oxidase-B inhibitors on rat striatal neuron responses to dopamine. Br. J. Pharmacol. 1994;113:1159–1166. doi: 10.1111/j.1476-5381.1994.tb17119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTCHER S.P., FAIRBROTHER I.S., KELLY J.S., ARBUTHNOTT G.W. Effects of selective monoamine oxidase inhibitors on the in vivo release and metabolism of dopamine in the rat striatum. J. Neurochem. 1990;55:981–988. doi: 10.1111/j.1471-4159.1990.tb04587.x. [DOI] [PubMed] [Google Scholar]
- CHEN L., HE M., SIBILLE E., THOMPSON A., SARNYAI Z., BAKER H., SHIPPENBERG T., TOTH M. Adaptive changes in postsynaptic dopamine receptors despite unaltered dopamine dynamics in mice lacking monoamine oxidase B. J. Neurochem. 1999;73:647–655. doi: 10.1046/j.1471-4159.1999.0730647.x. [DOI] [PubMed] [Google Scholar]
- COLZI A., D'AGOSTINI F., KETTLER R., BORRONI E., DA PRADA M. Effect of selective and reversible MAO inhibitors on dopamine outflow in rat striatum; a microdialysis study. J. Neural Transm. 1990;32 suppl:79–84. doi: 10.1007/978-3-7091-9113-2_9. [DOI] [PubMed] [Google Scholar]
- COOPER D.O., CARLSON K.R. Rapid determination of dopamine uptake in synaptosomal preparations. J. Neurosci. Methods. 1983;9:157–162. doi: 10.1016/0165-0270(83)90128-0. [DOI] [PubMed] [Google Scholar]
- FAIRBROTHER I.S., ARBUTHNOTT G.W., KELLY J.S., BUTCHER S.P. In vivo mechanisms underlying dopamine release from rat nigrostriatal terminals: II. Studies using potassium and tyramine. J. Neurochem. 1990;54:1844–1851. doi: 10.1111/j.1471-4159.1990.tb04881.x. [DOI] [PubMed] [Google Scholar]
- FANG J., YU P.H. Effect of 1-deprenyl, its structural analogues and some monoamine oxidase inhibitors on dopamine uptake. Neuropharmacol. 1994;33:763–768. doi: 10.1016/0028-3908(94)90116-3. [DOI] [PubMed] [Google Scholar]
- FINBERG J.P.M., TENNE M., YOUDIM M.B.H. Selective irreversible propargyl derivative inhibitors of monoamine oxidase (MAO) without the cheese effect Monoamine Oxidase Inhibitors – The State of the Art 1981Chichester: Wiley; 31–43.ed. Youdim, M.B.H. & Paykel, E.S. pp [Google Scholar]
- FINBERG J.P.M., TENNE M., YOUDIM M.B.H. Pharmacological effects of selective type A and type B MAO inhibitors New Vistas in Depression 1982Oxford: Pergamon Press; 219–225.ed. Langer S.Z. pp [Google Scholar]
- HAYASHI I., MIWA S., LEE K., KOSHIMURA K., KAMEI A., HAMAHATA H., FUJIWARA M. A nonisotopic method for determination of the in vivo activities of tyrosine hydroxylase in the rat adrenal gland. Anal. Biochem. 1988;168:176–183. doi: 10.1016/0003-2697(88)90026-7. [DOI] [PubMed] [Google Scholar]
- JUORIO A.V., PATERSON I.A., ZHU M.Y. Dopamine metabolism in the guinea pig striatum: role of monoamine oxidase A and B. Eur. J. Pharmacol. 1994;254:213–220. doi: 10.1016/0014-2999(94)90457-x. [DOI] [PubMed] [Google Scholar]
- KATO T., DONG B., ISHII K., KINEMUCHI H. Brain dialysis: In vivo metabolism of dopamine and serotonin by monoamine oxidase A but not B in the striatum of unrestrained rats. J. Neurochem. 1986;46:1277–1282. doi: 10.1111/j.1471-4159.1986.tb00650.x. [DOI] [PubMed] [Google Scholar]
- KNOLL J. R-(-)-deprenyl (selegiline, Movergan R) facilitates the activity of the nigrostriatal dopaminergic neuron. J. Neural. Transm. 1987;25 suppl:45–66. [PubMed] [Google Scholar]
- KNOLL J., MIKLYA I., KNOLL B., MARKO R., KELEMEN K. (-)Deprenyl and (-)1-phenyl-2-propylaminopentane, [(-)PPAP], act primarily as potent stimulants of action potential-transmitter release coupling in the catecholaminergic neurons. Life Sci. 1996;58:817–827. doi: 10.1016/0024-3205(96)00014-8. [DOI] [PubMed] [Google Scholar]
- KOPIN I.J., LAKE R.C., ZIEGLER M. Plasma levels of norepinephrine. Ann. Int. Med. 1978;88:671–680. doi: 10.7326/0003-4819-88-5-671. [DOI] [PubMed] [Google Scholar]
- LAMENSDORF I., YOUDIM M.B.H., FINBERG J.P.M. Effect of long-term treatment with selective monoamine oxidase A and B inhibitors on dopamine release from rat striatum in vivo. J. Neurochem. 1996;67:1532–1539. doi: 10.1046/j.1471-4159.1996.67041532.x. [DOI] [PubMed] [Google Scholar]
- LEVITT P., PINTAR J.E., BREAKEFIELD X.O. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc. Nat. Acad. Sci. U.S.A. 1982;79:6385–6389. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERCURI N.B., BONCI A., SINISCALCHI A., STEFANI A., CALABRESI P., BERNARDI G. Electrophysiological effects of monoamine oxidase inhibition on rat midbrain dopaminergic neurones: an in vitro study. Br. J. Pharmacol. 1996;117:528–532. doi: 10.1111/j.1476-5381.1996.tb15222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYLLYLA V.V., SOTANIEMI K.A., VUORINEN J.A., HEINONEN E.H. Selegiline as initial treatment in de novo parkinsonian patients. Neurology. 1992;42:339–343. doi: 10.1212/wnl.42.2.339. [DOI] [PubMed] [Google Scholar]
- O'CARROL A.M., FOWLER C.J., PHILLIPS J.P., TOBBIA I., TIPTON K.F. The deamination of dopamine by human brain monoamine oxidase: Specificity for the two enzyme forms in seven brain regions. Naunyn-Schmiedeberg's Arch. Pharmacol. 1983;332:198–202. doi: 10.1007/BF00500765. [DOI] [PubMed] [Google Scholar]
- OLANOW C.W. Deprenyl in the treatment of Parkinson's disease; clinical effects and speculation on mechanism of action. J. Neural Transm. 1996;48 suppl:75–84. doi: 10.1007/978-3-7091-7494-4_7. [DOI] [PubMed] [Google Scholar]
- OTSUKA S., KOBAYASHI Y. A radioisotopic assay for monoamine oxidase determinations in human plasma. Biochem. Pharmacol. 1964;13:995–1006. doi: 10.1016/0006-2952(64)90096-6. [DOI] [PubMed] [Google Scholar]
- PATERSON I.A., JUORIO A.V., BERRY M.D., ZHU M.Y. Inhibition of monoamine oxidase-B by (-)-deprenyl potentiates neuronal responses to dopamine agonists but does not inhibit dopamine catabolism in the rat striatum. J. Pharmacol. Exp. Ther. 1991;258:1019–1024. [PubMed] [Google Scholar]
- ROSS S.B. Distribution of the two forms of monoamine oxidase within monoaminergic neurons of the guinea-pig brain. J. Neurochem. 1987;48:609–614. doi: 10.1111/j.1471-4159.1987.tb04136.x. [DOI] [PubMed] [Google Scholar]
- SESACK S.R., HAWRYLAK V.A., MATUS C., GUIDO M.A., LEVEY A.I. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J. Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH A.D., JUSTICE J.B. The effect of inhibition of synthesis, release, metabolism and uptake on the microdialysate extraction fraction of dopamine. J. Neurosci. Meth. 1994;54:75–82. doi: 10.1016/0165-0270(94)90161-9. [DOI] [PubMed] [Google Scholar]
- WETHERELL J.R., FOSBRAEY P., FRENCH M.C. A comparison of the distribution of neurotransmitters in brain regions of the rat and guinea-pig using a chemiluminescent method and HPLC with electrochemical detection. J. Neurochem. 1989;53:1519–1526. doi: 10.1111/j.1471-4159.1989.tb08547.x. [DOI] [PubMed] [Google Scholar]


