Abstract
The present study was undertaken to investigate the intestinal anti-inflammatory effects of UR-12746 on the acute and chronic stages of a trinitrobenzene sulphonic acid (TNBS) experimental model of inflammatory bowel disease (IBD) in the rat. UR-12746 is a novel, locally-acting compound which combines, through an azo bond, 5-aminosalicylic (5-ASA) and UR-12715, a potent platelet activating factor (PAF)-antagonist.
UR-12746 oral pretreatment of colitic rats (50 and 100 mg kg−1) reduced acute colonic damage when evaluated 2 days after colonic insult.
Postreatment for 4 weeks with UR-12746 (50 and 100 mg kg−1) resulted in a faster recovery of the damaged colonic mucosa, which was macroscopically significant from the third week.
The intestinal anti-inflammatory effect of UR-12746 was associated with a decrease in leukocyte infiltration in the colonic mucosa, which was evidenced both biochemically, by a reduction in myeloperoxidase activity, and histologically, by a lower leukocyte count after morphometric analysis. This effect was higher than that seen with sulphasalazine, when assayed at the same doses and in the same experimental conditions.
Several mechanisms can be involved in the beneficial effects showed by UR-12746: inhibition of leukotriene B4 synthesis in the inflamed colon, improvement of the altered colonic oxidative status, and reduction of colonic interleukin-1β production.
The results suggest that the intestinal anti-inflammatory activity of UR-12746 can be attributed to the additive effects exerted by 5-ASA and UR-12715, the PAF antagonist compound, that are released in the colonic lumen after reduction of the azo bond by the intestinal bacteria.
Keywords: UR-12746, 5-ASA, UR-12715, intestinal anti-inflammatory activity, TNBS-induced rat colitis, PAF antagonist
Introduction
Crohn's disease (CD) and ulcerative colitis (UC) are chronic inflammatory bowel diseases of unknown aetiology, which are currently difficult to treat pharmacologically. Sulphasalazine, a diazo compound with 5-aminosalicylic acid (5-ASA) (mesalamine) linked to sulphapyridine that acts as a carrier, has been long used as therapy for both intestinal conditions. Thus, this agent has been shown to be useful in managing mild-to-moderate active UC and mild active CD, as well as in maintaining remission by prevention of relapses in patients with inflammatory bowel disease (IBD) (Egan & Sandborn, 1998). Unfortunately, long-term administration of sulphasalazine is accompanied by a considerable number of side-effects, either dose-dependent such as headache, nausea, vomiting, etc. or allergic, such as cutaneous rashes, exanthema, fever, diarrhoea, etc. Since most of these adverse reactions were initially attributed to the sulphapyridine moiety, a second generation of salicylates arose with the aim of delivering 5-aminosalicylic acid, which was shown to be the active moiety of sulphasalazine, into the intestinal lumen without the need for the sulphapyridine carrier. The so-called new salicylates, such as olsalazine, balsalazide and several 5-ASA release systems, have not been proven to be more effective than sulphasalazine in the medical management of inflammatory bowel disease (IBD) (Sutherland et al., 1993; 1997). However, they are better tolerated, even though they are not devoid of side-effects, especially when used at high doses (Enns & Sutherland, 1998). Several mechanisms have been postulated to be involved in the intestinal anti-inflammatory effect exerted by 5-ASA derivates, including antioxidant and/or radical scavenging properties (Keshavarzian et al., 1990), inhibition of leukocyte chemotaxis (Nielsen et al., 1988) and inhibition of interleukin-1 (IL-1) synthesis (Rachmilewitz et al., 1992).
Many mediators have been involved in the pathogenesis of IBD: eicosanoids, reactive oxygen metabolites, cytokines and platelet activating factor (PAF). PAF is a proinflammatory lipid mediator involved in hypersensitivity and inflammatory reactions such as platelet and neutrophil aggregation, vasodilation, increased vascular permeability and leukocyte adhesion. In addition to its direct effects, PAF also stimulates the release of eicosanoids (leukotrienes and prostaglandins) as well as cytokines such as IL-1 and tumour necrosis factor (TNF). All these actions contribute to the genesis and amplification of inflammatory processes, including IBD (Nassif et al., 1996). In fact, it has been shown that colonic mucosa from patients with UC or CD produces high levels of PAF (Ferraris et al., 1993; Wardle et al., 1996; Hocke et al., 1999). Since PAF may have an important role in the pathogenesis of IBD, its inhibition by specific antagonists may have a potential therapeutic benefit in the treatment and management of these inflammatory diseases of the gut, as has been previously proposed (Wallace, 1988; Meenan et al., 1996).
UR-12746 is a novel, locally-acting compound which combines 5-ASA and UR-12715 through an azo link, the latter being a carrier that also possesses PAF antagonist activity (Figure 1). After oral administration, the azo bond of UR-12746 is cleaved by azoreductases from colonic bacteria, thus delivering 5-ASA and UR-12715. This is the same mechanism that applies to other compounds including several 5-ASA derivates which contain the same type of link (Travis & Jewell, 1994a). As a consequence, both compounds may act additively in controlling the inflammatory response that occurs in IBD. The aim of the present study was to test the intestinal anti-inflammatory activity of UR-12746 in the acute and chronic stages of trinitrobenzene sulphonic acid (TNBS) model of rat colitis. This is a well established model of intestinal inflammation which has some histological and biochemical features of the human disease, specially with regard to the involvement of different inflammatory mediators, including eicosanoids, reactive oxygen (and nitrogen) species, PAF and cytokines (Morris et al., 1989; Stenson, 1994).
Figure 1.

Structure of UR-12746.
Methods
Inhibition of PAF-induced platelet aggregation in rabbit plasma in vitro
PAF-induced platelet aggregation was performed following the techinque described by Lalau Keraly et al. (1984). Briefly, blood was collected into 3.8% sodium citrate (1 volume for 9 volumes of blood) by cardiac puncture from anaesthetized (sodium pentobarbital 40 mg kg−1 i.p.) male New Zealand rabbits (2–2.5 kg body weight) provided by B & K Universal (Sant Vicenç dels Horts, Spain). Platelet rich plasma (PRP) was prepared by centrifugation of the blood (250×g, 10 min., 4°C). PRP was diluted with platelet poor plasma obtained by further centrifugation of PRP (3000×g, 10 min., 4°C). The platelet count was adjusted to 3.5×108 cells per ml. PRP was incubated with the inhibitors for 4 min before addition of the aggregation inducer. Platelet aggregation was induced by C18-PAF (1.5×10−8 M) and measured with a dual-channel aggregometer Chrono-log 560 (Havertown, PA, U.S.A.). Test substances were dissolved at a initial concentration of 10 mM in dimethylsulphoxide (DMSO) (UR-12746, WEB-2086, sulphasalazine, sulphapyiridine and 5-ASA) or in saline solution acidified with HCl 0.1 N (UR-12715), and subsequent dilutions were made with saline solution to obtain the different concentrations assayed. The per cent inhibition of platelet aggregation in the presence of different drug concentrations compared to the control curve were evaluated and the IC50 value for each inhibitor was determined. Preliminary assays showed that PAF aggregation was not modified in the presence of the two solvents used to dissolve the test compounds at the maximum concentrations reached.
Inhibition of PAF-induced hypotension in normotensive rats
PAF-induced hypotension was performed according to the method previously described by Baranes et al. (1986). Male Sprague-Dawley rats provided by Harlan Iberica (Sant Feliu de Codines, Spain) and weighing 180–220 g were anaesthetized with sodium pentobarbital (50 mg kg−1 i.p.). Blood pressure was recorded from the left carotid artery using a Statham pressure transducer coupled to a Letica 2006 recorder (Barcelona, Spain). The right and left femoral veins were catheterized to inject the test compounds and C18-PAF (0.5 mg kg−1). Test compounds were dissolved as described above and different doses were administered by i.v. injection (vol: 1 ml kg−1) 3 min before PAF injection. Mean blood pressure was monitored, and percentage inhibition of PAF-induced hypotension compared to controls was calculated. The results are expressed as ED50 values, i.e., the dose of test compound required to inhibit hypotension by 50%, and these were calculated by linear regression from a single experimental curve with no less than four points, each point being the mean of the percentage inhibition at a given dose obtained for three or more independent experiments.
Induction of experimental colitis
Colitis was induced by the method originally described by Morris et al. (1989) with minor modifications. Female Wistar rats (180–220 g) obtained from the Laboratory Animal Service of the University of Granada, (Granada, Spain) were randomly distributed in several experimental groups. Animals were housed in makrolon cages (3–4 rats per cage) and maintained in air-conditioned animal quarters with a 12 h light–dark cycle, and they were provided with free access to tap water and food (Panlab A.04). Animals were fasted overnight and anaesthetized with ethyl ether. Under anaesthesia, animals were given 30 mg of TNBS dissolved in 0.25 ml of 50% ethanol (v v−1) by means of a Teflon cannula inserted 8 cm through the anus. During and after TNBS administration rats were kept in a head-down position until they recovered from anaesthesia, and then returned to their cage. Rats from the non-colitic (normal) group received 0.25 ml of phosphate buffered saline.
Two different protocols were followed: (1) Acute colitis: rats were given 10, 25, 50 or 100 mg kg−1 of UR-12746 or sulphasalazine orally for 5 days before colitis induction, as well as 24 h thereafter. In another set of experiments, rats were administered intracolonically either UR-12715 (37.5 or 75 mg kg−1), 5-ASA (12.5 or 25 mg kg−1) or UR-12715 plus 5-ASA (37.5+12.5 mg kg−1 or 75+25 mg kg−1), following the same time-dosing protocol. The doses of UR-12715 and 5-ASA were equivalent to those which theoretically could be achieved in the rat colon after complete cleaving of UR-12746 at 50 and 100 mg kg−1 when administered orally and adjusted for the molecular weights of the different compounds. All compounds assayed were suspended in 1% (w v−1) methylcellulose and administered (vol: 1 ml) by means of an oesophageal catheter (when given orally) or by a Teflon cannula inserted 8 cm through the anus (when administered intracolonically). Animals were sacrificed 48 h after colitis induction. (2) Chronic colitis: rats were rendered colitic by TNBS administration and then treated orally with 50 or 100 mg kg−1 day−1 of UR-12746 or sulphasalazine starting 1 day after colitis induction until the day before the animals were put down. Animals were sacrificed at 1, 2, 3 and 4 weeks of colitis. In both protocols a TNBS control group and a normal (non-colitic) group were included for reference. These animals received only the vehicle (1 ml of 1% w v−1 methylcellulose). Animal body weight and total food intake for each group were recorded daily. All experimental groups had eight rats per treatment and time point, except the TNBS control and normal groups, which had 12 rats each.
Assessment of colonic damage
Animals were sacrificed with an overdose of ethyl ether, and the entire colon was removed. The colonic segments were placed on an ice-cold plate, cleaned of fat and mesentery, and blotted on filter paper. Each specimen was weighed and its length measured under a constant load (2 g). The colon was longitudinally opened and scored for macroscopically visible damage on a 0–10 scale by two observers unaware of the treatment, according to the criterion described by Bell et al. (1995), which takes into account the extension as well as the severity of colonic damage. The colon was subsequently divided longitudinally in four pieces for biochemical determinations. Two fragments were frozen at −30°C for myeloperoxidase (MPO) and malonyldialdehyde (MDA) determinations and another sample was weighed and frozen in 1 ml of 5% (w v−1) trichloroacetic acid for total glutathione content determination. The remaining sample was immediately processed for the measurement of leukotriene B4 (LTB4) and interleukin-1β (IL-1β) synthesis.
All biochemical measurements were completed within 1 week from the time of sample collection and were performed in duplicate. MPO activity was measured according to the technique described by Krawisz et al. (1984); the results were expressed as MPO units per gram of wet tissue and one unit of MPO activity was defined as that degrading 1 mmol min−1 of hydrogen peroxide at 25°C. Total glutathione content was quantified with the recycling assay described by Anderson (1985) and the results were expressed as nmol g−1 wet tissue. Colonic MDA content was evaluated according to the method proposed by Esterbauer & Cheeseman (1990), and expressed as μmol g−1 wet tissue. Samples for LTB4 and IL-1β synthesis determinations were immediately weighed, minced on an ice-cold plate and suspended in a tube with 10 mM sodium phosphate buffer (pH=7.4) (1 : 20 w v−1). The tubes were placed in a shaking water bath (37°C) for 20 min and centrifuged at 9000×g for 30 s at 4°C. The supernatants were frozen at −80°C until assay, which was performed within the next 10 days. LTB4 and IL-1β was quantified by enzyme-linked immunosorbent assay (Amersham, Madrid, Spain), and the results expressed as ng g−1 wet tissue.
Histological analysis
Three additional animals from each group were used for colonic histological studies. Samples from the distal colon of each animal were fixed in 4% paraformaldehyde and embedded in paraffin. Sections of 5 μm thickness were stained with hematoxylin and eosin. The number of leukocyte cells infiltrated in the the lamina propria at the apical level in the colonic mucosa of each animal was determined by an observer unaware of the treatment. All counts were made with a grid (6400 μm2) fitted in the eyepiece of the microscope at the 100×magnification. Three random fields in each of five sections were counted, and the mean values were calculated for each animal. The results are expressed as number of infiltrate cells per field.
Drugs
UR-12746 ((Z) -2-hydroxy -5- [[4-[3-[4-[ (2-methyl-lH-imidazo [4,5-c]pyridin-1-yl) methyl -1- piperidinyl]-3-oxo-1-phenyl-1-propenyl]phenyl]azo] benzoic acid), UR-12715 ((Z)-1-[4-(3-aminophenyl) -1-oxo-3-phenyl-2-propenyl]-4-[(2-methyl-lH-imidazo[4,5-c]pyridin-1-yl)methyl]piperidine) and WEB-2086 (3-[4-(2-chlorophenyl)-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo [4,3-a][1,4]diazepin-2-yl]-1-(4-morpholinyl)-1-propanone) were supplied by J. Uriach and Cia S.A. (Barcelona, Spain). Sulphasalazine, sulphapyridine, 5-ASA and C18-PAF were obtained from Sigma (Madrid, Spain). All other reagents, including TNBS, were also obtained from Sigma (Madrid, Spain), except glutathione reductase (Boehringer Mannheim, Barcelona, Spain).
Statistical analysis
All results are expressed as mean±s.e.mean. Differences among means were tested for statistical significance using one-way analysis of variance (ANOVA) and post hoc least significance tests. Nonparametric data (score) are expressed as median (range) and were analysed with the Mann–Whitney U test. Differences among proportions were analysed with the χ2 test. Statistical significance was set at P<0.05. In chronic experiments, data from non-colitic animals, which did not differ significantly from one another, were pooled together and presented as a single group.
Results
PAF antagonist activity
The IC50 value of UR-12715 as an inhibitor of PAF-induced aggregation in rabbit PRP was 0.008±0.002 μM, which was 500 and 11 times lower than those obtained for UR-12746 (IC50=4.1±0.8 μM) and WEB-2086 (IC50=0.091±0.020 μM) respectively, the latter being a PAF-antagonist which was used for reference (Casals-Stenzel et al., 1987). When sulphasalazine, sulphapyridine and 5-ASA were tested in this assay, concentrations up to 200 μM were devoid of any inhibitory effect on PAF-induced aggregation.
Administration of C18-PAF (0.5 μg kg−1, i.v.) to normotensive rats (106.0±3.2 mm Hg; n=12) induced a 59.3±0.8% drop in basal mean blood pressure. When the compounds were tested for their ability to inhibit PAF-induced hypotension in normotensive rats, UR-12715 showed an ED50 value of 0.029±0.012 mg kg−1, which was about 100 and six times more potent than UR-12746 (ED50=2.8±1.0 mg kg−1) and WEB-2086 (ED50=0.17±0.05 mg kg−1), respectively.
Effect of UR-12746 and sulphasalazine on the acute phase of the TNBS-induced colitis
The acute phase of colitis, i.e. 48 h after TNBS/ethanol administration, was characterized by severe necrosis of the colonic mucosa typically extending 4–6 cm along the colon accompanied by hyperemia, adhesions to adjacent organs and bowel wall thickening with a 2 fold increase in the wet weight/length ratio of the rat colon compared to normal animals (P<0.01; Table 1). This colonic damage resulted in the presence of signs of diarrhoea in 75% of animals from the TNBS control group. The histological sections (Figure 2) from colonic specimens showed hyperemia, oedema of the submucosa, epithelial disruption, mucosal erosions with goblet cell depletion, and a mixed inflammatory infiltrate consisting of polymorphonuclear leukocytes, lymphocytes and eosinophils (34.5±1.6 cells per field vs 6.8±0.5 in non-colitic animals; P<0.01). In addition, the biochemical determinations showed that the colonic levels of inflammatory markers such as MPO and LTB4 were increased by 14 and 6.5 fold, respectively, whereas there was a 55% fall in total glutathione levels compared to non-colitic animals (P<0.01; Table 2).
Table 1.
Effects of UR-12746 and sulphasalazine (SAZ) treatment on damage score, changes in colonic weight, incidence of adhesions and diarrhoea in the acute phase of TNBS colitis

Figure 2.

Histological sections of colonic mucosa from colitic rats 48 h after TNBS instillation (acute phase). (a) Non-colitic group; (b) TNBS-control group; (c and d:) UR-12746 treated groups at doses of 50 and 100 mg kg−1, respectively. Original magnification: 100×. Calibration bar=150 μm.
Table 2.
Effects of UR-12746 and sulphasalazine (SAZ) treatment on myeloperoxidase (MPO) activity, glutathione (GSH) content, and LTB4 synthesis in the acute phase of TNBS colitis
Pretreatment of colitic animals with UR-12746 significantly reduced the acute inflammatory response induced by the colonic administration of TNBS. Thus, UR-12746 decreased the colonic weight/length ratio, at doses of 50 and 100 mg kg−1, compared to control animals (P<0.05, Table 1). At a dose of 100 mg kg−1, UR-12746 was also able to reduce the area of haemorrhagic necrosis in the colonic mucosa by 20%, as shown by a lower macroscopic score than that assigned to control animals (P<0.05, Table 1). In addition, UR-12746 treatment (100 mg kg−1) resulted in a significantly lower incidence of diarrhoea and of adhesions between the colon and adjacent organs in comparison with the TNBS control group (P<0.05, Table 1). Histological sections obtained from treated rats displayed grossly similar changes to those observed in the TNBS control group, i.e. the colonic wall appeared thickened and inflammatory cells were present, especially in the mucosa; however there were no signs of epithelial sloughing, and goblet cell depletion seemed to be less severe (Figure 2). Morphometric analysis revealed that the drug treatment was able to reduce the number of infiltrated cells at the doses of 50 and 100 mg kg−1 (20.1±1.3 and 14.0±1.5 cells per field, respectively) in comparison with non-treated animals (34.5±1.6 cells per field, P<0.01). Colonic levels of MPO, an enzymatic marker of neutrophil infiltration, were significantly reduced after UR-12746 administration at these doses (P<0.05 and P<0.01 vs control group, respectively; Table 2). This effect was accompanied, at 100 mg kg−1, by a significant inhibition of LTB4 synthesis (P<0.01 vs control group; Table 2). Finally, drug pretreatment was also able to partially prevent colonic glutathione depletion compared to TNBS control animals, although this effect was obtained only at the dose of 100 mg kg−1 (P<0.05; Table 2) and was incomplete, i.e. the values were still low compared to those obtained in non-colitic animals.
Analysis of the data obtained from colitic rats treated with sulphasalazine, at doses ranging from 10–100 mg kg−1, showed no significant modification in macroscopic parameters of inflammation such as score or weight/length ratio compared to non-treated animals (Table 1). Furthermore, this drug treatment was not able to reduce significantly the incidence of diarrhoea in colitic animals at any of the doses assayed (Table 1). The biochemical parameters studied (Table 2) revealed that colonic MPO activity was also not affected by sulphasalazine treatment. However, pretreatment with 100 mg kg−1 significantly reduced leukocyte infiltration (23.7±1.4 cells, P<0.05) as well as LTB4 synthesis (P<0.01) when compared to the TNBS control group. An increase in glutathione content was observed in sulphasalazine treated colitic animals at doses of 25, 50 and 100 mg kg−1 (P<0.01 vs non-treated colitic group).
Effect of intracolonic administration of UR-12715, 5-ASA and their combination on the acute phase of TNBS-induced colitis
Intracolonic administration of UR-12715, at doses of 37.5 and 75 mg kg−1 for 5 days before colonic challenge as well as 1 day after, resulted in an intestinal anti-inflammatory effect, as evidenced by reduction in colonic MPO activity compared to non-treated colitic animals (P<0.01) (Table 3), suggesting a lower neutrophil infiltration in the inflamed colon. This effect was not accompanied by a significant reduction of the macroscopic score, although the lowest dose assayed significantly decreased the weight/length ratio (P<0.01) (Table 3). No significant modification was observed in colonic glutathione content or LTB4 production.
Table 3.
Effects of UR-12715, 5-aminosalicyclic acid (5-ASA) and UR-12715 plus 5-ASA treatment on damage score, changes in colonic weight, myeloperoxidase (MPO) activity, glutathione (GSH) content, and LTB4 synthesis in the acute phase of TNBS colitis

An intestinal anti-inflammatory effect was also observed when rats were intracolonically pretreated with 5-ASA, at the doses of 12.5 and 25 mg kg−1(Table 3). Thus, the colonic segments showed a lower weight/length ratio than those from the non-treated control group (P<0.01), but no significant reduction was shown in the macroscopic score or in the colonic MPO activity. No beneficial effect was observed on colonic LTB4 synthesis either. However, the highest dose assayed was able to significantly increase colonic glutathione content in colitic animals (P<0.05).
Concurrent intracolonic administration of UR-12715 and 5-ASA, at both dose combinations, showed intestinal anti-inflammatory activity, as evidenced both macroscopically, by a significant reduction in the colonic weight/length ratio (P<0.01), and biochemically, by a significant decrease in colonic MPO activity (P<0.01), compared to the corresponding control group (Table 3). Simultaneous pretreatment with both compounds also resulted in a partial preservation of colonic glutathione depletion (P<0.05 and P<0.01 at the lowest and highest dose combinations, respectively) and in a reduction of colonic LTB4 synthesis, which was significant only at the highest dose (Table 3).
Effect of UR-12746 and sulphasalazine on the chronic phase of TNBS-induced colitis
Macroscopic damage at 1 and 2 weeks after colitis induction was characterized by mucosal necrosis and ulceration extending 3–4 cm along the colon, with bowel wall thickening. The involved areas of the colon appeared rigid, with multiple adhesion points to neighbouring small intestinal loops, and functional obstruction of the colon was evident in 50% of the rats. From that time on the colon showed smaller ulcers in different degrees of healing. Adhesions and strictures persisted, and obstruction decreased gradually. Both the colonic weight/length ratio and the damage score remained significantly elevated by week 4 compared to normal animals (Table 4, P<0.05). Diarrhoea was present in the majority of colitic animals for 3 weeks (75%) but almost vanished by the fourth week (16.6%).
Table 4.
Effects of UR-12746 and sulphasalazine (SAZ) treatment (50 and 100 mg kg−1) on damage score and changes on colonic weight in the chronic phase of TNBS colitis

Histological assessment of distal colon revealed severe transmural disruption of the normal architecture of the colon. In the first week, extensive ulcers with necrosis of mucosa and submucosa, goblet cell depletion and infiltration with polymorphonuclear leukocytes were observed. From this time point on, the histological sections showed a progressive restauration of the intestinal cytoarchitecture, although at week 4 colonic histology was still altered (Figure 3), showing partially reepithelizated ulcers and an important chronic inflammatory infiltrate in the mucosa and submucosa; also, the restoration of the glandular structure was not complete, since goblet cells were not completely replenished with their mucin content. The existence of dilated cripts was still evident. The counts of leukocytes infiltrating the colonic mucosa were significantly higher in the TNBS-control group than in the saline group throughout the experiment (P<0.01; Figure 4).
Figure 3.

Histological sections of colonic mucosa from colitic rats 4 weeks after TNBS instillation (chronic phase) (a) TNBS-control group: (b and c) UR-12746 treated groups at doses of 50 and 100 mg kg−1, respectively. Original magnification: 100×. Calibration bar=150 μm.
Figure 4.

Effects of UR-12746 (UR) and sulphasalazine (SAZ) treatment (50 and 100 mg kg−1) on leukocyte infiltration in colonic tissue in the chronic phase of TNBS colitis. Data are expressed as mean±s.e.mean. * P<0.05, ** P<0.01 vs TNBS control group. All groups differ significantly from the non-colitic group (P<0.01, not shown) except those marked with # (P>0.05).
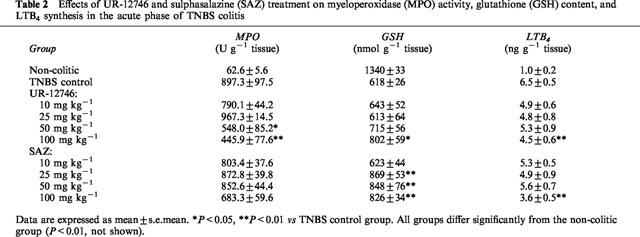
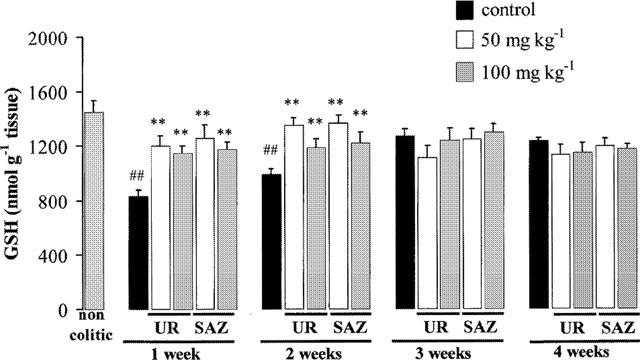
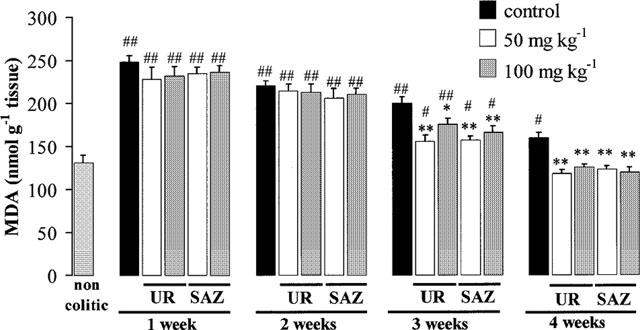
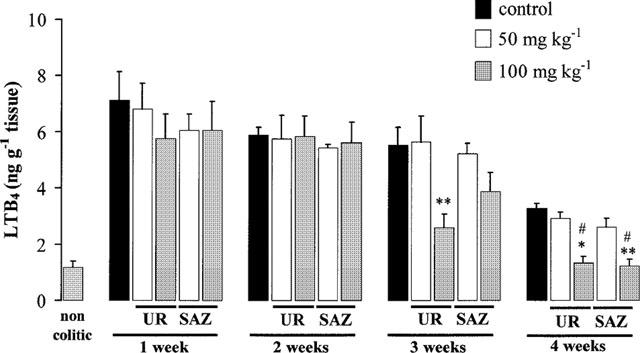
The biochemical markers of inflammation were affected in this chronic stage of TNBS rat colitis. The results revealed that MPO levels were increased the first week and then decreased slowly, being still significantly higher than normal by week 4 (Figure 5). Colonic oxidative status, as evaluated by colonic glutathione (Figure 6) and MDA (Figure 7) contents, was also affected in colitic animals. Thus, colonic glutathione content was significantly reduced compared to noncolitic rats at 1 and 2 weeks, but returned to normal values thereafter, whereas MDA levels were significantly increased at all time points studied. Colonic LTB4 and IL-1β synthesis were also regularly affected. IL-1β levels showed an almost 4 fold increase at week 1 and remained elevated by 2 fold afterwards (Figure 8). On the contrary, increased colonic LTB4 synthesis showed a trend to normalize with time (Figure 9).
Figure 5.

Effects of UR-12746 (UR) and sulphasalazine (SAZ) treatment (50 and 100 mg kg−1) on colonic myeloperoxidase (MPO) activity in the chronic phase of TNBS colitis. Data are expressed as mean±s.e.mean. *P<0.05, **P<0.01 vs TNBS control group. All groups differ significantly from the non-colitic group (P<0.01, not shown) except those marked with # (P>0.05).
Figure 6.
Effects of UR-12746 (UR) and sulphasalazine (SAZ) treatment (50 and 100 mg kg−1) on colonic glutathione (GSH) contents in the chronic phase of TNBS colitis. Data are expressed as mean±s.e.mean. *P<0.05, **P<0.01 vs TNBS control group # P<0.05, ## P<0.01 vs non-colitic group.
Figure 7.
Effects of UR-12746 (UR) and sulphasalazine (SAZ) treatment (50 and 100 mg kg−1) on colonic malonyldialdehyde (MDA) levels in the chronic phase of TNBS colitis. Data are expressed as mean±s.e.mean. *P<0.05, **P<0.01 vs TNBS control group. # P<0.05, ## P<0.01 vs. non-colitic group.
Figure 8.

Effects of UR-12746 (UR) and sulphasalazine (SAZ) treatment (50 and 100 mg kg−1) on interleukin-1β (IL-1β) levels in the chronic phase of TNBS colitis. Data are expressed as mean±s.e.mean. *P<0.05, **P<0.01 vs TNBS control group. ## P<0.01 vs non-colitic group.
Figure 9.
Effects of UR-12746 (UR) and sulphasalazine (SAZ) treatment (50 and 100 mg kg−1) on leukotrinene B4 (LTB4) levels in the chronic phase of TNBS colitis. Data are expressed as mean±s.e.mean. *P<0.05, **P<0.01 vs TNBS control group. All groups differ significantly from the non-colitic group (P<0.01, not shown) except those marked with # (P>0.05).
Treatment with 50 or 100 mg kg−1 of UR-12746 resulted in a significantly lower colonic damage score than the control at week 3, but at week 4 only the higher reduced this parameter significantly (P<0.05, Table 4). This beneficial effect was not accompanied by a significant reduction in colonic weight/length ratio, although a trend to decrease was observed (Table 4). However, UR-12746 treatment reduced the incidence of diarrhoea from the second week, this reduction being significant after 2 weeks of treatment at the dose of 50 mg kg−1 (25% of the animals showed signs of diarrhoea, P<0.05 vs TNBS control group) and after 3 weeks at the doses of 50 and 100 mg kg−1 (0% and 12.5% of the animals, respectively) compared to the control group (P<0.01). No significant reduction in the percentage of animals showing adhesions was observed throughout the experiment (data not shown).
Sulphasalazine showed a similar activity to that obtained with UR-12746 (Table 4), with the difference that only the highest dose assayed, 100 mg kg−1, significantly lowered macroscopic damage score at 3 and 4 weeks compared to the TNBS control group (P<0.05). The antidiarrhoeic effect was evidenced at 3 weeks with both doses of sulphasalazine, as the incidence was reduced to 12.5% (P<0.01 vs TNBS control group).
Histological analysis of the colonic specimens from rats treated with UR-12746 showed a less damaged intestinal cytoarchitecture which was already evident from the first week of treatment, showing decreased goblet cell depletion; however, several zones of ulcers with necrosis of the colonic mucosa were observed. After 4 weeks of treatment, tissue recovery was evident in comparison to non-treated animals (Figure 3). Thus, colonic sections showed an absence of dilated cripts as well as the presence of intact, mucin-repleted goblet cells, when compared with colonic sections from control colitic rats. In addition, UR-12746 treated animals, at the dose of 100 mg kg−1, showed an almost completely restored epithelial cell layer. Sulphasalazine also improved colonic histology compared to control animals after 4 weeks of treatment. However, recovery was not as complete as in UR-12746 treated animals since specimens still showed evidence of dilated cripts and inmature goblet cells, as well as a higher leukocyte infiltrate. Furthermore, UR-12746 treatment resulted in a progressive reduction of the anormal leukocyte infiltrate compared to non-treated colitic animals, so that no significant differences compared to the non-colitic group were observed at both doses assayed by the end of the experiment (Figure 4). Sulphasalazine also decreased significantly the number of inflammatory cells in the mucosa from the second week on compared to the TNBS control group, but this was of lower magnitude than that of UR-12746 and never achieved a normalization of the inflammatory infiltrate (Figure 4).
The intestinal anti-inflammatory effects exerted by UR-12746 or sulphasalazine were confirmed biochemically. MPO activity was reduced in both drug treatments at all doses assayed (Figure 5). However, while UR-12746 showed an increased percentage inhibition of MPO activity with time (from aproximately 50% inhibition at 1 and 2 weeks up to 70 and 80% at 3 and 4 weeks), the inhibition observed after sulphasalazine treatment was lower (ranging from 20–50%), and did not show a correlation with time. Both drug treatments showed a similar behaviour in restoring the altered colonic oxidative status in colitic animals, since they were able to counteract the colonic glutathione depletion that occurred during the first 2 weeks after colitis induction (Figure 6), as well as to significantly inhibit MDA production after 3 or 4 weeks of treatment compared to the TNBS control group (P<0.01; Figure 7). Colonic LTB4 and IL-1β synthesis were significantly inhibited after treatment with either drug. The inhibition in LTB4 synthesis was observed at 3 and 4 weeks with UR-12746, but only at week 4 with sulphasalazine, this inhibition being achieved only with the highest of the doses studied (Figure 9). On the contrary, IL-1β production by colitic animals was already inhibited from the first week after treatment with UR-12746 or sulphasalazine, and was restored to normal values from the second week of treatment (P<0.01 vs TNBS control group; Figure 8).
Discussion
The complicate pattern of proinflammatory mediators that are involved in intestinal inflammation, including cytokines, reactive oxygen and nitrogen metabolites, eicosanoids and PAF, suggests that the best chance of effectively downregulating the intestinal inflammatory response may be to interfere with multiple stages of the inflammatory cascade, preferably with an unique drug treatment. This could be the case with UR-12746, a novel conjugate compound which contains a molecule of 5-ASA linked through an azo bond to UR-12715, a molecule designed for its PAF antagonist activity, which presumably would be able to improve the well-known efficacy of 5-ASA in these intestinal conditions (Sutherland et al., 1993; 1997). The aim of the present study was to evaluate the intestinal anti-inflammatory activity of this new compound in the TNBS model of rat colitis. This is the animal model most extensively used over the past few years to evaluate the potential use of a given compound in IBD, since the patterns of cytokine and mediator expression in this model parallel those seen in human IBD (Stenson, 1994), and thus it provides a good model of gut injury, inflammation and repair. Yamada et al. (1992) described that TNBS has direct cytotoxic effects on the colonic epithelium barrier, which provokes a disruption in its cytoarchitecture and allows the entry of luminal products that, in turn, promotes the upregulation of local immune cells in the lamina propria, with the consequent overproduction of several pro-inflammatory mediators. There is also a chronic immune component in this model that involves activation of macrophages and/or infiltrating monocytes in the lamina propria by the trinitrobenzene sulphonic acid hapten. Margination and extravasation of circulating granulocytes into the distal colon contributes markedly to the acute and chronic injury in this model of IBD.
The main purpose of the synthesis of UR-12746 was to target the release of 5-ASA and UR-12715 into the colonic lumen, with minimal absorption of both compounds in the stomach and small intestine. UR-12746 has increased size and hydrophobicity relative to both separate components. These properties render it poorly absorbed in the small intestine after oral administration (less than 1% absorption both in rats and in humans, unpublished observations), and thus the conjugate reaches the large intestine where the azo bond is cleaved by the action of microbial azoreductases, resulting in the delivery of the two components, both of which could act locally through different mechanisms and thus be potentially beneficial in IBD. In fact, preliminary experiments (unpublished observations) have revealed that oral administration to rats of 14C-UR-12746 (sodium salt) at a dose of 50 mg kg−1 resulted in a 84.5% azoreduction when this was measured as radioactivity after recovery of UR-12715 from the rat stools. This colon-specific delivery has been already documented in the case of aminosalicylates such as sulphasalazine and olsalazine, from which the active drug 5-ASA is released in the colon by the action of the same bacterial enzymatic system (Travis & Jewell, 1994a). However, UR-12715 cannot be considered an inert carrier, as is the case with sulphapyridine in the molecule of sulphasalazine, since it is an active compound due to its PAF-antagonist activity. This has been shown in the present study by using two different assays, i.e. inhibition of PAF-induced platelet aggregation in rabbit PRP and reversal of the PAF-induced hypotension in normotensive rats. In both assays, UR-12715 displayed a potent PAF-antagonist activity, showing a potency aproximately one order of magnitude higher than that obtained with WEB-2086, a PAF-antagonist compound which was used as reference (Casals-Stenzel et al., 1987).
The present study demonstrates the therapeutic efficacy of UR-12746 when administered orally at doses of 50 and 100 mg kg−1, in both the acute and chronic stages of the TNBS model of rat colitis. It is important to note the different protocol followed to test this activity in each phase of the inflammatory process: a preventive treatment was used to evaluate the intestinal anti-inflammatory activity in the acute phase, while in the chronic phase drug administration started 24 h after colitis induction, once the inflammatory response had been already established. UR-12746 administration was able to reduce colonic macroscopic damage, as scored according to the severity and extension of involved tissue (Bell et al., 1995), as well as to improve the histological lesions that characterize this experimental model of colitis, i.e. epithelial sloughing, goblet cell depletion or intense granulocyte infiltration. These beneficial effects were achieved either by preventing the initial inflammatory response that took place 48 h after intracolonic administration of TNBS or by accelerating the healing process that occurred during the following 4 weeks after colonic insult.
The anti-inflammatory effect exerted by UR-12746 was associated with a decrease in colonic MPO activity, a marker of neutrophil infiltration, which has been previously described to be upregulated in experimental colitis inflammation (Yamada et al., 1992). For this reason MPO activity has been widely used to detect and follow intestinal inflammatory processes, and a reduction in the activity of this enzyme can be interpreted as a manifestation of the anti-inflammatory activity of a given compound (Veljaca et al., 1995). The lowering of this enzymatic activity suggests that UR-12746 is able to reduce granulocyte infiltration into the inflamed colon. This was confirmed histologically, because the number of leukocytes infiltrating the colonic mucosa was decreased in animals treated with UR-12746 at both active doses compared to the corresponding control groups. This inhibitory effect on the infiltration of inflammatory cells into the colonic mucosa can account for the beneficial effect of this drug against tissue injury, possibly through one or the combination of several mechanisms.
One of these mechanisms could be the inhibition in LTB4 synthesis in the inflamed colon, which occurred with the highest dose (100 mg kg−1) studied, both in the acute and chronic stages of this experimental model, although only from the third week on in the latter. LTB4 is a potent neutrophil chemotactic agent, which induces neutrophil adherence to the vascular wall and potentiates the effects of other mediators, like PAF, in promoting neutrophil migration across the endothelial monolayer as well as in increasing mesenteric vascular permeability (Kubes et al., 1991). In fact, LTB4 synthesis has been shown to be enhanced in colonic mucosa of patients with ulcerative colitis and Crohn's disease compared to normal tissues (Sharon & Stenson, 1984) and it has been proposed that inhibition in LTB4 synthesis may contribute in the therapeutic effect exerted by different drugs used in the treatment of IBD like sulphasalazine and 5-ASA (Stenson & Lobos, 1982; Peskar et al., 1987). The results obtained in the present study confirms these observations, since treatment with sulphasalazine (100 mg kg−1) was also able to significantly reduce colonic LTB4 synthesis compared to non-treated animals, in both protocols used, to a similar extent to that achieved with UR-12746 treatment.
Another mechanism that may be involved in the intestinal anti-inflammatory effect of UR-12746 is an inhibitory effect on free radical generation that occurs in these intestinal conditions. Free radical production has been shown to be increased in the colonic mucosa of patients with IBD (Grisham, 1994; McKenzie et al., 1996), although it is a matter of debate whether this is a cause or a consequence of intestinal inflammation. This colonic oxidative stress has been also observed in the present study, since TNBS insult resulted in a depletion of colonic glutathione content during the first 2 weeks of intestinal inflammation. The fact that colitic control animals showed no significant reduction in glutathione content 3–4 weeks after TNBS administration compared to normal animals could indicate that, at these time points, the colonic oxidant insult has vanished. For this reason we evaluated another marker of colonic oxidative status in the chronic stage of this experimental model of colitis, i.e. MDA levels, a product derived from the lipoperoxidative processes that take place as a consequence of the colonic oxidative insult after TNBS administration to rats (Loguercio et al., 1996). Our results show that MDA levels were significantly increased over the 4 weeks of experiment. Treatment with UR-12746 (100 mg kg−1) in the acute stage and at both doses assayed in the chronic stage, was able to ameliorate the altered colonic oxidative status by significantly increasing glutathione content and by reducing MDA levels from week 3. This could lead us to think that the positive effect exerted by this drug in this experimental model of rat colitis could also be ascribed to an antioxidant mechanism. The results obtained in the present study show that sulphasalazine treatment also resulted in an improvement in colonic oxidative status, supporting the fact, reported previously, that antioxidant activity can contribute to its beneficial effects in these intestinal conditions (Grisham, 1994).
Colonic IL-1β was also evaluated in the chronic stage in this model of rat colitis. IL-1β is a proinflammatory cytokine that has been considered as one of the primary triggers of intestinal inflammation (Morteau et al., 1993), the production of which has been also reported to increase during active disease in both ulcerative colitis and Crohn's disease (Ligumsky et al., 1990). UR-12746 administration to colitic rats resulted in a significant decrease in colonic IL-1β production after 1 week of treatment, and a normalization of this parameter from week 2. A similar effect was obtained with sulphasalazine, confirming previous studies with other salicylates like 5-ASA (Rachmilewitz et al., 1992), in which the authors proposed that the beneficial effects exerted by oral administration of this salicylate to TNBS-colitic rats were associated with a reduction in colonic IL-1β production, due to an inhibition of the synthesis and/or release of this cytokine from different cells in the inflamed colon.
Considering the similar activity shown by UR-12746 and sulphasalazine in reducing LTB4 and IL-1β colonic synthesis as well as in improving colonic oxidative status, it is conceivable that the presence of the 5-ASA moiety in the molecule of UR-12746 can collaborate in both the preventative and healing effects showed by UR-12746 in this model of experimentally induced colitis. However, UR-12746 was more effective in reducing colonic granulocyte infiltration and in restoring the intestinal cytoarchitecture than sulphasalazine. These activities were accompanied by a lower percentage of animals showing signs of diarrhoea during the first 2 weeks in the UR-12746 treated groups than in the corresponding sulphasalazine treated groups, as a sign of amelioration of the colonic absorptive capacity. The latter effect may be important because colonic hydroelectrolytic transport has been described to be one of the last parameters to recover from inflammation (Sanchez de Medina et al., 1996), remaining altered even when the inflammatory status has been essentially resolved (Asfaha et al., 1999). The higher efficacy of UR-12746 cannot be attributed to enhanced 5-ASA colonic delivery. Sulphasalazine is poorly absorbed in the rat gastrointestinal tract (Peppercorn & Goldman, 1972) and almost completely azoreduced by intestinal microflora, releasing 5-ASA in the colon lumen (Schröder & Gustafsson, 1973). The behaviour of UR-12746 is rather similar, systemic absorption after oral administration being less than 1% of the total administered dose and colonic azoreduction being higher than 80% (unpublished observations). Nevertheless, and taking into account the molecular weights of the respective 5-ASA carriers, i.e. sulphapyridine and UR-12715 (249.3 vs 465.6, respectively), sulphasalazine delivers more 5-ASA than UR-12746 when the same dose, expressed as mg kg−1, is given. Thus, the higher efficacy showed by UR-12746 can most likely be explained on the basis of the presence of UR-12715, the PAF antagonist moiety, in its structure. The PAF antagonist activity exerted by UR-12715 upon release in the colonic lumen or even by the conjugate itself, would seem to play a key role counteracting the different deleterious effects that have been ascribed to PAF in these intestinal pathologies. (Eliakim et al., 1988; Travis & Jewell, 1994b; Wardle et al., 1996). For this reason, the inhibition of the effects exerted by this lipid mediator by specific antagonists can be useful in the management of these intestinal conditions, as has been demonstrated in the present study and was previously proposed for another PAF antangonist, BN52021, which was effective in the same model of intestinal inflammation (Wallace et al., 1989). However, UR-12746 has the additional advantage in comparison with a pure PAF antagonist that it features in its structure the 5-ASA compound, which is supposed to contribute to the beneficial effects showed by this product in this model of experimental colitis. Additional experiments were performed in order to corroborate the additive effect of both compounds in the inflamed colon. For this purpose, colitic rats were intracolonically pretreated with UR-12715 or 5-ASA or UR-12715 plus 5-ASA, following the same dosing protocol used previously to test the intestinal anti-inflammatory activity of the compounds in the acute phase of this colitis model. The doses of the compounds assayed were equivalent to those which theoretically could be achieved in the rat colon after complete cleaving of UR-12746 at both active doses, i.e. 50 and 100 mg kg−1, when administered orally and having adjusted for the molecular weights of the different compounds. The results obtained show that pretreatment of colitic animals with the combination of UR-12715 plus 5-ASA, at both doses assayed, resulted in a higher intestinal anti-inflammatory effect than that obtained when the compounds were tested separately. This was evidenced by the beneficial effects exerted by the combination in all the biochemical markers of intestinal inflammation studied (MPO activity, glutathione content and LTB4 synthesis), compared to the effects obtained by UR-12715, which only reduced MPO activity, or by 5-ASA, which only prevented colonic glutathione depletion. In conclusion, the present study demonstrates the intestinal anti-inflammatory activity of UR-12746 in the TNBS model of rat colitis, and that both components present in its structure, UR-12715 and 5-ASA, additively participate in this beneficial effect once they are released in the colon of the colitic rats.
Acknowledgments
The authors want to thank the technical help of Dr González Herrera and of Dr Sánchez de Medina. This study was supported by the Spanish Ministry of Education and Science with CICYT (SAF98-0157) funds and by J. Uriach and Cia, S.A.
Abbreviations
- 5-ASA
5-aminosalicylic acid
- CD
Crohn's disease
- DMSO
dimethylsulphoxide
- IBD
inflammatory bowel disease
- IL-1β
interleukin 1β
- MDA
malonyldialdehyde
- MPO
myeloperoxidase
- PRP
platelet rich plasma
- TNBS
trinitrobenzene sulphonic acid
- TNF
tumour necrosis factor
- UC
ulcerative colitis
References
- ANDERSON M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- ASFAHA S., BELL C.J., WALLACE J.L., MACNAUGHTON W.K. Prolonged colonic epithelial hyporesponsiveness after colitis: role of inducible nitric oxide synthase. Am. J. Physiol. 1999;276:G703–G710. doi: 10.1152/ajpgi.1999.276.3.G703. [DOI] [PubMed] [Google Scholar]
- BARANES J., HELLEGOUARCH A., LE HEGARAT M., VIOSSAT I., AUGUET M., CHABRIER P., BRAQUET F., BRAQUET P. The effects of PAF-acether on the cardiovascular system and their inhibition by a new highly specific PAF-acether receptor antagonist BN-52021. Pharmacol. Res. Commun. 1986;18:717–737. doi: 10.1016/0031-6989(86)90114-1. [DOI] [PubMed] [Google Scholar]
- BELL C.J., GALL D.G., WALLACE J.L. Disruption of colonic electrolyte transport in experimental colitis. Am. J. Physiol. 1995;268:G622–G630. doi: 10.1152/ajpgi.1995.268.4.G622. [DOI] [PubMed] [Google Scholar]
- CASALS-STENZEL J., MUACEVIC G., WEBER K. Pharmacological actions of WEB-2086, a new specific antagonist of platelet-activating factor. J. Pharmacol. Exp. Ther. 1987;241:974–981. [PubMed] [Google Scholar]
- EGAN L.J., SANDBORN W.J. Drug therapy of inflammatory bowel disease. Drugs of Today. 1998;34:431–446. doi: 10.1358/dot.1998.34.5.485242. [DOI] [PubMed] [Google Scholar]
- ELIAKIM R., KARMELI F., RAZIN E., RACHMILEWITZ D. Role of platelet activating factor in ulcerative colitis. Gastroenterology. 1988;95:1167–1172. doi: 10.1016/0016-5085(88)90346-0. [DOI] [PubMed] [Google Scholar]
- ENNS R., SUTHERLAND L.R. Adverse events of medical therapy for treatment for inflammatory bowel disease Clinical Challenges in Inflammatory Bowel Diseases. Diagnosis, Prognosis and Treatment 1998London: Kluwer Academic Publishers; 113–123.ed. Campieri, M., Bianchi-Porro, G., Fiocchi, C. & Schölmerich, J. pp [Google Scholar]
- ESTERBAUER H., CHEESEMAN K.H. Determination of aldehydic lipid peroxidation products: Malonaldehide and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- FERRARIS L., KARMELI F., ELIAKIM R., KLEIN J., FIOCCHI C., RACHMILEWITZ D. Intestinal epithelial cells contribute to the enhanced generation of platelet activating factor in ulcerative colitis. Gut. 1993;34:665–668. doi: 10.1136/gut.34.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRISHAM M.B. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- HOCKE M., RICHTER L., BOSSECKERT H., EITNER K. Platelet activating factor in stool from patients with ulcerative colitis and Crohn's disease. Hepatogastroenterology. 1999;46:2333–2337. [PubMed] [Google Scholar]
- KESHAVARZIAN A., MORGAN G., SEDGHI S., GORDON J.H., DORIA M. Role of reactive oxygen metabolities in experimental colitis. Gut. 1990;31:786–790. doi: 10.1136/gut.31.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAWISZ J.E., SHARON P., STENSON W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- KUBES P., GRISHAM M.B., BARROWMAN J.A., GAGINELLA T., GRANGER D.N. Leukocyte-induced vascular protein leakage in cat mesentery. Am. J. Physiol. 1991;261:H1872–H1879. doi: 10.1152/ajpheart.1991.261.6.H1872. [DOI] [PubMed] [Google Scholar]
- LALAU KERALY C., DELAUTIER D., DELABASEE D., CHIGNARD M., BENVENISTE J. Inhibition by ticlopidine of PAF-acether-induced in vitro aggregation of rabbit and human platelets. Thromb. Res. 1984;34:463–466. doi: 10.1016/0049-3848(84)90251-2. [DOI] [PubMed] [Google Scholar]
- LIGUMSKY M., SIMON P.L., KARMELI F., RACHMILEWITZ D. Role of interleukin 1 in inflammatory bowel disease-enhanced production during active disease. Gut. 1990;31:686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOGUERCIO C., D'ARGENIO G., DELLE CAVE M., COSENZA V., DELLA VALLE N., MAZZACCA G., DEL VECCHIO BLANCO C. Direct evidence of oxidative damage in acute and chronic phases of experimental colitis in rats. Dig. Dis. Sci. 1996;6:1204–1211. doi: 10.1007/BF02088238. [DOI] [PubMed] [Google Scholar]
- MCKENZIE S.J., BAKER M.S., BUFFINTON G.D., DOE W.F. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J. Clin. Invest. 1996;98:136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEENAN J., GROOL T.A., HOMMES D.W., DIJKHUIZEN S., TEN KATE F.J., WOOD M., WHITTAKER M., TYTGAT G.N., VAN DEVENTER S.J. Lexipafant (BB-882). a platelet activating antagonist, ameliorates mucosal inflammation in ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 1996;8:569–583. doi: 10.1097/00042737-199606000-00014. [DOI] [PubMed] [Google Scholar]
- MORRIS G.P., BECK P.L. , HERRIDGE W., DEPEW W., SZEWCZUK M.R., WALLACE J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- MORTEAU O., MORE J., PONS L., BUENO L. Platelet-activating factor and interleukin 1 are involved in colonic dysmotility in experimental colitis in rats. Gastroenterology. 1993;104:47–56. doi: 10.1016/0016-5085(93)90834-y. [DOI] [PubMed] [Google Scholar]
- NASSIF A., LONGO W.E., MAZUSKI J.E., VERNAVA A.M., KAMINSKI D.L. Role of cytokines and platelet-activating factor in inflammatory bowel disease. Dis. Colon Rectum. 1996;39:217–223. doi: 10.1007/BF02068079. [DOI] [PubMed] [Google Scholar]
- NIELSEN O.H., VESPARGET H.W., ELMGREEN J. Inhibition of intestinal macrophage chemotaxis to leukotriene B4 by sulphasalazine, olsalazine and 5-aminosalicylic acid. Aliment. Pharmacol. Ther. 1988;2:203–211. doi: 10.1111/j.1365-2036.1988.tb00689.x. [DOI] [PubMed] [Google Scholar]
- PEPPERCORN M.A., GOLDMAN P. The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J. Pharmacol. Exp. Ther. 1972;181:555–562. [PubMed] [Google Scholar]
- PESKAR B.M., DREYLING K.W., MAY B., SCHAARSCHMIDT K., GOEBELL H. Possible mode of action of 5-aminosalicylic acid. Dig. Dis. Sci. 1987;32:51S–56S. doi: 10.1007/BF01312464. [DOI] [PubMed] [Google Scholar]
- RACHMILEWITZ D., KARMELI F., SCHWARTZ L.W. Effect of aminophenols (5-ASA and 4-ASA) on colonic interleukin-1 generation. Gut. 1992;33:929–932. doi: 10.1136/gut.33.7.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ DE MEDINA F., GALVEZ J., ROMERO J.A., ZARZUELO A. Effect of quercitrin on acute and chronic experimental colitis in the rat. J. Pharmacol. Exp. Ther. 1996;278:771–779. [PubMed] [Google Scholar]
- SCHRÖDER H., GUSTAFSSON B.E. Azo reduction of salicyl-azo-sulphapyridine in germ-free and conventional rats. Xenobiotica. 1973;3:225–231. doi: 10.3109/00498257309151518. [DOI] [PubMed] [Google Scholar]
- SHARON P., STENSON W.F. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984;86:453–460. [PubMed] [Google Scholar]
- STENSON W.F. Animal models of inflammatory bowel disease Inflammatory Bowel Disease: From Bench To Bedside 1994Baltimore: Williams & Wilkins; 180–192.ed. Targan, S.R. & Shanahan, F. pp [Google Scholar]
- STENSON W.F., LOBOS E. Sulfasalazine inhibits the synthesis of chemotactic lipids by neutrophils. J. Clin. Invest. 1982;69:494–497. doi: 10.1172/JCI110474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTHERLAND L.R., MAY G.R., SHAFFER E.A. Sulfasalazine revisited: a meta-analysis of 5-aminosalicylic acid in the treatment of ulcerative colitis. Ann. Intern. Med. 1993;118:540–549. doi: 10.7326/0003-4819-118-7-199304010-00009. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND L.R., ROTH D.E., BECK P.L. Alternatives to sulfasalazine: a meta-analysis of 5-ASA in the treatment of ulcerative colitis. Inflamm. Bowel Dis. 1997;3:65–78. [PubMed] [Google Scholar]
- TRAVIS S.P.L., JEWELL D.P. Salicylates for ulcerative colitis–Their mode of action. Pharmac. Ther. 1994a;63:135–161. doi: 10.1016/0163-7258(94)90042-6. [DOI] [PubMed] [Google Scholar]
- TRAVIS S.P.L., JEWELL D.P. The role of platelet-activating factor in the pathogenesis of gastrointestinal disease. Prostaglandins Leukotrienes Essent. Acids. 1994b;50:105–113. doi: 10.1016/0952-3278(94)90092-2. [DOI] [PubMed] [Google Scholar]
- VELJACA M., LESCH C.A., PLLANA R., SANCHEZ B., CHAN K., GUGLIETTA A. BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J. Pharmacol. Exp. Ther. 1995;272:417–422. [PubMed] [Google Scholar]
- WALLACE J.L. Release of platelet-activating factor (PAF) and accelerated healing induced by a PAF antagonist in an animal model of chronic colitis. Can. J. Physiol. Pharmacol. 1988;66:422–425. doi: 10.1139/y88-071. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., BRAQUET P., IBBOTSON G.C., MACNAUGHTON W.K., CIRINO G. Assessment of the role of platelet-activating factor in an animal model of inflammatory bowel disease. J. Lipid Med. 1989;1:13–23. [PubMed] [Google Scholar]
- WARDLE T.D., HALL L., TURNBERG L.A. Platelet activating factor: release from colonic mucosa in patients with ulcerative colitis and its effect on colonic secretion. Gut. 1996;38:355–361. doi: 10.1136/gut.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA T., MARSHALL S., SPECIAN R.D., GRISHAM M.B. A comparative study of two models of experimental colitis in rats. Gastroenterology. 1992;102:1524–1534. doi: 10.1016/0016-5085(92)91710-l. [DOI] [PubMed] [Google Scholar]


