Abstract
Stable clones of HEK293 cells expressing either FLAGTM epitope-tagged, wild type human β1- and β2-adrenoceptors or C-terminally green fluorescent protein (GFP)-tagged forms of these receptors were established.
The binding affinity of [3H]-dihydroalprenolol and other ligands was little affected by addition of GFP to the C-terminal of either receptor.
Isoprenaline induced the internalisation of both β1-adrenoceptor-GFP and β2-adrenoceptor-GFP and following removal of the agonist both constructs were able to recycle to the cell surface.
The extent of internalisation of β2-adrenoceptor-GFP produced by isoprenaline was substantially greater than for β1-adrenoceptor-GFP.
C-terminal addition of GFP slowed markedly the rate of internalization of both the β1-adrenoceptor and the β2-adrenoceptor in response to isoprenaline.
Sustained exposure to isoprenaline (24 h) produced substantially greater levels of downregulation of native β2-adrenoceptor compared to β2-adrenoceptor-GFP although both were equally effectively removed from the plasma membrane.
Sustained exposure to isoprenaline resulted in a large fraction of β2-adrenoceptor-GFP becoming trapped in internal vesicles/lysosomes but not degraded.
Even after sustained exposure to isoprenaline a significant fraction of β1-adrenoceptor-GFP remained at the cell surface.
These results indicate that although GFP tagging of β-adrenoceptors can provide qualitative visual patterns of agonist-induced receptor trafficking and regulation in HEK293 cells the quantitative details vary markedly from those obtained with the unmodified receptors.
Keywords: β-adrenoceptor, green fluorescent protein, internalization, trafficking, HEK293 cells
Introduction
A desire to visualize the cellular location and trafficking of G protein-coupled receptors (GPCRs) following their activation has produced a number of studies utilising forms of GPCRs which have been altered by addition of the Green Fluorescent Protein (GFP) from the jellyfish Aequorea victoria to their C-terminal tail (see Milligan, 1999 for review). Such studies have been highly informative, providing information about the mechanism and kinetics of internalization of a range of GPCRs. However, in most cases there have not been direct comparisons with effects of agonists on the unmodified GPCR. C-terminal addition of GFP to a range of GPCRs does not prevent agonist-induced activation of G proteins and downstream effectors nor does it prevent agonist-induced phosphorylation of GPCRs or their interactions with members of the arrestin family of proteins involved in their desensitization and internalization via clathrin-coated vesicles (Milligan, 1999).
Using co-immunoprecipitation and co-localization approaches, for both the β1-adrenoceptor (Tang et al., 1999), and the β2-adrenoceptor (Hall et al., 1998a,1998b; Cao et al., 1999; Luttrell et al., 1999), a number of other proteins, apart from G proteins, G protein-coupled receptor kinases and arrestins, have been shown to interact directly with these GPCRs (see Hall et al., 1999 for review). Interestingly, a number of these proteins have been shown to interact with the β2-adrenoceptor via amino acids at the extreme C-terminus of the GPCR. These include a protein named EBP50 (for ezrin radixin-moesin (ERM)-binding phosphoprotein-50) (Cao et al., 1999) which has been shown to play an important role in determining whether internalized β2-adrenoceptor is recycled to the plasma membrane or targeted to a separate set of vesicles which may lead to lysosomal degradation. As little as a single amino acid alteration at the C-terminal tail of the β2-adrenoceptor is sufficient to disrupt such interactions and thus potentially to modify the trafficking of the GPCR (Hall et al., 1998a; Cao et al., 1999). Addition of GFP to the C-terminus of the GPCR would be anticipated to prevent such interactions. We have thus generated clones of HEK293-cells stably expressing either wild type or C-terminally GFP-tagged forms of the β1 - and β2-adrenoceptors to explore the basic characteristics and kinetics of internalization and recycling of these GPCRs using combinations of confocal microscopy and intact cell [3H]-ligand binding studies. The addition of GFP to the C-terminus of both GPCRs substantially slowed their rate of internalization and the capacity of the cell to dispose of the proteins during long-term exposure to agonist. Some of this data has previously been presented in preliminary form (McLean & Milligan, 1999)
Methods
Materials and methods
[3H]-DHA (64 Ci mmol−1), [3H]-CGP-12177 (44 Ci mmol−1), [3H]-adenine and [3H]-cyclic AMP were purchased from Amersham International. All reagents for cell culture were from Life Technologies (Paisley, Strathclyde, U.K.). Receptor ligands were from RBI. All other general laboratory reagents were from Sigma or Fisons and were of the highest purity available.
Construction of GFP tagged forms of the β1- and β2-adrenoceptors
Human β1-adrenoceptor in pcDNA3 was amplified by PCR using a HindIII-FLAG forward primer, 5′-AAAAAAAAGC-TTGCCAC CATGG ACTA CAAGGACGAC GATGATAA-GGGCGCGGGGGTGCTC-3′, and a BamHI reverse primer, 5′-AAAAAGGATCCTCCCGCCACCTTGGATTCCGAGG-C-3′. This removed the stop codon and the initiating methionine (start codon) of the β1-adrenoceptor, with an initiator ATG being present in the N-terminally added FLAGTM epitope tag (ATG GAC TAC AAG GAC GAC GAT GAT AAG). The PCR product was digested with HindIII and BamHI and the resulting fragment ligated into pcDNA3. A modified form of GFP (Zernicka-Goetz et al., 1997) was also amplified by PCR using a BamHI forward primer, 5′-AAAAA GGATCC AGT AAA GGA GAA GAA CTT TTC-3′, and an XbaI reverse primer, 5′-TGCTCTAGATTATTTGTATAGTTCATCCATGCCATG-3′. This removed the initiating methionine of GFP and the resulting PCR product was digested and linked inframe to generate the FLAGTM β1-adrenoceptor-GFP construct. Construction of the human β2-adrenoceptor-GFP construct has been described previously (McLean et al., 1999).
Transient and stable transfection of HEK293 cells
HEK293 cells were maintained in Minimum Essential Medium (MEM, Sigma) supplemented with 0.292 g l−1 L-glutamine, and 10% newborn calf serum at 37°C. Cells were grown to 60–80% confluency prior to transient transfection. Transfection was performed using LipofectAMINE reagent (Life Technology, Inc.) according to the manufacturers' instructions. To generate cell lines stably expressing the various constructs, two days after transfection cells were seeded/diluted and maintained in MEM medium supplemented with 1 mg ml−1 Geneticin sulphate (Life Technology, Inc.). Medium was replaced every 3 days with MEM medium containing 1 mg ml−1 Geneticin sulphate.
Confocal laser scanning microscopy
Cells were observed using a laser scanning confocal microscope (Zeiss Axiovert 100) using a Zeiss Plan-Apo 63×1.40 NA oil immersion objective, pinhole of 20, and electronic zoom 2 or 3. The GFP was excited using a 488 nm argon/krypton laser and detected with 515–540 nm band pass filter. The images were manipulated with Zeiss LSM or MetaMorph software. When examining the time course of internalization and recycling cells were grown on glass coverslips and mounted on the imaging chamber. Cells were maintained in KRH buffer and temperature was maintained at 37°C.
[3H]-ligand binding studies
Stably transfected cells were plated into 24 well microtitre plates the day before the experiment. After appropriate drug treatments the cells were washed twice with ice-cold phosphate-buffered saline (PBS (mM): KCl 2.7, NaCl 137, KH2PO4 1.5, Na2HPO4 8, pH 7.4) followed by addition of 200 μl of binding mix ([3H]-CGP-12177 (10 nM) or [3H]-DHA (2 nM) in Krebs-Ringer-HEPES buffer (KRH (mM): NaCl 130, KCl 5, MgSO4 1.2, CaCl2 1.2, HEPES 20, Na2PO4 1.2, glucose 10, 0.1% BSA; pH 7.4) buffer). Non-specific binding was assessed by the presence of 10 μM betaxolol (β1-adrenoceptor) or propranolol (β2-adrenoceptor). [3H]-DHA binding assays were performed at 30°C for 45 min and [3H]-CGP-12177 binding at 4°C for 90 min in KRH buffer. All experiments were terminated by removal of the binding medium and washing of the cells with ice-cold PBS. 0.5 ml of 0.5 mM EDTA in PBS was added to detach cells from the plate and this volume plus a further 0.5 ml wash of the wells of the microtitre plate were counted. For each assay equivalent unlabelled cells were removed and counted to calculate receptor levels.
Intact cell adenylyl cyclase activity measurements
Were performed essentially as described in (Wong, 1994; Merkouris et al., 1997) Cells were split into wells of a 12-well plate and the cells were allowed to reattach. Cells were then incubated in medium containing [3H]-adenine (1.5 μCi well−1) for 16–24 h. The generation of [3H]-cyclic AMP in response to treatment of the cells with various ligands and other reagents was then assessed.
Electrophoresis and immunoblot analysis
Membrane samples were resolved by SDS–PAGE. Proteins were then electrophoretically transferred to nitrocellulose. The nitrocellulose filter was blocked for 1 h in 3% fat-free milk in PBS-T buffer (PBS containing 0.1% Tween 20). After a brief wash in PBS-T buffer, it was incubated overnight at 4°C with an appropriate primary antiserum diluted in PBS-T buffer containing 1% fat-free milk. Polyclonal antisera to either the β1- or the β2- adrenoceptor (Santa Cruz) were used for detection of the constructs. The primary antiserum was then removed and the blot washed extensively in PBS-T buffer. Subsequent incubation with secondary antibody (donkey anti-rabbit IgG conjugated with horseradish peroxidase, (Scottish Antibody Production Unit, Carluke, Scotland) proceeded for 2 h at RT and after extensive washing in PBS-T buffer the blot was visualised by ECL (Amersham).
Results
A cDNA encoding a modified form of GFP from Aequorea victoria was ligated to the 3′ end of cDNAs encoding the human forms of each of the β1- and β2-adrenoceptors using a polymerase chain reaction-based approach which allowed removal of the stop codons from the GPCR cDNAs. Although not utilized directly within the current studies, both the β1- and β2-adrenoceptor cDNAs were also modified at the 5′ end to introduce the FLAGTM epitope tag sequence (Asp-Tyr-Lys-Asp-Asp-AspAsp-Lys) at the N-terminus of the proteins. cDNAs encoding the FLAGTM-tagged β1- and β2-adrenoceptors and the GFP-tagged forms of these (Figure 1), ligated to the mammalian expression vector pCDNA3, were transfected into HEK293 cells. This plasmid also expresses a genetecin resistance marker and clones which displayed resistance to genetecin sulphate (1 mg ml−1) were selected and expanded. Initial screening of clones for the expression of the β1-adrenoceptor-GFP and β2-adrenoceptor-GFP was performed by visual examination in a confocal microscope to detect the autofluorescence of GFP. A significant number of such clones were selected based on clear, plasma membrane-delineated, autofluorescence of the GPCR-GFP fusion protein. Initial screening of the non-GFP tagged forms of the GPCRs was performed by monitoring the specific binding of a single, near saturating, concentration of the β-adrenoceptor antagonist [3H]-dihydroalprenolol ([3H]-DHA). Subsequent saturation [3H]-DHA binding studies were performed on membrane preparations from selected clones to obtain both Bmax values and measures of ligand affinity for the various constructs (Table 1). Although the measured Kd for [3H]-DHA at the β1-adrenoceptor constructs was approximately twice that at the β2-adrenoceptor constructs, the addition of GFP to the C-terminus of either GPCR had little effect on the binding affinity of [3H]-DHA (Table 1). However, clones expressing the GFP-tagged forms of the GPCRs generally expressed considerably higher levels of [3H]-DHA binding sites than those expressing the equivalent untagged GPCRs. We have previously also noted this feature in clones expressing wild type and GFP-tagged forms of the hamster α1b-adrenoceptor (Steven, P.A. and Milligan, G., unpublished) and believe this to reflect the well appreciated long half-life of GFP. These membranes were also immunoblotted with both β1- and β2-adrenoceptor selective antisera to confirm the identity of the correct GPCRs in each clone (Figure 2). The β-adrenoceptor agonist isoprenaline stimulated adenylyl cyclase activity in a concentration-dependent manner in all clones tested, further confirming (Barak et al., 1997; Kallal et al., 1998) that addition of GFP to the C-terminal tail of either the β1- or β2-adrenoceptor did not prevent productive interactions of the GPCRs with Gs (data not shown).
Figure 1.
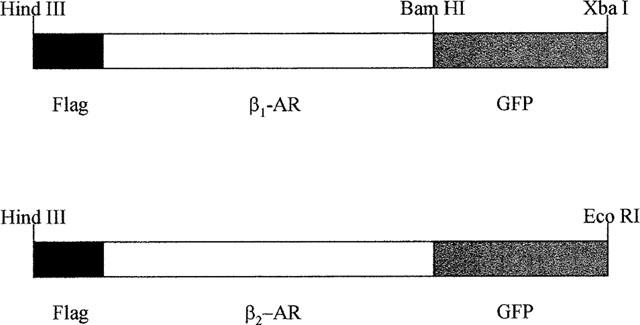
Schematic diagram of the constructs which were stably expressed in HEK293 cells. cDNAs encoding the human β1- and β2-adrenoceptor (AR) were modified at their N-terminus to encode a FLAGTM epitope. Following removal of the stop codon from the receptor sequences GFP was added in-frame.
Table 1.
Expression and ligand binding characteristics of GFP and non-GFP-tagged forms of the human β1- and β2-adrenoceptors
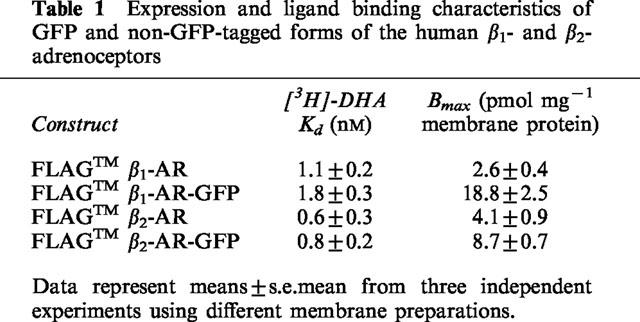
Figure 2.
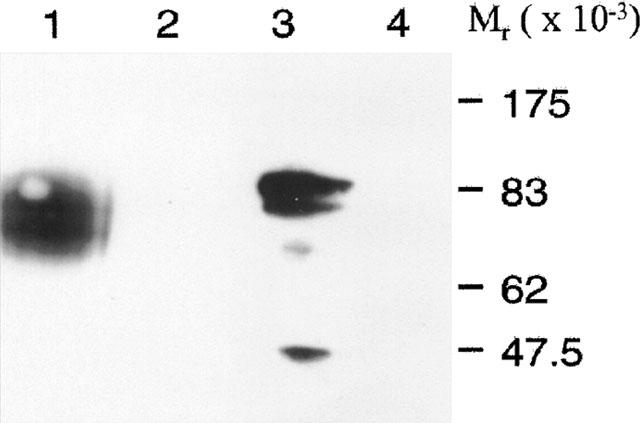
Immunoblots of β1-adrenoceptor and β2-adrenoceptor constructs. Membranes expressing FLAGTM β2-adrenoceptor-GFP (1 and 4) or FLAGTM β1-adrenoceptor-GFP (2 and 3) were immunoblotted using either anti-β2-adrenoceptor (1 and 2) or anti-β1-adrenoceptor (3 and 4) antisera. Similar results were obtained in another set of immunoblots.
When grown on a glass cover slip and then imaged on a confocal microscope clones expressing the β1-adrenoceptor-GFP construct displayed an essentially homogenous plasma membrane distribution of this protein. Addition of isoprenaline (10 μM) at 37°C for varying times resulted in the plasma membrane signal becoming reduced in intensity and the construct appearing in punctate intracellular vesicles (Figure 3). However, exposure of the cells to isoprenaline for greater than 10 min was required to observe a significant level of internalization. A similar pattern and time-course of internalization was also observed in other clones expressing this construct (data not shown). Betaxolol is a selective β1-adrenoceptor blocker. Competition binding experiments in membranes expressing either the β1-adrenoceptor or β1-adrenoceptor-GFP with [3H]-DHA confirmed that the high affinity of betaxolol for the β1-adrenoceptor was not compromized by addition of GFP (Table 2). Following pretreatment of β1-adrenoceptor-GFP expressing cells with isoprenaline (10 μM, 30 min, 37°C), removal of the agonist and its replacement with betaxolol (10 μM) allowed recycling of the GPCR construct to the plasma membrane (Figure 4). Equivalent studies were performed with a clone expressing β2-adrenoceptor-GFP. Again, isoprenaline (10 μM) produced internalization of the construct from the plasma membrane into punctate intracellular vesicles and removal of the agonist and replacement with the antagonist alprenolol (10 μM) allowed efficient recycling of the construct to the plasma membrane (Figure 5).
Figure 3.
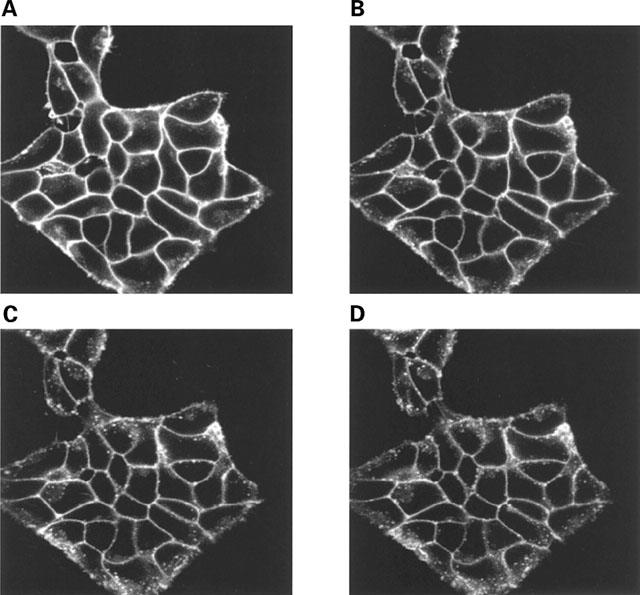
The β1-adrenoceptor-GFP construct is internalized following addition of isoprenaline. A group of cells stably expressing the FLAGTM β1-adrenoceptor-GFP construct were exposed to isoprenaline (10 μM) for 0 (A), 10 (B), 20 (C) or 40 (D) min during visualization in the confocal microscope. Three separate experiments produced similar results.
Table 2.
Ligand binding characteristics of GFP and non-GFP-tagged forms of the human β1-adrenoceptor
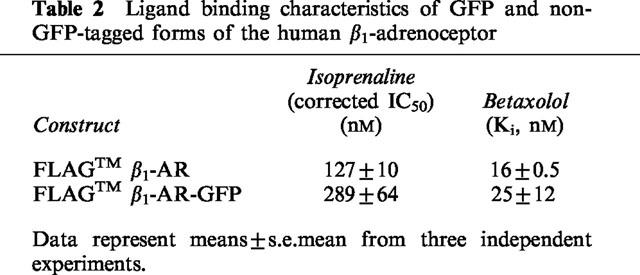
Figure 4.
Replacement of isoprenaline with betaxolol allows the β1-adrenoceptor-GFP construct to recycle to the plasma membrane. Cells stably expressing the FLAGTM β1-adrenoceptor-GFP construct were exposed to isoprenaline (10 μM) for 0 (A) or 30 (B–D) min. Following washing in the presence of betaxolol (10 μM) the distribution of the receptor was visualized 10 (C) and 30 (D) min later. Three separate experiments produced similar results.
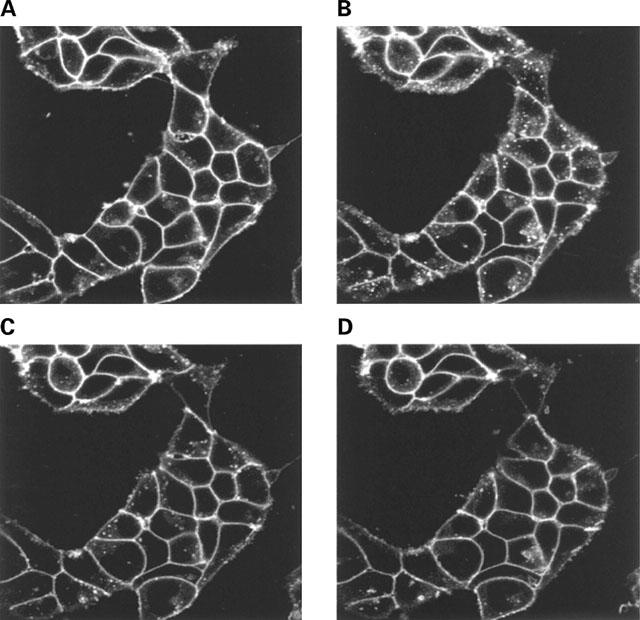
Figure 5.
Internalization and recycling of β2-adrenoceptor-GFP. Cells stably expressing the FLAGTM β2-adrenoceptor-GFP construct were exposed to isoprenaline (10 μM) for 0 (A) or 30 (B–D) min. Following washing with buffer containing alprenolol (10 μM) the distribution of the receptor was monitored 30 (C) and 40 (D) min later. Three separate experiments produced similar results.
Despite the ease of visualization of agonist-induced internalization of these constructs it is difficult to use this approach to provide more than a qualitative analysis. [3H]-DHA is a hydrophobic antagonist and thus in intact cell binding studies it can cross the plasma membrane. In this situation, specific binding of this ligand represents a combination of cell surface and internal GPCR. By contrast, [3H]-CGP-12177 is a hydrophilic antagonist which in intact cell binding studies labels only cell surface GPCRs. In preliminary experiments the specific binding at 4°C of [3H]-CGP-12177 to intact cells expressing each of the β1- and β2-adrenoceptor constructs achieved steady-state within 60 min. All the subsequent binding experiments with this ligand were thus incubated for 90 min at 4°C to ensure maximal binding but to prevent recycling of any internalized GPCRs to the cell surface during the course of the assay as this is an energy and temperature-dependent process. Following incubation of cells expressing either the β2-adrenoceptor or the β2-adrenoceptor-GFP with isoprenaline (10 μM) for 30 min at 4°C to allow the agonist to bind but at a temperature at which GPCR internalization would not proceed, the cells were washed with buffer between 0–4 times prior to initiating a specific [3H]-CGP-12177 (10 nM) binding assay. With only a single wash the levels of specific [3H]-CGP-12177 binding achieved was the same as in cells which had not been pre-exposed to isoprenaline, indicating effective removal of isoprenaline. However, two washes of the cells were routinely applied in subsequent studies to ensure this would be the case. Isoprenaline (10 μM) was then added to cells at 37°C for between 10–60 min and, following cooling and washing to remove the agonist, the specific binding of either [3H]-CGP-12177 (at 4°C) or [3H]-DHA (at 30°C, to allow ligand to penetrate the cells and bind both cell surface and internal receptors) was determined. For both the β2-adrenoceptor and the β2-adrenoceptor-GFP expressing cells specific [3H]-DHA binding was unaltered over this time period of isoprenaline treatment indicating total cellular levels of the GPCRs were not reduced (Figure 6). However, for both cell lines cell surface levels of the GPCRs, monitored by the binding of [3H]-CGP-12177, were reduced substantially. Although cell surface levels of the β2-adrenoceptor were reduced by some 40% within 10 min of exposure to isoprenaline, significant loss of the β2-adrenoceptor-GFP construct from the cell surface took longer (Figure 7). However, following 60 min exposure to isoprenaline some 60% of both the β2-adrenoceptor and β2-adrenoceptor-GFP had become internalized (Figure 7). Similar results were obtained for the cells expressing the β1-adrenoceptor and β1-adrenoceptor-GFP (Figure 8). Internalization of the wild type β1-adrenoceptor in response to isoprenaline was more rapid than internalization of β1-adrenoceptor-GFP, although following a 60 min exposure the two constructs achieved similar (between 30–40%) extents of internalization which was substantially lower than for the β2-adrenoceptor constructs.
Figure 6.
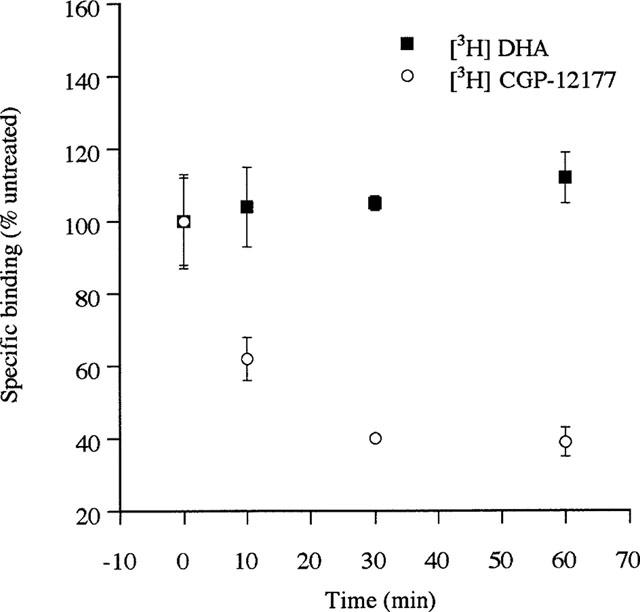
Short-term treatment with isoprenaline internalizes but does not downregulate the FLAGTM β2-adrenoceptor-GFP construct. Cells stably expressing the FLAGTM β2-adrenoceptor-GFP construct were exposed to isoprenaline (10 μM) for varying times. Specific binding of a single concentration of either [3H]-DHA (2 nM) or [3H]-CGP-12177 (10 nM) to intact cells was then measured as described in Methods.
Figure 7.
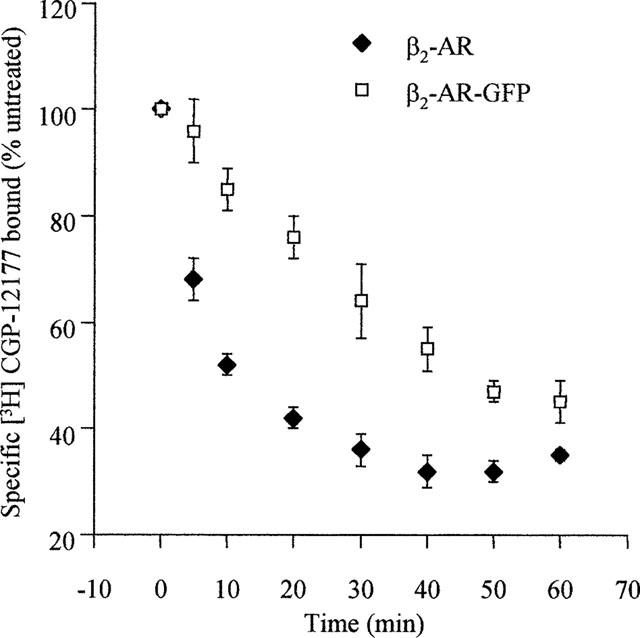
Comparison of the kinetics and extent of isoprenaline-induced internalization of the β2-adrenoceptor and β2-adrenoceptor-GFP. Cells stably expressing either the FLAGTM β2-adrenoceptor or the FLAGTM β2-adrenoceptor-GFP construct were exposed to isoprenaline (10 μM) for varying times. Specific binding of a single concentration of [3H]-CGP-12177 (10 nM) to intact cells was then measured as described in Methods. Results are presented as means±s.e.m. for five (FLAGTM β2-adrenoceptor) or four (FLAGTM β2-adrenoceptor-GFP) experiments.
Figure 8.
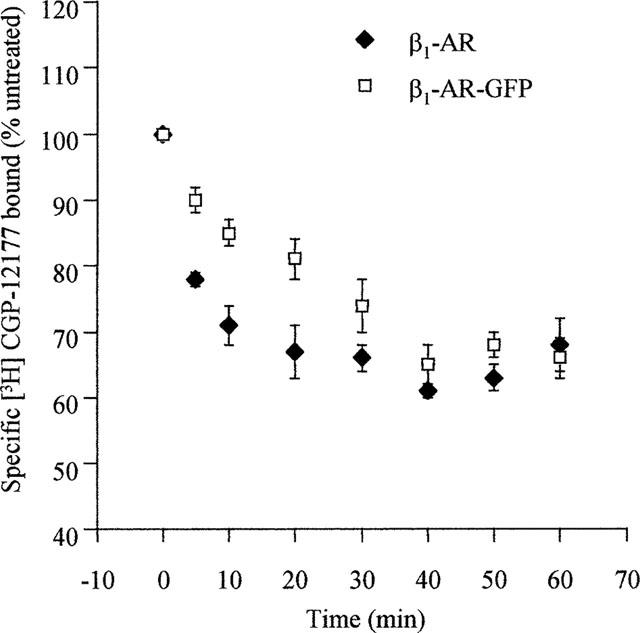
Comparison of the kinetics and extent of isoprenaline-induced internalization of the β1-adrenoceptor and β1-adrenoceptor-GFP. Cells stably expressing either the FLAGTM β1-adrenoceptor or the FLAGTM β1-adrenoceptor-GFP construct were exposed to isoprenaline (10 μM) for varying times. Specific binding of a single concentration of [3H]-CGP-12177 (10 nM) to intact cells was then measured as described in Methods. Results are presented as means±s.e.m. for three (FLAGTM β1-adrenoceptor) or five (FLAGTM β1-adrenoceptor-GFP) experiments.
Long-term exposure of cells to agonist often results in a substantial downregulation of total cellular levels of a GPCR. Indeed, following exposure of wild type β2-adrenoceptor expressing cells to isoprenaline (10 μM) for 24 h, levels of intact cell [3H]-DHA binding were reduced by 40% and the vast majority of the remaining GPCR was now internalized because specific [3H]-CGP-12177 binding was reduced by 80% (Figure 9A). However, when equivalent experiments were performed on cells expressing β2-adrenoceptor-GFP, although very little cell surface GPCR could be measured as [3H]-CGP-12177 binding sites, there was little downregulation of the construct as [3H]-DHA binding was reduced by less than 20% (Figure 9A). To examine this further, membrane fractions were prepared from untreated and isoprenaline (10 μM, 24 h) treated cells expressing either the β2-adrenoceptor or β2-adrenoceptor-GFP and [3H]-DHA binding monitored. These confirmed that isoprenaline produced a large downregulation of the β2-adrenoceptor but not of β2-adrenoceptor-GFP (Figure 9B). To examine the basis for this, β2-adrenoceptor-GFP expressing cells were grown on glass cover slips and exposed to isoprenaline (10 μM, 24 h). Untreated and agonist-treated cells were then visualized (Figure 10). As anticipated from the intact cell binding studies, although virtually all the construct was intracellular it was still present in high levels. Rather than being present in small, discrete, punctate vesicles as seen with short periods of exposure to isoprenaline, it was predominantly located in large, perinuclear, vesicles. In similar experiments with cells expressing β1-adrenoceptor-GFP, 24 h after treatment with isoprenaline (10 μM) internalization of the construct could be observed but a large amount of cell surface GPCR remained (Figure 11) and parallel ligand binding studies indicated virtually no downregulation of the protein had occurred (data not shown).
Figure 9.
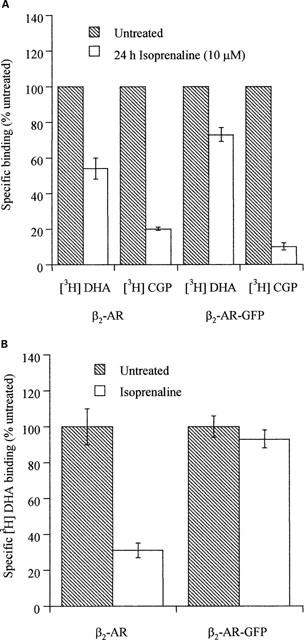
Long-term treatment with isoprenaline. Effects on distribution and downregulation of the β2-adrenoceptor and β2-adrenoceptor-GFP monitored by ligand binding studies. (A) Intact Cells. Cells stably expressing either the FLAGTM β2-adrenoceptor or the FLAGTM β2-adrenoceptor-GFP construct were either untreated or exposed to isoprenaline (10 μM) for 24 h. Specific binding of a single concentration of either [3H]-DHA (2 nM) or [3H]-CGP-12177 (10 nM) to intact cells was then measured as described in Methods. Results are presented as means±s.e.m. for three separate experiments. (B) Membranes. Cells stably expressing either the FLAGTM β2-adrenoceptor or the FLAGTM β2-adrenoceptor-GFP construct were either untreated or exposed to isoprenaline (10 μM) for 24 h. Membranes were prepared and the specific binding of a single concentration of [3H]-DHA (2 nM) was then measured as described in Methods. Results are presented as means±s.e.m. for three separate experiments.
Figure 10.
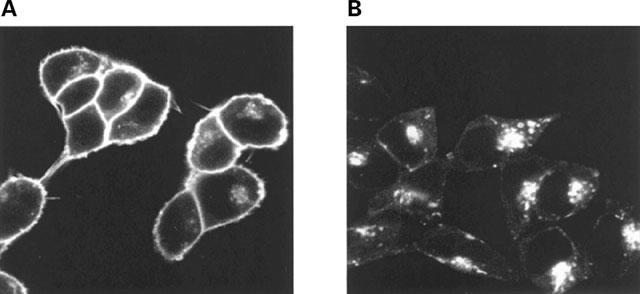
Visualization of the location of β2-adrenoceptor-GFP following long-term challenge with isoprenaline. Cells stably expressing the FLAGTM β2-adrenoceptor-GFP construct were either untreated (A) exposed to isoprenaline (10 μM) for 24 h (B). The distribution of the construct was then visualized. Two further experiments produced similar patterns.
Figure 11.
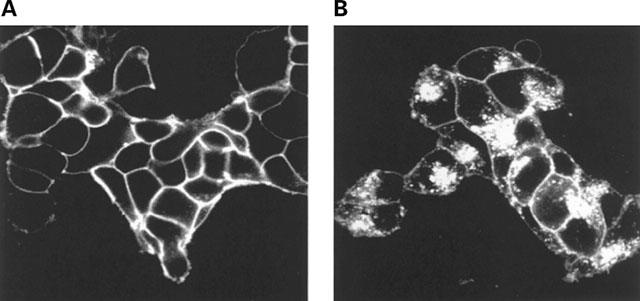
Visualization of the location of β1-adrenoceptor-GFP following long-term challenge with isoprenaline. Cells stably expressing the FLAGTM β1-adrenoceptor-GFP construct were either untreated (A) exposed to isoprenaline (10 μM) for 24 h (B) The distribution of the construct was then visualized. Two further experiments produced similar patterns.
Discussion
C-terminal attachment of forms of GFP to the C-terminal tail of GPCRs has recently become a popular strategy to visualize their cellular location to investigate the processes of agonist-induced internalization and recycling to the plasma membrane. GPCRs which have been modified in this manner include the β1- (McLean & Milligan, 1999) and β2-adrenoceptors (Barak et al., 1997; Kallal et al., 1998; Conway et al., 1999; McLean et al., 1999), the α1a- (Hirasawa et al., 1997) and α1b-adrenoceptors (Awaji et al., 1998), the CXCR1 receptor (Barlic et al., 1999), the parathyroid hormone receptor (Conway et al., 1999), the thyrotropin-releasing hormone receptor-1 (Drmota et al., 1998; 1999; Groarke et al., 1999), the Ca2+ sensing receptor (Gama & Brietwieser, 1998), the V2 vasopressin receptor (Schulein et al., 1998) and the cholecystokinin type A receptor (Tarasova et al., 1997). This strategy has many obvious benefits, the most useful being the capacity to monitor the location and movement of the construct in living cells over an extended period of time. However, there are also potential drawbacks. These include that the GPCR is generally increased in size by at least 50% by attachment of the 27 kDa GFP. Furthermore, limitations of resolution of GFP visualization in living cells makes it unclear whether true co-localization rather than overlapping distribution with a second protein is observed (see Drmota et al., 1999; Groarke et al., 1999 for examples). It is generally accepted that addition of GFP does not prevent GPCR interaction with G proteins and this was again confirmed in the present studies for both the β1-adrenoceptor-GFP and β2-adrenoceptor-GFP constructs as both were able to produce a robust, concentration-dependent, stimulation of adenylyl cyclase upon addition of isoprenaline. However, recent studies have indicated the capacity of GPCRs, including the β1- and β2-adrenoceptors (see Hall et al., 1999 for review) to interact with a range of other intracellular proteins. The C-terminal tail of the β2-adrenoceptor appears vital for a number of these interactions including with EBP50 (Cao et al., 1999) which relies on the presence of Ser411. This amino acid is three amino acids from the C-terminal tail of the β2-adrenoceptor and is a target for phosphorylation by GRK-5 (Cao et al., 1999). Furthermore, single amino acid alterations at the extreme C-terminus of the β2-adrenoceptor have been reported to alter such interactions (Hall et al., 1998a,1998b). Addition of GFP to the C-terminal tail of a GPCR clearly must modify this region. Indeed, in many cases the sequence of the extreme C-terminal tail is altered during this process, predominantly for expediency and ease of the molecular manipulations, without considering the effects this might have on protein function. Purely in terms of ligand binding, and often when monitoring G protein and effector activation, such sequence alterations are unlikely to be a major issue. Indeed, herein we observed little variation in the binding affinity of either agonist or antagonist ligands following addition of GFP to the C-terminal tail of either the β1- or β2-adrenoceptor (Table 2). Moreover, isoprenaline was able to stimulate adenylyl cyclase activity via all of the constructs. However, we observed two key alterations in ligand regulation of the C-terminally GFP-tagged form of the β-adrenoceptors compared to the wild type forms. Firstly, isoprenaline-induced internalisation of the GFP-tagged constructs was substantially slower than of the wild type forms of both GPCRs. The molecular basis for this effect has not been established. However, a number of C-terminal truncations of GPCRs are known to slow ligand-induced internalization, e.g. for the rat thyrotropin releasing hormone receptor-1 (Drmota & Milligan, 2000). Indeed, in the case of the gonadotrophin releasing-hormone receptors from mammalian species, the absence of a C-terminal tail appears responsible for their very slow rates of agonist-induced internalization (Vrecl et al., 1998). The equivalent GPCR from catfish has a C-terminal tail and both this GPCR and the mammalian version with the tail of the rat thyrotropin releasing hormone receptor-1 appended internalize rapidly in response to agonist when expressed in HEK293 cells (Heding et al., 2000). It is also interesting to note that although C-terminal truncation of the rat thyrotropin releasing hormone receptor-1 slows the rate of internalization it does not affect the recycling rate constant of the GPCR once it has been internalized (Drmota & Milligan, 2000), suggesting that the C-terminal tail of the GPCR is not directly involved in controlling recycling to the cell surface. The current studies indicate that a substantial addition to the C-terminal tail of a GPCR can also markedly slow the rate of internalization. In studies on gonadotrophin releasing-hormone receptors and the thyrotropin releasing hormone receptor-1, the capacity of arrestins to interact with the C-terminal of the GPCR appears intimately involved in the effectiveness of internalization. However, addition of GFP to the C-terminal tail of the rat thyrotropin releasing hormone receptor-1 certainly does not prevent interactions with arrestin-2 (Groarke et al., 1999) but whether it alters the affinity of these interactions has not been explored.
The β1- and β2-adrenoceptors are highly suitable GPCRs for these type of studies because well characterized radiolabelled ligands are available to measure either total receptor levels or only those at the cell surface in intact cell binding studies. These were then used to provide a quantitative monitor of the rate of internalization of both the GFP-tagged and unmodified GPCRs. Direct visualization of the GPCRs was limited to the GFP-tagged forms and without the use of complex algorithms for analysis (Conway et al., 1999) can provide only qualitative pictures of agonist effects on GPCR localization. However, there are still distinct benefits in being able to directly visualize the presence and distribution of the GFP-tagged forms of the GPCRs.
The second feature of ligand regulation of the GFP-tagged β1- and β2-adrenoceptors which was altered was the effectiveness of downregulation of the GPCRs during sustained cellular exposure to agonist. Following sustained challenge of the β2-adrenoceptor expressing cells with a high concentration of isoprenaline, intact cell ligand binding studies confirmed both physical loss of some 40% of the receptor population and the removal of most of the receptor from the cell surface. However, although a similar removal of β2-adrenoceptor-GFP from the plasma membrane was evident the protein was apparently not effectively downregulated. Visualisation of such cells confirmed the maintained presence of high levels of β2-adrenoceptor-GFP inside the cells and the combination of binding studies and imaging was thus able to confirm that the GFP-linked protein still maintained ligand binding capacity. GFP has a long half-life and it may well be this feature which resulted in the maintained presence of the GFP-tagged β2-adrenoceptor in large intracellular vesicles which appear to be lysosomes. It is thus noteworthy that the majority of the clones we were able to isolate following transfection with the β2-adrenoceptor-GFP cDNA expressed the protein to relatively high levels. Indeed, clones expressing the protein at less than 3–4 pmol mg−1 membrane protein were not found. This also has been a feature of clones of cells we have isolated expressing GFP-tagged forms of other GPCRs, including the α1b-adrenoceptor. Whilst this is useful in many regards, it may be more difficult to study certain aspects of the regulation of these GPCRs if levels of expression start to outstrip endogenous levels of various, required, assessory proteins. We anticipated that addition of GFP to the C-terminus of the β2-adrenoceptor might modulate some aspects of its regulatory cycle as this is a known site of protein–protein interactions which can link this GPCR to the actin cytoskeleton (Cao et al., 1999). The current studies demonstrate again some of the benefits of the use of GFP-tagging of proteins but also suggest that a range of comparative studies with the unmodified protein are required in parallel to obtain a useful picture of the kinetics of their regulation and movement.
Acknowledgments
A.J. McLean thanks the Biotechnology and Biosciences Research Council for a studentship.
Abbreviations
- DHA
dihydroalprenolol
- EBP50
ezrin radixin-moesin (ERM)-binding phosphoprotein-50
- GFP
green fluorescent protein
- GPCR
G protein-coupled receptor
- KRH
Krebs-Ringer-HEPES
- PBS
phosphate-buffered saline
References
- AWAJI T., HIRASAWA H., KATAOKA M., SHINOURA H., NAKAYAMA Y., SUGAWARA T., IZUMI S., TSUJIMOTO G. Real-time optical monitoring of ligand-mediated internalisation of α1b-adrenoceptor with green fluorescent protein. Mol. Endocrinol. 1998;12:1099–1111. doi: 10.1210/mend.12.8.0149. [DOI] [PubMed] [Google Scholar]
- BARAK L.S., FERGUSON S.S., ZHANG J., MARTENSON C., MEYER T., CARON M.G. Internal trafficking and surface mobility of a functionally intact beta2-adrenergic receptor-green fluorescent protein conjugate. Mol. Pharmacol. 1997;51:177–184. doi: 10.1124/mol.51.2.177. [DOI] [PubMed] [Google Scholar]
- BARLIC J., KHANDAKER M.H., MAHON E., ANDREWS J., DEVRIES M.E., MITCHELL G.B., RAHIMPOUR R., TAN C.M., FERGUSON S.S.G., KELVIN D.J. β-arrestins regulate interleukin-8-induced CXCR1 internalization. J. Biol. Chem. 1999;274:16287–16294. doi: 10.1074/jbc.274.23.16287. [DOI] [PubMed] [Google Scholar]
- CAO T.T., DEACON H.W., RECZEK D., BRETSCHER A., VON ZASTROW M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- CONWAY B.R., MINOR L.K., XU J.Z., GUNNET J.W., DEBIASIO R., D'ANDREA M.R., RUBIN R., DEBIASIO R., GIULIANO K., ZHOU L., DEMAREST K.T. Quantification of G-protein coupled receptor internalization using G-protein coupled receptor-green fluorescent protein conjugates with the Array ScanTM high-content screening system. J. Biomol. Screening. 1999;4:75–86. doi: 10.1177/108705719900400207. [DOI] [PubMed] [Google Scholar]
- DRMOTA T., GOULD G.W., MILLIGAN G. Real time visualization of agonist-mediated redistribution and internalisation of a green fluorescent protein-tagged form of the thyrotropin-releasing hormone receptor. J. Biol. Chem. 1998;273:24000–24008. doi: 10.1074/jbc.273.37.24000. [DOI] [PubMed] [Google Scholar]
- DRMOTA T., MILLIGAN G. Kinetic analysis of the internalization and recycling of [3H]TRH and C-terminal truncations of the long isoform of the rat thyrotropin-releasing hormone receptor-1. Biochem. J. 2000;346:711–718. [PMC free article] [PubMed] [Google Scholar]
- DRMOTA T., NOVOTNY J., GOULD G.W., SVOBODA P., MILLIGAN G. Visualization of distinct patterns of subcellular redistribution of the thyrotropin-releasing hormone receptor and Gqα/G11α induced by agonist stimulation. Biochem. J. 1999;340:529–538. [PMC free article] [PubMed] [Google Scholar]
- GAMA L., BRIETWIESER G.E. A carboxyl-terminal domain controls the cooperativity for extracellular Ca2+ activation of the human calcium sensing receptor. A study with receptor-green fluorescent protein fusions. J. Biol. Chem. 1998;273:29712–29718. doi: 10.1074/jbc.273.45.29712. [DOI] [PubMed] [Google Scholar]
- GROARKE D.A., WILSON S., KRASEL C., MILLIGAN G. Visualization of agonist-induced association and trafficking of green fluorescent protein-tagged forms of both β-arrestin-1 and the thyrotropin releasing hormone receptor-1. J. Biol. Chem. 1999;274:23263–23269. doi: 10.1074/jbc.274.33.23263. [DOI] [PubMed] [Google Scholar]
- HALL R.A., OSTEDGAARD L.S., PREMONT R.T., BLITZER J.T., RAHMAN N., WELSH M.J., LEFKOWITZ R.J. A C-terminal motif found in the β2-adrenergic receptor, P2Y 1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc. Natl. Acad. Sci. U.S.A. 1998a;95:8496–8501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL R.A., PREMONT R.T., CHOW C.W., BLITZER J.T., PITCHER J.A., CLAING A., STOFFEL R.H., BARAK L.S., SHENOLIKAR S., WEINMAN E.J., GRINSTEIN S., LEFKOWITZ R.J. The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998b;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- HALL R.A., PREMONT R.T., LEFKOWITZ R.J. Heptahelical receptor signaling: beyond the G protein paradigm. J. Cell Biol. 1999;145:927–932. doi: 10.1083/jcb.145.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDING A., VRECL M., HANYALOGLU A.C., SELLAR R., TAYLOR P.L., EIDNE K.A. The rat gonadotropin-releasing hormone receptor internalizes via a beta-arrestin-independent, but dynamin-dependent, pathway: addition of a carboxyl-terminal tail confers beta-arrestin dependency. Endocrinology. 2000;141:299–306. doi: 10.1210/endo.141.1.7269. [DOI] [PubMed] [Google Scholar]
- HIRASAWA A., SUGAWARA T., AWAJI T., TSUMAYA K., ITO H., TSUJIMOTO G. Subtype-specific differences in subcellular localization of α1-adrenoceptors: Chlorethylclonidine preferentially alkylates the accessible cell surface α1-adrenoceptors irrespective of the subtype. Mol. Pharmacol. 1997;52:764–770. doi: 10.1124/mol.52.5.764. [DOI] [PubMed] [Google Scholar]
- KALLAL L., GAGNON A.W., PENN R.B., BENOVIC J.L. Visualization of agonist-induced sequestration and down-regulation of a green fluorescent protein-tagged β2-adrenergic receptor. J. Biol. Chem. 1998;273:322–328. doi: 10.1074/jbc.273.1.322. [DOI] [PubMed] [Google Scholar]
- LUTTRELL L.M., FERGUSON S.S., DAAKA Y., MILLER W.E., MAUDSLEY S., DELLA ROCCA G.J., LIN F., KAWAKATSU H., OWADA K., LUTTRELL D.K., CARON M.G., LEFKOWITZ R.J. β-arrestin-dependent formation of β2-adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- MCLEAN A.J., BEVAN N., REES S., MILLIGAN G. Visualizing differences in ligand regulation of wild type and constitutively active mutant β2-adrenoceptor-green fluorescent protein fusion proteins. Mol. Pharmacol. 1999;56:1182–1191. doi: 10.1124/mol.56.6.1182. [DOI] [PubMed] [Google Scholar]
- MCLEAN A.J., MILLIGAN G. Receptor-GFP fusion proteins: a study of receptor internalisation and trafficking Molecular Mechanisms of Transcellular Signalling: From Membane Receptors to Transcription Factors 1999247–252.(ed. Thiery, J.P.). NATO Science Series.A.S.I. Series A: Life Sciences, 309
- MERKOURIS M., MULLANEY I., GEORGOUSSI Z., MILLIGAN G. Regulation of spontaneous activity of the δ-opioid receptor: Studies of inverse agonism in intact cells. J. Neurochem. 1997;69:2115–2122. doi: 10.1046/j.1471-4159.1997.69052115.x. [DOI] [PubMed] [Google Scholar]
- MILLIGAN G. Exploring the dynamics of regulation of G protein-coupled receptors using green fluorescent protein. Br. J. Pharmacol. 1999;128:501–510. doi: 10.1038/sj.bjp.0702824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULEIN R., HERMOSILLA R., OKSCHE A., DEHE M., WIESNER B., KRAUSE G., ROSENTHAL W. A dileucine sequence and an upstream glutamate residue in the intracellular carboxyl terminus of the vasopressin V2 receptor are essential for cell surface transport in COS.M6 cells. Mol. Pharmacol. 1998;54:525–535. doi: 10.1124/mol.54.3.525. [DOI] [PubMed] [Google Scholar]
- TANG Y., HU L.A., MILLER W.E., RINGSTAD N., HALL R.A., PITCHER J.A., DECAMILLI P., LEFKOWITZ R.J. Identification of the endophilins (SH3p4/p8/p13) as novel binding partners for the β1-adrenergic receptor. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12559–12564. doi: 10.1073/pnas.96.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARASOVA N.I., STAUBER R.H., CHOI J.K., HUDSON E.A., CZERWINSKI G., MILLER J.L., PAVLAKIS G.N., MICHEJDA C.J., WANK S.A. Visualization of G protein-coupled receptor trafficking with the aid of the green fluorescent protein. Endocytosis and recycling of cholecystokinin receptor type A. J. Biol. Chem. 1997;272:14817–14824. doi: 10.1074/jbc.272.23.14817. [DOI] [PubMed] [Google Scholar]
- VRECL M., ANDERSON L., HANYALOGLU A., MCGREGOR A.M., GROARKE A.D., MILLIGAN G., TAYLOR P.L., EIDNE K.A. Agonist-induced endocytosis and recycling of the gonadotropin-releasing hormone (GnRH) receptor. Effect of β-arrestin on internalization kinetics. Mol. Endocrinol. 1998;12:1818–1829. doi: 10.1210/mend.12.12.0207. [DOI] [PubMed] [Google Scholar]
- WONG Y.G. Gi assays in transfected cells. Methods Enzymol. 1994;238:81–94. doi: 10.1016/0076-6879(94)38008-2. [DOI] [PubMed] [Google Scholar]
- ZERNICKA-GOETZ M., PINES J., MCLEAN HUNTER S., DIXON J.P.C., SIEMERING K., HASELOFF J., EVANS M.J. Following cell fate in the living mouse embryo. Development. 1997;124:1133–1137. doi: 10.1242/dev.124.6.1133. [DOI] [PubMed] [Google Scholar]


