Abstract
Single channel recordings were obtained from a Drosophila S2 cell line stably expressing the wild-type RDLac Drosophila melanogaster homomer-forming ionotropic GABA receptor subunit, a product of the resistance to dieldrin gene, Rdl. GABA (50 μM) was applied by pressure ejection to outside-out patches from S2-RDL cells at a holding potential of −60 mV. The resulting inward current was completely blocked by 100 μM picrotoxin (PTX). The unitary current-voltage relationship was linear at negative potentials but showed slight inward rectification at potentials more positive than 0 mV. The reversal potential of the current (EGABA=−1.4 mV) was close to the calculated chloride equilibrium potential.
The single channel conductance elicited by GABA was 36 pS. A 71 pS conductance channel was also observed when the duration of the pulse, used to eject GABA, was longer than 80 ms. The mean open time distribution of the unitary events was fitted best by two exponential functions suggesting two open channel states.
When either 1 μM fipronil or 1 μM BIDN was present in the external saline, the GABA-gated channels were completely blocked. When BIDN or fipronil was applied at a concentration close to the IC50 value for suppression of open probability (281 nM, BIDN; 240 nM, fipronil), the duration of channel openings was shortened. In addition, the blocking action of BIDN resulted in the appearance of a novel channel conductance (17 pS).
The effects of co-application of BIDN and fipronil were examined. Co-application of BIDN (300 nM) with various concentrations (100–1000 nM) of fipronil resulted in an additional BIDN-induced dose-dependent reduction of the maximum Po value.
Thus both BIDN and fipronil shorten the duration of wild-type RDLac GABA receptor channel openings but appear to act at distinct sites.
Keywords: GABA receptor, BIDN, fipronil, Drosophila melanogaster, stable cell line, single channel
Introduction
GABAA and GABAC-type ionotropic receptors gate distinct chloride channels and mediate the fast actions of the neurotransmitter γ-aminobutyric acid (GABA) in vertebrates. GABAA receptors are the target of many drugs acting on the central nervous system and several distinct drug binding sites have been identified (Sieghart, 1995; Whiting et al., 1995; Mehta & Ticku, 1999). Although insect ionotropic GABA receptors do not fit readily into the current vertebrate classification, sites of action of benzodiazepines, steroids and convulsants have been described (Sattelle 1990; Casida 1993; Bloomquist 1996; Narahashi et al., 1998). Furthermore, insect GABA-gated chloride channels are sites of action for several classes of insecticides including cyclodienes, cyclohexanes and phenylpyrazoles (Rauh et al., 1990; Sattelle et al., 1991; Schnee et al., 1997). A range of electrophysiological techniques, radioligand-binding assays and measurements of GABA-induced 36Cl− flux have been employed to compare the pharmacology of insect and vertebrate GABA-gated chloride channels (Rauh et al., 1990; Sattelle 1990). To these, have been added molecular cloning of vertebrate (see for review Mehta & Ticku, 1999) and insect (Hosie et al., 1997) GABA receptor subunits. In the case of insects, this has facilitated investigation of the mechanism of action of insecticides acting on GABA receptors. For example, studies on native (Olsen et al., 1987; Cole et al., 1993; 1995; Buckingham et al., 1994; Gant et al., 1998) and recombinant (Buckingham et al., 1994; Hosie et al., 1995a, 1995b; Hamon et al., 1998) receptors have shown that 3,3-bis - trifluromethyl - bicyclo - [2,2,1]heptane - 2,2 -dicarbonitrile, BIDN (a bicyclic dinitrile with insecticidal activity) and fipronil (a phenylpyrazole insecticide) act on insect neuronal GABA receptors as well as on the cloned Drosophila GABA receptor, RDL (the product of the resistance to dieldrin gene: Rdl) expressed in Xenopus oocytes and Drosophila cell lines. The radioligand [3H]-BIDN has been shown to be a probe of insect and vertebrate GABA-gated chloride channels and the unlabelled form of BIDN is an extremely effective blocker of functional ionotropic GABA receptors (Rauh et al., 1997; Hamon et al., 1998). Fipronil is an insecticide acting at the GABA receptor (Colliot et al., 1992; Bloomquist, 1996; Hainzl et al., 1998). It is deployed mainly as an animal health drug and is also used in crop protection. However, although both BIDN and fipronil block GABA-gated chloride channels, little is known of their detailed mechanism of action.
The homomer-forming ionotropic GABA receptor subunit RDL is a product of the Rdl gene originally identified in the Maryland strain of Drosophila melanogaster (ffrench-Constant, 1993; ffrench-Constant et al., 1990; 1993) and is the most comprehensively characterized insect GABA receptor subunit (Hosie et al., 1997; McGurk et al., 1998; ffrench-Constant et al., 1998). Messenger RNA for the RDLac subunit has been expressed successfully in Xenopus oocytes which has permitted studies on the properties of macroscopic GABA-gated chloride currents (ffrench-Constant et al., 1993; Buckingham et al., 1994; Zhang et al., 1994; McGurk et al., 1998). RDLac forms a homomeric, picrotoxin-sensitive, GABA-gated chloride channel which is sensitive to cyclodiene insecticides (e.g. dieldrin), phenylpyrazoles (e.g. fipronil) and other convulsants (e.g. BIDN; 4-n-propyl-4′-ethynylbicycloorthobenzoate, EBOB; t-butylbicyclo-phosphorothionate, TBPS). Here we have worked on a Drosophila cell line (S2-RDL) expressing stably the wild-type cloned Drosophila Rdlac GABA receptor subunit under the control of an inducible metallothionein promoter. Previous studies on this splice variant cell line, using the whole-cell patch-clamp technique, have demonstrated the presence of functional GABA-gated chloride channels which are blocked by a number of convulsants and insecticides such as picrotoxinin (PTXN), BIDN, fipronil and EBOB (Millar et al., 1994; Buckingham et al., 1996). The present study was undertaken to elucidate the detailed mechanism of action of BIDN and fipronil on the RDL receptor using steady-state single channel recordings. This study describes the single channel properties of the wild-type RDLac receptor stably expressed in S2 cells. It also provides the first unitary current investigation of the blocking actions of BIDN and fipronil on insect GABA-gated chloride channels. Preliminary accounts of some of these results have been published in abstract form (Grolleau & Sattelle, 1998).
Methods
Cells
Electrophysiological experiments were performed on the Drosophila melanogaster S2-RDL cell line expressing a functional homomer-forming GABA receptor subunit, the product of the Rdlac splice variant of the Rdl gene. Detailed procedures for transfection and maintenance of the S2-RDL cell line are given elsewhere (Millar et al., 1994). Cells were assayed 1–3 days after induction of receptor expression by the addition of CuSO4 to the growth medium to a final concentration 0.6 mM. Cells were transferred to a glass cover slip and allowed to settle for 10 min, then washed gently with normal saline containing (in mM): NaCl 120, KCl 5, CaCl2 2, MgCl2 8, N-tris(hydroxymethyl)-methyl-2aminoethane-sulphonic acid (TES) 10, pH 7.2.
Patch-clamp recording
The outside-out configuration of the patch-clamp recording technique was employed (Hamill et al., 1981). Patch electrodes were drawn from borosilicate glass capillary tubes (Clark Electromedical Instruments, Reading, U.K.) on a two-stage puller (Narashige PP-83, Tokyo, Japan) and coated with Sylgard (Dow Corning Corporation, Midland, U.S.A.) to reduce capacitance. The tip of the patch pipette was heat-polished on a Narishige (MF-83) microforge and had a resistance of 7–8 MΩ when filled with the pipette solution (for composition see below). The liquid junction potential between the saline and the internal pipette solution was always corrected before the formation of a gigaohm seal (>30 GΩ). The membrane currents were recorded using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA, U.S.A.). The cut-off frequency of the low-pass filter (−3 dB, 4-pole low-pass Bessel filter) was set at 5 kHz. The currents were digitized at 20 kHz and stored directly on the hard disk of an IBM Pentium 100 computer connected to a 125 kHz labmaster DMA data acquisition system (TL-1-125 interface, Axon Instruments). All patches were voltage-clamped at a membrane potential of −60 mV unless stated otherwise. The pClamp software (version 6.03, Axon Instruments) was used for data acquisition and analysis. All experiments were performed at room temperature (20–22°C).
Single channel analysis
The Fetchan program from the pClamp software package was used to construct unitary current amplitude histograms from original current traces which were filtered at 3–5 kHz before final display. Events were detected on the basis of crossing a threshold level equal to 50% of the unitary current amplitude. The threshold was adjusted visually prior to acceptance and each individual opening was examined. The histograms were then fitted (least-squares) by a Gaussian fit distribution to determine the mean amplitude of single channel openings. The mean open channel probability was calculated using patches with only one channel open and using segments of recording that were at least 40 s in duration. Dose-response curves were fitted to a sigmoïdal function with a four-parameter logistic equation with a variable slope. The equation used is the following:
where Ymax is the maximum Po, EC50 is the concentration required for a half-maximal response and n is the Hill coefficient (or slope factor). Data were expressed as mean±s.e.m.
Solutions
The composition of the saline bathing the extracellular patch surface was (in mM): NaCl 100, tetraethylammonium chloride (TEACl) 20, KCl 5, CaCl2 2, MgCl2 8, TES 10, 4-aminopyridine 5, pH 7.2. The patch pipette solution contained (in mM): KCl 130, MgCl2 4, TES 10, ethylene glycol-bis (β-aminoethyl ether) tetra-acetic acid (EGTA) 10, pH 7.2. These solutions resulted in a predicted equilibrium potential for chloride ions close to zero (ECl=−1.3 mV). GABA was applied to the membrane patch by pressure ejection from a glass pipette (resistance=4 MΩ when filled with 50 μM GABA) connected to a pneumatic pressure system (Miniframe PPS-2, Medical Systems Corporation, U.S.A.). The tip of the GABA-containing micropipette was placed 60 μm from the excised patch using a hydraulic micromanipulator (Narishige MW-3, Tokyo, Japan). Unless otherwise indicated, GABA was applied from the delivery pipette with repeated pressure ejection pulses of 50 ms (15 psi kg−1, 1 pulse s−1). To examine the dose-dependence of GABA-induced currents, the pressure duration of the repetitive gas pulse was increased in stages from 20 to 200 ms. The cells or outside-out patches were continuously perfused with the saline (before, during and after ejection of GABA) with a constant rate (0.1 ml min−1). The saline was supplied by a gravity perfusion system through one of an array of parallel outlet tubes placed close (100 μm) to the excised patch. The antagonists tested were added in the saline and applied by displacing the array of tubes laterally so that the membrane patch was exposed to the test solution flowing through an adjacent tube, at the same rate as the normal saline. To avoid interference from any use-dependent effect, the different concentrations of antagonist tested were never cumulative, but applied by using the same protocol based on a single dose per cell.
Chemicals
BIDN was obtained from DuPont Agricultural Products, U.S.A. Fipronil was supplied from ChemServ, U.S.A. Concentrated stock solutions of fipronil (5 mM) were prepared in acetone, whereas the BIDN stock solution (10 mM) was prepared in dimethylsulfoxide (DMSO). Final dilutions contained at most 0.2% acetone or 0.1% DMSO. These concentrations of solvent were found to be without effect on the channel properties. All others compounds were purchased from Sigma Chemicals (L'Isles d'Abeau Chesnes, France).
Results
GABA-induced unitary currents
Figures 1 and 2 illustrate some of the properties of RDL receptor channels in excised outside-out patches obtained from S2-RDL cells. Repeated application of 50 ms-pulses of GABA (50 μM pipette concentration; 900 ms intervals) resulted in the appearance of unitary currents which rapidly disappeared on washout of GABA. Figure 1a shows examples of single-channel openings recorded in the presence of GABA over a range of holding potentials (+80 to −80 mV). The unitary currents were inwardly directed for potentials more negative than 0 mV and of greater amplitude at negative holding potentials. Above 0 mV, the GABA-induced unitary currents were outwardly directed. No such unitary events were observed when GABA was applied to patches from untransfected S2 cells or from uninduced S2-RDL cells. An amplitude histogram for data recorded at −60 mV was generated (Figure 1b) and fitted by a mixture of two Gaussian components with mean peak amplitudes of 0.110±0.002 pA and −2.126±0.003 pA respectively for the closed and the open levels. Pooling data from 10 experiments, in which recordings were made at a range of steady-state membrane potentials, enabled the construction of a current-voltage (I/V) relationship (Figure 1c). The I/V curve was linear in the potential range −100 to −20 mV and exhibited inward rectification at potentials more positive than 0 mV. Only the mean data points beyond 0 mV were fitted by linear regression (correlation coefficient r=0.997) giving a slope conductance of 36 pS and an extrapolated reversal potential of −1.4 mV, a value close to the calculated chloride equilibrium potential (ECI=−1.3 mV) predicted by the Nernst equation under our experimental conditions. This indicated that the GABA-induced current was mainly carried by chloride ions. Picrotoxin (PTX), a known chloride channel blocker acting on RDL and also on vertebrate GABAA receptors, was tested. As illustrated in Figure 1d, PTX (10 μM) inhibited the unitary currents evoked by GABA (n=5). The effects were partially reversible. These results are in agreement with those described previously for S2-RDL cells using whole-cell patch-clamp recordings (Buckingham et al., 1996) and are consistent with the conclusion that GABA-activated currents are due to the activation of a PTX-sensitive chloride conductance.
Figure 1.
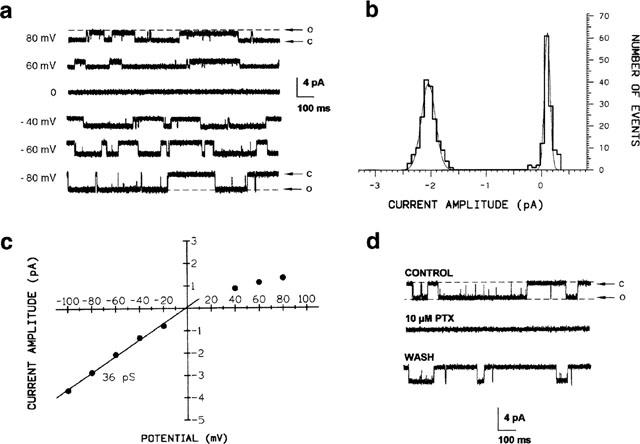
GABA-induced unitary currents recorded from an outside-out patch from cultured S2-RDL cells. (a) Unitary currents activated by repeated 50-ms pulses of GABA (50 μM; intervals: 900 ms) are shown for different membrane potentials indicated next to each trace. The sample frequency is 20 kHz (c: closed-state; o: open-state). (b) Current amplitude histogram derived from a segment of channel activity recorded when the patch was held at −60 mV. The solid line is a fit to the sum of two Gaussian functions (see text for parameters). (c) Plot of current-voltage relationship of GABA-activated unitary currents from 10 patches. Bars indicating±one s.e.m. are shown when larger than the symbol. Only data at negative potentials are fitted by linear regression giving a slope conductance of 36 pS. The single channel current reverses at −1.4 mV, a value close to the calculated ECl of −1.3 mV. (d) Single channel currents recorded in the absence and presence of picrotoxin (PTX) are shown. Membrane potential −60 mV.
Figure 2.
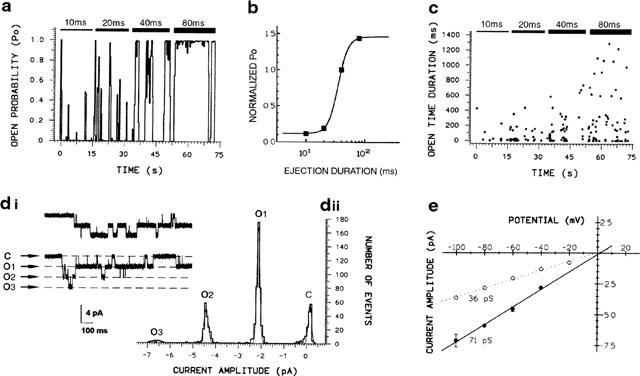
Study of the dependence of channel activity on GABA concentration. Continuous single channel recordings were divided into consecutive 20-s segments while GABA (50 μM) was applied through the pipette at various pressure-pulse durations. (a) Plot of Po versus time for unitary currents activated by GABA at −60 mV. During data acquisition, the duration of the pressure ejection pulse from the pipette was increased as indicated above the graph. (b) Representative dose-response curve constructed from data shown in (a). Each point is the Po value measured on the same patch for each individual segment and corresponds to data which were normalized to the Po value obtained in response to a 40-ms pulse of GABA. (c) Corresponding open time duration plotted versus time of application, showing that the duration of channel opening depends on the dose of ejected GABA. (di) Example of single channel currents observed at −60 mV under repeated application of GABA (pulse of 150 ms; intervals: 900 ms). The dotted lines represent the amplitude of the different conductance levels obtained from Gaussian fits of the amplitude distribution displayed to the right of the current traces. (dii; c: closed-state; o1 to o3: open-states). Means of the Gaussian components are given in the text. (e) Single channel I/V plot for the second conductance level is superimposed on the main conductance state (indicated by the dashed line). The slope conductance is 71 pS. Each data point represents the mean±s.e.m. (n=5).
The single-channel activity observed in response to pressure-application of GABA increased in a dose-dependent manner (Figure 2). To demonstrate this, the continuous single-channel recordings were divided into consecutive, equally-spaced time intervals (20 s-segments). Increasing GABA doses were achieved by progressively increasing, within each segment, the length of the pressure ejection pulse from the delivery pipette containing 50 μM GABA (Figure 2a). For each 20 s-data segment, the probability of the channel being open (Po) was calculated and plotted versus the duration of the repeated ejection pulse (Figure 2b). Dose-dependence was observed as an increase in open probability as a function of increasing duration of the GABA ejection pulse. For example, Po calculated at the steady-state membrane potential of −60 mV increased from 0.074 to 0.926 when the ejection pulse duration was changed from 10 to 80 ms. When open-time duration was plotted against time of application, open time was also found to be dependent on the dose of ejected GABA (Figure 2c). Long openings appeared when GABA ejection pulse length was increased whereas the occurrence of the short openings was unaffected. This suggested two open times for the RDL receptor.
In general, channel activity with a single amplitude was observed but multiple, well-defined open states could be distinguished by application of GABA for longer than 80 ms. Part of the recording shown in Figure 2di illustrates the multi-conductance state behaviour of GABA receptor channels at −60 mV when the duration of the GABA ejection pulse was 150 ms. Under these conditions, openings with at least three different current amplitudes were recorded. The corresponding histogram in Figure 2dii shows the frequency with which the three current levels were detected. Both closed and open levels were fitted by Gaussian distributions. In addition to the main peak at −2.14±0.01 pA (54.1%), other peaks were detected at −4.43±0.01 pA (22.5%) and −6.62±0.18 pA (3.2%). The values of the mean amplitudes at the peaks of the Gaussian curves appeared to be simple multiples (×2,×3) of the smallest unitary current level recorded in the patch (i.e. 2.14 pA). The I/V relationships for the two most prominent conductance states were studied over the voltage range −100 to 0 mV (Figure 2e). The linear regression (correlation coefficient r=0.995) through mean data points of the second conductance level crossed the potential axis at+1.4 mV, a value close to the chloride reversal potential and yielded a slope conductance of 71 pS, approximately twice the value of the predominant conductance. In addition, direct transitions to the second level from the main level or to the third from the second were always observed (Figure 2di), suggesting that the supraconductance states could be considered as additional conductance levels arising from a single channel type. Thus the 71 pS conductance state is attributable to simultaneous openings of two identical channels.
Dose dependent effects of BIDN and fipronil on single GABA-activated channel activity
Figure 3 illustrates the effects of BIDN and fipronil on unitary currents evoked by pressure ejection of 50 μM GABA at a holding potential of −60 mV. As illustrated in Figure 3 (left panel), channel activity disappeared completely in the presence of 1 μM BIDN. The timecourse of BIDN-antagonism was demonstrated by measuring Po before, during and after application of BIDN. An example of a Po plot versus time is shown in Figure 3b. The block by 1 μM BIDN showed a rapid onset and was rapidly reversible upon rebathing the cell in normal saline. The dose-response curve reflecting the blocking action on RDLac channels was constructed by noting the reduction in Po at different concentrations of BIDN (Figure 3c). Each data point represents the mean of 3–5 separate patches. Fitting the data to the Hill equation yielded a concentration of BIDN resulting in a half maximum inhibition of Po (IC50=281 nM). A similar analysis was performed for fipronil. Figure 3a (right panel) showed an example of the effect of applying 1 μM fipronil. At this concentration, the unitary currents activated by GABA were completely blocked. Unlike the case for BIDN, the steady-state level of block by fipronil was reached only after a delay, varying from 1–5 min. This is shown in the analysis of Po distribution before and during fipronil application (Figure 3b). In addition, whereas block by BIDN was reversible, this was not the case for fipronil. Channel activity rarely recovered even after prolonged wash in normal saline (more than 30 min) and then only brief isolated openings were detected. However channel activity following fipronil block was partially recoverable if the GABA ejection pulse was increased in duration from 80 ms to 250 ms (Figure 3a, asterix). When mean values for percentage inhibition of Po (measured after a 5 min application) were plotted against log [fipronil], the resulting sigmoid curve fit (correlation coefficient r=0.996) to the Hill equation (Figure 3c) yielded an estimated IC50 for fipronil of 240 nM.
Figure 3.
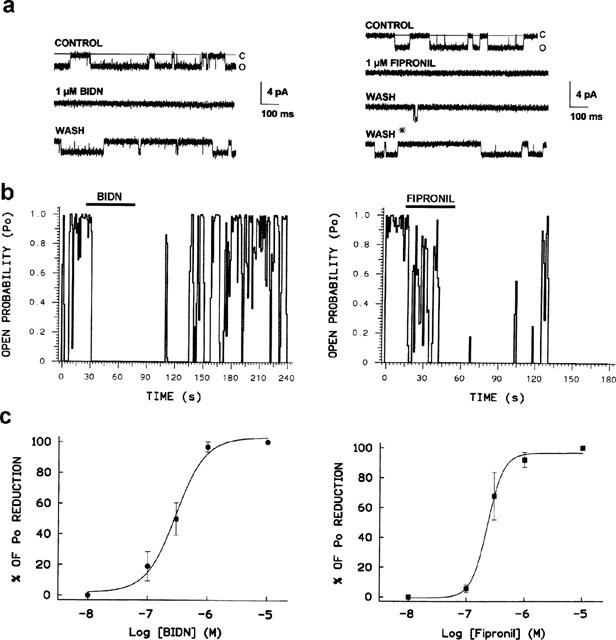
Blocking effect of BIDN (left) and fipronil (right) on single GABA-gated currents in an outside-out patch excised from S2-RDL cells. (a) Each panel shows records evoked by 50-ms pulses of GABA at a holding potential of −60 mV in the absence (CONTROL) and in the presence of drug. Channel openings are indicated by downward deflections (o) from the closed level indicated by the solid line (c). The lower traces show channel activity during the wash. The asterix means that channel activity could be recovered, during the saline wash following fipronil application, but only if the duration of repeated agonist ejection pulses is increased from 50 to 200 ms. (b) Po distribution versus time showing change in Po of GABA-activated chloride channel following application of either BIDN (1 μM) or fipronil (1 μM). The solid bars indicate the duration of drug application. Note that the effects of BIDN on Po occur without delay and are fully reversible. By contrast, the effects resulted from fipronil application are progressive and the Po does not recover following the wash out of the blocker. (c) Semi-logarithmic dose-response curves for BIDN (left) and fipronil (right). The smooth line represents the best fit to the mean data according to the Hill equation, giving an IC50 for BIDN and fipronil block at −60 mV of 281 nM and 240 nM respectively. Each point is the mean of the percentage of Po measured from at least three patches and for a data acquisition time of 40 s.
Changes in single channel conductance and channel kinetics
Kinetic analysis showed that BIDN and fipronil both resulted in a marked reduction of the mean channel open time (τo). As shown in Figure 4a, the distribution of open times in control conditions, at a holding potential of −60 mV, was best fitted by the sum of two exponentials. The mean time constants of the fast (τo1) and slow (τo2) exponential components yielded values of 1.54±0.27 and 125.6±32.9 ms (mean±s.e.m., n=8) accounting for 24.8±6.5 and 72.2±6.5% respectively of channel openings. All histograms were constructed from data sets in which events consisting of overlapping multiple single units were systematically excluded. Thus, as previously suggested (Figure 2C), the observation that two exponentials are required to describe the open times is consistent with two kinds of openings (i.e. long- and short-lived openings) of the channel under normal conditions. Similar analysis of patches exposed to pressure-ejected GABA during superfusion of 300 nM BIDN (close to the IC50 value) revealed that a bi-exponential distribution was still required to fit the data (Figure 4b). However, if τo1 was unaffected by BIDN (τo1=1.67±0.45 ms, 26.7±5.7%), τo2 was reduced (τo2=76.65±9.51 ms, 73.3±5.7%) indicating that most BIDN-modified channels have shorter duration open states. In a typical experiment (Figure 4b), BIDN (300 nM) reduced τo2 by almost 50% (τo2=69.6 ms). By comparison, fipronil shortened τo2 from 130.7 to 26.3 ms (a reduction of 80%, Figure 4c). This result was representative of the open-time modification by fipronil with the average time constants τo1=1.33±0.42 ms (29.5±8.4%) and τo2=56.3±30.0 ms (70.5±8.4%) (n=3). Thus BIDN and fipronil, at a concentration (300 nM) close to their IC50 values for Po suppression of unitary GABA-induced current, reduced channel activity mainly by lowering the time for which the channels stay in the longer open states. Furthermore, it is important to note that the occurrence of the two components was not modified by either BIDN or fipronil.
Figure 4.
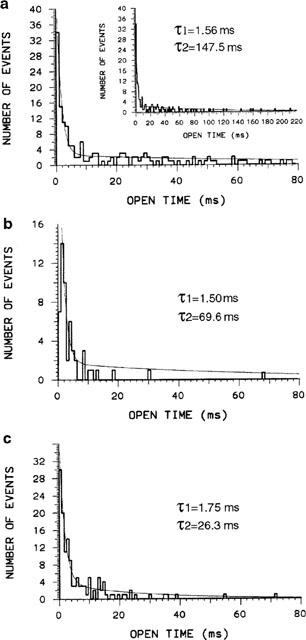
Open-time distribution of GABA-activated channels recorded from outside-out patches excised from S2-RDL cells. (a) control, (b) BIDN (300 nM), fipronil (300 nM). Single-channel data derived from a patch held at −60 mV and activated by repeated 50 ms-pulses of GABA (50 μM). Simultaneous openings of more than one channel have been excluded. Histograms are fitted by least-squares to double exponentials. The fits (smooth curves) were superimposed on the histograms and the time-constants (τ1, τ2) are indicated. Note the reduction of the second time constant (τ2) and the absence of effect on the first (τ1) in the presence of BIDN or fipronil. These histograms are representative of eight patches for the control and three patches with BIDN or fipronil.
To obtain more precise information on the mechanism of block, the effects of a lower concentration of BIDN (100 nM) were analysed using the scatter plot distribution of all channel amplitudes versus time (Figure 5). Repeated 100 ms-duration pulses of GABA were applied to obtain maximum channel opening. As shown in Figure 2d,e, such experimental conditions resulted in the appearance of more than one open state. Two open states (36 and 71 pS) were characterized in the experiment shown in Figure 5ai. After bath application of 100 nM BIDN, the distinction between the open states was not so clear and at least six different amplitude levels could be detected (Figure 5aii). After a 10 min BIDN application, the total number of events from each patch was reduced and only two open-state conductance levels persisted including an intermediate open-state level never observed in the controls (Figure 5aiii). Figure 5b shows the time-dependent effect of a low concentration of BIDN (100 nM) on channel activity illustrating the progressive appearance of this intermediate open level. To determine the corresponding conductance, we constructed a histogram of single channel amplitudes measured in the presence of BIDN (Figure 5c). The histogram has three peaks including the closed state at 0.131+0.002 pA (c) and two open states at −1.00±0.01 pA (o1, 14.6%) and at −2.05±0.05 pA (o1, 39.2%) corresponding to a conductance of 17 and 35 pS respectively. As shown in Figure 5b, the channel is capable of entering a subconductance state from the channel closed state. Transitions between this intermediate level to the main conductance level were observed suggesting that they probably correspond to opening of the same channel at a different amplitude level. Such behaviour has been observed only occasionally with fipronil.
Figure 5.
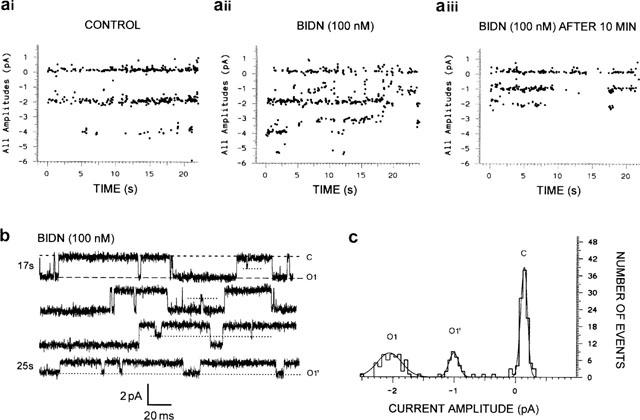
Change in conductance of the RDL receptor channel in the presence of BIDN. (a) Scatter plots of unitary current amplitude versus time constructed from data recorded before (ai), at the beginning (aii) and after 10 min of BIDN superfusion (aiii). GABA is repeatedly pressure applied (50 ms on; 900 ms off). (b) Example of single channel currents recorded from a single outside-out patch excised from S2-RDL cell showing the time-course of the effect of 100 nM BIDN on GABA receptor activity at −60 mV. (c) Amplitude histogram constructed from a record similar to that shown in (b). The solid line is the fit of a function consisting of three Gaussian components. The current level amplitudes at the Gaussian peaks derived from the curve fitting are given in the text.
Co-application of BIDN and fipronil
To investigate whether or not BIDN and fipronil act on the same site on the RDL receptor, we explored the effects of co-applying fipronil and BIDN on excised S2-RDL cell membrane patches. The channel block by 250 nM fipronil (reduction of Po) was affected by subsequent addition of 300 nM BIDN (Figure 6). In experiments where fipronil was added before BIDN, the application of BIDN resulted in an additional reduction in channel Po (n=5). For example, in the experiment shown in Figure 6a, 250 nM fipronil (a concentration close to the estimated IC50 value) applied alone reduced channel Po by 51.8±4.2% (n=4). Po was further reduced to 11.5±4.4% (n=3) by the addition of 300 nM of BIDN. To confirm that blocking the RDL receptor channel by fipronil did not prevent the blocking effect of BIDN, we carried out an additional set of experiments in which patches were perfused with saline containing of a mixture of 300 nM BIDN and increasing fipronil concentrations in the range 100–1000 nM (Figure 6b). As illustrated in Figure 6b, co-application of BIDN with fipronil resulted in an additional fipronil-induced dose-dependent reduction of Po. These results argue against a competitive interaction indicating that BIDN and fipronil do not share a common binding site on the RDL receptor.
Figure 6.
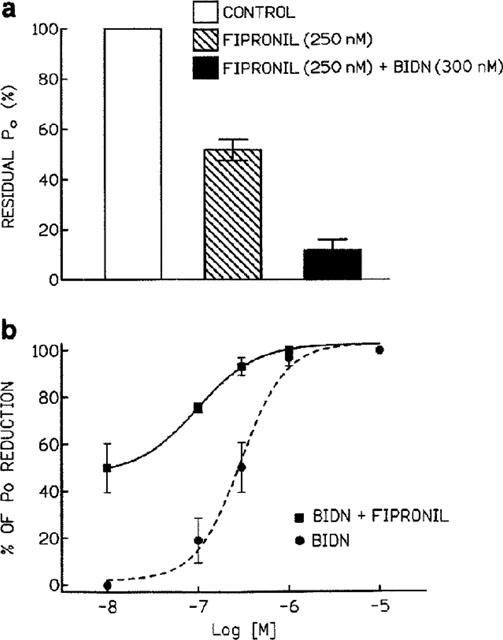
Effect of co-application of fipronil and BIDN on the RDL receptor. (a) Comparative histogram of the percentage of residual Po measured after application of 250 nM fipronil and a solution containing 250 nM fipronil+300 nM BIDN. These concentrations are close to the IC50 values calculated from the semi-logarithmic dose-response curve. (b) Dose-response curve for the inhibitory actions of simultaneously applied fipronil and BIDN on Po. The smooth line represents the best fit to the mean data points according to the Hill equation (n=3). The dotted line represents the dose-response curve for BIDN alone.
Discussion
This is the first study describing the properties of single GABA-gated Cl− channels stably expressed in S2-RDL cells. Although both fipronil and BIDN have previously been shown to have potent insecticidal activity against RDL receptors, their modes of action are unknown.
We have utilized cultured S2-RDL cell lines as a stable expression system in which large numbers of identical cells consistently express RDL GABA receptors. Stable cell lines used for the expression of receptors of known molecular composition have proved valuable for studying the pharmacology of receptor subtypes and establishing the functional roles of particular subunits (Moss et al., 1990; Grisshammer & Tate, 1995). The homogeneity, accessibility and the facility with which single channel patch clamping can be applied are some of the evident technical advantages of such stable cell lines. Millar et al. (1994) have demonstrated that there are many similarities between the pharmacology of the RDL receptor when expressed in Drosophila S2 cells and in Xenopus oocytes. In both expression systems, the RDL GABA receptor is blocked by micromolar concentrations of PTX, TBPS, EBOB and fipronil but is not blocked by 100 μM bicuculline. In this regard, the expressed RDL GABA receptor preserves many of the pharmacological properties of certain native insect GABA receptor (Rauh et al., 1990; ffrench-Constant et al., 1993; Chen et al., 1994; Buckingham et al., 1994). Consequently, the expressed RDL receptor represents an excellent model with which GABA-receptor-insecticide interactions in insect pest species could be investigated.
The single channel properties of the RDL receptor expressed in S2 cells described in this study seem to match many of those reported in only few studies of oocytes expressing RDL (Zhang et al., 1995) or cultured wild-type Drosophila neurones (Zhang et al., 1994). The rectification property of the I/V relationship is one of the common characteristics of the RDL receptor in all expression system studied. In our case, GABA (50 μM) activated mainly one conductance level 36 pS in negative potential range, a value which differs slightly from the 21.4 pS conductance of the RDL channel observed in oocytes (Zhang et al., 1995) and from the corresponding single channel conductance (28 pS) obtained from cultured Drosophila larval neurones (Zhang et al., 1994). Kinetic properties of RDL receptors expressed in S2 cells resemble those of the neuronal Drosophila GABA receptor in that the channel open-time distribution required fitting by two exponentials. In cultured neurones from wild-type Drosophila (Zhang et al., 1994), the open time was fitted by the sum of two exponentials with time constants of 6.6±1.1 and 174±98 ms (based on single channel recording at −70 mV in the presence of 500 μM GABA). In contrast to the similarity in the slow component, the value of the fast time constant we measured was four times shorter than that of the GABA-gated channel in Drosophila larvae neurones. In fact, variability in the duration of open times of GABA-activated chloride channel has been well documented (Sakman et al., 1983; Martin, 1985; MacDonald et al., 1989). This could be explained in part by the large differences in the electrophysiological approaches (e.g., potential, agonist concentration, GABA application) that make direct comparisons of our data with those found in the literature very difficult. In addition, the discrepancies might be due to (a) the existence in Drosophila neurones of receptors composed of different subunits since the existence of hetero-oligomeric GABA receptors resulting from the co-assembly of RDL and other subunits is known (Zhang et al., 1995; Hosie et al., 1997); (b) effects of host cell on ion channel properties as seen for certain nicotinic acetylcholine receptors. For example, Lewis et al. (1997) showed that single channel conductance and kinetics of rat recombinant neuronal nicotinic receptor could be influenced by the choice of heterologous expression system.
Previous electrophysiological studies have reported that many of the commonly used GABA receptor antagonists affect channel kinetics. Recent single channel analysis on rat dorsal root ganglion neurones has shown that the insecticide dieldrin modulates the GABAA receptor-channels in the same manner as PTX by reducing the channel open probability and by prolonging the mean closed time (Ikeda et al., 1998). Neither dieldrin nor PTX have been shown to alter the open time distribution on this preparation. However dieldrin competes with [3H]-BIDN binding competitively on rootworm membranes but PTX does not (Rauh et al., 1997). GABA channel opening frequency on cockroach neurones was also reduced by dieldrin without changing the mean open time (Bermudez et al., 1991). In accordance with a prolongation to the closed time, Newland & Cull-Candy (1992) have discussed several mechanisms of inhibition by PTX on vertebrate neuronal GABA receptor. In the present study, we have shown that, at the single-channel level, the reduction of the RDL receptor channel activity in the presence of either BIDN or fipronil was due to a reduction in open probability. Both compounds produced a concentration-dependent reduction of Po and shortened the slower open time constant by more than 40% when applied at a concentration close to the IC50. In the presence of BIDN or fipronil, the gradual shortening of long duration opening at the main conductance level may reflect the reduction in open probability. By contrast, the value of the fast time constant obtained for GABA-gated channel was unaffected by bath application of either of the two compounds. The blocking effects of BIDN or fipronil were not associated with increases in the proportion of brief openings since the shortened-component measured with BIDN and fipronil accounted for the same fraction of that of the unmodified component. This observation together with the reduction of the long-lived openings (estimated by the slow time-constant) suggests that, for both compounds, the channel needs to be open for inhibition.
In addition, the present work has shown that when 50 μM GABA is applied to outside-out patches in the presence of 100 nM BIDN, a novel conductance level is observed. The question arises as to whether or not this subconductance state and the dominant 36 pS conductance result from the activity of one type of channel which could open to several different conductance levels in different experimental conditions (i.e. before and after modification by BIDN). Because the 17 pS conductance has never been observed in controls, it suggests that there is only one type of channel. In addition, some direct transitions between the larger and smaller conductance levels are detected in the presence of BIDN discounting the possible existence of two different types of channel. Consequently, it is reasonable to assume that the subconductance state may correspond to a different conformation of the ion pore. This conformation would be unmasked by BIDN rather than being the direct result of modification of the channel. Assuming that the fast time-constant which is unaffected by BIDN corresponds to the opening at the subconductance state, we speculate that, because openings become very short in the presence of BIDN, the transition of the modified channel between the fully open state and the substate unmasked by BIDN can then be detected. In this context, a mechanism governing substate selection has been suggested to explain differences in elementary conductance between recombinant homo- and hetero-oligomeric glycine receptor isoforms expressed in HEK-293 cells (Borman et al., 1993). These authors have shown that particular residues within the M2 segment, known to line the ion channel, determined the chloride conductance. Thus, if channel openings at a lowest conductance level do not favour openings at the highest level, this may reflect an allosteric effect of BIDN on residues bordering the M2 segment. Another hypothesis to explain openings at a lowest level may involve a desynchronization of channel clustering (Guyon et al., 1999). In this case, the highest conductance level has been related to the number of co-operative GABA receptor channels.
One intriguing aspect of the fipronil block of GABA-gated chloride channels is that the final extent of the block is reduced as the agonist concentration increases. Occasionally, recovery is facilitated by applying longer GABA ejection pulses suggesting a competitive antagonist mechanism. These observations are inconsistent with the view that fipronil and BIDN are open channel blockers as suggested above. A channel blocking mechanism would be expected to produce a reduction in open time with an increase in agonist concentration. This has previously been shown for the PTX blocking action on rat hippocampal neurones (Yoon et al., 1993). In fact, previous studies have demonstrated that the potency of fipronil and BIDN on wild-type RDL receptor transiently expressed in oocyte was dependent upon the concentration of GABA used (Hosie et al., 1995a). In earlier studies on insect GABA receptors, fipronil was found to be a non-competitive antagonist (Millar et al., 1994; Bloomquist, 1996) but studies on RDLac showed that it did not exhibit a simple non-competitive blocking action (Hosie et al., 1995a). In our study, after fipronil application, a slowly dissociating channel blocker could be expected to make its binding irreversible and to explain the absence of recovery prior to increasing the dose of agonist.
Although BIDN and fipronil seem to have similar blocking effects, fipronil further suppresses GABA activity recorded in the presence of BIDN and the combination of fipronil and BIDN in the saline (both at concentration close to their IC50) almost completely blocks single channel activity. Co-applying fipronil with BIDN results in an inhibitory effect, which is always higher than that expected if the concentration of BIDN is simply increased. In addition, the dose response curve for inhibitory action of simultaneously applied fipronil and BIDN does not show any reduction of the apparent potency of BIDN. This suggests that fipronil does not bind to the BIDN site. This could explain why, unlike BIDN, fipronil does not normally show a subconductance state whilst both compounds are capable of modifying the open time properties of the RDL receptor. Biochemical studies including radioligand binding assays and measurements of 36Cl− influx indicate that, in insects, fipronil is active at a chloride channel site distinct from the TBPS site (Lunt et al., 1985; Olsen et al., 1987; Gant et al., 1998). In addition, [3H]-BIDN binding is competitively displaced by dieldrin (Rauh et al., 1997). However, because no significant inhibition by fipronil at the housefly head [3H]-BIDN site has been found (Gant et al., 1998), it suggests that fipronil binds at a distinct site within the chloride channel from BIDN and cyclodienes. These results are consistent with our own conclusion that BIDN and fipronil bind to different sites on RDL. Nevertheless, the similarity of their actions leads us to suggest a closely related binding site on the receptor. This was previously postulated based on finding that the A302→S mutation in Rdl confers resistance to both BIDN (Shirai et al., 1995) and fipronil (Hosie et al., 1995a).
Acknowledgments
The authors would like thank Professors B. Hue and B. Lapied for their support, helpful discussions and their comments on the manuscript.
Abbreviations
- BIDN
3,3-bis-trifluromethyl-bicyclo-[2,2,1]heptane-2,2-dicarbonitrile
- DMSO
dimethyl-sulfoxide
- EBOB
4-n-propyl-4′-ethynylbicycloorthobenzoate
- EGTA
ethylene glycol-bis (β-aminoethyl ether)tetra-acetic acid
- GABA
γ-aminobutyric acid
- PTX
picrotoxin
- PTXN
picrotoxinin
- RDL
resistance to dieldrin
- TBPS
t-butylbicyclophosphorothionate
- TEA-Cl
tetraethyl-ammonium chloride
- TES
N-tris(hydroxymethyl)-methyl-2aminoethane-sulphonic acid
References
- BERMUDEZ I., HAWKINS C.A., TAYLOR A.M., BEADLE D.J. Actions of insecticides on the insect GABA receptor complex. J. Receptor Research. 1991;11:221–232. doi: 10.3109/10799899109066401. [DOI] [PubMed] [Google Scholar]
- BLOOMQUIST J.R. Ion channels as targets for insecticides. Annu. Rev. Entomol. 1996;41:163–190. doi: 10.1146/annurev.en.41.010196.001115. [DOI] [PubMed] [Google Scholar]
- BORMANN J., RUNDSTRÖM N., BETZ H., LANOSCH D. Residues within transmembrane segment M2 determine chloride conductance of glycine receptor homo- and hetero-oligomers. EMBO J. 1993;12:3729–3737. doi: 10.1002/j.1460-2075.1993.tb06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCKINGHAM S.D., HOSIE A.M., ROUSH R.L., SATTELLE D.B. Actions of agonists and convulsant antagonists on a Drosophila melanogaster GABA receptor (Rdl) homo-oligomer expressed in Xenopus oocytes. Neurosc. Letts. 1994;181:137–140. doi: 10.1016/0304-3940(94)90578-9. [DOI] [PubMed] [Google Scholar]
- BUCKINGHAM S.D., MATSUDA K., HOSIE A.M., BAYLIS H.A., SQUIRE M.D., LANDSELL S.J., MILLAR N.S., SATTELLE D.B. Wild-type and insecticide-resistant homo-oligomeric GABA receptors of Drosophila melanogaster stably expressed in a Drosophila cell line. Neuropharmacol. 1996;35:1393–1401. doi: 10.1016/s0028-3908(96)00087-1. [DOI] [PubMed] [Google Scholar]
- CASIDA J.E. Insecticide action at the GABA-gated chloride channel: recognition, progress and prospects. Arch. Insect Biochem. Physiol. 1993;22:13–23. doi: 10.1002/arch.940220104. [DOI] [PubMed] [Google Scholar]
- CHEN C.A., BELETTI D., LAMBERT J.J., PETERS J.A., REYES A., LAN C.C. Cloning and functional expression of a Drosophila γ-amino-butyric acid receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6069–6073. doi: 10.1073/pnas.91.13.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE L.M., NICHOLSON R.A., CASIDA J.E. Action of phenylpyrazole insecticides at the GABA-gated chloride channel. Pestic. Biochem. Physiol. 1993;46:47–54. [Google Scholar]
- COLE L.M., ROUSH R.T., CASIDA J.E. Drosophila GABA-gated chloride channel: modified [3H]EBOB binding site associated with ALA ® SER or GLY mutants of Rdl subunit. Life Sci. 1995;56:757–765. doi: 10.1016/0024-3205(95)00006-r. [DOI] [PubMed] [Google Scholar]
- COLLIOT F., KUKOROWSKI K.A., ROBERT D.A. Fipronil: A new soil and foliar broad spectrum insecticide. Proc Brighton crop Protection Conf. Pests and diseases. 1992. pp. 29–34.
- FFRENCH-CONSTANT R.H. Cloning of the Drosophila cyclodiene insecticide resistance gene: a novel GABAA receptor subtype. Comp. Biochem. Physiol. 1993;104:9–12. doi: 10.1016/0742-8413(93)90103-r. [DOI] [PubMed] [Google Scholar]
- FFRENCH-CONSTANT R.H., PITTENDRIGH B., VAUGHAN A., ANTHONY N. Why are there so few resistance-associated mutations in insecticide target genes. Philos. Trans. R. Soc. Lond. 1998;353:1685–1693. doi: 10.1098/rstb.1998.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FFRENCH-CONSTANT R.H., ROCHELEAU T.A., STEICHEN J.C., CHALMERS A.E. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature. 1993;363:449–451. doi: 10.1038/363449a0. [DOI] [PubMed] [Google Scholar]
- FFRENCH-CONSTANT R.H., ROUSH R.T., MORTLOCK D., DIVELY G.P. Isolation of dieldrin resistance from field populations of Drosophila melanogaster (Diptera: Drosophilidae) J. Econ. Entomol. 1990;83:1733–1737. doi: 10.1093/jee/83.5.1733. [DOI] [PubMed] [Google Scholar]
- GANT D.B., CHALMERS A.E., WOLFF M.A., HOFFMAN H.B., BUSHEY D.F. Fipronil: action at the GABA receptor. Rev. Toxicol. 1998;2:147–156. [Google Scholar]
- GRISSHAMMER R., TATE R. Overexpression of integral membrane proteins for structural studies. Q. Rev. Biophys. 1995;28:315–422. doi: 10.1017/s0033583500003504. [DOI] [PubMed] [Google Scholar]
- GROLLEAU F., SATTELLE D.B. BIDN and fipronil affect single-channel properties of a Drosophila GABA receptor (RDL) stably expressed in a Drosophila cell line. J. Physiol. 1998;513:134P. doi: 10.1038/sj.bjp.0703507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUYON A., LAURENT S., PAUPARDIN-TRITSCH D., ROSSIER J., EUGENE D. Incremental conductance levels of GABAA receptors in dopaminergic neurones of the rat substantia nigra pars compacta. J. Physiol. 1999;516:719–737. doi: 10.1111/j.1469-7793.1999.0719u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAINZL D., COLE L.M., CASIDA J.E. Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem. Res. Toxicol. 1998;11:1529–1535. doi: 10.1021/tx980157t. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HAMON A., LE CORRONC H., HUE B., RAUH J.J., SATTELLE D.B. BIDN, a bicyclic dinitrile convulsant, selectively blocks GABA-gated Cl-channels. Brain Res. 1998;780:20–26. doi: 10.1016/s0006-8993(97)00895-0. [DOI] [PubMed] [Google Scholar]
- HOSIE A.M., ARONSTEIN K., SATTELLE D.B., FFRENCH-CONSTANT R.H. Molecular biology of insect neuronal GABA receptors. Trends Neurosci. 1997;20:578–583. doi: 10.1016/s0166-2236(97)01127-2. [DOI] [PubMed] [Google Scholar]
- HOSIE A.M., BAYLIS H.A., BUCKINGHAM S.D., SATTELLE D.B. Actions of the insecticide fipronil, on dieldrin-sensitive and -resistant GABA receptors of Drosophila melanogaster. Br. J. Pharmacol. 1995a;115:909–912. doi: 10.1111/j.1476-5381.1995.tb15896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSIE A.M., SHIRAI Y., BUCKINGHAM S.D., RAUH J.J., ROUSH R.T., BAYLIS H.A., SATTELLE D.B. Blocking actions of BIDN, a bicyclic dinitrile convulsant compound, on wild-type and dieldrin-resistant GABA receptor homo-oligomers of Drosophila melanogaster expressed in Xenopus oocytes. Brain Research. 1995b;693:257–260. doi: 10.1016/0006-8993(95)00605-p. [DOI] [PubMed] [Google Scholar]
- IKEDA T., NAGATA K., SHONO T., NARAHASHI T. Dieldrin and picrotoxin modulation of GABAA receptor single channels. Neuroreport. 1998;9:3189–3195. [PubMed] [Google Scholar]
- LEWIS T.M., HARKNESS P.C., SIVILOTTI L.G., COLQUHOUN D., MILLAR N.S. The ion channel properties of a rat recombinant neuronal nicotinic receptor are dependent on the host cell type. J. Physiol. Lond. 1997;502:299–306. doi: 10.1111/j.1469-7793.1997.299bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNT G.G., ROBINSON T.N., MILLER T., KNOWLES W.P., OLSEN R.W. The identification of GABA receptor binding sites in insect ganglia. Neurochem. Int. 1985;7:751–754. doi: 10.1016/0197-0186(85)90028-2. [DOI] [PubMed] [Google Scholar]
- MACDONALD R.L., ROGERS C.J., TWYMAN R.E. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J. Physiol. Lond. 1989;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R.J. γ-Aminobutyric acid- and piperazine-activated single-channel currents from Ascaris suum body muscle. Br. J. Pharmacol. 1985;84:445–461. doi: 10.1111/j.1476-5381.1985.tb12929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGURK K.A., PISTIS M., BELELLI D., HOPE A.G., LAMBERT J.J. The effect of a transmembrane amino acid on etomidate sensitivity of an invertebrate GABA receptor. Br. J. Pharmacol. 1998;124:13–20. doi: 10.1038/sj.bjp.0701787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEHTA A.K., TICKU M.K. An update on GABA A receptors. Brain Research Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- MILLAR N.S., BUCKINGHAM S.D., SATTELLE D.B. Stable expression of a functional homo-oligomeric Drosophila GABA receptor in a Drosophila cell line. Proc. R. Soc. Lond. B. 1994;258:307–314. doi: 10.1098/rspb.1994.0178. [DOI] [PubMed] [Google Scholar]
- MOSS S.J., SMART T.G., PORTER N.M., NAYEEM N., DEVINE J., STEPHENSEN F.A., MACDONALD R.L., BARNARD E.A. Cloned GABA receptors are maintained in a stable cell line : allosteric and channel properties. Eur. J. Pharmacol. 1990;189:77–88. doi: 10.1016/0922-4106(90)90232-m. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., GINSBURG K.S., NAGATA K., SONG J.H., TATEBAYASHI H. Ion channels as targets for insecticides. Neurotoxicol. 1998;19:581–590. [PubMed] [Google Scholar]
- NEWLAND C.F., CULL-CANDY S.G. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones. J. Physiol. Lond. 1992;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSEN R.W., SZAMRAJ O., MILLER T. T-[35S]Butylbicyclophosphorothionate binding sites in invertebrate tissues. J. Neurochem. 1987. pp. 1311–1318. [DOI] [PubMed]
- RAUH J.J., BENNER E., SCHNEE M.E., CORDOVA D., HOLYOKE C.W., HOWARD M.H., BAI D., BUCKINGHAM S.D., HUTTON M.L., HAMON A., ROUSH R.T., SATTELLE D.B. Effects of [3H]-BIDN, a novel bicyclic dinitrile radioligand for GABA-gated chloride channels of insects and vertebrates. Br. J. Pharmacol. 1997;121:1496–1505. doi: 10.1038/sj.bjp.0701215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAUH J.J., LUMMIS S.C.R., SATTELLE D.B. Pharmacological and biochemical properties of insect GABA receptors. Trends Pharmacol. Sci. 1990;11:325–329. doi: 10.1016/0165-6147(90)90236-2. [DOI] [PubMed] [Google Scholar]
- SAKMANN B., HAMILL O.P., BORMANN J. Patch-clamp measurements of elementary chloride currents activated by the putative inhibitory transmitter GABA and glycine in mammalian spinal neurons. J. Neural Trans. Supple. 1983;18:83–95. [PubMed] [Google Scholar]
- SATTELLE D.B. GABA Receptors of Insects. Adv. Insect Physiol. 1990;22:1–113. [Google Scholar]
- SATTELLE D.B., LUMMIS S.C.R., WONG J.F.H., RAUH J.J. Pharmacology of insect GABA receptors. Neurochem. Res. 1991;16:363–374. doi: 10.1007/BF00966100. [DOI] [PubMed] [Google Scholar]
- SCHNEE M.E., RAUH J.J., BUCKINGHAM S.D., SATTELLE D.B. Pharmacology of skeletal muscle GABA-gated chloride channels in the cockroach Periplaneta Americana. J. Exp. Biol. 1997;200:2947–2955. doi: 10.1242/jeb.200.23.2947. [DOI] [PubMed] [Google Scholar]
- SHIRAI Y., HOSIE A.M., BUCKINGHAM S.D., HOLYOKE C.W., Jr, BAYLIS H.A., SATTELLE D.B. Actions of picrotoxinin analogues on an expressed, homo-oligomeric GABA receptor of Drosophila melanogaster. Neurosci. Letts. 1995;189:1–4. doi: 10.1016/0304-3940(95)11432-v. [DOI] [PubMed] [Google Scholar]
- SIEGHART W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol. Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- WHITING P.J., McKERNAN R.M., WAFFORD K.A. Structure and pharmacology of vertebrate GABAA receptor subtypes. Int. Rev. Neurobiol. 1995;38:96–138. doi: 10.1016/s0074-7742(08)60525-5. [DOI] [PubMed] [Google Scholar]
- YOON K., COVEY D.F., ROTHMAN S.M. Multiple mechanisms of picrotoxin block of GABA-induced currents in rat hippocampal neurons. J. Physiol. 1993;464:423–439. doi: 10.1113/jphysiol.1993.sp019643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H.G., FFRENCH-CONSTANT R.H., JACKSON M.B. A unique amino acid of the Drosophila GABA receptor with influence on drug sensitivity by two mechanisms. J. Physiol. 1994;479:65–75. doi: 10.1113/jphysiol.1994.sp020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H.G., LEE H.J., ROCHELEAU T., FFRENCH-CONSTANT R.H., JACKSON M.B. Subunit composition determines picrotoxin and bicuculline sensitivity of Drosophila γ-aminobutyric acid receptors. Mol. Pharmacol. 1995;48:835–840. [PubMed] [Google Scholar]


