Abstract
Ginsenoside, an extract of Panax ginseng, is an essential constituent of anti-asthmatic Chinese herbal medicine. To elucidate whether ginsenoside affects airway smooth muscle tone and, if so, what the mechanism of action is, we studied relaxant responses of human bronchial strips under isometric condition in vitro, and directly measured the release of nitric oxide (NO) by an amperometric sensor for this molecule.
Addition of ginsenoside relaxed the tissues precontracted with acetylcholine in a dose-dependent manner, the maximal relaxation and the ginsenoside concentration required to produce 50% relaxation being 67±8% and 210±29 μg ml−1, respectively.
The relaxant responses to ginsenoside were inhibited by NG-nitro-L-arginine methylester (L-NAME) and removal of the epithelium, but not by NG-nitro-D-arginine methylester (D-NAME) or tetrodotoxin. This inhibitory effect of L-NAME was reversed by L-arginine but not by D-arginine.
Addition of ginsenoside to the medium containing bronchial tissues dose-dependently increased NO-selective electrical current, and this effect was greatly attenuated by the epithelial removal or Ca2+-free medium.
Ginsenoside also increased tissue cyclic GMP contents, an effect that was abolished in the presence of L-NAME.
It is concluded that ginsenoside induces relaxation of human bronchial smooth muscle via stimulation of NO generation predominantly from airway epithelium and cyclic GMP synthesis. This action might account for the anti-asthmatic effect of Panax ginseng.
Keywords: Chinese herbal medicine, asthma, airway smooth muscle, nitric oxide synthase
Introduction
Nitric oxide (NO) is an endogenous multifunctional bioregulatory molecule generated from the amino acid L-arginine by nitric oxide synthase (NOS). In the respiratory system, NOS-like immunoreactivities are constitutively expressed in a variety of cell types including airway epithelial cells and nonadrenergic noncholinergic (NANC) nerve fibres (Barnes & Belvisi, 1993; Kobzik et al., 1993), and the NO released by these cells protect against the development of bronchoconstriction, airway hyperresponsiveness and, possibly, airway inflammation (Nijkamp et al., 1993; Wink et al., 1993).
Anti-asthmatic Chinese herbal medicine has long been used in the treatment of asthma in Asian countries. As to the mechanism of the efficacy, inhibition of IgE-mediated release of histamine from basophils (Koda et al., 1982) and platelet-activating factor from neutrophils (Miyamoto et al., 1990), and prevention of down-regulation of glucocorticoid and β-adrenergic receptors (Miyamoto et al., 1990) have been reported, but the direct action on airway smooth muscle contractility remains unknown. Ginsenoside, an extract of the root of Panax ginseng, is one of the essential constituents of traditional Chinese anti-asthma drugs and aphrodisiacs. Previous studies have shown that ginsenoside relaxes rabbit pulmonary blood vessels (Chen et al., 1984) and corpus cavernosum (Chen & Lee, 1995), and that the relaxant responses are endothelium-dependent and can be inhibited by the NOS inhibitors. These findings suggest that vasodilation induced by ginsenoside may be associated with NO released from vascular endothelial cells. In the present experiments, to elucidate whether ginsenoside also possesses a bronchodilating property and, if so, to determine possible contribution of NO to this action, we studied relaxant responses and NO generation in human bronchial smooth muscle in vitro.
Methods
Preparation of bronchial strips
Written informed consent was obtained from each patient, and the study was approved by the Tokyo Women's Medical University Medical Board and a local ethics committee. Human lung tissues were obtained from 23 patients at thoracotomies performed because of carcinoma. After surgical removal, macroscopically normal lung tissues were rapidly immersed in Krebs-Henseleit solution (composition in mM: NaCl, 118; KCl, 5.9; CaCl2, 2.5; MgSO4, 1.2; NaH2PO4, 1.2; NaHCO3, 25.5 and D-glucose, 5.6). Cartilaginous bronchi, 2–4 mm in internal diameter, were then dissected free of parenchyma, fat and surrounding connective tissues, and cut helically at a 45°-pitch to obtain bronchial strips measuring 2–3 mm in width and ∼15 mm in length. Between two and eight strips were dissected from each specimen and mounted in 7 ml organ chambers containing Krebs-Henseleit solution maintained at 37°C and continuously aerated with a gas mixture of 95% O2 and 5% CO2 to obtain a pH of 7.4, a PCO2 of 38 Torr, and a PO2 of >500 Torr. Contractile responses were continuously measured isometrically with a force-displacement transducer (Nihon Kohden, TB-652T, Tokyo, Japan) and were recorded on a pen recorder (Nihon Kohden, WT-685G).
Measurement of relaxant responses
The bronchial strips were allowed to equilibrate in the baths for 60 min, while they were washed with Krebs-Henseleit solution every 15 min and the resting tension was adjusted to 1 g. A contractile response was measured as the difference between peak developed tension and resting tension. Because airway smooth muscle and epithelium release prostaglandin E2, which may alter bronchoconstrictor responses, indomethacin (3×10−6 M) was present in the chamber throughout the experiments.
To determine whether ginsenoside causes airway smooth muscle relaxation, acetylcholine (10−6 M) was added to the chamber and, when the contraction reached a plateau, various concentrations of ginsenoside (10–1000 μg ml−1) were cumulatively added to the chamber in half-molar increments at 5-min intervals or 2 min after a stable plateau was achieved, whichever was the longer period, and the contractile response to each concentration was determined. Using paired tissues from the same patient, to assess the role of endogenous NO in the ginsenoside-induced relaxation, the tissues were incubated for 15 min with NG-nitro-L-arginine methylester (L-NAME, 10−3 M), an inhibitor of NO synthase (Rees et al., 1990), or its inactive enantiomer NG-nitro-D-arginine methylester (D-NAME, 10−3 M), and the concentration response curves for ginsenoside were generated in a similar manner. Moreover, in some experiments, L-arginine (10−2 M) or D-arginine (10−2 M) was added 5 min before the addition of L-NAME.
To elucidate the source of NO involved in the ginsenoside-induced relaxation, the relaxant responses to ginsenoside (100, 300, and 1000 μg ml−1) were determined in the tissues that had been pretreated for 15 min with tetrodotoxin (TTX, 10−6 M) and the tissues whose epithelium had been mechanically denuded. To do so, the epithelium was removed by passage of a moistened cotton-wrapped pipe cleaner through the bronchial lumen, and the absence of epithelial layer was confirmed after the experiment by staining tissues with Masson's trichrome. In our preliminary experiment, epithelial removal and L-NAME each potentiated the contractile responses to acetylcholine (10−6 M), whereas TTX or D-NAME had no effect. Therefore, to obtain a same magnitude of contraction as that observed with the epithelium-intact tissues, we used 4×10−7 and 6×10−7 M acetylcholine in the epithelium-denuded tissues and L-NAME-treated tissues, respectively.
Measurement of NO release
The measurement of NO concentration using the NO-selective electrode has been described in detail previously (Ichimori et al., 1994). Briefly, bronchial tissues were suspended in a glass chamber containing Krebs-Henseleit solution (1 ml per 10 mg of tissue weight), which was continuously stirred and maintained at 37°C by a water-jacketed perfusion apparatus (Yellow Springs Instruments Co., model 5301, Yellow Springs, OH, U.S.A.). Using an NO meter (Inter Medical Co., NO-501, Tokyo, Japan), the concentration of NO in the medium bathing the tissues was determined by measuring a redox current between an NO-selective electrode and a counter electrode (NOE-55 and NOR-20, respectively, Inter Medical Co.). The NO-selective electrode was supplied with 0.8 V for the electrochemical oxidation of NO, and the NO diffusing through the membrane was detected as an electrical current based on the following reaction: NO+2H2O→NO3−+4H++3e−. The current flow was proportional to the rate of diffusing through the membrane, which was, in turn, proportional to the concentration of NO at the outer surface of the membrane.
The working electrode and the counter electrode were placed 5 mm apart in the medium, and after a 30-min incubation when the baseline current became stable, ginsenoside (1000 μg ml−1) was added to the medium. The response of polarographic current was detected by a current-voltage converter circuit and continuously recorded for 30 min on a pen recorder (Graphtec Co., Model SR-6355, Tokyo, Japan). To confirm that the electrical current was generated by NO, when the response reached a plateau, L-NAME or D-NAME at 10−3 M was added to the medium. To test whether the generation of NO is Ca2+-dependent, tissues were incubated with Ca2+-free medium containing EGTA (10−3 M) for 30 min and the response of electrical current to ginsenoside was determined. In addition, to assess the involvement of NANC nerve fibres and airway epithelial cells, the responses were likewise determined in TTX (10−6 M)-treated tissues and epithelium-denuded tissues. In evaluating the concentration-dependent effect on NO generation, various concentrations of ginsenoside (10–1000 μg ml−1) were added to the medium, and the response of electrode current to each concentration was monitored. Moreover, the tissues were incubated for 15 min with L-NAME (10−3 M) and ginsenoside was added in a similar manner.
Calibration of the electrode was performed daily prior to the experiments. Briefly, using the nitrosothiol NO donor S-nitroso-N-acetyl-DL-penicillamine (SNAP) as a standard (Ignarro et al., 1981), the relationship between the magnitude of electrical current and the concentration of SNAP in the medium was determined. The current increased linearly as SNAP concentration increased, so that the concentration of NO could be determined from the electrical current recorded. In addition, immersion of the electrode in the medium containing ginsenoside (1000 μg ml−1) but not bronchial tissues was without effect on the current, indicating that ginsenoside per se had no effect on the electrode.
Measurement of cyclic GMP contents
Because NO causes smooth muscle relaxation by elevating intracellular cyclic GMP levels, the changes of cyclic GMP contents in response to ginsenoside were determined. After the dissection of the epithelial layer and connective tissues, bronchial strips were incubated for 30 min with various concentrations of ginsenoside (10–1000 μg ml−1) in the absence or presence of L-NAME (10−3 M). The tissues were then homogenized in a glass homogenizer and placed in ice-cold 10% trichloroacetic acid with [3H]-cyclic GMP added as a tracer of recovery determination. After the extraction of trichloroacetic acid with ether, the residue was dissolved in acetate buffer. The cyclic GMP levels were determined in duplicate by radioimmunoassay according to the method of Brooker et al. (1979), corrected for ether extraction of 87% recovery, and normalized for protein contents of the tissues as determined by the method of Lowry et al. (1951).
Materials
Indomethacin, acetylcholine hydrochloride, EGTA, L-NAME, D-NAME, L-arginine and D-arginine were purchased from Sigma (St. Louis, MO, U.S.A.), SNAP was from WAKO Pure Chemical Co. (Osaka, Japan), and [3H]-cyclic GMP was from Amersham (Tokyo, Japan). Ginsenoside was a gift from Tsumura Co. (Tokyo, Japan).
Statistics
All values were expressed as means±s.e.mean. Statistical analysis was performed by Students' t-test or Newman-Keuls multiple comparison test. Statistical significance was accepted at a P value of less than 0.05.
Results
Ginsenoside-induced relaxation
As demonstrated in Figure 1, addition of ginsenoside caused a concentration-dependent relaxation of human bronchial smooth muscle that had been contracted with acetylcholine, the maximal relaxation and the ginsenoside concentration required to produce 50% relaxation being 67±8% and 210±29 μg ml−1, respectively (n=11 for each). Pretreatment of tissues with L-NAME attenuated the relaxant responses to ginsenoside, the maximal relaxation being only 15±4% (P<0.001 vs ginsenoside alone, n=11), whereas D-NAME had no effect. This inhibition was reversed by L-arginine but not by D-arginine. Addition of TTX tended to reduce the relaxant responses to various concentrations of ginsenoside, but this reduction did not reach a significant level (Figure 2). On the other hand, the ginsenoside-induced relaxation was reduced by epithelial removal. Combination with TTX and epithelial removal reduced the relaxation, but the values were not significantly different from those for epithelial removal alone.
Figure 1.
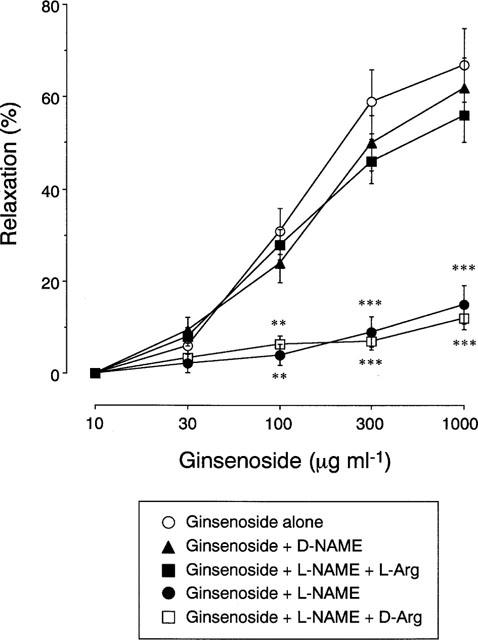
Concentration-dependent relaxation of human bronchial strips induced by ginsenoside. Ginsenoside was cumulatively applied to the tissues precontracted with acetylcholine in the absence or presence of the following drug(s): L-arginine (L-Arg, 10−2 M), D-arginine (D-Arg, 10−2 M), NG-nitro-L-arginine methylester (L-NAME, 10−3 M), and NG-nitro-D-arginine methylester (D-NAME, 10−3 M). Responses are expressed as per cent of relaxation. Data are means±s.e.mean, n=11 for each point. **P<0.01, ***P<0.001, significantly different from the response to ginsenoside alone.
Figure 2.
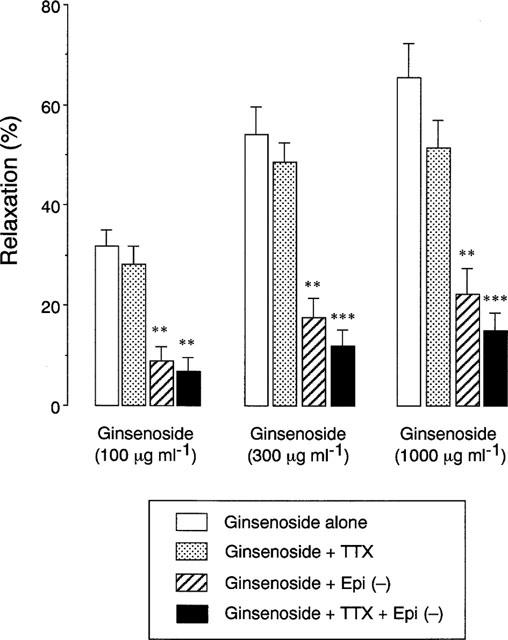
Effects of tetrodotoxin (TTX, 10−6 M) and mechanical removal of epithelium (Epi (−)) on the relaxant responses of human bronchial strips to various concentrations of ginsenoside. Responses are expressed as per cent of relaxation of acetylcholine-contracted tissues. Data are means±s.e.mean, n=10 for each column. **P<0.01, ***P<0.001, significantly different from the response to ginsenoside alone.
Release of NO
The output current of the NO-selective electrode in the medium bathing human bronchial tissues is shown in Figure 3. When the electrodes were immersed in the medium bathing the tissues, the baseline current was detected with a variation of 18–42 pA, which corresponded to the NO concentration at 23±5 nM (n=16). Addition of ginsenoside elicited an increase in the current, which peaked within 2 min and remained elevated at least for a 30-min observation period. After the response of electrical current reached a plateau, addition of L-NAME rapidly decreased the current by 109±7% (P<0.001, n=5), but D-NAME was without effect. The ginsenoside-induced electrical current was not altered by TTX but reduced by 91±5% (P<0.001, n=5) by Ca2+-free medium and 78±9% (P<0.001, n=5) in the epithelium-denuded tissues. The effect of ginsenoside on NO generation was concentration-dependent, with the maximal increase from the baseline NO concentration in the medium was 119±14 nM (P<0.001, n=8) (Figure 4). This effect was abolished by pretreatment of tissues with L-NAME.
Figure 3.
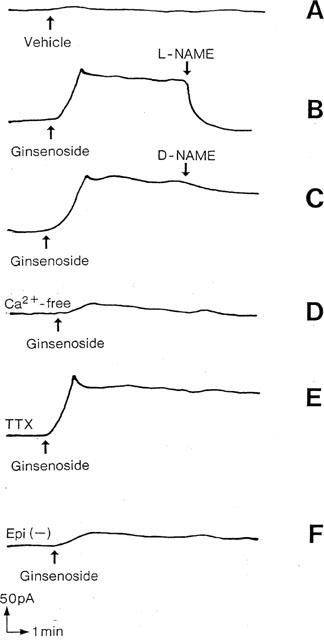
Representative tracing of the current detected by an NO-selective electrode in the medium containing human bronchial strips. (A) response of electrical current in the medium after addition of the vehicle of ginsenoside (Krebs-Henseleit solution). (B and C) ginsenoside (1000 μg ml−1) was added and, when the response reached a plateau, NG-nitro-L-arginine methylester (L-NAME, 10−3 M) or NG-nitro-D-arginine methylester (D-NAME, 10−3 M) was added. (D and E) the tissues were incubated with Ca2+-free medium containing EGTA (10−3 M) or with tetrodotoxin (TTX, 10−6 M) and ginsenoside (1000 μg ml−1) was added. (F) ginsenoside (1000 μg ml−1) was applied to epithelium-denuded tissues (Epi(−)).
Figure 4.
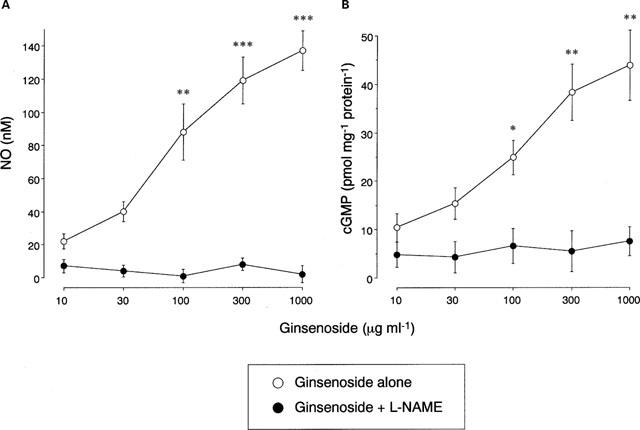
Concentration-dependent effect of ginsenoside on nitric oxide (NO) generation (A) and cyclic GMP synthesis (B) in human bronchial tissues. Various concentrations of ginsenoside were applied in the absence or presence of NG-nitro-L-arginine methylester (L-NAME, 10−3 M). Values are expressed as the concentrations of NO in the medium and the tissue cyclic GMP contents. Data are means±s.e.mean, n=8 for each point. *P<0.05, **P<0.01, ***P<0.001, significantly different from the response to ginsenoside alone.
Tissue cyclic GMP levels
Incubation of bronchial tissues with ginsenoside increased intracellular cyclic GMP contents in a concentration-dependent manner (Figure 4). Significant effects were observed at the ginsenoside concentrations of 100 μg ml−1 and higher. Incubation with L-NAME decreased the baseline levels of cyclic GMP contents and abolished the ginsenoside-induced increase in cyclic GMP synthesis.
Discussion
Our in vitro studies demonstrate that ginsenoside, an essential constituent of Chinese anti-asthmatic herbal medicine and aphrodisiacs, relaxes human bronchial smooth muscle presumably through a stimulation of NO generation by airway epithelium.
The multipurpose messenger molecule NO is generated from the amino acid L-arginine by NOS and has been identified as part of the transduction mechanism of soluble guanylyl cyclase-dependent pathway. Among a variety of cell types present in the respiratory tract, constitutive isoform of NOS and NOS mRNA have been found in airway epithelial cells and NANC nerve fibres in humans (Barnes & Belvisi, 1993; Kobzik et al., 1993), and the former cell type may be the principal source of NO in exhaled air (Kharitonov et al., 1994). In the present study, addition of ginsenoside relaxed human bronchial tissues in a concentration-dependent manner, and this relaxation was greatly inhibited by pretreatment of tissues with the NOS inhibitor L-NAME (Rees et al., 1990), whereas D-NAME had no effect. Furthermore, this inhibition was reversed by L-arginine but not by D-arginine. These findings suggest that bronchodilating effect of ginsenoside depends on NO generation by these tissues. We also found that the ginsenoside-induced relaxation was not altered by pretreatment with TTX that suppresses the neural release of NO, whereas it was reduced by mechanical removal of the epithelium. Thus, the effect of ginsenoside may be mediated by NO derived from airway epithelium rather than NANC nerve fibres.
Because NO is formed in small amounts and can rapidly decay by oxidation in the biological environment, the measurement of this molecule has been difficult. Previous studies have shown that NO may be involved in the ginsenoside-induced vasodilation in pulmonary artery (Chen et al., 1984) and corpus cavernosum (Chen & Lee, 1995). In these studies, the notion that NO is generated was derived from the enantiomer specific effects of methylester derivatives of arginine analogues, including L-NAME, and the potentiating effect of superoxide dismutase, a scavenger of superoxide anion which is known to inactivate NO. However, this conclusion is based on the premise that these drugs are specific inhibitors of NOS and a specific enhancer of NO action, respectively. In the present study, to ensure that NO is actually released, we directly measured NO concentration using a specific amperometric sensor for this molecule (Ichimori et al., 1994). Immersion of the NO-selective electrode in the medium bathing human bronchial tissues detected the electrical current, and the addition of ginsenoside to the medium increased the current in a concentration-dependent manner. In addition, L-NAME decreased the current to the level below the baseline, and the epithelium-denuded tissues generated only small amounts of NO in response to ginsenoside. It is therefore likely that NO is spontaneously released from bronchial tissues, especially from airway epithelium, in our experimental condition, and that the release is stimulated by ginsenoside.
It is known that endogenous NO stimulates soluble guanylyl cyclase, which in turn generates intracellular cyclic GMP and causes airway smooth muscle relaxation (Bohme et al., 1978). In our experiment, ginsenoside dose-dependently increased cyclic GMP contents, where the threshold concentration (100 μg ml−1) was similar to that observed with smooth muscle relaxation and NO generation. Therefore, the ginsenoside-induced bronchodilation is most likely attributable to the NO-mediated cyclic GMP synthesis.
To date, three main isoforms of NOS have been identified: type I (constitutive NOS, primarily soluble), type II (inducible NOS, primarily soluble), and type III (constitutive NOS, primarily particulate), and airway epithelium contains all subtypes (Asano et al., 1994; Shaul et al., 1994). Although we did not clarify these isoforms in this experiment, immediate detection of NO-selective current after the addition of ginsenoside and its abolition in Ca2+-free medium suggest that type I and/or type III NOS may be involved in the effect of ginsenoside. However, further studies are needed to clarify the mechanism whereby ginsenoside stimulates the epithelial NOS activity.
In conclusion, ginsenoside, an essential constituent of Chinese anti-asthmatic medicine, relaxes human bronchial smooth muscle. This effect is predominantly mediated by NO generation from airway epithelium and may, in part, account for the anti-asthmatic action of Panax ginseng. It should be noted however that Zizyphi fructus, an extract of nutlets of Chinese date tree, appears to be another important constituent of anti-asthmatic herbal medicine. In contrast to ginsenoside, Zizyphi fructus has been shown to have cyclic AMP-like bioactivities (Cyong et al., 1979), suggesting that this compound may also be involved in the anti-asthmatic effects through a different mechanism.
Acknowledgments
The authors thank Dr Kiyoshi Takeyama for helpful discussions during the course of this work and comments on the manuscript. We also thank Masayuki Shino and Yoshimi Sugimura for their technical assistance. This work was supported in part by Grant No 06670632 from the Ministry of Education, Science and Culture, Japan.
Abbreviations
- D-NAME
NG-nitro-D-arginine methylester
- L-NAME
NG-nitro-L-arginine methylester
- NANC
nonadrenergic noncholinergic
- NO
nitric oxide
- NOS
nitric oxide synthase
- SNAP
S-nitroso-N-acetyl-DL-penicillamine
- TTX
tetrodotoxin
References
- ASANO K., CHEE C.B.E., GASTON B., LILLY C.M., GERARD C., DRAZEN J.M., STAMLER J.S. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1994;91:10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES P.J., BELVISI M.G. Nitric oxide and lung disease. Thorax. 1993;48:1034–1043. doi: 10.1136/thx.48.10.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOHME E., GRAF H., SCHULTZ G. Effects of sodium ntiroprusside and other smooth muscle relaxants on cyclic GMP formation in smooth muscle and platelets. Adv. Cyclic Nucleotide Res. 1978;9:131–143. [PubMed] [Google Scholar]
- BROOKER G., HARPER J.F., TERASAKI W.L., MOYLAN R.D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv. Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- CHEN X., GILLS C.N., MAOLLI R. Vascular effects of ginsenosides in vitro. Br. J. Pharmacol. 1984;82:485–491. doi: 10.1111/j.1476-5381.1984.tb10784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN X., LEE T.J.-F. Ginsenosides-induced nitric oxide-mediated relaxation of the rabbit corpus cavernosum. Br. J. Pharmacol. 1995;115:15–18. doi: 10.1111/j.1476-5381.1995.tb16313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CYONG J.-C., HANABUSA K., OTSUKA Y. Studies on the cyclic AMP-like substance found in Zizyphi fructus. Wakan-Yaku. 1979;12:1–7. [Google Scholar]
- ICHIMORI K., ISHIDA H., FUKABORI M., NAKAZAWA H., MURAKAMI E. Practical nitric oxide measurement employing a nitric oxide-sensitive electrode. Rev. Sci. Instrum. 1994;65:1–5. [Google Scholar]
- IGNARRO L.J., LIPPTON H., EDWARDS J.C., BARICOS W.H., HYMAN A.L., KADOWITZ P.J., GRUETTER C.A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrothiols as active intermediates. J. Pharmacol. Exp. Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- KHARITONOV S.A., YATES D., ROBBINS R.A., LOGAN-SINCLAIR R., SHINEBOURNE E.A., BARNES P.J. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- KOBZIK L, , BREDT D.S., LOWENSTEIN C.J., DRAZEN J., GASTON B., SUGARBAKER D., STAMLER J.S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am. J. Respir. Cell Mol. Biol. 1993;9:371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- KODA A., NISHORI T., NAGAI H., MATSUURA T., TSUCHIYA H. Anti-allergic actions of crude drugs and blended Chinese traditional medicine: effect on type I and type IV allergic reactions. Folia Pharmacol. Japon. 1982;80:31–41. [PubMed] [Google Scholar]
- LOWRY O.H., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MIYAMOTO T., TAKAISHI T., MORITA H., NURIYAMA M., NAKAMURA T. The actions of Saiboku-to (TJ-96) on histamine release and the production of platelet-activating factor in human leukocytes Medicines of Plant Origin in Modern Therapy 1990Oxford: Oxford Clinical Communications; 12–13.ed. Miyamoto T. pp [Google Scholar]
- NIJKAMP F.P., VAN DER LINDE H.J., FOLKERS G. Nitric oxide synthesis inhibitors induce airway hyperresponsiveness in the guinea pig in vivo and in vitro: role of epithelium. Am. Rev. Respir. Dis. 1993;148:727–734. doi: 10.1164/ajrccm/148.3.727. [DOI] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M.J., SHULZ R., HODSON H.F., MONCADA S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAUL P.W., NORTH A.J., WU J.C., WELLS L.B., BRANNON T.S., LAU K.S., NICHEL T., MARGRAF L.R., STAR R.A. Endothelial nitric oxide synthase is expressed in cultured human bronchiolar epithelium. J. Clin. Invest. 1994;94:2231–2236. doi: 10.1172/JCI117585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINK D.A., HANBAUER I., KRISHNA M.C., DEGAFF W., GAMSON J., MITCHELL J.B. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]


