Abstract
The possible role of the endothelium in modulating responses to human urotensin-II (U-II) was investigated using isolated segments of rat thoracic aorta, small mesenteric artery, left anterior descending coronary artery and basilar artery.
Human U-II was a potent vasoconstrictor of endothelium-intact isolated rat thoracic aorta (EC50=3.5±1.1 nM, Rmax=103±10% of control contraction induced by 60 mM KCl and 1 μM noradrenaline). However the contractile response was not significantly altered by removal of the endothelium or inhibition of nitric oxide synthesis with L-NAME (100 μM). Human U-II did not cause relaxation of noradrenaline-precontracted, endothelium-intact rat aortae.
Human U-II contracted endothelium-intact rat isolated left anterior descending coronary arteries (EC50=1.3±0.8 nM, Rmax=20.1±4.9% of control contraction induced by 10 μM 5-HT). The contractile response was significantly enhanced by removal of the endothelium (Rmax=55.4±16.1%). Moreover, human U-II caused concentration-dependent relaxation of 5-HT-precontracted arteries, which was abolished by L-NAME or removal of the endothelium.
No contractile effects of human U-II were found in rat small mesenteric arteries. However the peptide caused potent, concentration- and endothelium-dependent relaxations of methoxamine-precontracted vessels. The relaxant responses were potentiated by L-NAME (300 μM) but abolished in the additional presence of 25 mM KCl (which inhibits the actions of endothelium-derived hyperpolarizing factor).
The present study is the first to show that human U-II is a potent endothelium-dependent vasodilator in some rat resistance vessels, and acts through release of EDHF as well as nitric oxide. Our findings have also highlighted clear anatomical differences in the responses of different vascular beds to human U-II which are likely to be important in determining the overall cardiovascular activity of this peptide.
Keywords: Human urotensin-II, orphan receptor, mesenteric artery, endothelium, EDHF, nitric oxide
Introduction
Urotensin-II (U-II) is a cyclic 12-amino acid residue neuropeptide originally isolated from fish spinal cords, that has some sequence similarity, although not homology, with somatostatin-14 (Pearson et al., 1980). U-II has also recently been cloned from the human genome, where it has been shown to be an 11-amino acid residue peptide, but with absolute conservation of the C-terminal cyclic hexapeptide that has been observed in all species from which U-II has been isolated (Coulouarn et al., 1998). This study showed human U-II precursor mRNA to be expressed at high levels in human spinal cord, indicating a possible neuromodulatory role for the peptide.
Ames et al. (1999) have recently shown that human U-II is an agonist for the orphan receptor GPR 14, and represents the most potent vasoconstrictor agonist yet identified, being an order of magnitude more potent than endothelin-1 in isolated monkey arteries. Human U-II triggered Ca2+ mobilization in transfected cells expressing GRP14, and was a potent vasoconstrictor in monkey arteries and rat thoracic aorta, whilst systemic administration to anaesthetized monkeys of human U-II caused circulatory collapse as a result of the profound vasoconstrictor effects of the peptide (Ames et al., 1999).
Binding sites for fish U-II have also been identified in membrane preparations isolated from rat thoracic aorta, where the peptide is a potent vasoconstrictor, but not mesenteric arterial smooth muscle (Itoh et al., 1988). In contrast to its effects in primates, previous studies have shown that administration of (fish) U-II to anaesthetized rats does not cause cardiovascular collapse, rather that this peptide has a vasodepressor effect (Gibson et al., 1986; Hasegawa et al., 1992). Interestingly, Ames et al. (1999) noted that sub-lethal doses of human U-II decreased total peripheral resistance in anaesthetized monkeys, whilst fish U-II exerts modest vasodilator effects in isolated rat aorta (Gibson, 1987).
The aim of the present study was to investigate the role of the endothelium in responses to human U-II in three rat small arteries (small mesenteric, left anterior descending coronary and basilar), and also to further the isolated artery studies of Ames et al. (1999) by examining the effect of endothelium in the rat aorta. We show that human U-II is an endothelium-dependent vasodilator in rat mesenteric and coronary arteries, which would seem likely to be a crucial mechanism in the overall effects of this peptide in vivo, but clearly shows marked regional specificity of action since significant relaxations were not observed in either rat aorta or basilar artery.
Methods
Rat isolated proximal descending thoracic aorta
Following sodium pentobarbitone overdose (Steris Laboratories Inc. Phoenix, AZ, U.S.A.), proximal descending thoracic aortae were isolated from adult male Sprague-Dawley rats (400 g; Charles River Breeding Labs, Wilmington, MA, U.S.A.), cleaned of adherent tissue and rings suspended in 10 ml organ baths containing Krebs solution of the following composition (mM): NaCl 112, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, NaHCO3 25, dextrose, 11. The Krebs solution was maintained at 37±1°C and gassed with 95% O2/5% CO2 (pH 7.4). Unless stated to the contrary, preparations were endothelium-intact (where necessary, aortae were denuded of the endothelium using a pair of fine forceps and loss of endothelial function confirmed using 10 μM carbachol).
Variations in isometric tension were recorded under 1 g (9.81 mN) optimal resting tension using FT03 force-displacement transducers (Grass Instrument Co., Quincy, MA, U.S.A.) coupled to Grass Model 7C polygraphs. Vessels were equilibrated for 90 min before being exposed sequentially to standard concentrations of 60 mM KCl (followed by washing) and 1 μM noradrenaline. All subsequent responses were normalized to the contraction induced by 60 mM KCl.
Myograph studies
Male Wistar rats (250–350 g; Tucks, Rayleigh, Essex, U.K.) were killed with an overdose of sodium pentobarbitone (120 mg kg−1, i.p., Sagatal, Rhone Merieux, Harlow, U.K.). The basilar, left anterior descending coronary or small (third generation) mesenteric arteries were then removed and cleaned of adherent tissue (Otley et al., 1997). Segments (2 mm) were mounted in a Mulvany-Halpern type wire myograph and normalized as described previously (White & Hiley, 1997) in gassed (95% O2/5% CO2) Krebs-Henseleit solution of the following composition (mM): NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, CaCl2 2.5, D-glucose 11 (mesenteric and basilar arteries) or 22 (coronary arteries). All experiments were carried out in the presence of 10 μM indomethacin and the presence of a functional endothelium was tested by precontracting the vessels with either methoxamine (mesenteric arteries), 5-HT (coronary) or 40 mM KCl (basilar), and adding 10 μM carbachol. Relaxations of greater than 90% (mesenteric) or 65% (coronary) denoted endothelium-intact vessels. Basilar arteries never relaxed by more than 20% upon addition of carbachol. Where the endothelium was not required, vessels were denuded by rubbing the intimal surface with a human hair.
Experimental protocols
Once equilibrated, concentration-response curves were generated to human U-II (0.03–300 nM) following 30 min pretreatment of aortae with either DMSO vehicle (0.1% v v−1) or the NOS inhibitor Nω-L-nitro-arginine methyl ester (L-NAME; 100 μM). Contractile effects of human U-II in rat small coronary, mesenteric and basilar arteries were investigated similarly by cumulative addition of the peptide to resting vessels in either the presence or absence of endothelium as indicated.
The relaxant action of human U-II was examined in rat aorta using tissues preconstricted with noradrenaline (0.1 μM). Human U-II was administered either as a single concentration (0.05–2 nM) added to the organ bath or by cumulative addition (0.03–10 nM) of peptide. The vasodilator effects of human U-II were investigated in myograph studies in both endothelium-intact and -denuded tissues following precontraction of the vessels with 5-HT (coronary arteries), methoxamine (mesenteric) or 40 mM KCl (basilar). The peptide was added cumulatively to the precontracted vessels in order to establish a concentration-response curve for the relaxation. In some experiments, nitric oxide synthesis was inhibited by preincubating vessels with L-NAME (300 μM for 30 min).
Data and statistical analysis
Data are given as the mean±s.e.mean and n indicates the number of animals used. All concentration-response data were fitted to the logistic function
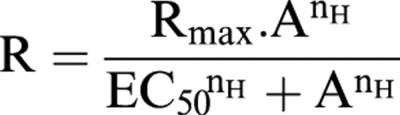 |
where R is response, A the concentration of the agonist, Rmax the maximum response, nH the slope function and EC50 the concentration of agonist giving half the maximal response (White & Hiley, 1998). The curve fitting was carried out using KaleidaGraph software (Synergy Software, Reading, PA, U.S.A.) running on a Macintosh computer. Curve fitting parameters were compared by unpaired t-test, and P values less than 0.05 were considered to be statistically significant.
Drugs
Human U-II (acetate salt, stored as 1 mM aqueous solution at −20°C) was synthesized in the Departments of Protein Biochemistry and Medicinal Chemistry, SmithKline Beecham. Methoxamine, carbachol, 5-HT and L-NAME (all from Sigma) were dissolved in distilled water. Indomethacin (Sigma) was dissolved in 5% (w v−1) NaHCO3. All other reagents were of analytical grade. All drugs were made freshly on the day of experimentation and stored in a light-tight container on ice.
All experiments were performed in accredited facilities in strict accordance with the institutional guidelines of SmithKline Beecham (Animal Care and Use Committee, ACUC), which follow the guidelines of the American Association of Laboratory Animal Care (AALAC; DHSS publication NIH 85-23).
Results
Rat aorta
Human U-II was a potent vasoconstrictor of resting, endothelium-intact aortic segments (EC50=3.5±1.1 nM, Rmax=103±10% of control contraction induced by 60 mM KCl; n=18). However the contractile effects of human U-II were not significantly altered either by inhibition of nitric oxide synthesis (with L-NAME, EC50=3.7±1.3 nM, Rmax=124± 9%; n=11) or by mechanical disruption of the endothelium (EC50=3.3±1.0 nM, Rmax=99±6%; n=12; Figure 1).
Figure 1.
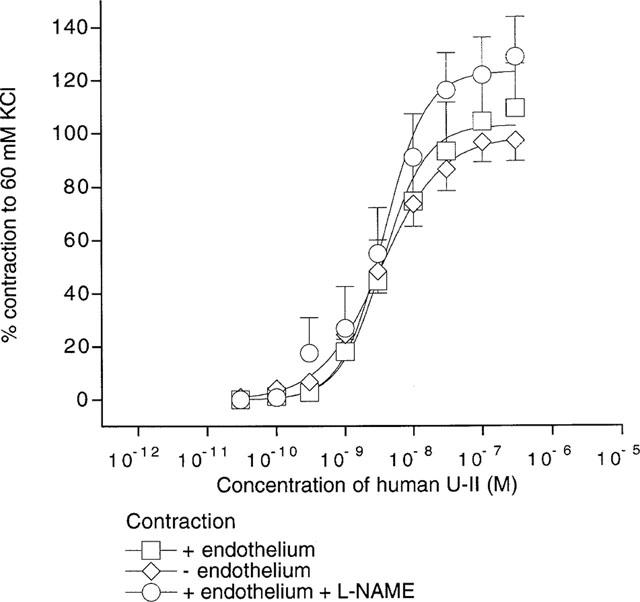
Contractile effects of human U-II in rat isolated proximal descending thoracic aorta. Data (presented as mean±s.e.mean) indicate the contractile effects of the peptide in resting arteries, expressed as a percentage of the initial test contraction induced by 60 mM KCl. Data were fitted to the logistic equation and the parameters are given in the text.
The putative vasorelaxant properties of human urotensin-II were examined in endothelium-intact aortae preconstricted with noradrenaline (100 nM). In accordance with the lack of effect of endothelium removal observed in the above experiments, human urotensin-II did not elicit any detectable vasorelaxant activity when administered either as a single concentration (0.05–2 nM) or when added to the organ bath in a cumulative (0.03–10 nM) manner (data not shown).
Rat left anterior descending coronary artery
Human U-II caused concentration-dependent vasoconstriction of resting rat left anterior descending coronary arteries and, in some endothelium-intact preparations, also caused vasorelaxant effects at higher concentrations (see Figure 2a). The vasoconstrictor effect of the peptide in endothelium-intact vessels was modest (EC50=1.3±0.8 nM, Rmax=20.1±4.9% of control contraction induced by 10 μM 5-HT; n=7). However, removal of the endothelium significantly enhanced the vasoconstrictor action of human U-II (EC50=32±33 nM, Rmax=55.4±16.1% of control contraction induced by 5-HT; n=5; Figure 2b).
Figure 2.
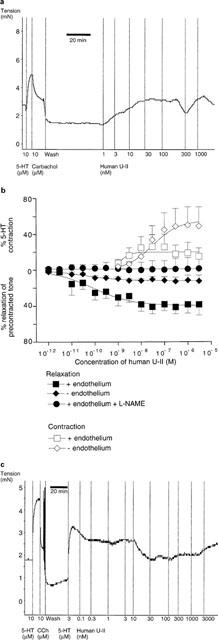
(a) Original experimental record showing the contractile effect of human U-II in rat isolated left anterior descending coronary artery. Vertical lines denote addition of drugs at the concentrations indicated. Note that the addition of carbachol shows this to be an endothelium-intact vessel, and also that some relaxant effects were observed at high concentrations of human U-II (100 and 1000 nM). (b) Contractile and relaxant effects of human U-II in rat isolated left anterior descending coronary artery. Data (presented as mean±s.e.mean) above zero indicate the contractile effects of the peptide in resting arteries, whilst the data below zero denote the relaxant effects of human U-II in 5-HT-precontracted vessels. Data were fitted to the logistic equation and the parameters are given in the text. (c) Original experimental record showing the relaxant effect of human U-II in rat isolated coronary artery precontracted with 5-HT (3 μM). Vertical lines denote addition of drugs at the concentrations indicated. Note that the addition of carbachol shows this to be an endothelium-intact vessel, and also that some contractile effects were observed at high concentrations of human U-II (above 100 nM). The apparent ‘stepped' nature of the tension responses is due to a lack of resolution in the recording system at the high gain required to measure these small responses.
Although some variation was observed in the response of individual vessels to the peptide, human U-II generally caused vasorelaxation of endothelium-intact coronary arteries precontracted with 5-HT (EC50=80±34 pM, Rmax=39.5±1.9% relaxation of 5-HT-induced tone; n=7). A typical response is shown in Figure 2c. The relaxant effect was essentially abolished in the presence of nitric oxide synthesis inhibition by L-NAME (300 μM), or by removal of the endothelium (n=4; Figure 2b).
Rat small mesenteric artery
Figure 3a shows that human U-II did not cause marked vasoconstriction of rat small mesenteric arteries either in the presence (2.7±2.3% of control contraction induced by 10 μM methoxamine, n=4) or absence of a functional endothelium (4.1±1.8%, n=4). Nevertheless human U-II was a potent relaxant of intact mesenteric arteries precontracted with methoxamine (EC50=1.6±1.6 nM, Rmax=76.2±10.5%, n=6). The peptide exerted only small relaxant effects in endothelium-denuded vessels (Rmax=10.5±3.2%, n=4). A typical experimental record from a relaxation experiment is shown in Figure 3b, from which it can be seen that the responses were generally not sustained. Furthermore, some intermediate concentrations caused little or no additional relaxation, however it is notable that complete and sustained relaxation was obtained at the highest concentrations.
Figure 3.
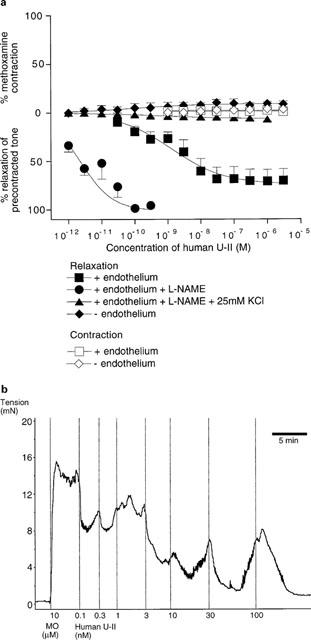
(a) Contractile and relaxant effects of human U-II in rat isolated mesenteric artery. Data above zero indicate the contractile effects of the peptide in resting arteries, whilst the data below zero denote the relaxant effects of human U-II in methoxamine-precontracted vessels. Where appropriate, data were fitted to the logistic equation and the parameters are given in the text. (b) Original experimental record showing the relaxant effect of human U-II in rat isolated mesenteric artery precontracted with methoxamine (10 μM). Vertical lines denote addition of drugs at the concentrations indicated.
Incubation of endothelium-intact vessels with the nitric oxide synthase inhibitor L-NAME (300 μM for 30 min) potentiated the vasorelaxant effects of human U-II (EC50=2.3±0.6 pM, Rmax=101.0±3.5%, n=5), however the additional presence of 25 mM KCl effectively abolished the relaxations (Rmax=6.6±2.2%, n=4).
Rat basilar artery
Figure 4 shows that human U-II was a very weak vasoconstrictor of rat basilar arteries, causing a maximum response of only 11.7±8.0% of the control contraction to 40 mM KCl (n=4). Basilar arteries do not exhibit marked endothelium-dependent relaxations (test relaxations to 10 μM carbachol were 16.6±2.9%, n=8), nevertheless human U-II caused a maximum relaxation of 15.4±7.9% (n=4) of arteries precontracted with 40 mM KCl.
Figure 4.
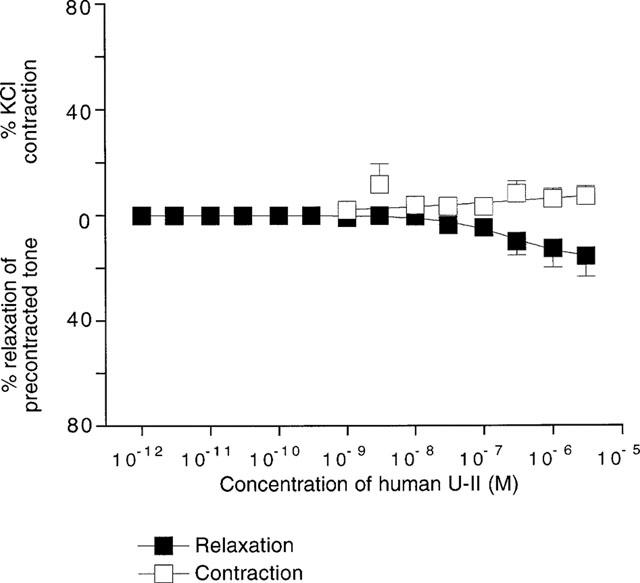
Contractile and relaxant effects of human U-II in rat isolated basilar artery. Data above zero indicate the contractile effects of the peptide in resting arteries, whilst the data below zero denote the relaxant effects of human U-II in vessels precontracted with 40 mM KCl. Responses were too small for data to be fitted to the logistic equation.
Discussion
The present study is, to our knowledge, the first to demonstrate a potent vasodilator effect of human U-II in isolated resistance arterial preparations, and this is mediated through the release of endothelium-derived relaxing factors. It is also of interest that the relaxant effects of human U-II in rat mesenteric arteries are not accompanied by contractile activity. These findings suggest that the overall cardiovascular actions of this peptide may be influenced by the endothelium, and also by regional factors such as receptor distribution.
Initial experiments showed that human U-II was a potent vasoconstrictor of endothelium-intact isolated rat thoracic aorta acting in the nanomolar concentration range, and this is consistent with previous findings using this preparation (Gibson, 1987; Ames et al., 1999; Nothacker et al., 1999). We have extended these observations by showing that neither removal of the endothelium, nor inhibition of nitric oxide synthesis by L-NAME significantly affected the contractions induced by human U-II. Moreover, human U-II failed to cause relaxation of noradrenaline-precontracted endothelium-intact aortae. Clearly human U-II does not cause endothelium-dependent relaxation of this vessel, and the presence of a functional endothelium does not modulate the contractile effects of the peptide. These results differ from those obtained in the same preparation by Gibson (1987) with fish U-II, which caused modest endothelium-dependent relaxations of precontracted aortae; they also noted that the vasoconstrictor effects of the peptide were modified to a small extent by the endothelium. It is therefore clear that the results of previous studies using fish U-II cannot necessarily be extrapolated to the human form of the peptide.
Human U-II caused concentration-dependent contractions of rat left anterior descending coronary artery, with some variation between individual animals (two of five endothelium-denuded vessels did not respond to human U-II, conversely two of seven intact vessels responded with large contractions). The mean data, however, indicate that the presence of a functional endothelium suppresses the contractile effect of human U-II, hence investigations were made for a possible endothelium-dependent vasodilator effect of the peptide. Endothelium-dependent relaxations in response to U-II have previously been demonstrated in isolated aortae from rabbits (Muramatsu et al., 1979) and rats (Gibson, 1987), whilst relaxations to U-II are also seen in non-vascular smooth muscle preparations (Gibson et al., 1984).
Consistent with the contractile data, human U-II caused endothelium- and concentration-dependent relaxations of 5-HT-precontracted coronary arteries. The relaxant effect of human U-II was abolished by L-NAME, in accordance with previous reports that endothelium-dependent vasorelaxation of isolated rat coronary arteries is mediated entirely through release of nitric oxide (Richard et al., 1994). The observation that human U-II can both constrict and dilate coronary microvessels is entirely novel, and may have relevance to humans since the expression of GPR14 (the orphan receptor of which U-II is likely to be the endogenous ligand) has been detected in human coronary artery smooth muscle and endothelial cell cultures (Ames et al., 1999).
Interestingly, whilst human U-II did not contract rat small mesenteric arteries, either in the presence of absence of a functional endothelium (consistent with previous work by Itoh et al., 1988), the peptide was a very potent endothelium-dependent relaxant of methoxamine-precontracted arteries. The relaxant effects were potentiated in the presence of the nitric oxide synthase inhibitor, L-NAME, but were abolished in the additional presence of 25 mM KCl, which treatment inhibits the effects of endothelium-derived hyperpolarizing factor (EDHF) in rat mesenteric arteries (Waldron & Garland, 1994; White & Hiley, 1997). It therefore seems likely that, although nitric oxide may play a minor role in relaxation of mesenteric arteries by human U-II, the major mechanism is the release of EDHF. The results obtained using rat mesenteric arteries are also of great importance since they represent the first demonstration of a blood vessel subtype in which human U-II is a potent vasorelaxant, but not a vasoconstrictor.
Human U-II was also tested in rat basilar arteries, which exhibit very limited endothelium-dependent relaxant responses. The peptide caused only small contractions in resting arteries, but (at high concentrations) did induce vasodilatation of vessels precontracted with 40 mM KCl that was similar in magnitude to that induced by carbachol.
Ames et al. (1999) showed that human U-II may be an endogenous ligand for the orphan receptor GPR14. In their study, the peptide caused concentration-dependent increases in intracellular Ca2+ in HEK-293 cells expressing human GPR14. Just as mobilization of intracellular Ca2+ clearly explains the contractile effect of U-II on vascular smooth muscle, the activation of receptors for U-II on vascular endothelial cells, causing Ca2+ mobilization in the same way, would induce endothelium-dependent relaxation through release of relaxing factors such as nitric oxide and EDHF (Fukao et al., 1997). Most endothelium-dependent vasodilators such as carbachol, however, show similar potency at causing vasorelaxation in either the absence or presence of L-NAME in the rat mesenteric artery (White & Hiley, 1997), and it is not clear why human U-II should be more potent in the presence of nitric oxide synthase inhibition. It may be of note that, in the absence of L-NAME, large relaxations were often elicited by an initial low, but not subsequent, concentration of the peptide. This may indicate that some tachyphylaxis to the peptide might occur, but this was not investigated further in the present study. It is of interest that although NG-monomethyl-L-arginine and indomethacin both partially block the hypotensive effects of fish U-II in anaesthetized rats, neither alone caused complete inhibition (Hasegawa et al., 1992), which suggests that the overall response may involve the complex release of a combination of mediators, such as nitric oxide and EDHF.
The findings of these experiments reconcile previous data suggesting that (fish) U-II exerts vasodepressor effects upon administration to rats (Gibson et al., 1986; Hasegawa et al., 1992), whilst high doses of human U-II caused profound vasoconstriction and cardiovascular collapse when administered to anaesthetized monkeys; however it should be noted that lower doses actually reduced total peripheral resistance (Ames et al., 1999). It therefore seems possible that the human peptide may exert similar endothelium-dependent vasodilator effects in primate arteries as were found here in rat arteries. It should be noted that small, resistance-sized vessels such as the mesenteric and coronary arteries examined in the present study are more important in determining vascular resistance than the conduit vessels in which vasodilator effects of U-II have previously been reported (Muramatsu et al., 1979; Gibson, 1987).
Our results also indicate that the overall cardiovascular effects of human U-II are likely to show considerable anatomical variations, depending on whether particular branches of the vasculature show both marked vasodilator and vasoconstrictor responses (such as the coronary arteries examined in this study), or only vasodilator responses (such as in the rat small mesenteric artery) or little of either (rat basilar artery). Such regional variation renders systemic depressor response data of limited use in interpreting the effects of U-II.
In summary, we have shown that human U-II causes endothelium-dependent relaxation in some rat small arteries, although there are clear anatomical differences in the relative importance of the vasodilator and vasoconstrictor mechanisms. Since administration of low doses of this peptide caused a decrease in total peripheral resistance in anaesthetized monkeys (Ames et al., 1999), similar to the effects of fish U-II in rats (Gibson et al., 1986; Hasegawa et al., 1992), we conclude that the endothelium may play an important role in determining the cardiovascular responses to human U-II.
Acknowledgments
R. White is a Junior Research Fellow of Sidney Sussex College, Cambridge.
Abbreviations
- EDHF
endothelium-derived hyperpolarizing factor
- L-NAME
NG-nitro-L-arginine methyl ester
- U-II
urotensin-II
References
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMELCH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.-S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W.P., OHLSTEIN E.H., BERSGMA D.J., DOUGLAS S.A. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- COULOUARN Y., LIHRMANN I., JEGOU S., ANOUAR Y., TOSTIVINT H., BEAUVILLIAN J.C., CONLON J.M., BERN H.A., VAUDRY H. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15803–15808. doi: 10.1073/pnas.95.26.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKAO M., HATTORI Y., KANNO M., SAKUMA I., KITABATAKE A. Sources of Ca2+ in relation to generation of acetylcholine-induced endothelium-dependent hyperpolarization in rat mesenteric artery. Br. J. Pharmacol. 1997;120:1328–1334. doi: 10.1038/sj.bjp.0701027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A. Complex effects of Gillichthys urotensin II on rat aortic strips. Br. J. Pharmacol. 1987;91:205–212. doi: 10.1111/j.1476-5381.1987.tb09000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., BERN H.A., GINSBURG M., BOTTING J.H. Neuropeptide-induced contraction and relaxation of the mouse anococcygeus muscle. Proc. Natl. Acad. Sci. U.S.A. 1984;81:625–629. doi: 10.1073/pnas.81.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., WALLACE P., BERN H.A. Cardiovascular effects of urotensin II in anaesthetised and pithed rats. Gen. Comp. Endocrinol. 1986;64:435–439. doi: 10.1016/0016-6480(86)90080-8. [DOI] [PubMed] [Google Scholar]
- HASEGAWA J., KOBAYASHI Y., KOBAYASHI H. Vasodepressor effects of urotensin II in rats. Neuroendocrinol. Lett. 1992;14:357–363. [Google Scholar]
- ITOH H., MCMASTER D., LEDERIS K. Functional receptors for fish neuropeptide urotensin II in major rat arteries. Eur. J. Pharmacol. 1988;149:61–66. doi: 10.1016/0014-2999(88)90042-8. [DOI] [PubMed] [Google Scholar]
- MURAMATSU I., FUJIWARA M., HIDAKA H., AKUTAGAWA H. Pharmacological analysis of urotensin-induced contraction and relaxation in isolated rabbit aortas The Caudal Neurosecretory System of Fishes 1979Maebashi, Japan: Gunma University; 39–47.16th Gunma Symposium in Endocrinologyed. Yamamoto, K. pp [Google Scholar]
- NOTHACKER H.-P., WANG Z., MCNEILL A.-M., SAITO Y., MERTEN S., O'DOWD B., DUCKLES S.P., CIVELLI O. Identification of the natural ligand of an orphan G-protein-coupled receptor involved in the regulation of vasoconstriction. Nature Cell Biology. 1999;1:383–385. doi: 10.1038/14081. [DOI] [PubMed] [Google Scholar]
- OTLEY C.E., CRAWFORD S.P., DAVIDSON H.J., HILEY C.R. Effects of peroxynitrite on contraction and relaxation responses in the small mesenteric, coronary and basilar arteries of the rat Br. J. Pharmacol. 199712055P [Google Scholar]
- PEARSON D., SHIVELY J.E., CLARK B.R., GESCHWIND I.I., BARKLEY M., NISHIOKA R.S., BERN H.A. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc. Natl. Acad. Sci. U.S.A. 1980;77:5021–5024. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARD V., KNEEFER N., TRON C., THUILLEZ C. Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation. 1994;89:1254–1261. doi: 10.1161/01.cir.89.3.1254. [DOI] [PubMed] [Google Scholar]
- WALDRON G.J., GARLAND C.J. Contribution of both nitric oxide and a change in membrane potential to acetylcholine-induced relaxation in the rat small mesenteric artery. Br. J. Pharmacol. 1994;112:831–836. doi: 10.1111/j.1476-5381.1994.tb13154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br. J. Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. Modulation of relaxation to levcromakalim by S-nitroso-N-acetylpenicillamine (SNAP) and 8-bromo cyclic GMP in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998;124:1219–1226. doi: 10.1038/sj.bjp.0701973. [DOI] [PMC free article] [PubMed] [Google Scholar]


