Abstract
Sphingolipids such as sphingosine-1-phosphate (SPP) and sphingosylphosphorylcholine (SPPC) can act both intracellularly and at G-protein-coupled receptors, some of which were cloned and designated as Edg-receptors.
Sphingolipid-induced vascular effects were determined in isolated rat mesenteric and intrarenal microvessels. Additionally, sphingolipid-induced elevations in intracellular Ca2+ concentration were measured in cultured rat aortic smooth muscle cells.
SPPC and SPP (0.1–100 μmol l−1) caused concentration-dependent contraction of mesenteric and intrarenal microvessels (e.g. SPPC in mesenteric microvessels pEC50 5.63±0.17 and Emax 49±3% of noradrenaline), with other sphingolipids being less active. The vasoconstrictor effect of SPPC in mesenteric microvessels was stereospecific (pEC50 D-erythro-SPPC 5.69±0.08, L-threo-SPPC 5.31±0.06) and inhibited by pretreatment with pertussis toxin (Emax from 44±5 to 19±4%), by chelation of extracellular Ca2+ with EGTA and by nitrendipine (Emax from 40±6 to 6±1 and 29±6%, respectively). Mechanical endothelial denudation or NO synthase inhibition did not alter the SPPC effects, while indomethacin reduced them (Emax from 87±3 to 70±4%).
SPP and SPPC caused transient increases in intracellular Ca2+ concentrations in rat aortic smooth muscle cells in a pertussis toxin-sensitive manner.
Our data demonstrate that SPP and SPPC cause vasoconstriction of isolated rat microvessels and increase intracellular Ca2+ concentrations in cultured rat aortic smooth muscle cells. These effects appear to occur via receptors coupled to pertussis toxin-sensitive G-proteins. This is the first demonstration of effects of SPP and SPPC on vascular tone and suggests that sphingolipids may be an hitherto unrecognized class of endogenous regulators of vascular tone.
Keywords: Sphingosine-1-phosphate, sphingosylphosphorylcholine, calcium, G-protein, microvessel, rat, vasoconstriction
Introduction
Sphingolipid metabolites such as sphingosine-1-phosphate (SPP) and sphingosine (SPH) have recently gained much attention as bioactive molecules (Ghosh et al., 1997; Meyer zu Heringdorf et al., 1997; Spiegel & Milstien, 1995). SPP is produced from SPH by sphingosine kinase and rapidly degraded by a phosphatase or a specific lyase (for review see Spiegel & Milstien, 1995). SPP is a rather unusual signalling molecule since it can act as an intracellular second messenger as well as an extracellular ligand at plasma membrane receptors (Hla et al., 1999; Meyer zu Heringdorf et al., 1998a; Van Brocklyn et al., 1998; van Koppen et al., 1996). Intracellular SPP can release Ca2+ from intracellular stores, and the sphingosine kinase/SPP signal transduction pathway might play a similar role in Ca2+ signalling as the phospholipase C/inositol-1,4,5-trisphosphate pathway (Meyer zu Heringdorf et al., 1998a). In contrast, extracellular SPP activates specific G-protein-coupled receptors, which belong to the family of endothelial differentiation gene (Edg) receptors (for review see Goetzl & An, 1998). While Edg-2 and -4 are activated by lysophosphatidic acid, Edg-1, -3 and -5 are activated by SPP at nanomolar concentrations. These receptors are also activated by sphingosylphosphorylcholine (SPPC), however at much higher (i.e. micromolar) concentrations. SPPC, similar to SPP, also can release Ca2+ from intracellular stores; the effects of SPPC on G-protein-coupled receptors can be distinguished from its intracellular activity by the stereospecificity of the former (Meyer zu Heringdorf et al., 1998b). While many but not all receptor-mediated sphingolipid effects are inhibited by pertussis toxin (PTX), PTX sensitivity indicates receptor-mediated rather than intracellular sphingolipid actions (Goetzl & An, 1998).
SPP has been identified in human serum and plasma, where it occurs in concentrations of 484±82 and 191±79 nmol l−1, respectively (Yatomi et al., 1997). One source of extracellular SPP are platelets, which release the lipid upon activation (Yatomi et al., 1995). Thus, it is tempting to assume a role for SPP in cardiovascular function. In fact, both endothelial cells and vascular smooth muscle cells express Edg receptors, e.g. Edg-1 in differentiating human endothelial cells (Hla & Maciag, 1990) and Edg-5 in rat aortic smooth muscle cells (Okazaki et al., 1993). However, a systematic analysis of Edg receptor expression in the cardiovascular system is still lacking. Functional data obtained with cultivated cells show that vascular endothelial as well as smooth muscle cells are activated by SPP (Bornfeldt et al., 1995; Meyer zu Heringdorf et al., 1996; Xia et al., 1998). In bovine aortic endothelial cells, SPP increases intracellular Ca2+ concentrations in a PTX-sensitive manner (Meyer zu Heringdorf et al., 1996), which strongly argues for the involvement of G protein-coupled SPP receptors. SPP-induced increases of intracellular Ca2+ concentration have also been demonstrated in human arterial smooth muscle cells (Bornfeldt et al., 1995), but it is not known whether this is PTX-sensitive. Whether sphingolipids affect vascular tone is not known. Therefore, the functional responses to SPP, SPPC and related sphingolipids in isolated rat mesenteric and intrarenal microvessels were investigated.
Methods
Assessment of microvessel contraction
Mesenteric microvessels and renal microvessels (interlobar arteries) were prepared from adult male Wistar rats (280–380 g) as previously described (Chen et al., 1996, 1997). Rats were killed by decapitation except for the experiments with PTX treated rats which were killed by an overdose of thiobutabarbitone. Experiments were performed according to the procedure of Mulvany & Halpern (1977), with minor modifications. Briefly, the vessels were mounted on 40 μm diameter stainless steel wires in a myograph chamber for isometric recording of tension development. They were bathed in Krebs-Henseleit buffer of the following composition (mmol l−1): NaCl 119, NaHCO3 25, KCl 4.7, KH2PO4 1.18, MgSO4 1.17, CaCl2 2.5, EDTA 0.026, D-glucose 5.5. The buffer temperature was maintained at 37°C, and the chamber was gassed with 5% CO2/95% O2 to maintain pH at 7.4. Additionally, 5 μmol l−1 cocaine and 1 μmol l−1 (±)-propranolol were added to block neuronal catecholamine uptake and β-adrenoceptor activation by high noradrenaline concentrations. Unless otherwise noted, the following experimental design was used: Following equilibration, the vessels were challenged several times with 125 mmol l−1 KCl and 10 μmol l−1 noradrenaline. Thereafter, the vessels were treated with 100 μmol l−1 carbachol; only vessels with a relaxation response of at least 50% were accepted, indicating a functionally intact endothelium. Following washout and another 30 min of equilibration, a concentration-response curve for noradrenaline was generated. After another washout and 30 min equilibration, a concentration-response curve for a sphingolipid was performed.
In some experiments, nitrendipine and EGTA were added to the organ bath 30 min prior to the sphingolipid, and indomethacin and/or NG-nitro-L-arginine 20 min prior to the sphingolipid. In some other experiments, the endothelium was mechanically removed by gently rubbing a rat whisker hair through the lumen prior to mounting the vessel in the myograph chamber. In some other experiments, rats were treated with 10 μg kg−1 PTX or vehicle given via the jugular vein under light ketamine (100 mg kg−1, i.p.) anaesthesia 3 days before the experiment.
Measurement of intracellular free Ca2+ concentrations in cultured rat aortic smooth muscle cells
Vascular smooth muscle cells were prepared from rat thoracic aorta according to Rosskopf et al. (1995). Briefly, freshly prepared aortae were incubated for 30 min at room temperature with 125 u ml−1 collagenase I in Hank's balanced salt solution (HBSS) of the following composition (mmol l−1): NaCl 118, KCl 5, CaCl2 1, MgCl2 1, D-glucose 5, HEPES 15, pH 7.4. Thereafter, remaining connective tissue and endothelium were removed, the aortae were cut into small pieces and incubated for 4–6 h at 37°C in DMEM/F12 medium with 100 u ml−1 penicillin, 100 μg ml−1 streptomycin, 250 ng ml−1 amphotericin B. Treatment with collagenase (125 u ml−1) and elastase (0.5 mg ml−1) in HBSS without Ca2+ and Mg2+ followed for 2 h at 37°C. The reaction was stopped by addition of DMEM/F12 medium containing 20% foetal calf serum and penicillin, streptomycin and amphotericin B, and cells were plated onto 60-mm cell culture plates. The cells were used between passage 3 and 6. The Ca2+ concentration measurements were performed as described previously (Meyer zu Heringdorf et al., 1996). The cells were loaded with 1 μM fura2/AM for 1 h at room temperature in HBSS, washed with HBSS, and used for fluorescence measurements within the next hour. Ca2+ concentrations were measured in a continuously stirred cell suspension at room temperature in a Hitachi F2000 spectrofluorometer as described (Meyer zu Heringdorf et al., 1996).
Data analysis
In the microvessel studies, force of contraction data were normalized to % of the maximal noradrenaline effect in the respective vessel (Chen et al., 1997) unless otherwise noted; maximum noradrenaline-induced contraction was ≈18 mN and ≈6 mN in the mesenteric and intrarenal microvessels, respectively. Data are shown as mean±s.e.mean for n experiments. Whenever sphingolipid concentration-response curves approached saturating maximal responses, pEC50 and Emax were calculated by fitting sigmoidal functions to the experimental data using the Prism program (GraphPAD Software, San Diego, CA, U.S.A.).
Statistical significance of differences between two groups was analysed by two-tailed t-tests, while for comparison of multiple groups a one-way analysis of variance followed by a Dunnett multiple comparison test was used. In some cases entire concentration-response curves were compared by two-way analysis of variance testing for the overall treatment effect. All statistical calculations were performed with the Prism program, and P<0.05 was considered significant.
Chemicals
SPP and SPPC were obtained from Biomol (Hamburg, Germany); SPH, glucopsychosine (GLU) and psychosine (PSY) were obtained from Matreya (Bad Homburg, Germany). The SPPC stereoisomers were a kind gift from the Bayer AG (Leverkusen, Germany). For all studies, sphingolipids were dissolved in methanol, dried in a SpeedVac concentrator and redissolved in 1 mg ml−1 bovine serum albumin. Within each series of experiments only one SPPC batch was used, but multiple batches were used for the overall study. (−)-Noradrenaline bitartrate, methoxamine HCl, carbachol HCl and (±)-propranolol HCl (all dissolved in 10 mmol l−1 HCl), indomethacin (dissolved in 25 mmol l−1, NaH2PO4/25 mmol l−1, K2HPO4/1 mmol l−1, MgCl2 buffer), NG-nitro-L-arginine (dissolved in destilled H2O) and collagenase I were obtained from Sigma (Deisenhofen, Germany). Nitrendipine (dissolved in ethanol) was obtained from RBI (Natick, MA, U.S.A.), PTX was from ICN (Eschwege, Germany), elastase from Fluka (Deisenhofen, Germany) and cell culture media supplements from Gibco (Karlsruhe, Germany).
Results
Since ceramide can cause vasodilatation (Johns et al., 1997) and SPP is a ceramide metabolite (Meyer zu Heringdorf et al., 1997), it was initially tested whether SPP can cause vasodilatation. Mesenteric microvessels were precontracted with 100 μmol l−1 methoxamine to reach a force of contraction of 13.0±0.8 mN (n=13). Addition of 100 μmol l−1 SPP did not cause relaxation but rather a contraction which took ≈5 min to fully develop (data not shown) to maximal values of 29±6% above the methoxamine responses. In contrast, addition of 100 μmol l−1 carbachol reduced the tone of these microvessels by 57±5%. Similar data were obtained with SPPC (contraction of 16±1% above methoxamine values; n=8). Therefore, our further studies focused on vasoconstricting effects of the sphingolipids.
In initial experiments, the vasoconstricting effects of SPP, SPPC, SPH, PSY (i.e. galactosyl-sphingosine), and GLU (i.e. glucosyl-sphingosine) on mesenteric and intrarenal microvessels (n=8–10 each) were compared. All five sphingolipids caused concentration-dependent vasoconstriction in both vessel types which reached maximum values after 5–7 min (Figure 1). In mesenteric microvessels the calculated pEC50 and Emax for SPPC and SPH were 5.63±0.17 and 5.36±0.19, respectively, and 49±3 and 32±2% of maximum noradrenaline values (n=8 each). In intrarenal microvessels the calculated pEC50 and Emax for SPPC and SPH were 4.75±0.16 and 5.06±0.58, respectively, and 68±8 and 42±10% of maximum noradrenaline values (n=8 for SPPC, only five of 10 SPH curves could be fitted). In both vessel types pEC50 and Emax of the other sphingolipids could not be determined reliably by curve fitting since their curves did not yield clear plateau effects. However, at a concentration of 100 μmol l−1, the order of efficacy was SPPC≈SPP>SPH⩾PSY>GLU in mesenteric vessels and SPPC>SPP≈SPH≈PSY≈GLU in intrarenal vessels (Figure 1). Since in vitro vasoconstriction was greater and more consistent in the mesenteric than in the intrarenal vessels, mesenteric microvessels with SPPC as the agonist were used in all further in vitro experiments.
Figure 1.
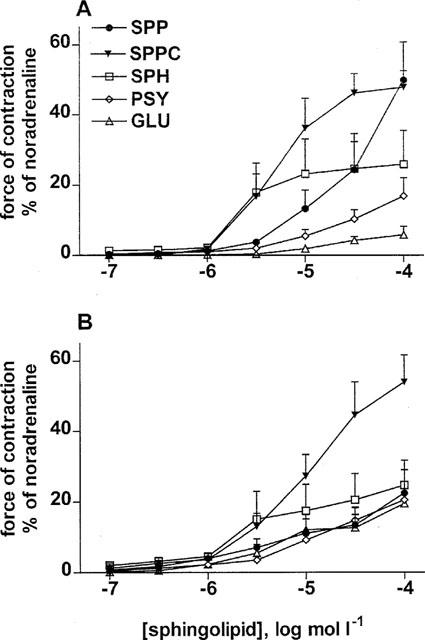
Effects of sphingolipids on rat mesenteric and renal microvessels in vitro. Data are mean±s.e.mean (n=8–10) and normalized as % of maximal noradrenaline values (≈18 and 6 mN for mesenteric and intrarenal vessels, respectively). SPP, SPPC, SPH, PSY, and GLU concentration-dependently constricted isolated mesenteric (A) and intrarenal (B) microvessels.
Two series of experiments were performed to determine whether sphingolipid-induced vasoconstriction is receptor-mediated. As SPPC effects on membrane receptors, but not intracellular SPPC effects, exhibit stereospecificity (Meyer zu Heringdorf et al., 1998b), a possible stereospecifity of SPPC was investigated first. The SPPC diastereoisomer D-erythro-SPPC was significantly more potent and efficient than L-threo-SPPC to cause contraction of rat mesenteric microvessels (pEC50 5.69±0.08 and 5.13±0.06, n=14 and 8, respectively, P<0.001; Emax 76±4 and 56±8%; P<0.005), while racemic SPPC had intermediate values (pEC50 5.59±0.13, Emax 59±4%, n=9; Figure 2). To determine a role for G-proteins in sphingolipid-induced vasoconstriction, mesenteric microvessels from rats which had been treated with 10 μg kg−1 PTX or its vehicle 3 days before the experiment (n=16–17) were studied. PTX treatment did not significantly affect the ability of carbachol (100 μmol l −1) to induce relaxation (85±4 vs 93±2% for vehicle and PTX treatment, respectively) and the maximal contractile response to noradrenaline (20.2±0.9 vs 19.6±1.2 mN) and only slightly but significantly decreased pEC50 values for noradrenaline (6.36±0.07 vs 6.10±0.05, P<0.01). In contrast, PTX treatment greatly reduced the SPPC-induced vasoconstriction (n=16 and 18, respectively; pEC50 from 5.03±0.05 to 4.80±0.04, P<0.01; Emax from 44±5 to 18±3%, P<0.0001; Figure 3). In comparison, vasoconstriction induced by 100 nmol l−1 neuropeptide Y, which acts via receptors coupled to PTX-sensitive G-proteins (Michel et al., 1998), was reduced by PTX treatment from 6.9±0.7 to 3.6±1.3 mN (P<0.05). To further demonstrate that our conditions of PTX treatment did not non-specifically alter microvessel responsiveness, the vasoconstriction evoked by 125 mmol l−1 KCl was studied in a separate series of experiments and found to be not significantly altered (9.7±1.4 vs 8.6±1.4 mN; n=11 each).
Figure 2.
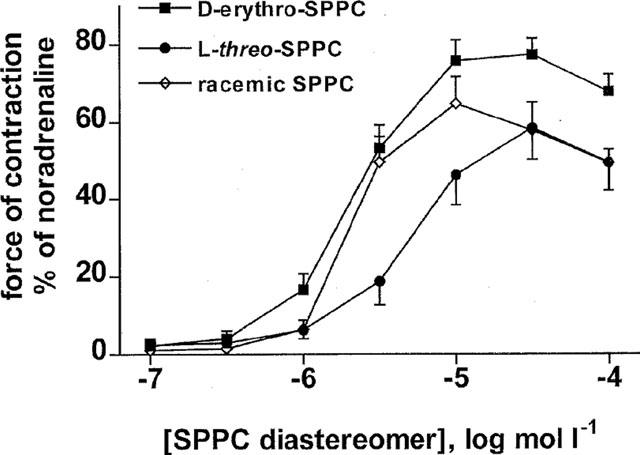
Effects of SPPC diastereoisomers on rat mesenteric microvessels in vitro. Data are mean±s.e.mean (n=8–14) and normalized as % of maximal noradrenaline values (≈16 mN). D-erythro-SPPC was significantly more potent and efficient than L-threo-SPPC (P<0.05, see results) with intermediate values for racemic SPPC.
Figure 3.
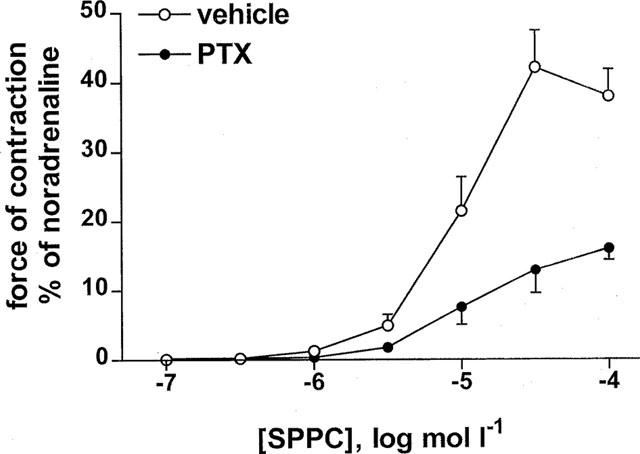
Effect of PTX treatment (10 μg kg−1 3 days before the experiment) on SPPC-induced vasoconstriction in rat mesenteric microvessels in vitro. Data are mean±s.e.mean (n=16–17) and normalized as % of maximal noradrenaline values (≈20 mN). In microvessels of PTX-treated rats, the SPPC-induced vasoconstriction was significantly reduced compared to vehicle-treated animals (P<0.05, see results).
The role of extracellular Ca2+ and L-type Ca2+ channels in SPPC-induced vasoconstriction was investigated by adding EGTA (0.5 mmol l−1 with nominally Ca2+-free buffer in the organ bath) or nitrendipine (300 nmol l−1) 30 min prior to SPPC. Addition of nitrendipine slightly and that of EGTA markedly reduced vasoconstriction at all effective SPPC concentrations (e.g. contraction at 100 μmol l−1 SPPC control 40±6%, nitrendipine 29±6%, EGTA addition 6±1%, n=13, 11 and 12, respectively; P<0.05 for nitrendipine and P<0.0001 for EGTA in a two-way analysis of variance testing for overall treatment effect based on the entire concentration-response curve; Figure 4). When 5 mmol l−1 EGTA was added 1 min prior to SPPC in standard buffer to prevent depletion of intracellular Ca2+ stores prior to SPPC addition, EGTA addition also markedly reduced SPPC-induced contraction almost completely (e.g. at 100 μmol l−1 control 75±10% and EGTA 10±2%, n=4 and 8, respectively, P<0.0001).
Figure 4.
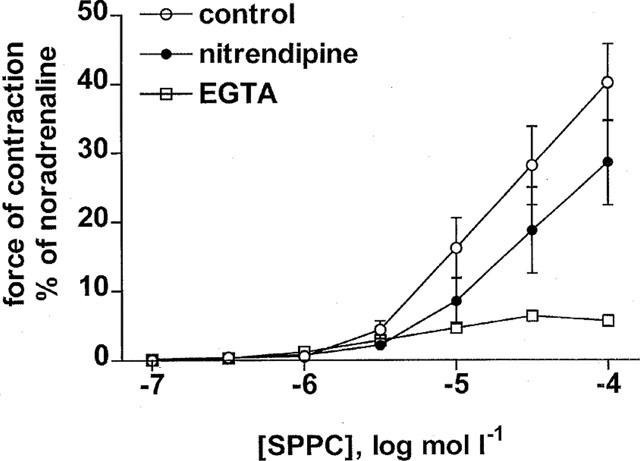
Effects of EGTA (0.5 mmol l−1 with nominally Ca2+-free buffer in the organ bath) and nitrendipine (300 nmol l−1) treatment on SPPC-induced contraction of rat mesenteric microvessels in vitro. Data are mean±s.e.mean (n=11–13) and normalized as % of maximal noradrenaline values (≈18 mN). Nitrendipine significantly attenuated SPPC-induced vasoconstriction (P<0.05 in a two-way analysis of variance) compared to controls, while EGTA abolished it.
The possible role of endothelium-derived modifiers or mediators of sphingolipid effects in the microvessels was tested using the NO synthase inhibitor, NG-nitro-L-arginine (1 mmol l−1, n=9), the cyclooxygenase inhibitor, indomethacin (10 μmol l−1, n=8), both (n=12) or vehicle (n=8) added 20 min prior to SPPC, and mechanical endothelium denudation (n=16). None of the treatments significantly affected the pEC50 of SPPC (control 5.62±0.11, NG-nitro-L-arginine 5.60±0.12, indomethacin 5.48±0.12, indomethacin plus NG-nitro-L-arginine 5.82±0.10). The Emax of SPPC (87±3%) was not significantly altered by NG-nitro-L-arginine (96±5%), tended to be reduced by indomethacin (70±4%; P<0.01 in t-test but P>0.05 in one-way ANOVA followed by Dunnett test) and was significantly reduced by indomethacin plus NG-nitro-L-arginine (66±6%, P<0.05 in one-way ANOVA followed by Dunnett test; Figure 5). Mechanical endothelium removal did not significantly alter the SPPC-induced vasoconstriction (pEC50 control 5.36±0.12 and denuded 5.36±0.06, n=13 and 9, respectively, Emax control 47±5% and denuded 50±5%; Figure 5), while it reduced the vasorelaxant effects of 100 μmol l−1 carbachol from 77±4 to 14±3% (P<0.0001).
Figure 5.
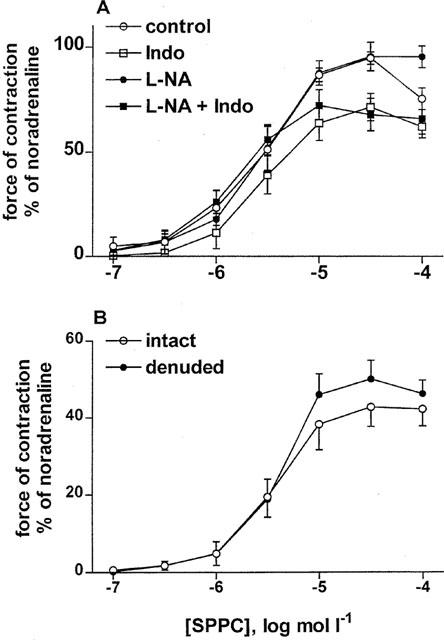
Effects of endothelial mediators on SPPC-induced contraction of rat mesenteric microvessels in vitro. Data are mean±s.e.mean (n=8–9) and normalized as % of maximal noradrenaline values (≈13 mN and 19 mN for A and B, respectively). Effects of NG-nitro-L-arginine (L-NA, 1 mmol l−1) and indomethacin (10 μmol l−1). L-NA alone did not affect SPPC-induced vasoconstriction, while indomethacin alone or in combination with L-NA slightly but significantly decreased the response (P<0.05 in a two-way analysis of variance (A). Effects of SPPC in intact and denuded mesenteric microvessels which did not differ significantly in their responsiveness in a two-way analysis of variance (B).
Since it is unknown whether previously reported SPP-induced elevations of intracellular Ca2+ in vascular smooth muscle cells (Bornfeldt et al., 1995) are PTX-sensitive and since sufficient numbers of cells could not be obtained from the microvessels, this question was studied in cultured rat aortic smooth muscle cells. SPP and SPPC concentration-dependently elevated intracellular Ca2+ concentrations (EC50≈1 nmol l−1 vs ≈0.6 μmol l−1 and maximum increases ≈700 nmol l−1 vs ≈250 nmol l−1 in two experiments). The Ca2+ elevations consisted of transient peak elevations followed by much smaller but sustained plateau elevations (Figure 6). Smooth muscle cell treatment with PTX largely abolished the Ca2+ elevations by 1 μmol l−1 SPP or 10 μmol l−1 SPPC but had little effect on the plateau elevations (Figure 6).
Figure 6.
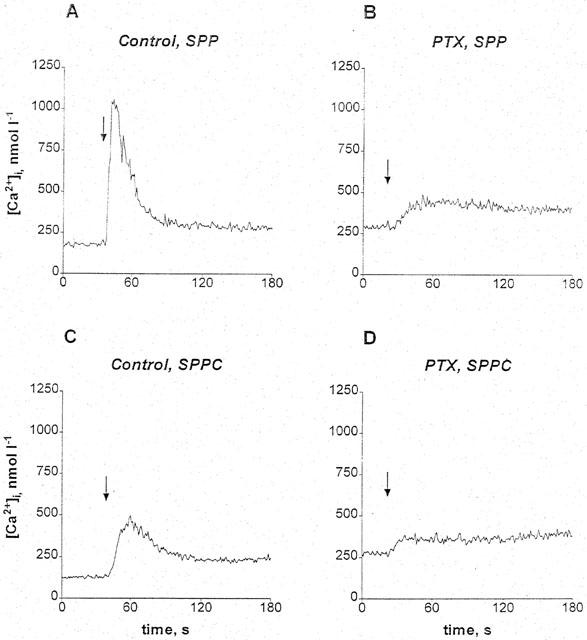
Original recordings of elevations of intracellular Ca2+ concentrations by SPP and SPPC in cultured rat aortic smooth muscle cells. Intracellular Ca2+ concentrations were measured in Fura2-loaded cells pretreated with or without 100 ng ml−1 PTX for 16 h. The arrows indicate time points of addition of SPP (1 μmol l−1) or SPPC (10 μmol l−1). SPP (A) and SPPC (C) rapidly and transiently elevated intracellular Ca2+ concentrations with a larger increase upon stimulation with SPP compared to SPPC. The effect of SPP and SPPC was strongly attenuated by PTX (B, D).
Discussion
Recent studies have identified SPP as an endogenous sphingolipid metabolite in platelets, plasma and serum (Yatomi et al., 1995, 1997). The reported plasma and serum concentrations are in the high nanomolar range, and since sphingolipids are believed to act in a paracrine rather than hormonal manner, it can be expected that their concentrations in the effect compartment may be in the micromolar range. These data suggest that sphingolipids may play a mediator role in the vascular system. Therefore, the present study was designed to investigate vascular sphingolipid effects in isolated mesenteric and intrarenal microvessels. All tested sphingolipids caused concentration-dependent contraction of isolated mesenteric and intrarenal microvessels with pEC50 values in the low micromolar range. Together with estimated endogenous sphingolipid concentrations (see above), these data indicate that sphingolipids are an hitherto unrecognized class of endogenous vasoactive agents. The maximal effects of all tested sphingolipids in both vascular beds were smaller and developed more slowly than α1A-adrenoceptor-mediated vasoconstriction by noradrenaline, but their magnitude and slow time course resembled those of neuropeptide Y Y1 receptor-mediated vasoconstriction (Chen et al., 1996, 1997). In both vessel types, SPPC was the most and PSY und GLU the least effective vasoconstrictor, with SPP and SPH having intermediate effects. This order, particularly the high potency of SPPC, does not match the sphingolipid order of potency in cultured cells expressing endogenous or cloned Edg receptors (Ancellin & Hla, 1999; Meyer zu Heringdorf et al., 1996; van Koppen et al., 1996; Xu et al., 2000). Therefore, additional experiments were designed to determine whether sphingolipid effects on vascular tone were receptor-mediated.
For the mechanistic experiments, isolated mesenteric microvessels and SPPC as the agonist were used primarily, since this combination caused the most prominent vasoconstriction. The SPPC effects were stereospecific, the naturally occurring D-erythro-SPPC being significantly more potent than L-threo-SPPC. A similar stereospecificity has been demonstrated for SPPC-induced increases of Ca2+ concentrations in cultured human embryonic kidney cells (Meyer zu Heringdorf et al., 1998b) and activation of IK(ACh) in rat atrial cardiomyocytes (Bünemann et al., 1996). Since receptor-mediated but not intracellular SPPC effects are stereospecific (Meyer zu Heringdorf et al., 1998b), our data strongly suggest that SPPC-induced vasoconstriction is receptor-mediated.
Sphingolipid receptors of the Edg family can couple to PTX-sensitive G-proteins (An et al., 1997, 1999; Goetzl & An, 1998; Hla et al., 1999; Liu et al., 1999; Meyer zu Heringdorf et al., 1997; Okamoto et al., 1999; Van Brocklyn et al., 1998; Zondag et al., 1998). Therefore, the hypothesis of a receptor-mediated sphingolipid effect on the vasculature was tested further by using PTX. Our conditions of in vivo PTX treatment did not cause toxic effects, since they had no major effects on noradrenaline- or KCl-induced vasoconstriction or on carbachol-induced vasodilatation. On the other hand, the PTX treatment was effective since it significantly reduced the vasoconstriction caused by neuropeptide Y, which acts via receptors coupled to PTX-sensitive G-proteins (Michel et al., 1998). PTX treatment greatly reduced the SPPC-induced vasoconstriction, indicating that it occurs predominantly, if not exclusively, via receptors coupled to PTX-sensitive G-proteins. Possible candidates are receptors belonging to the Edg receptor family which are activated by SPP and SPPC. However, the previously cloned Edg receptors are generally more sensitive to SPP than SPPC. Hence, the high potency of SPPC in our in vitro studies suggests that additional as yet unrecognized subtypes of Edg receptors may exist. Indeed very recent studies have identified the orphan receptor OGR1 as a SPPC-prefering receptor (Xu et al., 2000). Alternatively, it is possible that vasoconstriction is mediated by one of the known Edg receptors but that the relative potencies of SPP and SPPC differ between isolated cells and intact tissues. In this context it should be noted that SPP was much more potent and efficient than SPPC in raising intracellular Ca2+ concentrations in our cultured rat smooth muscle cells. While a resolution of this problem was beyond the scope of the present study, further experiments were designed to determine the roles of extracellular Ca2+ and the vascular endothelium in sphingolipid-induced vasoconstriction.
Enhancement of vascular smooth muscle tone largely relies on elevation of intracellular Ca2+ concentrations caused by mobilization of Ca2+ from intracellular stores and/or by the influx of extracellular Ca2+. Sphingolipid-induced elevation of intracellular Ca2+ concentrations has been demonstrated in various types of cultured cells (Bornfeldt et al., 1995; Ghosh et al., 1990, 1994; Gijsbers et al., 1999; Meyer zu Heringdorf et al., 1996, 1998a; Törnquist & Ekokoski, 1994). Our data extend these observations by demonstrating that SPP and SPPC can concentration-dependently elevate intracellular Ca2+ concentrations in cultured rat aortic smooth muscle cells. While the phasic component of this elevation was PTX-sensitive, the tonic component, which matches the time course of vasoconstriction more closely, was not. Hence, the findings in the mesenteric microvessels and in the isolated vascular smooth muscle cells differ with regard to SPPC potency and possibly the role of PTX-sensitive G-proteins. Thus, the Ca2+ elevations in the cultured cells may occur via a receptor subtype distinct from that in the mesenteric microvessels (see above). Nevertheless, these data are consistent with a role of sphingolipid receptor-mediated elevations of intracellular Ca2+ concentrations in vasoconstriction. The data with chelation of extracellular Ca2+ and the Ca2+-channel blocker nitrendipine indicate that SPPC-induced vasoconstriction largely relies on influx of extracellular Ca2+, which at least partly occurs via L-type Ca2+ channels.
Since the vascular endothelium can modulate smooth muscle contractility by releasing vasodilatating and vasoconstricting agents, the role of the endothelium in sphingolipid-induced vasoconstriction was investigated. For this purpose, mechanical endothelium removal as well as pharmacological inhibition of two important groups of endothelium-derived vasoactive substances, i.e. NO and cyclo-oxygenase-dependent mediators, were used. The present data demonstrate that SPPC-induced vasoconstriction is not attenuated by a concomitant release of endothelium-derived vasodilatators in mesenteric microvessels. The small inhibitory effect of indomethacin suggests that intermediary prostaglandin formation may contribute to SPPC-induced vasoconstriction. Since mechanical endothelium removal did not affect SPPC-induced vasoconstriction, such prostaglandins are unlikely to be endothelium-derived. Although the role of prostaglandins in this regard appears to be small, this observation is consistent with a previous report that SPPC can activate phospholipase A2 (Orlati et al., 1998).
Taken together, the present data for the first time demonstrate effects of SPP and SPPC in isolated tissues. These actions appear to be mediated by receptors localized on the vascular smooth muscle cells and coupled to PTX-sensitive G-proteins. Activation of these receptors causes vasoconstriction which is dependent on the influx of extracellular Ca2+, at least partly through L-type Ca2+ channels. Since SPP can be detected in plasma and serum and is released from activated platelets (Yatomi et al., 1995, 1997), we propose that SPP and possibly also SPPC belong to an hitherto unrecognized class of endogenous vasoactive agents. Hence, sphingolipid receptors may represent not only possibly pathophysiologically relevant factors but also targets for a pharmacological manipulation of the vascular system. In vivo vascular effects of SPP and SPPC are described in the accompanying paper (Bischoff et al., 2000).
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (Bi 544/2-1) and the intramural grant program of the Universitätsklinikum Essen (IFORES). We thank Dr S. Busch for providing the vascular smooth muscle cells.
Abbreviations
- Edg
endothelial differentiation gene
- GLU
glucopsychosine
- HBSS
Hank's balanced salt solution
- PSY
psychosine
- PTX
pertussis toxin
- SPH
sphingosine
- SPP
sphingosine-1-phosphate
- SPPC
sphingosylphosphorylcholine
References
- AN S., BLEU T., HUANG W., HALLMARK O.G., COUGHLIN S.R., GOETZL E.J. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–282. doi: 10.1016/s0014-5793(97)01301-x. [DOI] [PubMed] [Google Scholar]
- AN S., BLEU T., ZHENG Y. Transduction of intracellular calcium signals through G protein-mediated activation of phospholipase C by recombinant sphingosine 1-phosphate receptors. Mol. Pharmacol. 1999;55:787–794. [PubMed] [Google Scholar]
- ANCELLIN N., HLA T. Differential pharmacological properties and signal transduction of the sphingosine 1-phosphate receptors EDG-1, EDG-3, and EDG-5. J. Biol. Chem. 1999;274:18997–19002. doi: 10.1074/jbc.274.27.18997. [DOI] [PubMed] [Google Scholar]
- BISCHOFF A., CZYBORRA P., MEYER ZU HERINGDORF D., JAKOBS K.-H., MICHEL M.C. Sphingosine-1-phosphate reduces renal and mesenteric blood flow in anaesthetized rats in a pertussis toxin-sensitive manner. Br. J. Pharmacol. 2000;130:1878–1883. doi: 10.1038/sj.bjp.0703516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORNFELDT K.E., GRAVES L.M., RAINES E.W., IGARASHI Y., WAYMAN G., YAMAMURA S., YATOMI Y., SIDHU J.S., KREBS E.G., HAKOMORI S., ROSS R. Sphingosine-1.phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: Spatial and temporal modulation of PDGF chemotactic signal transduction. J. Cell Biol. 1995;130:193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÜNEMANN M., LILIOM K., BRANDTS B.K., POTT L., TSENG J.-L., DESIDERIO D.M., SUN G., MILLER D., TIGYI G. A novel membrane receptor with high affinity for lysosphingomyelin and sphingosine-1-phosphate in atrial myocytes. EMBO J. 1996;15:5527–5534. [PMC free article] [PubMed] [Google Scholar]
- CHEN H., BISCHOFF A., SCHÄFERS R.F., WAMBACH G., PHILIPP T., MICHEL M.C. Vasoconstriction of rat renal interlobar arteries by noradrenaline and neuropeptide Y. J. Auton. Pharmacol. 1997;17:137–146. doi: 10.1046/j.1365-2680.1997.00452.x. [DOI] [PubMed] [Google Scholar]
- CHEN H., FETSCHER C., SCHÄFERS R.F., WAMBACH G., PHILIPP T., MICHEL M.C. Effects of noradrenaline and neuropeptide Y on rat mesenteric microvessel contraction. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;353:314–323. doi: 10.1007/BF00168634. [DOI] [PubMed] [Google Scholar]
- GHOSH S., STRUM J.C., BELL R.M. Lipid biochemistry: functions of glycerolipids and sphingolipids in cellular signalling. FASEB J. 1997;11:45–50. doi: 10.1096/fasebj.11.1.9034165. [DOI] [PubMed] [Google Scholar]
- GHOSH T.K., BIAN J., GILL D.L. Intracellular calcium release-mediated by sphingosine derivatives generated in cells. Science. 1990;248:1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- GHOSH T.K., BIAN J., GILL D.L. Sphingosine 1-phosphate generated in the endoplasmatic reticulum membrane activates release of stored calcium. J. Biol. Chem. 1994;269:22628–22635. [PubMed] [Google Scholar]
- GIJSBERS S., MANNAERTS G.P., HIMPENS B., VAN VELDHOVEN P.P. N-Acetyl-sphingenine-1-phosphate is a potent calcium mobilizing agent. FEBS Lett. 1999;453:269–272. doi: 10.1016/s0014-5793(99)00735-8. [DOI] [PubMed] [Google Scholar]
- GOETZL E.J., AN S. Diversity of cellular receptors and functions for the lysopholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J. 1998;12:1589–1598. [PubMed] [Google Scholar]
- HLA T., LEE M.-J., ANCELLIN N., LIU C.H., THANGADA S., THOMPSON B.D., KLUK M. Sphingosine-1-phosphate:Extracellular mediator or intracellular second messenger. Biochem. Pharmacol. 1999;58:201–207. doi: 10.1016/s0006-2952(99)00086-6. [DOI] [PubMed] [Google Scholar]
- HLA T., MACIAG T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G protein-coupled receptors. J. Biol. Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- JOHNS D.G., OSBORN H., WEBB R.C. Ceramide: A novel cell signaling mechanism for vasodilation. Biochem. Biophys. Res. Commun. 1997;237:95–97. doi: 10.1006/bbrc.1997.7084. [DOI] [PubMed] [Google Scholar]
- LIU C.H., THANGADA S., LEE M.-J., VAN BROCKLYN J.R., SPIEGEL S., HLA T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER ZU HERINGDORF D., LASS H., ALEMANY R., LASER K.T., NEUMANN E., ZHANG C., SCHMIDT M., RAUEN U., JAKOBS K.H., VAN KOPPEN C.J. Sphingosine kinase-mediated Ca2+ signalling by G protein-coupled receptors. EMBO J. 1998a;17:2830–2837. doi: 10.1093/emboj/17.10.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER ZU HERINGDORF D., NIEDERDRÄING N., NEUMANN E., FRÖDE R., LASS H., VAN KOPPEN C.J., JAKOBS K.H. Discrimination between plasma membrane and intracellular target sites of sphingosylphosphorylcholine. Eur. J. Pharmacol. 1998b;354:113–122. doi: 10.1016/s0014-2999(98)00436-1. [DOI] [PubMed] [Google Scholar]
- MEYER ZU HERINGDORF D., VAN KOPPEN C.J., JAKOBS K.H. Molecular diversity of sphingolipid signalling. FEBS Lett. 1997;410:34–38. doi: 10.1016/s0014-5793(97)00320-7. [DOI] [PubMed] [Google Scholar]
- MEYER ZU HERINGDORF D., VAN KOPPEN C.J., WINDORFER B., HIMMEL H.M., JAKOBS K.H. Calcium signalling by G protein-coupled sphingolipid receptors in bovine aortic endothelial cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:397–403. doi: 10.1007/BF00168428. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A.G., COX H., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., SCHWARTZ T.W., WESTFALL T.C. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- OKAMOTO H., TAKUWA N., YATOMI Y., GONDA K., SHIGEMATSU H., TAKUWA Y. EDG3 is a functional receptor specific for sphingosine 1-phosphate and sphingosylphosphorylcholine with signalling characteristics distinct from EDG1 and AGR16. Biochem. Biophys. Res. Commun. 1999;260:203–208. doi: 10.1006/bbrc.1999.0886. [DOI] [PubMed] [Google Scholar]
- OKAZAKI H., ISHIZAKA N., SAKURAI T., KUROKAWA K., GOTO K., KUMADA M., TAKUWA Y. Molecular cloning of a novel putative G protein-coupled receptor expressed in the cardiovascular system. Biochem. Biophys. Res. Commun. 1993;190:1104–1109. doi: 10.1006/bbrc.1993.1163. [DOI] [PubMed] [Google Scholar]
- ORLATI S., PORCELLI A.M., HRELIA S., LORENZINI A., RUGOLO M. Intracellular calcium mobilization and phospholipid degradation in sphingosylphosphorylcholine-stimulated human airway epithelial cells. Biochem. J. 1998;334:641–649. doi: 10.1042/bj3340641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSKOPF D., HAIDER N., QUEDNAU B., SIFFERT W. Expression and activity of the Na+/H+exchanger NHE-1 in various tissues of spontaneously hypertensive rats and normotensive Wistar-Kyoto rats. Cell Physiol. Biochem. 1995;5:276–285. [Google Scholar]
- SPIEGEL S., MILSTIEN S. Sphingolipid metabolites: Members of a new class of lipid second messengers. J. Membrane Biol. 1995;146:225–237. doi: 10.1007/BF00233943. [DOI] [PubMed] [Google Scholar]
- TÖRNQUIST K., EKOKOSKI E. Effect of sphingosine derivatives on calcium fluxes in thyroid FRTL-5 cells. Biochem. J. 1994;299:213–218. doi: 10.1042/bj2990213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN BROCKLYN J.R., LEE M.-J., MENZELEEV R., OLIVERA A., EDSALL L., CUVILLIER O., THOMAS D.M., COOPMAN P.J.P., THANGADA S., LIU C.H., HLA T. Dual actions of sphingosine-1-phosphate:Extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN KOPPEN C.J., MEYER ZU HERINGDORF D., LASER K.T., ZHANG C., JAKOBS K.H., BÜNEMANN M., POTT L. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J. Biol. Chem. 1996;271:2082–2087. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- XIA P., GAMBLE J.R., RYE K.-A., WANG L., HII C.S.T., COCKERILL P., KHEW-GOODALL Y., BERT A.G., BARTER P.J., VADAS M.A. Tumor necrosis factor-α induces adhesion molecule expression through the sphingosine kinase pathway. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14196–14201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU Y., ZHU K., HONG G., WU W., BAUDHUIN L.M., XIAO Y., DAMRON D.S. Sphingosylphosphorylcholine is a ligand for ovarian cancer G-protein-coupled receptor 1. Nature Cell Biol. 2000;2:261–267. doi: 10.1038/35010529. [DOI] [PubMed] [Google Scholar]
- YATOMI Y., IGARASHI Y., YANG L., HISANO N., QI R., ASAZUMA N., SATOH K., OZAKI Y., KUME S. Sphingosine-1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- YATOMI Y., RUAN F., HAKOMORI S., IGARASHI Y. Sphingosine-1-phosphate: A platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- ZONDAG G.C.M., POSTMA F.R., VAN ETTEN I., VERLAAN I., MOOLENAAR W.H. Sphingosine-1-phosphate signalling through the G protein-coupled receptor Edg-1. Biochem. J. 1998;330:605–609. doi: 10.1042/bj3300605. [DOI] [PMC free article] [PubMed] [Google Scholar]


