Abstract
Sphingolipids such as sphingosine-1-phosphate (SPP) and sphingosylphosphorylcholine constrict isolated rat intrarenal and mesenteric microvessels in vitro. The present study investigates their effects on the cardiovascular system in vivo in anaesthetized rats.
The animals were given intravenous or intrarenal arterial bolus injections of sphingolipids (0.1–100 μg kg−1) with subsequent measurements of mean arterial pressure, heart rate and renal and mesenteric blood flows (RBF, MBF) using a pressure transducer and electromagnetic flow probes, respectively.
Intravenous injection of SPP rapidly (within 30 s), transiently and dose-dependently reduced RBF (maximally −4.0±0.3 ml min−1) and MBF (maximally −1.4±0.2 ml min−1), without affecting mean arterial pressure or heart rate. Other sphingolipids had no significant effect.
Intrarenal arterial SPP administration caused greater blood flow reductions (maximally −6.4±0.3 ml min−1) than systemic administration. Upon intrarenal administration, sphingosylphos- phorylcholine also lowered RBF (maximally −2.8±0.6 ml min−1), while the other sphingolipids remained without effect.
Pretreatment with pertussis toxin (PTX, 10 μg kg−1) 3 days before the acute experiment abolished the SPP-induced reductions of RBF and MBF.
These data demonstrate, that SPP is a potent vasoconstrictor in vivo, particularly in the renal vasculature, while the other structurally related sphingolipids had little if any effects. The PTX-sensitivity strongly suggests that the effects of SPP on renal and mesenteric blood flow are mediated by receptors coupled to Gi-type G-proteins.
Keywords: Sphingosine-1-phosphate, sphingosylphosphorylcholine, rat, vasoconstriction, kidney, renal blood flow
Introduction
Sphingosine-1-phosphate (SPP) has been identified in human serum and plasma, where it occurs in concentrations of 484±82 and 191±79 nmol l−1, respectively (Yatomi et al., 1997a). One source of extracellular SPP are platelets, which release the lipid upon activation (Yatomi et al., 1995; 1997a). SPP and related sphingolipids can affect the function of cultured cells in many ways via receptor-mediated and receptor-independent mechanisms (Hla et al., 1999; Meyer zu Heringdorf et al., 1997; Van Brocklyn et al., 1998). Furthermore, it has been demonstrated that SPP, sphingosylphosphorylcholine (SPPC) and other sphingolipids can constrict mesenteric and intrarenal microvessels in vitro in a concentration-dependent manner (Bischoff et al., 2000). The vasoconstrictor effect of SPPC on mesenteric microvessels was stereospecific, sensitive to pertussis toxin (PTX) and dependent on the influx of extracellular Ca2+ but not on the presence of a functionally intact endothelium (Bischoff et al., 2000). Despite a large number of recent studies of sphingolipid effects on cultured cells (Bornfeldt et al., 1995; Goetzl & An, 1998; Im et al., 1997; MacDonell et al., 1998; Mattie et al., 1994; Meyer zu Heringdorf et al., 1996; van Koppen et al., 1996), little is known about their effects in vivo, particularly with regard to the cardiovascular system. Therefore, the cardiovascular responses to SPP and other sphingolipids in anaesthetized rats in vivo have been investigated.
Methods
Animal surgery and experimental protocol for anaesthetized rat experiments
All animal studies were performed in accordance with the NIH guidelines for the care and use of laboratory animals following approval by the state animal welfare board. Male Wistar rats (strain: Hsd/Cpb:WU; 272–430 g in study 1; 300–496 g in study 2; 320–470 g in study 3) were obtained from Harlan (Borchem, Germany). In studies 1 and 2 naive rats were used. In study 3 rats were pretreated with PTX (10 μg kg−1) or its vehicle 3 days before the experiment. For this the rats were injected with PTX or its vehicle under a light ketamine anaesthesia (100 mg kg−1 i.p.) via the jugular vein. For haemodynamic measurements, the rats were prepared as previously described (Bischoff et al., 1996) with minor modifications. Briefly, the rats were anaesthetized with a single dose of thiobutabarbitone (Inactin®, 100 mg kg−1 i.p.). The animals were placed on a heating pad to maintain the body temperature at 37°C. Following tracheotomy to facilitate ventilation, the left femoral artery was cannulated for monitoring mean arterial pressure (MAP) via a Statham pressure transducer. The femoral vein was catheterized for systemic infusion and bolus injections. Following an abdominal midline incision, the connective tissue was carefully dissected from the right renal artery and the cranial mesenteric artery, and electromagnetic blood flow sensors (Skalar MDL 1401, Föhr Medical Instruments GmbH, Seeheim/Oberbeerbach, Germany) were placed on the vessels for monitoring heart rate (HR), renal blood flow (RBF), and mesenteric blood flow (MBF). In some experiments, a catheter was placed into the suprarenal artery for intrarenal bolus injections. The signals from the flow sensors and the pressure transducer were continuously recorded online using the HDAS haemodynamic data aquisition system (Dept. of Bioengineering, Rijksuniversiteit Limburg, Maastricht, The Netherlands).
Following completion of the preparation, the animals were allowed a 120 min recovery period, during which 0.9% saline was infused via the femoral vein (study 1) or femoral vein plus suprarenal artery (study 2) at a rate of 60 μl min−1. In study 3, the rats were allowed 65 min of recovery. Thereafter, the effectiveness of the PTX treatment was tested with three bolus injections of neuropeptide Y (1, 3 and 10 μg kg−1) in 5 min intervals 60 min before the start of the SPP bolus injections. The fluid substitution was started immediately after completion of the surgery and maintained until the end of the experiment except during systemic bolus injections.
Systemic (1–100 μg kg−1) and intrarenal bolus injections (0.1–100 μg kg−1) of sphingolipids were administered in a volume of 100 μl per 100 g body weight and injected within 30 s. Bolus injections were given in 40 min intervals in studies 1 and 2 and in 3 min intervals in study 3. During the experimental period, MAP, HR, RBF and MBF were monitored every min from 3 min before until 10 min (studies 1 and 2) or 3 min (study 3) after the bolus injection. The maximum response to sphingolipid injection occurred within 20 s. At the end of the experiment, the rats were killed with an overdose of anaesthetic.
Data analysis
The averages of the haemodynamic parameters during the last 3 min before the first bolus injection were taken as baseline values (see Results); basal values remained stable throughout the experiments (data not shown). All other data are expressed as alterations relative to the baseline values. Data are shown as mean±s.e.mean for n experiments. Statistical significance of differences was determined by unpaired two-tailed t-tests when two groups were compared, e.g. for baseline values. When multiple groups were compared, one-way analysis of variance followed by a multiple comparison-corrected Dunnett test was performed. Statistical significance of the overall treatment effect (time or dose) relative to control animals was determined by a two-way analysis of variance. All statistical calculations were performed with the Prism program (GraphPAD Software, San Diego, CA, U.S.A.) and P<0.05 was considered significant.
Chemicals
SPP and SPPC were obtained from Biomol (Hamburg, Germany); sphingosine and glucopsychosine were obtained from Matreya (Bad Homburg, Germany). For all studies, sphingolipids were dissolved in methanol, dried in a SpeedVac concentrator and redissolved in 1 mg ml−1 bovine serum albumine (BSA). Thiobutabarbitone (Inactin®) was obtained from RBI (Natick, MA, U.S.A.), and PTX was from ICN (Eschwege, Germany). PTX was dissolved in a phosphate buffer (mol l−1): 0.5 NaCl, 0.1 NaH2PO4 and 0.1 Na2HPO4; this buffer served as vehicle.
Results
In study 1, the haemodynamic effects of systemically administered SPP, SPPC, sphingosine and glucopsychosine were tested. At the end of the equilibration period, basal MAP, HR, RBF and MBF were 112±3 mmHg, 353±6 b.p.m., 7.8±0.3 ml min−1 and 9.0±0.3 ml min−1, respectively (n=31 each). During constant saline infusion, these values remained stable throughout the experimental period (data not shown). Five groups of 4–8 rats received nine systemic bolus injections (1, 3, 5, 10, 20, 40, 60, 80 and 100 μg kg−1) of either SPP, SPPC, sphingosine, glucopsychosine or their vehicle (BSA 1 mg ml−1). Intravenous bolus injections of SPP caused rapid, transient (Figure 1) and dose-dependent (Figure 2) reductions of RBF and MBF by up to 50 and 16%, respectively (in the time course experiments P<0.01 for SPP vs vehicle at the 30 s time point for either RBF or MBF; in the dose-response experiments P<0.01 for the overall treatment effect of SPP vs vehicle for either RBF or MBF). Half-maximal and maximal reductions in both RBF and MBF were seen at about 5 and 60 μg kg−1 of SPP, respectively (Figure 2). The reductions occurred immediately after termination of the injection, and were reversible within the next minute and were not accompanied by detectable alterations of MAP or HR (Figure 3). In contrast to SPP, intravenous injections of SPPC, sphingosine or glucopsychosine in doses up to 100 μg kg−1 or vehicle did not alter RBF or MBF (Figures 1 and 2).
Figure 1.
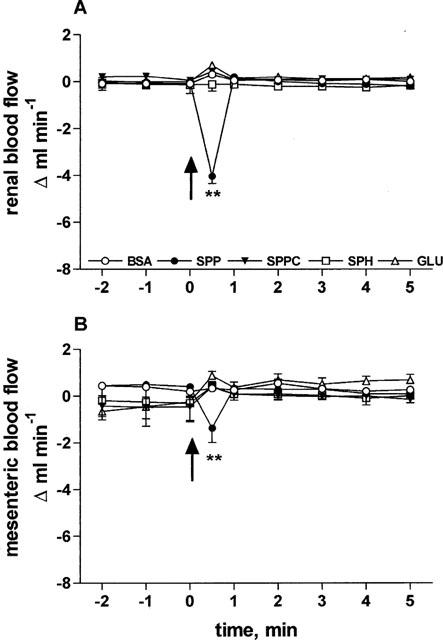
Effects of 100 μg kg−1 intravenously injected sphingolipids on RBF and MBF in anaesthetized rats. Data are mean±s.e.mean (n=4–8) of alterations relative to baseline values. Start of the bolus injections was indicated by an arrow. RBF (A) and MBF (B) were rapidly and transiently reduced by injection of 100 μg kg−1 of SPP (4.0±0.3 and 1.4±0.2 ml min−1, respectively, ** P<0.01 in a one-way analysis of variance). BSA, SPPC, sphingosine (SPH) or glucopsychosine (GLU) had no effect.
Figure 2.
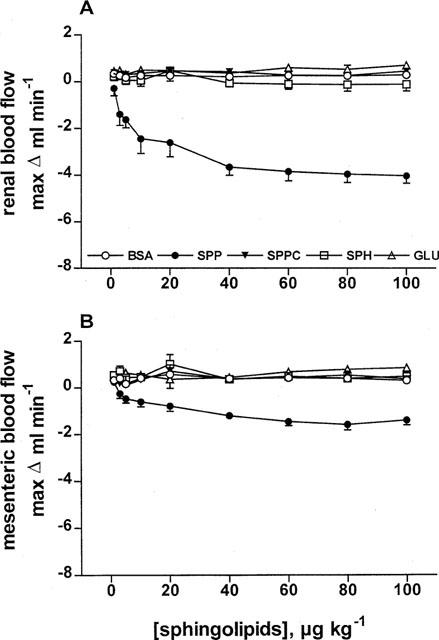
Dose-response curves of intravenously injected sphingolipids on RBF and MBF in anaesthetized rats. Data are mean±s.e.mean (n=4–8) of alterations relative to baseline values. RBF (A) and MBF (B) were dose-dependently reduced by SPP (P<0.0001 in a two-way analysis of variance) but not by BSA, SPPC, sphingosine (SPH) or glucopsychosine (GLU).
Figure 3.
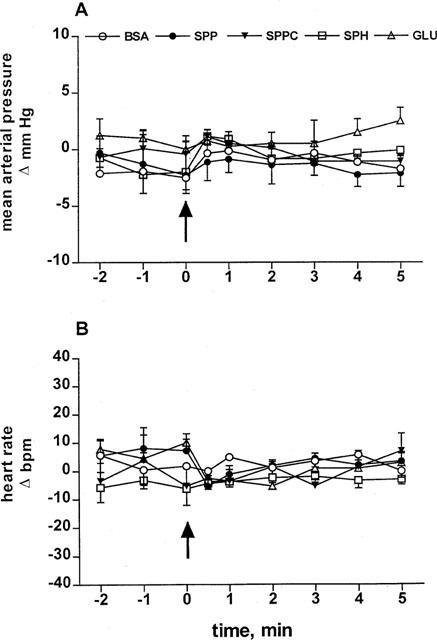
Effects of 100 μg kg−1 intravenously injected sphingolipids on MAP and HR in anaesthetized rats. Data are mean±s.e.mean (n=4–8) of alterations relative to baseline values. Start of the bolus injections was indicated by an arrow. Injection of BSA, SPP, SPPC, sphingosine (SPH) or glucopsychosine (GLU) did not detectably alter MAP (A) or HR (B) up to 100 μg kg−1.
To test whether the lack of effect of SPPC, sphingosine and glucopsychosine was related to dilution in the systemic circulation, the sphingolipids were administered intrarenally in study 2. Despite the more extensive preparation of the rats, basal values of MAP (113±3 mmHg), HR (345±6 b.p.m.), RBF (7.2±0.3 ml min−1), and MBF (8.3±0.5 ml min−1; n=25 each) were very similar to the values in study 1 and remained stable throughout the experiment. Four groups of 5–7 rats received seven intrarenal bolus injections of sphingolipids (0.1, 0.3, 1, 3, 10, 30 and 100 μg kg−1). Intrarenal injection of SPP rapidly, transiently (Figure 4) and dose-dependently (Figure 5) lowered RBF and MBF (in the time course experiments P<0.01 for SPP and SPPC vs vehicle at the 30 s time point for RBF and for SPP vs vehicle for MBF; in the dose-response experiments P<0.01 for the overall treatment effect of SPP vs vehicle for either RBF or MBF). In comparison with the effects of systemic injection, maximal reductions of RBF lasted longer, were almost twice as large (maximum reduction 4.0±0.3 vs 6.4±0.3 ml min−1, P=0.0001) and appeared to occur with smaller half-maximally effective SPP doses (approximately 0.3 μg kg−1, Figure 5). Upon intrarenal administration, RBF reductions also became detectable for SPPC, while sphingosine and BSA remained without effect (Figure 4). None of the sphingolipids affected MAP or HR upon intrarenal administration (data not shown).
Figure 4.
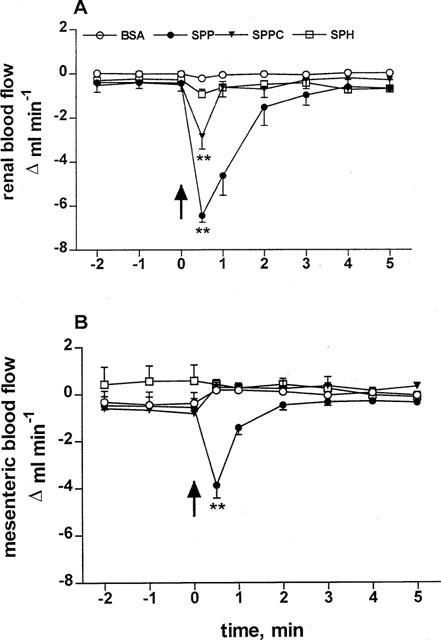
Effects of 100 μg kg−1 intrarenally injected sphingolipids on RBF and MBF in anaesthetized rats. Data are mean±s.e.mean (n=5–7) of alterations relative to baseline values. Start of the bolus injections was indicated by an arrow. RBF (A) and MBF (B) were rapidly and transiently reduced by injection of SPP (**P<0.01 in a one-way analysis of variance); the blood flow reduction was more pronounced than upon intravenous injection. SPPC also reduced RBF but not MBF to a lower extent compared to SPP (**P<0.01 in a one-way analysis of variance). BSA and sphingosine (SPH) had no effects.
Figure 5.
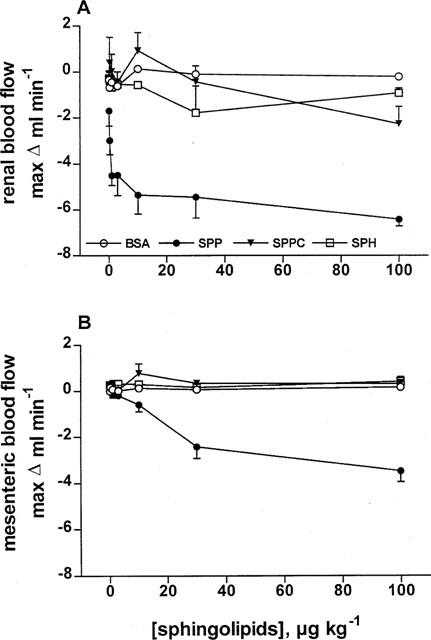
Dose-response curves of intrarenally injected sphingolipids on RBF and MBF in anaesthetized rats. Data are mean±s.e.mean (n=5–7) of alterations relative to baseline values. RBF (A) and MBF (B) were dose-dependently reduced by SPP (P<0.0001 in a two-way analysis of variance) but not by BSA or sphingosine (SPH). SPPC had a small inhibitory effect at 100 μg kg−1.
In study 3, two groups of rats (n=5–6 each) were investigated, one pretreated with vehicle and the other pretreated with PTX (10 μg kg−1) 3 days before the acute experiment. Pretreatment with PTX did not significantly alter basal values of MAP (101±10 vs 98±4 mmHg), HR (374±8 vs 359±11 b.p.m.), RBF (6.2±0.2 vs 7.2±0.5 ml min−1) or MBF (5.5±0.9 vs 4.7±1.0 ml min−1). On the other hand, reduction of RBF caused by 10 μg kg−1 neuropeptide Y was abolished in PTX-treated rats (1.9±0.4 vs 0.3±0.1 ml min−1, n=5–6, P=0.0022). SPP administered in seven consecutive systemic bolus injections (1, 3, 5, 10, 20, 30 and 100 μg kg−1) via the femoral vein, did not markedly alter MAP (Figure 6A) or HR (data not shown) in vehicle- and PTX-pretreated rats and dose-dependently reduced RBF and MBF in vehicle-treated control rats (P<0.0001 for SPP vs vehicle for RBF and MBF; Figure 6B,C). In contrast, in PTX-pretreated rats SPP was without effects on RBF and MBF at any studied dose. To demonstrate the lack of non-specific effects of the experimental conditions of PTX treatment on vascular reactivity, a second group of rats was treated with the same PTX dose or vehicle (n=6 each). Following equilibration these rats received intravenous bolus doses of methoxamine (40 μg kg−1) and thereafter angiotensin II (1 μg kg−1). Methoxamine and angiotensin II-induced elevations of MAP (15±4 vs 18±2 mmHg and 48±8 vs 35±6 mmHg, respectively) and reductions of RBF (1.0±0.1 vs 1.0±0.1 ml min−1 and 3.6±0.5 vs 3.6±0.6 ml min−1, respectively) were not significantly altered by PTX treatment.
Figure 6.
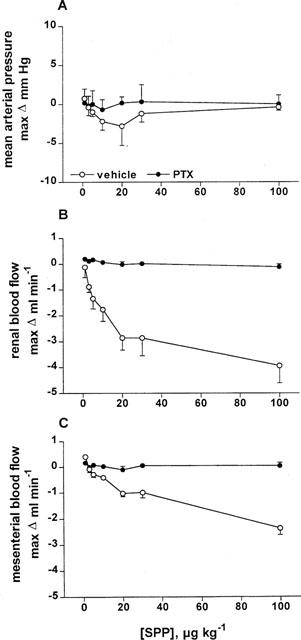
Dose-response curves of systemically injected SPP on MAP, RBF and MBF in PTX- and vehicle-treated anaesthetized rats. Data are mean±s.e.mean (n=5–6) of alterations relative to baseline values. SPP did not alter MAP (A), but dose-dependently reduced RBF (B) and MBF (C) in vehicle- but not in PTX-treated rats (P<0.0001 in a two-way analysis of variance).
Discussion
The sphingolipid SPP is stored in and released from human platelets (Yatomi et al., 1995; 1997a), suggesting that it may be a biologically active agent in the cardiovascular system. Sphingolipid effects have been studied in various types of cultured cells including those related to the cardiovascular system. In cultured rat cardiomyocytes, for example, sphingolipids such as sphingosine modulate Na+ and Ca2+ currents (Yasui & Palade, 1996) and tumour necrosis factor-induced negative inotropic effects (Oral et al., 1997). Furthermore, Bünemann et al. (1995) observed a SPP-induced activation of muscarinic K+ current in guinea-pig atrial myocytes. However, very little is known about the effects of sphingolipids on the cardiovascular system in vivo. It has also been reported that sphingolipids, including SPP and SPPC, cause vascoconstriction in rat isolated mesenteric and intrarenal microvessels; these in vitro effects were dose-dependent, stereospecific and PTX-sensitive, suggesting a mediation via receptors coupled to Gi-type G-proteins (Bischoff et al., 2000).
In the present study, systemic and intrarenal bolus injections of SPP dose-dependently reduced RBF and, to a smaller extent, MBF in anaesthetized rats. On the other hand, MAP and HR remained unaffected by any tested sphingolipid, indicating a preferential sphingolipid effect on the vasculature rather than the heart. The lack of MAP and HR alterations despite RBF and MBF reductions could be explained haemodynamically by a reduced resistance in other vascular beds and/or lowered cardiac output upon SPP administration; the latter could result from a reduced HR and/or a reduced stroke volume. A reduced HR cannot explain the lack of MAP change, since HR was unaltered in the present study and was even reported to be increased in a recent study with an isolated canine heart preparation. A reduced stroke volume, and hence a reduced cardiac output, should be considered, since SPP was a reported cause of coronary vasoconstriction and mild negative inotropic effects in an isolated canine heart preparation (Sugiyama et al., 2000). SPP was also reported to activate the muscarinic K+ current in guinea-pig atrial cardiomyocytes (Bünemann et al., 1995). Moreover, SPPC and sphingosine were reported to inhibit Na+ and Ca2+ channels in cardiomyocytes isolated from adult rats (Yasui & Palade, 1996), and sphingosine was reported to cause negative inotropy in isolated feline cardiomyocytes (Oral et al., 1997). Taken together, these data make a reduced cardiac output even with unchanged HR a likely cause of unaltered MAP, despite lowered RBF and MBF. However, the existence of SPP-induced vasodilatation in some vascular beds cannot be excluded, based on the present data, as an additional and/or alternative cause for unchanged MAP values upon SPP infusion. Moreover, differences between in vitro and in vivo studies should be considered when taking the above in vitro data on cardiac function into consideration as an explanation for the present in vivo findings.
The detectable SPP-induced alterations of local blood flows indicate that sphingolipids can cause vasoconstriction not only in vitro (Bischoff et al., 2000) but also in vivo. The pronounced SPP-induced RBF reduction may be surprising in light of the known autoregulation of RBF, but a similar situation has been described for another receptor coupling to PTX-sensitive G-proteins, the neuropeptide Y receptor (Bischoff et al., 1996). The direct effect of SPP on the renal vasculature was further demonstrated by enhanced RBF lowering upon direct intrarenal SPP administration. Upon intrarenal administration, RBF reduction by SPPC also became detectable, which was not seen upon systemic treatment.
SPP and other sphingolipids are bioactive molecules that can act on signal transduction pathways as second messengers and also as ligands at G-protein-coupled receptors of the Edg-family (Goetzl & An, 1998; Hla et al., 1999; Van Brocklyn et al., 1998). Many of the Edg receptor-mediated sphingolipid actions, in contrast to the intracellular effects of these agents, are dependent on PTX-sensitive Gi-type G-proteins (An et al., 1997; 1999; Goetzl & An, 1998; Hla et al., 1999; Liu et al., 1999; Meyer zu Heringdorf et al., 1997; Okamoto et al., 1999; Van Brocklyn et al., 1998; Zondag et al., 1998). To determine whether the in vivo effects of SPP are receptor-mediated, their sensitivity to PTX have been tested. The experimental conditions of in vivo PTX treatment were effective since they abolished the vasoconstriction by neuropeptide Y, which acts via receptors coupled to PTX-sensitive G-proteins (Michel et al., 1998), but did not cause obvious toxic effects since they did not affect vascular effects of angiotensin II or the α1-adrenoceptor agonist methoxamine. Pretreatment with PTX abolished SPP-induced vasoconstriction in anaesthetized rats. As PTX treatment also markedly attenuated the contractile effects of sphingolipids on microvessels in vitro (Bischoff et al., 2000), these data strongly suggest that sphingolipid-induced vasoconstriction both in vivo and in vitro is apparently mediated by receptors coupled to Gi-type G-proteins. Possible candidates are receptors belonging to the Edg- receptor family which are activated by SPP and SPPC. While Edg-receptors have been detected on cultured cells of cardiovascular origin and in several rat tissues (Yatomi et al., 1997b), to the best of our knowledge no data exist on Edg-receptor distribution in blood vessels.
Despite the similarities between sphingolipid-induced vasoconstriction in vitro (Bischoff et al., 2000) and in vivo (present study), several differences have also been noticed. First, sphingolipids had greater effects on RBF than on MBF in vivo but caused stronger vasoconstriction in mesenteric than in intrarenal microvessels. However, this difference should not be overinterpreted, since the physiological regulation of RBF is complex and the intralobar arteries used in the in vitro experiments represent only a part of the intrarenal resistance vasculature. More important, the order of agonist potency differed between the in vitro and the in vivo studies. In vivo, SPP had the greatest effects, SPPC was less active, and the other tested sphingolipids were inactive. In contrast, in vitro SPPC had the greatest effects, SPP was less active, and sphingosine and glucopsychosine had small but detectable effects (Bischoff et al., 2000). One possible explanation for these differences might be a rapid degradation of SPPC and sphingosine in vivo, e.g. by a bloodborn esterase. On the other hand, it should be noted that the observed in vivo effects are in line with findings on sphingolipid responses in various types of cultured cells where SPP usually is the most potent agonist (Meyer zu Heringdorf et al., 1996; van Koppen et al., 1996). The development of selective and chemically stable agonists and/or antagonists for sphingolipid receptors will hopefully allow to resolve these discrepancies.
Taken together, the present data demonstrate that the sphingolipid SPP reduces RBF and, to a smaller extent, MBF in anaesthetized rats. This effect appears to occur via receptors coupled to Gi-type G-proteins. To the best of our knowledge, this is the first report on sphingolipid action via Gi-protein-coupled receptors in vivo and identifies sphingolipids as a novel endogenous regulator of cardiovascular function.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (Bi 544/2-1) and the intramural grant program of the Universitätsklinikum Essen (IFORES).
Abbreviations
- BSA
bovine serum albumine
- HR
heart rate
- MAP
mean arterial pressure
- MBF
mesenteric blood flow
- PTX
pertussis toxin
- RBF
renal blood flow
- SPP
sphingosine-1-phosphate
- SPPC
sphingosylphosphorylcholine
References
- AN S., BLEU T., HUANG W., HALLMARK O.G., COUGHLIN S.R., GOETZL E.J. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–282. doi: 10.1016/s0014-5793(97)01301-x. [DOI] [PubMed] [Google Scholar]
- AN S., BLEU T., ZHENG Y. Transduction of intracellular calcium signals through G protein-mediated activation of phospholipase C by recombinant sphingosine 1-phosphate receptors. Mol. Pharmacol. 1999;55:787–794. [PubMed] [Google Scholar]
- BISCHOFF A., CZYBORRA P., FETSCHER C., MEYER ZU HERINGDORF D., JAKOBS K.-H., MICHEL M.C. Sphingosine-1-phosphate and sphingosylphosphorycholine constrict renal and mesenterial microvessels in vitro. Br. J. Pharmacol. 2000;130:1871–1877. doi: 10.1038/sj.bjp.0703515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCHOFF A., ERDBRÜGGER W., SMITS J., MICHEL M.C. Neuropeptide Y-enhanced diuresis and natriuresis in anaesthetized rats is independent of renal blood flow reduction. J. Physiol. 1996;495:525–534. doi: 10.1113/jphysiol.1996.sp021612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORNFELDT K.E., GRAVES L.M., RAINES E.W., IGARASHI Y., WAYMAN G., YAMAMURA S., YATOMI Y., SIDHU J.S., KREBS E.G., HAKOMORI S., ROSS R. Sphingosine-1 phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: Spatial and temporal modulation of PDGF chemotactic signal transduction. J. Cell Biol. 1995;130:193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÜNEMANN M., BRANDTS B., MEYER ZU HERINGDORF D., VAN KOPPEN C.J., JAKOBS K.H., POTT L. Activation of muscarinic K current in guinea pig atrial myocytes by sphingosine-1-phosphate. J. Physiol. 1995;489:701–707. doi: 10.1113/jphysiol.1995.sp021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOETZL E.J., AN S. Diversity of cellular receptors and functions for the lysopholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J. 1998;12:1589–1598. [PubMed] [Google Scholar]
- HLA T., LEE M.-J., ANCELLIN N., LIU C.H., THANGADA S., THOMPSON B.D., KLUK M. Sphingosine-1-phosphate:Extracellular mediator or intracellular second messenger. Biochem. Pharmacol. 1999;58:201–207. doi: 10.1016/s0006-2952(99)00086-6. [DOI] [PubMed] [Google Scholar]
- IM D.-S., FUJIOKA T., KATADA T., KONDO Y., UI M., OKAJIMA F. Characterization of sphingosine-1-phosphate-induced actions and its signalling pathways in rat hepatocytes. Am. J. Physiol. 1997;272:G1091–G1099. doi: 10.1152/ajpgi.1997.272.5.G1091. [DOI] [PubMed] [Google Scholar]
- LIU C.H., THANGADA S., LEE M.-J., VAN BROCKLYN J.R., SPIEGEL S., HLA T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONELL K.L., SEVERSON D.L., GILES W.R. Depression of excitability by sphingosine 1-phosphate in rat ventricular myocytes. AJP. 1998;275:H2291–H2299. doi: 10.1152/ajpheart.1998.275.6.H2291. [DOI] [PubMed] [Google Scholar]
- MATTIE M., BROOKER G., SPIEGEL S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J. Biol. Chem. 1994;269:3181–3188. [PubMed] [Google Scholar]
- MEYER ZU HERINGDORF D., VAN KOPPEN C.J., JAKOBS K.H. Molecular diversity of sphingolipid signalling. FEBS Lett. 1997;410:34–38. doi: 10.1016/s0014-5793(97)00320-7. [DOI] [PubMed] [Google Scholar]
- MEYER ZU HERINGDORF D., VAN KOPPEN C.J., WINDORFER B., HIMMEL H.M., JAKOBS K.H. Calcium signalling by G protein-coupled sphingolipid receptors in bovine aortic endothelial cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:397–403. doi: 10.1007/BF00168428. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A.G., COX H., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., SCHWARTZ T.W., WESTFALL T.C. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- OKAMOTO H., TAKUWA N., YATOMI Y., GONDA K., SHIGEMATSU H., TAKUWA Y. EDG3 is a functional receptor specific for sphingosine 1-phosphate and sphingosylphosphorylcholine with signalling characteristics distinct from EDG1 and AGR16. Biochem. Biophys. Res. Commun. 1999;260:203–208. doi: 10.1006/bbrc.1999.0886. [DOI] [PubMed] [Google Scholar]
- ORAL H., DORN G.W. , II, MANN D.L. Sphingosine mediates the immediate negative inotropic effects of tumor necrosis factor-α in the adult mammalian cardiac myocyte. J. Biol. Chem. 1997;272:4836–4842. doi: 10.1074/jbc.272.8.4836. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA F., YATOMI Y., OZAKI Y., HASHIMOTO K. Sphingosine 1-phosphate induces sinus tachycardia and coronary vasoconstriction in the canine heart. Cardiovasc. Res. 2000;46:119–125. doi: 10.1016/s0008-6363(00)00013-4. [DOI] [PubMed] [Google Scholar]
- VAN BROCKLYN J.R., LEE M.-J., MENZELEEV R., OLIVERA A., EDSALL L., CUVILLIER O., THOMAS D.M., COOPMAN P.J.P., THANGADA S., LIU C.H., HLA T. Dual actions of sphingosine-1-phosphate: Extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN KOPPEN C.J., MEYER ZU HERINGDORF D., LASER K.T., ZHANG C., JAKOBS K.H., BÜNEMANN M., POTT L. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J. Biol. Chem. 1996;271:2082–2087. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- YASUI K., PALADE P. Sphingolipid actions on sodium and calcium currents of rat ventricular myocyts. AJP. 1996;270:C645–C964. doi: 10.1152/ajpcell.1996.270.2.C645. [DOI] [PubMed] [Google Scholar]
- YATOMI Y., IGARASHI Y., YANG L., HISANO N., QI R., ASAZUMA N., SATOH K., OZAKI Y., KUME S. Sphingosine-1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. 1997a;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- YATOMI Y., RUAN F., HAKOMORI S., IGARASHI Y. Sphingosine-1-phosphate: A platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- YATOMI Y., WELCH R.J., IGARASHI Y. Distribution of sphingosine-1-phosphate, a bioactive sphingolipid, in rat tissues. FEBS Lett. 1997b;404:173–174. doi: 10.1016/s0014-5793(97)00121-x. [DOI] [PubMed] [Google Scholar]
- ZONDAG G.C.M., POSTMA F.R., VAN ETTEN I., VERLAAN I., MOOLENAAR W.H. Sphingosine-1-phosphate signalling through the G protein-coupled receptor Edg-1. Biochem. J. 1998;330:605–609. doi: 10.1042/bj3300605. [DOI] [PMC free article] [PubMed] [Google Scholar]


