Abstract
D-pinitol (3-O-methyl-chiroinositol), an active principle of the traditional antidiabetic plant Bougainvillea spectabilis, is claimed to exert insulin-like effects. This study investigates the effect of D-pinitol on glucose homeostasis in animal models of diabetes, and on glucose transport by cultured muscle cells.
Plasma glucose concentrations were measured in normal, obese-diabetic (ob/ob) and streptozotocin (STZ)-diabetic mice after oral (p.o.) and intraperitoneal (i.p.) administration of D-pinitol. Glucose transport was measured in L6 rat muscle cells by 2-deoxyglucose (2DG) uptake.
In STZ-diabetic mice, 100 mg kg−1 p.o. D-pinitol acutely decreased the hyperglycaemia (by 22% at 6 h). A similar decrease in plasma glucose (by 21%) was observed after 100 mg kg−1 i.p. D-pinitol. Insulin concentrations and the rate of insulin-induced (1 unit kg−1 actrapid i.p.) glucose disappearance were not altered by 100 mg kg−1 p.o. D-pinitol. Chronic administration of D-pinitol (100 mg kg−1 i.p. twice daily for 11 days) to STZ-diabetic mice maintained a reduction in plasma glucose concentrations from about 14 to 10 mmol l−1.
In normal non-diabetic and severely insulin resistant ob/ob mice, 100 mg kg−1 p.o. D-pinitol did not significantly affect plasma glucose or insulin during acute studies.
Incubation of L6 muscle cells with D-pinitol (10−3 M) increased basal 2DG uptake by 41% after 10 min and by 34% after 4 h. The effect of D-pinitol was inhibited by the phosphatidylinositol 3-kinase inhibitor LY294002. D-pinitol did not increase insulin-stimulated 2DG uptake by L6 cells.
The data support the view that D-pinitol can exert an insulin-like effect to improve glycaemic control in hypoinsulinaemic STZ-diabetic mice. D-pinitol may act via a post-receptor pathway of insulin action affecting glucose uptake.
Keywords: D-pinitol, insulin action, glucose transport, muscle L6 cells, streptozotocin-diabetic mice, obese-diabetic ob/ob mice, antidiabetic agent
Introduction
D-chiroinositol is structurally related to the phosphatidylinositol phosphates which participate in the insulin signalling pathways that stimulate glucose transport (Holman & Kasuga, 1997; White, 1997). Reduced urinary excretion of D-chiroinositol has been observed in rhesus monkeys and human subjects with impaired glucose tolerance, insulin resistance and type 2 diabetes mellitus (Kennington et al., 1990; Ortmeyer et al., 1993a; Suzuki et al., 1994). Acute administration of D-chiroinositol reduced plasma glucose concentrations in streptozotocin (STZ)-diabetic rats, and increased glucose utilization in insulin-resistant monkeys (Ortmeyer et al., 1993b; Fonteles et al., 1996). D-chiroinositol also improved glucose tolerance in normal rats and increased glycogenesis in diaphragm (Ortmeyer et al., 1993b; Huang et al., 1993).
The leaves of Bougainvillea spectabilis are used as a traditional treatment for diabetes in Asia and the West Indies (Narayanan et al., 1987). D-pinitol (1D-3-0-methyl-chiroinositol; Figure 1), a 3-methoxy analogue of D-chiroinositol, has been identified as an active principle, and is reported to reduce glucose concentrations in alloxan diabetic rats (Narayanan et al., 1987). Since this observation has not been confirmed or evaluated from a potential therapeutic perspective, the present study has investigated the effect of D-pinitol on blood glucose homeostasis in normal mice, hypoinsulinaemic STZ-diabetic mice and hyperinsulinaemic obese-diabetic ob/ob mice. The study has also examined the effect of D-pinitol on glucose transport by cultured L6 muscle cells.
Figure 1.
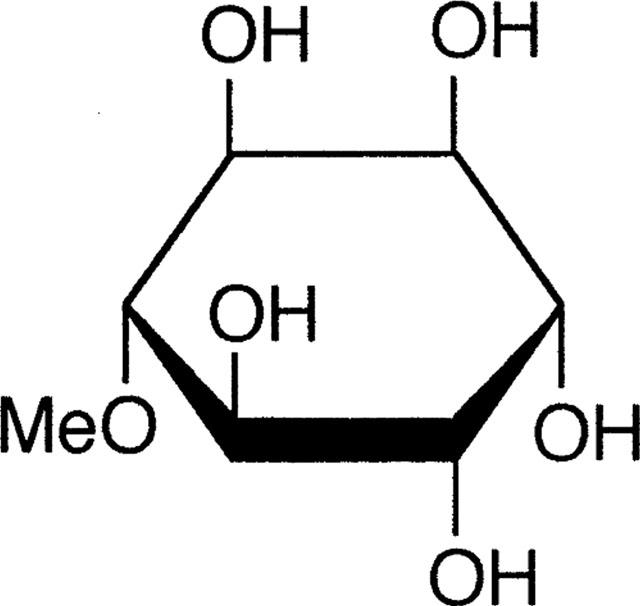
Structure of D-pinitol (1 D-3-O-methyl-chiroinositol).
Methods
The chemicals and their sources were: D-pinitol from Aldrich, Gillingham, U.K; human actrapid insulin from Novo Nordisk, Crawley, U.K.; 2-deoxy-D-[3H]-glucose (15.0 Ci mmol−1) from Amersham International, Amersham, U.K.; Hi-safe II scintillant from Fisons, Loughborough, U.K.; cell culture reagents from Gibco, Paisley, U.K.; other chemicals from Sigma, Poole, U.K. and BDH, Poole, U.K. Plastic ware was from Sarstedt, Leicester, U.K.
Animals
Obese-diabetic ob/ob mice and normal homozygous lean +/+ mice from the Aston colony were used at 12–16 weeks of age. The origin and characteristics of these mice have been described previously (Flatt & Bailey, 1981; Bailey et al., 1982). Mice were housed in an air-conditioned room at 22±2°C with a lighting schedule of 12 h light (0800–2000 h) and 12 h dark. A standard pellet diet (Economy Rodent Breeding Diet, SDS, Witham, Essex, U.K.) and tap water were provided ad libitum. Hypoinsulinaemic diabetes was induced in 5 h fasted lean mice by intraperitoneal (i.p.) injection of STZ 160 mg kg−1 in citrate buffer, pH 4.5. Food was returned 4 h after injection of STZ, and animals were accepted as diabetic if the basal plasma glucose concentration was >12 mmol l−1 after 9 days. For the 62 STZ-diabetic mice used in the present study the plasma glucose and insulin values (mean±s.e.mean) were 19.8±1.5 mmol l−1 and 160±51 pmol l−1. Values in the control non-diabetic state were 7.2±0.5 mmol l−1 and 283±33 pmol l−1.
Procedures with animals
D-pinitol was dissolved in phosphate buffered saline for oral (p.o.) administration by gavage, and by i.p. injection. The acute effect of D-pinitol p.o. was determined in STZ-diabetic and obese-diabetic ob/ob mice. D-pinitol was administered at doses up to 100 mg kg−1, and controls received vehicle only (5 ml kg−1). Blood samples (20 μl) were taken from the tail tip at intervals up to 6 h. Food was withheld for the duration of all acute tests. An insulin hypoglycaemia test was undertaken in STZ-diabetic mice 6 h after administration of D-pinitol (100 mg kg−1 p.o.). Human actrapid (1 u kg−1) was administered by i.p. injection and blood samples were taken at intervals up to 2 h thereafter. The acute effect of D-pinitol (5 and 100 mg kg−1 p.o.) was also studied in normal lean non-diabetic mice, and blood samples were taken up to 4 h. An i.p. glucose challenge was then given (2 g kg−1 glucose in a 40% w v−1 solution) and blood samples were taken up to 90 min thereafter. The acute effect of D-pinitol (100 mg kg−1) administered i.p. was studied over 6 h in STZ-diabetic mice.
The chronic effect of D-pinitol (100 mg kg−1 i.p. twice daily) was determined in STZ-diabetic mice. Mice were studied for 11 days on D-pinitol treatment, and for 10 days after D-pinitol treatment was stopped. A control group received injections of vehicle only. Blood samples were taken for plasma glucose analysis, and body weight and food intake were noted at intervals.
Analyses
Plasma glucose was measured by an automated glucose oxidase procedure (Stevens, 1971), and insulin was measured by radioimmunoassay using an Amerlex magnetic separation procedure (Amersham Life Science, Amersham, U.K.) with rat insulin as standard.
L6 muscle cells
Cultured L6 muscle cells (Yaffe, 1968) from the European Culture Collection, Porton Down, U.K. were grown as monolayers in Dulbecco's modified Eagle's medium (DMEM) containing 1 mM glutamate, 1 mM pyruvate and 25 mM glucose. The medium was supplemented with 5% foetal calf serum (FCS), 100 u ml−1 penicillin G, 100 μg ml−1 streptomycin and 25 μg ml−1 amphotericin B. Cells were maintained at 37°C with humidified 95% air and 5% CO2. Experiments were undertaken in 24-well plates seeded from pre-confluent flasks with 5×105 cells in 1 ml. The cells (passage 10) were grown to confluence and the medium was changed to DMEM containing 0.5% FCS and 2.5 mM glucose for 24 h to induce differentiation and fusion of myoblasts into myotubes and preclude further division (Poyner et al., 1992). Myotubes were then incubated with test substances (bovine insulin 10−8 M and/or D-pinitol 10−6–10−3 M and/or LY294002 10−5 M) for 10 min, 4 h or 24 h.
After exposure to test substances for incubations of 10 min to 24 h, uptake of 2-deoxyglucose (2DG) was determined during an additional incubation period of 10 min. The monolayers of myotubes were washed with glucose-free Krebs Ringer bicarbonate (KRB) buffer at 22°C, then incubated in 1 ml of this buffer supplemented with 0.1 mM 2-deoxy-D-glucose and 2-deoxy-D-[3H]-glucose (0.2 μCi ml−1) for 10 min at 22°C. Buffer was aspirated, and cells were washed twice with ice-cold KRB buffer, lysed with 0.5 ml 1 M NaOH, and 3H was counted in 5 ml Hi-safe II scintillant using a Packard 1900TR liquid scintillation counter. Most experiments were undertaken three times in multiples of six wells (ie. n=18). Six control wells and six wells treated with 10−8 M insulin were included in each 24 well plate. Uptake of 2DG was expressed as the percentage change compared with control (100%).
Statistical analyses
Data are presented as mean±s.e.mean. Treatment groups for animal studies were compared by ANOVA and data at individual time points were compared to time zero by Student's paired t-test. Comparisons between groups at the same time points, and between tissue culture treatments were made using Student's unpaired t-test with Bonferroni's correction. Probability values of P<0.05 were considered to be significant.
Results
STZ-diabetic mice
Acute oral administration of D-pinitol (5, 10 and 100 mg kg−1) to STZ-diabetic mice produced a progressive decrease in plasma glucose over 6 h (Figure 2). The greatest effect was observed with a dose of 100 mg kg−1 D-pinitol which significantly decreased plasma glucose by 12% (P<0.05) at 2 h and 22% (P<0.02) at 6 h. The plasma insulin concentration was unaltered by D-pinitol treatment (e.g. 168±34 and 153±17 pmol l−1, mean±s.e.mean, n=10, pretreatment and 6 h after 100 mg kg−1 p.o. D-pinitol respectively). Since 100 mg kg−1 D-pinitol produced the greatest glucose-lowering effect over 6 h, this dose was used for subsequent studies. To investigate the effect of D-pinitol on insulin sensitivity in STZ-diabetic mice, D-pinitol (100 mg kg−1 p.o.) was administered as above. After 6 h, when plasma glucose was decreased by 20% (P<0.05; Figure 3), insulin (1 u kg−1 i.p.) was injected. The rate of glucose disappearance over the 40 min after insulin injection was similar in the D-pinitol (1.1±0.2% per min) and control (1.3±0.2% per min) groups (Figure 3). Acute i.p. administration of D-pinitol (100 mg kg−1) to STZ-diabetic mice decreased plasma glucose at 6 h by 21% (P<0.05), similar to the decrease produced by oral administration of the same dose of D-pinitol.
Figure 2.
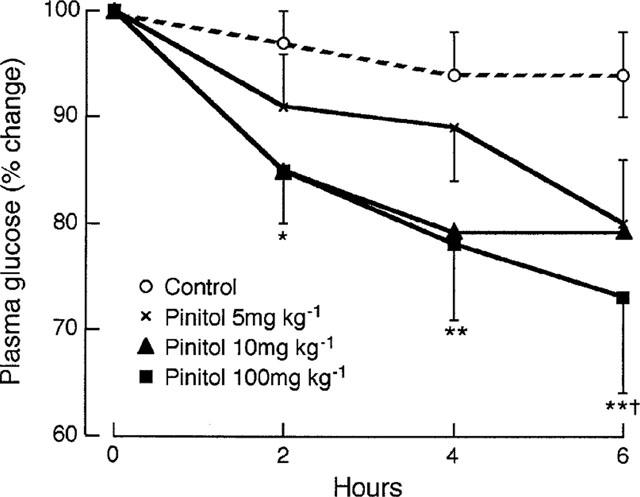
Acute effect of D-pinitol (5, 10 and 100 mg kg−1 p.o.) on plasma glucose in STZ-diabetic mice. Data are expressed as per cent change from time zero, mean±s.e.mean, n=10. Plasma glucose concentrations (mmol l−1) at time zero were 25.5±2.6, 23.6±2.0, 18.9±2.4 and 25.3±1.3 for control and 5, 10 and 100 mg kg−1 D-pinitol respectively *P<0.05, **P<0.02 versus time zero, †P<0.05 versus control at same time point.
Figure 3.
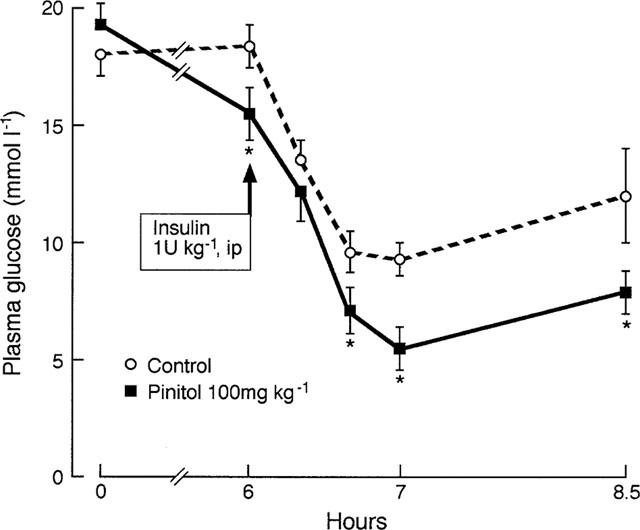
Acute effect of D-pinitol (100 mg kg−1 p.o.) on plasma glucose disappearance in response to insulin (1 u kg−1 i.p. at 6 h) in STZ-diabetic mice. Plasma glucose disappearance (6 h to 6 h 40 min) was 1.3±0.2 and 1.1±0.2% per min for control and D-pinitol respectively. Values are mean±s.e.mean, n=6. *P<0.05 versus control at same time point.
Chronic administration of D-pinitol (100 mg kg−1 i.p. twice daily) reduced the mean plasma glucose concentration from 14.2 mmol l−1 to 10 mmol l−1 after 1 day (Figure 4). The lowered plasma glucose concentration was maintained for the 11 days of D-pinitol treatment. When D-pinitol was stopped plasma glucose concentrations increased after 1 day to nearly the pretreatment value. Food intake and body weight were not significantly altered by the D-pinitol treatment (data not shown).
Figure 4.
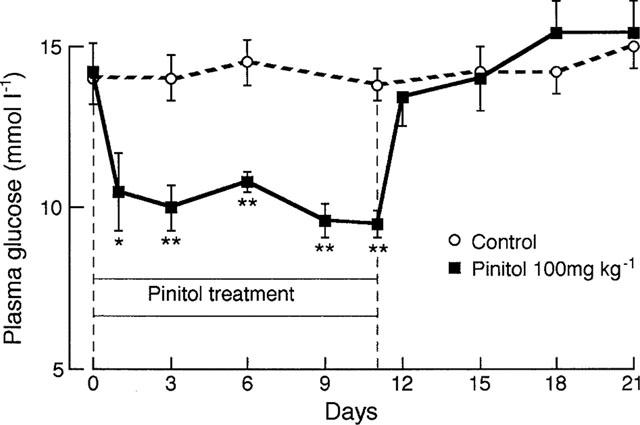
Chronic effect of D-pinitol (100 mg kg−1 i.p. twice daily) on plasma glucose in STZ-diabetic mice. Values are mean±s.e.mean, n=5. *P<0.05, **P<0.02 versus time zero and versus control at same time point.
Normal mice and obese-diabetic (ob/ob) mice
Acute oral administration of D-pinitol (100 mg kg−1) to normal non-diabetic mice did not significantly alter basal plasma glucose or insulin concentrations over 4 h, and an i.p. glucose tolerance test at 4 h was also unaffected by the D-pinitol treatment (data not shown). Also, acute oral administration of D-pinitol (100 mg kg−1) did not significantly alter plasma glucose or insulin concentrations over 6 h in ob/ob mice (data not shown).
L6 muscle cells
2-deoxyglucose (2DG) uptake by L6 myotubes was stimulated by insulin (24 h incubation) in a concentration-dependent manner with a maximum effect at 10−7 M, and an ED50 of 2.19×10−8 M (Figure 5). 10−8 M insulin increased 2DG uptake at 10 min, 4 h and 24 h by 25, 42 and 49% respectively (P<0.05 versus control; Figure 6). In the absence of added insulin, 10−3 M D-pinitol increased basal 2DG uptake at each of the three time points studied, but lower concentrations of D-pinitol (10−4–10−6 M) did not exert a significant effect. D-pinitol (10−3 M) produced a larger increase in 2DG uptake at 10 min and 4 h (by 41 and 34% respectively, P<0.01 versus control) than at 24 h (15% increase, P<0.05 versus control; P<0.05 versus 10 min and 4 h). At 10 min the increase in 2DG uptake produced by 10−3 M D-pinitol (by 41%) was slightly greater (P<0.05) than produced by 10−8 M insulin (by 25%). D-pinitol (10−3 M) did not alter insulin-stimulated 2DG uptake at 10 min or 4 h, but decreased insulin-stimulated 2DG uptake by 18% at 24 h (P<0.02 versus insulin alone).
Figure 5.
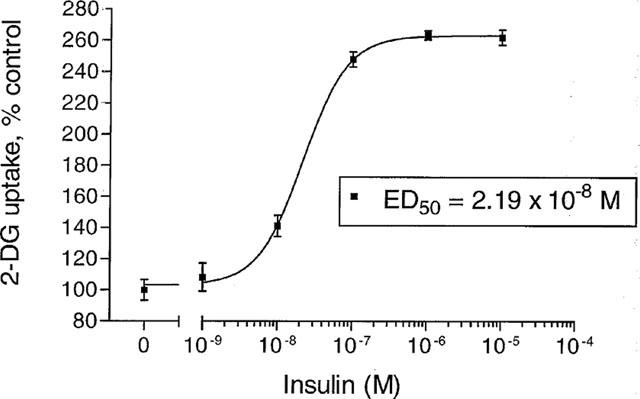
2-deoxy-[3H]-glucose (2DG) uptake by L6 myotubes incubated for 24 h with insulin (10−9–10−5 M). Data are expressed as per cent control (no added insulin), mean±s.e.mean, n=18.
Figure 6.
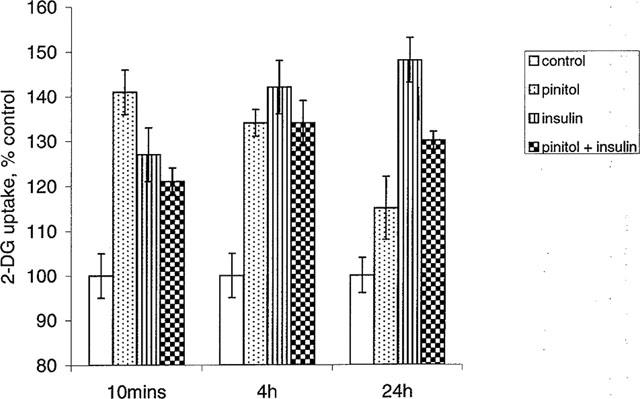
2-deoxy-[3H]-glucose (2DG) uptake by L6 myotubes incubated for 10 min, 4 h and 24 h with insulin (10−8 M) and/or D-pinitol (10−3 M). Data are expressed as per cent control (no addition of insulin or D-pinitol), mean±s.e.mean, n=18. *P<0.05 versus control at same time interval; †P<0.05 versus insulin alone.
Incubation of L6 cells for 4 h with LY294002 (10−5 M), an inhibitor of phosphatidylinositol 3-kinase (PI3K), prevented insulin-stimulated 2DG uptake (Figure 7). LY294002 also prevented the stimulation of 2DG uptake by D-pinitol (10−3 M).
Figure 7.
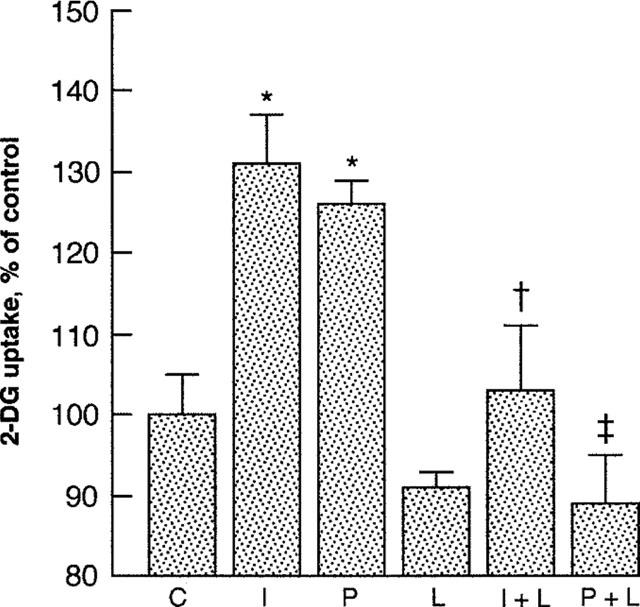
2-deoxy-[3H]-glucose (2DG) uptake by L6 myotubes incubated for 4 h with insulin (10−8 M), D-pinitol (10−3 M) and/or LY294002 (10−5 M). C, control, I, insulin; P, D-pinitol; L, LY294002. Data are expressed as per cent control (no addition of insulin or D-pinitol), mean±s.e.mean, n=6. *P<0.05 versus control; +P<0.05 versus insulin; ‡P<0.05 versus D-pinitol.
Discussion
In this study D-pinitol, the 3-methoxy analogue of D-chiroinositol, exerted an acute and chronically-sustained antihyperglycaemic effect in the murine STZ-induced model of hypoinsulinaemic diabetes. D-pinitol also acutely increased basal (but not insulin-stimulated) 2DG transport by L6 muscle cells.
The ability of D-pinitol to reduce the hyperglycaemia of STZ-diabetic mice to near-normal glucose concentrations cannot be attributed to increased insulin concentrations. A change in food consumption can also be excluded, since food was withheld during acute studies and was not significantly altered during chronic D-pinitol treatment. Augmentation of insulin action is also unlikely, because D-pinitol did not significantly increase insulin-stimulated glucose disappearance in STZ-diabetic mice or insulin-stimulated glucose uptake in cultured L6 muscle cells. However, a direct effect of D-pinitol on muscle glucose metabolism could contribute to the antihyperglycaemic effect, since D-pinitol increased basal glucose uptake by cultured L6 muscle cells. The concentration of D-pinitol (10−3 M) required to significantly increase glucose uptake by the L6 cells was not inconsistent with the most effective dosage used in vivo (100 mg kg−1) for a lipophilic agent with a large volume of distribution.
Thus D-pinitol might exert an insulin-like effect on glucose transport that is independent of insulin. This does not preclude the possibility that D-pinitol could interact with a pathway of insulin signalling. By analogy with D-chiroinositol (Ortmeyer et al., 1993b; Huang et al., 1993), there is a structural similarity of D-pinitol with inositol phosphates involved in the signalling of insulin via PI3K and protein kinase B (PKB) (Holman & Kasuga, 1997; White, 1997). Indeed the effect of D-pinitol was prevented by LY294002, an inhibitor of PI3K. If D-pinitol provided a substrate or surrogate signal for this pathway it might increase glucose transport without increasing the effect of insulin. However, D-pinitol slightly reduced the effectiveness of insulin in L6 muscle cells by 24 h. Hence it is conceivable that an acute interaction of D-pinitol with the signalling pathways of insulin to glucose transport could limit the extent to which these pathways can be stimulated subsequently by insulin. Consistent with this view there was no acute effect of D-pinitol on glucose or insulin concentrations in normal non-diabetic mice.
D-pinitol, like insulin (Bailey & Flatt, 1997), was not effective in severely insulin resistant ob/ob mice. These mice probably incur multiple lesions within the signalling pathways between the insulin receptor and glucose transport (Bailey & Flatt, 1997), which could involve rate-limiting defects distal to the site of interaction with D-pinitol.
It is concluded that D-pinitol exerts an acute and chronic insulin-like antihyperglycaemic effect in STZ-diabetic mice. The mechanism of action of D-pinitol does not augment the effect of insulin but might involve an interaction with part of a cellular signalling pathway that links insulin with glucose transport.
Abbreviations
- 2DG
2-deoxyglucose
- DMEM
Dulbecco's modified Eagle's Medium
- FCS
foetal calf serum
- i.p.
intraperitoneal
- PI3K
phosphatidylinositol 3-kinase
- PKB
protein kinase B
- p.o.
per oral
- STZ
streptozotocin
References
- BAILEY C.J., FLATT P.R. Animal syndromes of non-insulin dependent diabetes mellitus Textbook of Diabetes 1997Oxford: Blackwell; 23.1–23.25.2nd end. ed. Pickup, J.C. & Williams, G. pp [Google Scholar]
- BAILEY C.J., FLATT P.R., ATKINS T.W. Influence of genetic background and age on the expression of the obese hyperglycaemic syndrome in Aston ob/ob mice. Int. J. Obesity. 1982;6:11–21. [PubMed] [Google Scholar]
- FLATT P.R., BAILEY C.J. Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia. 1981;20:573–577. doi: 10.1007/BF00252768. [DOI] [PubMed] [Google Scholar]
- FONTELES M.C., HUANG L.C., LARNER J. Infusion of pH 2.0 D-chiroinositol glycan insulin putative mediator normalizes plasma glucose in streptozotocin diabetic rats at a dose equivalent to insulin without inducing hypoglycaemia. Diabetologia. 1996;39:731–734. doi: 10.1007/BF00418546. [DOI] [PubMed] [Google Scholar]
- HOLMAN G.D., KASUGA M. From receptor to transporter: insulin signalling to glucose transport. Diabetologia. 1997;40:991–1003. doi: 10.1007/s001250050780. [DOI] [PubMed] [Google Scholar]
- HUANG L.C., FONTELES M.C., HOUSTON D.B., ZHANG C., LARNER J. Chiroinositol deficiency and insulin resistance. III. Acute glycogenic and hypoglycemic effects of two inositol phosphoglycan insulin mediators in normal and streptozotocin diabetic rats. Endocrinol. 1993;132:652–657. doi: 10.1210/endo.132.2.8425485. [DOI] [PubMed] [Google Scholar]
- KENNINGTON A.S., HILL C.R., CRAIG J., BOGARDUS C., RAZ I., ORTMEYER H.K., HANSEN B.C., ROMERO G., LARNER J. Low urinary chiroinositol excretion in non-insulin dependent diabetes mellitus. N. Engl. J. Med. 1990;323:373–378. doi: 10.1056/NEJM199008093230603. [DOI] [PubMed] [Google Scholar]
- NARAYANAN C.R., JOSHI D.D., MUDJUMDAR A.M., DHEKNE V.V. Pinitol, a new anti-diabetic compound from the leaves of Bougainvillea spectabilis. Curr. Sci. 1987;56:139–141. [Google Scholar]
- ORTMEYER H.K., BODKIN N.L., LILLEY K., LARNER J., HANSEN B.C. Chiroinositol deficiency and insulin resistance. I. Urinary excretion rate of chiroinositol is directly associated with insulin resistance in spontaneously diabetic rhesus monkeys. Endocrinol. 1993a;132:640–645. doi: 10.1210/endo.132.2.8425483. [DOI] [PubMed] [Google Scholar]
- ORTMEYER H.K., HUANG L.C., ZHANG L., HANSEN B.C., LARNER J. Chiroinositol deficiency and insulin resistance. II. Acute effects of D-chiroinositol administration in streptozotocin diabetic rats, normal rats given a glucose load, and spontaneously insulin-resistant rhesus monkeys. Endocrinol. 1993b;132:646–651. doi: 10.1210/endo.132.2.8425484. [DOI] [PubMed] [Google Scholar]
- POYNER D.R., ANDREWS D.P., BROWN D., ROSE C., HANLEY M.R. Characterisation of receptors for calcitonin gene-related receptor on rat L6 skeletal myocytes. Br. J. Pharmacol. 1992;105:441–447. doi: 10.1111/j.1476-5381.1992.tb14272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEVENS J.F. Determination of glucose by an automatic analyser. Clin. Chim. Acta. 1971;32:199–201. doi: 10.1016/0009-8981(71)90332-9. [DOI] [PubMed] [Google Scholar]
- SUZUKI S., KAWASAKI H., SATOH Y. Urinary chiroinositol excretion is an index marker of insulin sensitivity in Japanese type II diabetes. Diabetes Care. 1994;17:1465–1468. doi: 10.2337/diacare.17.12.1465. [DOI] [PubMed] [Google Scholar]
- WHITE M. The insulin signalling system and IRS proteins. Diabetologia. 1997;40:S2–S17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- YAFFE D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc. Natl. Acad. Sci. U.S.A. 1968;61:477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]


