Abstract
The progestin and oestrogen component of oral contraceptives have been involved in the development of venous thromboembolic events in women. In the present study we determined the vasoactive effects of sex steroids used in oral contraceptives in isolated preconstricted rabbit jugular veins in the presence of diclofenac and examined the underlying mechanisms.
The natural hormone progesterone, the synthetic progestins levonorgestrel, 3-keto-desogestrel, gestodene and chlormadinone acetate, and the synthetic estrogen 17 α-ethinyloestradiol induced concentration-dependent relaxations of endothelium-intact veins constricted with U46619. Levonorgestrel also inhibited constrictions evoked by either a high potassium (K+) solution or phorbol myristate acetate (PMA) in the absence and presence of extracellular calcium (Ca2+). In addition, levonorgestrel depressed contractions evoked by Ca2+ and reduced 45Ca2+ influx in depolarized veins.
Relaxations to levonorgestrel in U46619-constricted veins were neither affected by the presence of the endothelium nor by the inhibitor of soluble guanylyl cyclase, NS2028, but were significantly improved either by the selective cyclic AMP phosphodiesterase inhibitor rolipram or in the absence of diclofenac, and decreased by the protein kinase A inhibitor, Rp-8-CPT-cAMPS. Rolipram also potentiated relaxations to levonorgestrel in PMA-constricted veins in the presence, but not in the absence of extracellular Ca2+. Levonorgestrel increased levels of cyclic AMP and inhibited PMA-induced activation of protein kinase C in veins.
These findings indicate that levonorgestrel caused endothelium-independent relaxations of jugular veins via inhibition of Ca2+ entry and of protein kinase C activation. In addition, the cyclic AMP effector pathway contributes to the levonorgestrel-induced relaxation possibly by depressing Ca2+ entry.
Keywords: Progestins, vein, protein kinase C, calcium entry, cyclic AMP
Introduction
Since the early 1960s it has been known that the use of oral contraceptives is associated with an increased risk of venous thromboembolism (Sartwell et al., 1969; Vessey et al., 1986; WHO, 1995a,1995b; Jick et al., 1995). Several studies have provided evidence that the risk of vascular events is related to the content of oestrogen but the type and concentration of progestin also seems to be important (Vessey et al., 1986; WHO, 1995a,1995b; Jick et al., 1995; Bloemenkamp et al., 1995; Spitzer et al., 1996). The pathogenesis of venous thromboembolism in users of oral contraceptives is unknown, but may relate to changes in the coagulation and fibrinolysis systems and may also involve mechanisms affecting platelets and the vasculature. The use of oral contraceptives has been shown to increase plasma concentration of coagulation factor II, VII and X, and also of fibrinogen (Sabra & Bonnar, 1983; Bonnar, 1987), to reduce those of the physiological inhibitors of the coagulation including protein S, antithrombin III, and is associated with a reduced sensitivity to activated protein C (Bloemenkamp et al., 1995; Sabra & Bonnar, 1983; Bonnar, 1987; Jesperern et al., 1990; Peters et al., 1992; Rosing et al., 1997). The enhancing effect of oral contraceptives on coagulation appears to be partially counterbalanced by a simultaneous activation of the endogenous fibrinolysis activity (Sabra & Bonnar, 1983; Jesperern et al., 1990; Gevers Leuven et al., 1987). An increased reactivity of platelets has also been found in users of oral contraceptives (Sabra & Bonnar, 1983; Bonnar, 1987). Moreover, oral contraceptives may directly affect vascular tone since both 17 β-oestradiol and progesterone induced concentration-dependent relaxations of isolated arteries (Jiang et al., 1991; 1992; Omar et al., 1995; Glusa et al., 1997; Ramirez et al., 1998; Crews et al., 1999). The inhibitory effect of oestrogens on vascular tone involves both endothelium-dependent and -independent mechanisms, whereas the effect of progesterone appears to be due to direct action at the smooth muscle (Jiang et al., 1991; 1992; Crews et al., 1999; McNeill et al., 1996; Mügge et al., 1993). Since thrombotic events following use of oral contraceptives are predominantly found in the venous circulation and veins have been reported to have different sensitivities to vasoactive agents compared to arteries (D'Orleans-Juste et al., 1989; Miller et al., 1989), a clarification of the effect of sex steroids used in oral contraceptives on venous tone is required. Therefore, the purpose of the present study was to determine whether sex steroids used in oral contraceptives (progesterone, levonorgestrel, gestodene, 3-keto-desogestrel, chlormadinone acetate, and 17 α-ethinyloestradiol) affect the tone of isolated veins and, if so, to identify the underlying mechanism of action.
Methods
Materials
HEPES and NG-nitro-L-arginine (L-NNA) were from Serva (Heidelberg, Germany), diclofenac (Voltaren® injection solution) from Ciba-Geigy (Wehr, Germany), cromakalim, acetylcholine, rolipram, phorbol-12-myristate-13-acetate (PMA), EGTA as well as the progestins levonorgestrel, progesterone, chlormadinone acetate and 17 α-ethinyloestradiol from Sigma (Deisenhofen, Germany), gestodene and 3-keto-desogestrel from Organon (Oberschleißheim, Germany), onapristone from Schering (Berlin, Germany), Rp-8-CPT-cAMPS from Biomol, and Ro-31-8220 from Calbiochem (Bad Soden, Germany). 9,11-dideoxy-11α,9α-epoxymethano-prostaglandin F2α (U46619) was kindly provided by Upjohn (Ann Arbor, MI, U.S.A.) and NS2028 by NeuroSearch (Glostrup, Denmark). The protein kinase C (PKC) assay was from Gibco BRL (Karlsruhe, Germany) and the cyclic AMP radioimmunoassay from RBI Biotrend (Köln, Germany). Stock solutions of the following compositions (mM) were prepared in DMSO: sex steroid (10), PMA (10), Ro-31-8220 (10), NS2028 (1), rolipram (10) and U46619 (1).
Preparation of jugular veins
Male New Zealand white rabbits (1.5–2.0 kg body weight) were anaesthetized with sodium pentobarbitone (105 mg kg−1 intravenous). After exsanguination, both jugular veins were removed, cleaned of adventitial adipose and connective tissue and cut into either rings of 3–4 mm length for organ chamber experiments or segments of 10–12 mm length for determination of PKC activity, 45Ca2+ influx, and cyclic AMP content.
Organ chamber studies
Rings were suspended between K30 force transducers (Hugo Sachs Elektronik, Germany) and a rigid support for measurement of isometric force in 10 ml-organ baths containing warm (37°C) and oxygenated (95% O2/5% CO2) Krebs-Henseleit solution (pH 7.4, mM composition): NaCl 119.0, NaHCO3 25.0, glucose 11.1, CaCl2 1.6, KCl 4.7, KH2PO4 1.2, and MgSO4 1.2, to which the cyclooxygenase inhibitor diclofenac (1 μM) was added. Passive tension was adjusted over a 30-min equilibration period to 0.5 g. Rings were constricted with potassium chloride (KCl, 80 mM) followed by a 30-min wash-out period. Thereafter, rings were constricted with U46619 (0.1–0.3 μM) and once a stable plateau was achieved acetylcholine (10 μM) was added to verify the presence of endothelium. Following a 30-min washout period rings were constricted with either KCl (80 mM), U46619 (0.1–0.3 μM) or PMA (a PKC activator, 0.1–1 μM) before construction of a concentration-relaxation curve to a sex steroid or solvent. In some experiments the extracellular calcium (Ca2+) was removed by changing three-times the Krebs-Henseleit solution with a Ca2+-free Krebs-Henseleit solution containing ethyleneglycol-bis-(β-aminoethyl ether)-tetraacetic acid (EGTA 100 μM) and then six-times with a Ca2+-free Krebs-Henseleit solution without EGTA. The removal of Ca2+ from the extracellular medium was confirmed by the absence of a constriction to KCl (80 mM). Thereafter, a concentration-constriction curve to calcium chloride in a Ca2+-free Krebs-Henseleit solution containing KCl (80 mM) was performed in the absence and presence of levonorgestrel. In some experiments, veins were constricted with either U46619 or PMA before addition of rolipram (100 nM), a selective inhibitor of the cyclic AMP phosphodiesterase (Lugnier et al., 1986) or Rp-8-CPT-cAMPS (300 μM), a selective protein kinase A inhibitor (Gjertsen et al., 1995), for 15 min followed by the construction of a relaxation curve to levonorgestrel or cromakalim, an opener of ATP-dependent K+ channels (Edwards & Weston, 1995).
45Ca2+ experiments
The uptake of Ca2+ into venous segments was estimated by measuring the increase in 45Ca2+ content in tissues during exposure in a Krebs-Henseleit solution containing 45Ca2+ (0.2 μCi ml−1) in the absence and presence of KCl (100 mM). Lanthanum, which has been shown to displace extracellularly bound Ca2+ while having little or no effect on the intracellular Ca2+ content (Godfraind, 1976), was used to remove the relatively large amount of 45Ca2+ in the extracellular space which would otherwise interfere with the determination of cellular 45Ca2+. The segments were equilibrated for at least 60 min in Krebs-Henseleit solution maintained at 37°C and aerated with a gas mixture of 95% O2 and 5% CO2. To measure stimulated influx of 45Ca2+, tissues were preincubated in Krebs-Henseleit solution containing 45Ca2+ for 5 min before being exposed to KCl (100 mM) in the presence of 45Ca2+ for 5 min. To test the effect of levonorgestrel on KCl-induced 45Ca2+ influx, levonorgestrel (100 μM) was added to the incubation medium 2 min before the addition of KCl. Control tissues, taken from the same vein, were incubated with 45Ca2+ in the course of the experiment. Thereafter, vein segments were washed for 5 min in 200 ml of a La3+ solution (mM): NaCl 122, KCl 5.9, MgCl2 0.38, LaCl3 50, glucose 11, Tris maleate 15; pH 6.8, to remove extracellular Ca2+ from the segments. After the La3+ wash, the vein segments were placed between two sheets of filter paper and pressed three times with a roller weighing 350 g. Each vein segment was weighed, dissolved in 0.1 ml of a solution composed of equal parts of perchloric acid (37% w v−1) and H2O2 (30 vol) by heating for 15 min at 75°C. After cooling, 4.5 ml scintillation fluid (Rotiszint® eco plus, Roth, Germany) was added and the radioactivity of the samples counted in a liquid scintillation counter.
Determination of PKC activity
Vein segments were equilibrated in HEPES-Tyrode (pH 7.4; mM composition: NaCl 132.0, HEPES 9.4, glucose 5.0, KCl 4.0, MgCl2 0.49, CaCl2 1.0), at 37°C prior to the addition of levonorgestrel (30 μM) or solvent (dimethyl sulphoxide, DMSO, 0.3%). After a 10-min incubation period, segments were either untreated or exposed to PMA (1 μM) for 5 min. Segments were immediately blotted on filter paper and then frozen in liquid nitrogen. Segments were homogenized in liquid nitrogen and the powder was collected in Eppendorf tubes containing 150 μl ice-cold extraction buffer for protein (composition: Tris 10 mM, pH 7.5, β-mercaptoethanol 10 mM, ethylenediaminetetraacetic acid (EDTA) 0.5 mM, EGTA 0.5 mM, Triton®X-100 0.5%, aprotinin and leupeptin each 25 μg ml−1). The homogenate was vortexed, incubated for 45 min at 4°C, and centrifuged (20 min, 13,000×g, 4°C). The supernatant was removed and the amount of protein was determined by the Bradford method. Each sample (40 μg protein) was incubated in the absence (duplicate) and presence of 10 μl PKC inhibitor solution [PKC (19–36) 100 μM, Tris 20 mM, pH 7.5] for 20 min at room temperature. The enzymatic reaction was started by addition of 10 μl of 32P-marked substrate solution [containing acetylated myeline basic protein (Ac-MBP 4-14) 250 μM, adenosine triphosphate (ATP) 100 μM, CaCl2 5 mM, MgCl2 100 mM, Tris 20 mM, pH 7.5, [γ-32P]ATP 25 μCi ml−1 substrate solution] at 37°C. After 15 min, 25 μl of the reaction mixture was removed from each tube and spotted onto a phosphocellulose disc paper. The phosphocellulose paper was washed three times with phosphoric acid (1%) for 5 min and then twice with distilled H2O for 3 min. The discs were put into scintillation vials containing 10 ml of scintillation fluid. The peptide incorporated 32P was counted using a β-counter. The background activity obtained in the presence of the PKC inhibitor was subtracted from each value to obtain the specific PKC activity of each sample.
Determination of cyclic AMP content
Vein segments were equilibrated in HEPES-Tyrode at 37°C for 1 h prior to the addition of rolipram (1 μM). After a 20-min incubation period segments were either treated with solvent (DMSO, 0.3%) or exposed to levonorgestrel (30 μM) for various times. Segments were immediately frozen in liquid nitrogen. After homogenization in liquid nitrogen, the powder was collected in Eppendorf tubes containing ice-cold trichloroacetic acid (TCA; 6%). The homogenate was incubated for 30 min at 4°C, mixed several times and then centrifuged (20 min, 13,000×g, 4°C). The protein pellet was kept for protein determination by the method of Lowry. The supernatant was extracted three times with H2O-saturated ether (5 vol ether/vol H2O). The cyclic AMP content in each sample was determined using a commercial radioimmunoassay kit.
Data analysis
All data are expressed as means±s.e.mean. n represents the number of different animals studied. The EC50 and IC50 values are defined as the concentration of the drug evoking half-maximal constriction and relaxation, respectively. Statistical evaluation was performed by Student's paired t-test or when more than two treatments were compared by ANOVA followed by Student-Newman-Keuls t-test. A P value <0.05 was considered statistically significant.
Results
The natural hormone progesterone, the synthetic progestins levonorgestrel, 3-keto-desogestrel, gestodene and chlormadinone acetate and the synthetic estrogen 17 α-ethinyloestradiol elicited concentration-dependent relaxations of endothelium-intact rabbit jugular veins constricted with U46619 (0.1–0.3 μM) in the presence of diclofenac (1 μM) (Figure 1 and Table 1). The onset of the relaxation to each sex steroid occurred within 1 min, and was maximal within 15 min. Further experiments were performed with levonorgestrel to characterize the mechanism underlying the relaxation. Relaxations to levonorgestrel were unaffected by onapristone, an antagonist of intracellular progesterone receptors (10 μM, data not shown, Edwards et al., 1995). Higher concentrations of onapristone could not be tested due to a marked reduction of U46619-induced constrictions. Levonorgestrel also inhibited constriction of vein rings induced by either KCl or PMA to a similar extent as those induced by U46619 (Figure 2a,b). Relaxations to levonorgestel in U46619-constricted veins were slightly but significantly greater in the absence of diclofenac (IC50 changed from 3.5±0.8 to 1.8±0.5 μM without affecting maximal relaxation to levonorgestel 30 μM which changed from 98.7±4.1 to 102.4±1.4, n=6) and were affected neither by the presence of the endothelium (Figure 3a) nor by the inhibitor of nitric oxide synthase, L-NNA (10 μM; data not shown), and also not by the inhibitor of soluble guanylyl cyclase, NS 2028 (Figure 3b, Olesen et al., 1998). Next, the possibility that levonorgestrel affects Ca2+-induced constrictions was examined. Addition of extracellular Ca2+ to depolarized vein rings caused a concentration-dependent constriction, which was significantly attenuated by levonorgestrel (Figure 4a). The EC50 changed from 291±50 to 676±67 μM CaCl2 and the maximal constriction was reduced from 100 to 69.7±4.5%. In addition, 45Ca2+ experiments were performed to test whether levonorgestrel affects Ca2+ influx in depolarized veins. Incubation of vein segments in a depolarizing solution (100 mM KCl) caused 45Ca2+ influx, and this effect was markedly reduced by levonorgestrel (100 μM; added 2 min prior to KCl; Figure 4b). Levonorgestrel, in addition to inhibiting PMA-induced constrictions in a Ca2+-containing Krebs-Henseleit solution (Figure 2b), also significantly inhibited those evoked in a Ca2+-free solution (Figures 5a and 6c). Since the PMA-induced constrictions in the absence of extracellular Ca2+ were abolished by Ro-31-8220 (1 μM; data not shown), experiments were performed to test whether levonorgestrel inhibits the activation of PKC. Exposure of vein segments to PMA (1 μM) for 5 min significantly increased PKC specific activity (Figure 5b), a response which was abolished by Ro-31-8220 (1 μM; data not shown). The stimulatory effect of PMA was significantly prevented by levonorgestrel (30 μM; added 10 min prior to PMA) whereas the progestin alone had only minimal effects (Figure 5b).
Figure 1.
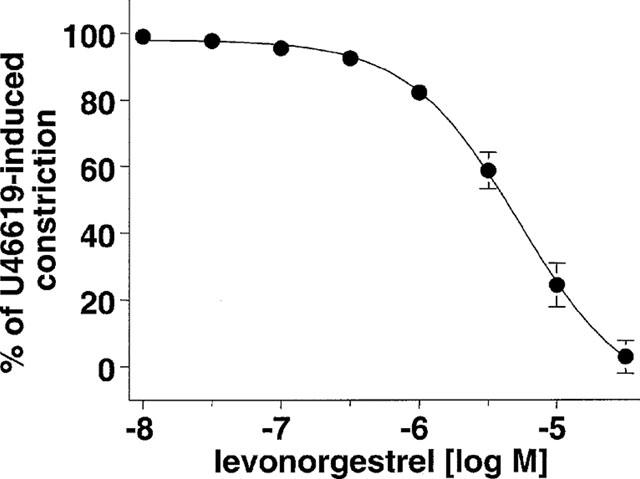
Concentration-relaxation curve to levonorgestrel in endothelium-intact rabbit jugular vein rings constricted with U46619 (0.1–0.3 μM). Experiments were performed in the presence of diclofenac (1 μM). Results are expressed as mean±s.e.mean of four different experiments.
Table 1.
Relaxation evoked by sex steroids on rabbit jugular vein rings with endothelium
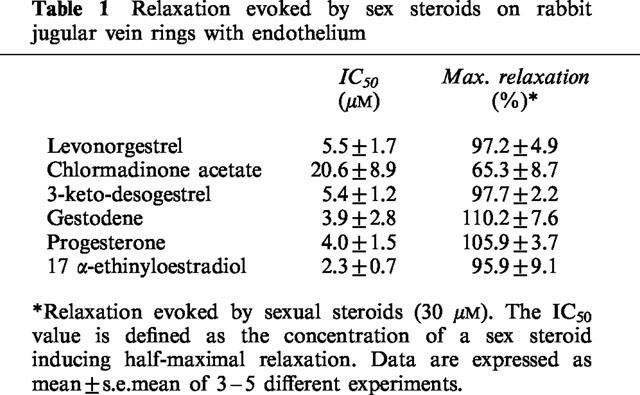
Figure 2.
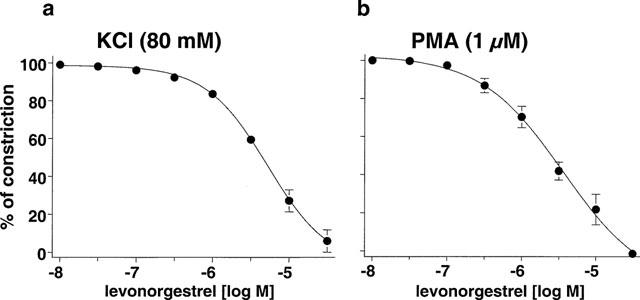
Concentration-relaxation curves to levonorgestrel in endothelium-intact jugular vein rings constricted with (a) potassium chloride (KCl) and (b) phorbol 12-myristate-13-acetate (PMA). Experiments were performed in the presence of diclofenac (1 μM). Results are expressed as mean±s.e.mean of (a) four, (b) three different experiments.
Figure 3.
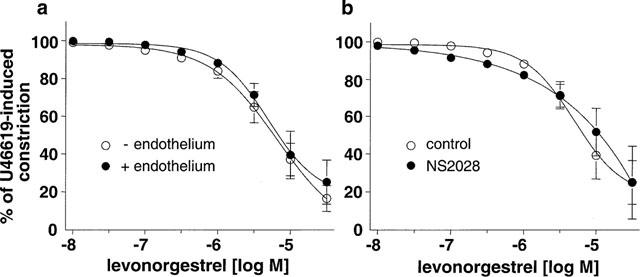
Concentration-relaxation curves to levonorgestrel in U46619 (0.1–0.3 μM)-constricted jugular vein rings (a) with and without endothelium and (b) with endothelium in the absence and presence of NS2028 (10 μM). Experiments were performed in the presence of diclofenac (1 μM). Results are expressed as mean±s.e.mean of 3–7 different experiments.
Figure 4.
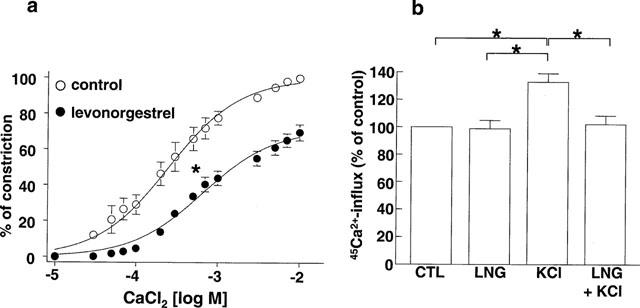
(a) Effect of levonorgestrel on the calcium chloride (CaCl2)-induced constriction of endothelium-intact jugular vein rings in a Ca2+-free Krebs-Henseleit solution containing potassium chloride (80 mM). Rings were preincubated either with solvent or levonorgestrel (10 μM) for 3 min prior to the construction of a concentration-constriction curve to CaCl2. (b) Effect of levonorgestrel on the KCl (100 mM for 5 min)-induced 45Ca2+ influx in endothelium-intact jugular vein segments. Segments were preincubated with either solvent or levonorgestrel (100 μM) for 2 min prior to the addition of KCl. Experiments were performed in the presence of diclofenac (1 μM). Results are expressed as mean±s.e.mean of seven different experiments. *P<0.05.
Figure 5.
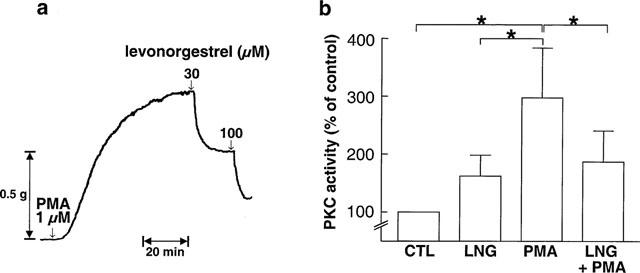
(a) Representative tracing demonstrating the levonorgestrel-induced relaxation of an endothelium-intact jugular vein ring constricted with PMA in a Ca2+-free Krebs-Henseleit solution, (b) effect of levonorgestrel on the PMA-induced protein kinase C specific activity in endothelium-intact jugular vein segments. Segments were exposed to either levonorgestrel (LNG, 30 μM) or solvent (DMSO 0.3%, CTL) for 10 min prior to the addition of PMA (1 μM) for 5 min. Results are expressed as mean±s.e.mean of four different experiments. *P<0.05.
Figure 6.
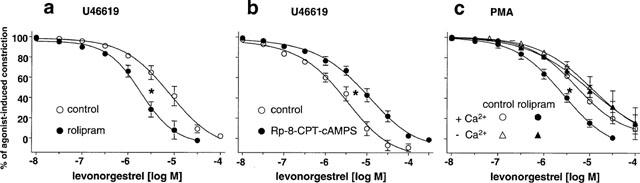
Effect of (a) rolipram (100 nM) and (b) Rp-8-CPT-cAMPS (300 μM) on the levonorgestrel-induced relaxation in endothelium-intact jugular vein rings constricted with U46619 (0.1–0.3 μM), and (c) effect of rolipram (100 nM) on the levonorgestrel induced relaxation in endothelium-intact jugular vein rings constricted with PMA (1 μM) in the absence and presence of extracellular Ca2+. Rings were exposed to either solvent or an agent for 15 min prior to construction of the concentration-relaxation curve to levonorgestrel. Experiments were performed in the presence of diclofenac (1 μM). Results are expressed as mean±s.e.mean of 3–6 different experiments respectively. *P<0.05 vs respective control.
Since estrogens have been shown to induce the formation of cyclic AMP in arteries and some non-vascular tissues (Mügge et al., 1993; Aronica et al., 1994), the role of the cyclic AMP relaxing pathway in the levonorgestrel-induced relaxation of vein rings was examined. In the presence of the selective cyclic AMP phosphodiesterase inhibitor, rolipram (100 nM), the levonorgestrel-induced concentration-relaxation curve of U46619-constricted veins was shifted significantly to the left (Figure 6a), whereas relaxations to cromakalim, an opener of ATP-sensitive K+ channels, were unaffected (IC50 values were 0.20±0.04 and 0.28±0.14 μM in the absence and presence of rolipram, respectively, n=6). In contrast, levonorgestrel-induced relaxations were significantly attenuated in the presence of the selective protein kinase A inhibitor, Rp-8-CPT-cAMPS (300 μM; IC50 values were 2.1±0.5 and 7.2±2.1 μM in the absence and presence of Rp-8-CPT-cAMPS, respectively, Figure 6b). A potentiation of the levonorgestrel-induced relaxation by rolipram was also observed in vein rings constricted with PMA in the presence, but not in the absence of extracellular Ca2+ (Figure 6c). In addition, levonorgestrel significantly increased the formation of cyclic AMP in veins. The maximal effect of 220.5±61.3% was observed after a 2-min treatment period (Figure 7). Incubation of veins with the adenylyl cyclase activator forskolin (1 μM for 5 min) markedly increased cyclic AMP levels by 2047.4±227.9% (n=5).
Figure 7.
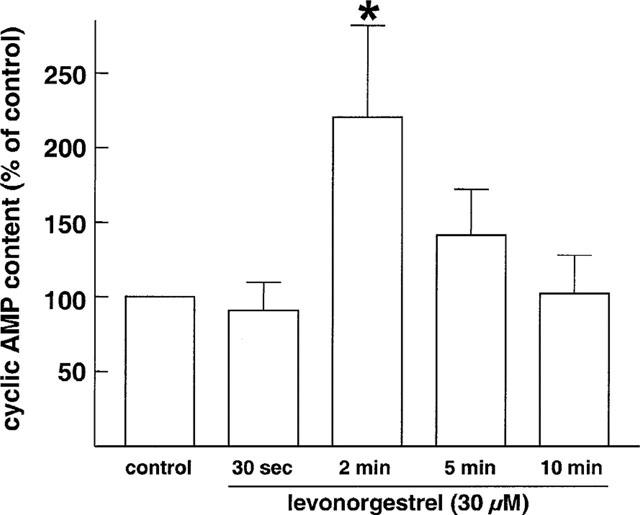
Effect of levonorgestrel on the formation of cyclic AMP in segments of endothelium-intact rabbit jugular veins. Segments were exposed to rolipram (1 μM) for 20 min prior to the addition of levonorgestrel or solvent. Results are expressed as mean±s.e.mean of five different experiments. *P<0.05 vs control.
Discussion
The present findings demonstrate that sex steroids used in oral contraceptives (progesterone, levonorgestrel, 3-keto-desogestrel, gestodene, chlormadinone acetate and 17 α-ethinyloestradiol) induce pronounced relaxations of isolated rabbit jugular veins constricted with U46619. These findings extend previous data obtained with isolated arteries that have shown acute vascular relaxations in response to progesterone in constricted blood vessels such as porcine and rabbit coronary arteries (Jiang et al., 1992; Crews and Khalil, 1999) and rat aorta (Glusa et al., 1997). Relaxation was also observed in response to the synthetic progestins norethisterone acetate and chlormadinone acetate in rat aorta (Glusa et al., 1997), and to 17 β-oestradiol in rat tail artery, and human, rabbit and pig coronary arteries (Jiang et al., 1991; Crews & Khalil, 1999; McNeill et al., 1996; Mügge et al., 1993; White et al., 1995). Moreover, the data indicate that most of the sex steroids investigated were equipotent in inducing relaxation in U46619-constricted veins. In addition, as expected, the prodrug of 3-keto-desogestel, desogestrel, caused only small relaxations (data not shown). In contrast to the findings obtained with isolated veins, estrogens have been reported to induce greater relaxations than progesterone and the synthetic progestins in isolated arteries. The relative rank order of potency for relaxation in phenylephrine-constricted rat aorta was 17 β-oestradiol>progesterone>synethetic progestins (Glusa et al., 1997), and in PGF2α-constricted porcine coronary arteries 17 β-oestradiol>progesterone (Crews & Khalil, 1999). These differences in sensitivity may imply that the tone of the arteria bed is regulated predominantly by oestrogens whereas progestins regulate in addition to oestrogens that of the venous bed. Alternatively, such changes in potencies could also reflect differences in the animal model, vascular bed, and/or contractile agents used. The findings obtained with isolated blood vessels are in good agreement with those obtained following acute administration of oestrogens in intact animals and in women. Subcutaneous administration of 17 β-oestradiol in female guinea pigs reversibly and significantly lowered both the resting systemic blood pressure and the peak systolic blood pressure induced by a pressor challenge of norepinephrine (McCaffrey & Czaja, 1989). In addition, an intravenous infusion of 17 β-oestradiol in ovariectomized nonpregnant sheep caused a significant decrease in systemic vascular resistance (Magness & Rosenfeld, 1989). Moreover, sublingual administration of 17 β-oestradiol increased blood flow in the peripheral vasculature in postmenopausal women (Volterrani et al., 1995). The possibility that, like oestrogens, progestins acutely regulate blood flow and vascular resistance in vivo still remains to be addressed.
The characterization of the sex steroid-induced relaxation in veins was next performed with levonorgestrel, a major synthetic progestin used in oral contraceptives. These studies indicate that the levonorgestrel-induced relaxation of jugular veins is not mediated by the endothelium but due to a direct action at the vascular smooth muscle. Moreover, experiments without the cyclooxygenase inhibitor diclofenac have indicated that vasorelaxing prostanoids contribute only minimally to the levonorgestrel-induced relaxation of jugular veins. The results are consistent with the experimental data that have shown that progesterone caused acute relaxations of isolated arteries in an endothelium- and cyclooxygenase-independent manner (Jiang et al., 1992; Crews & Khalil, 1999). In contrast to progestins, the ability of 17 β-oestradiol to acutely relax isolated arteries was shown to be dependent on an intact endothelium and mediated by nitric oxide in some arteries (McNeill et al., 1996; Collins et al., 1994), but independent in several other types of arteries (Jiang et al., 1991; Glusa et al., 1997; Mügge et al., 1993; White et al., 1995). Nitric oxide also appears to mediate the oestrogen-induced acute changes in haemodynamics in experimental animals since these effects were prevented by inhibitors of nitric oxide synthase (Van Buren et al., 1992; Rosenfeld et al., 1996). Moreover, endothelium-derived nitric oxide is also involved in the potentiating effect of 17 β-oestradiol on endothelium-dependent vasodilatation in postmenopausal women (Gilligan et al., 1994a,1994b).
In vitro studies have suggested the involvement of several molecular mechanisms in the sex steroid-induced acute relaxation of blood vessels. It has been proposed that progesterone induces relaxations in human cerebral artery by opening ATP-dependent K+ channels (Leathard & Eccles, 1987), and that 17 β-oestradiol induces relaxations in porcine coronary arteries by opening Ca2+-dependent K+ channels (White et al., 1995). However, in the present study, changes in K+ conductivity are unlikely to play a major role in the levonorgestrel-induced relaxation since similar relaxations were obtained in veins constricted with a high or normal extracellular [K+]. It has also been suggested that the relaxing effect of sex steroids involves modulation of the activator Ca2+ signal in the vascular smooth muscle. Indeed, 17 β-oestradiol and progesterone inhibited high K+-induced 45Ca2+ influx in arteries and also the increase in constriction induced by addition of Ca2+ to depolarized arteries, whereas the release of Ca2+ from intracellular stores was unaffected (Crews & Khalil, 1999; Han et al., 1995; Shan et al., 1994). The inhibitory effect appears to be due to a decreasing effect of 17 β-oestradiol on voltage-dependent Ca2+ channels without changing Ca2+ sensitivity of contractile elements in vascular smooth muscle cells (Han et al., 1995; Shan et al., 1994). The present findings indicate that an inhibition of Ca2+ entry from the extracellular space also appears to contribute to the levonorgestrel-induced relaxation since the progestin significantly depressed Ca2+-induced contractions and 45Ca2+ influx in veins exposed to high K+. However, since levonorgestrel inhibited extracellular Ca2+-independent constrictions of veins induced by PMA, the progestin, in addition to the regulation of the activator Ca2+ signal, also appears to affect protein kinase C-dependent mechanisms. Such an assumption is confirmed by the present biochemical studies indicating that levonorgestrel significantly reduced the PMA-induced activation of protein kinase C in veins. The present findings also indicate that levonorgestrel significantly stimulated the formation of cyclic AMP in veins and that this effect contributes to inhibit vascular tone. Indeed, relaxations to levonorgestrel in both U46619- and PMA-constricted veins were significantly increased by the selective cyclic AMP phosphodiesterase inhibitor rolipram and decreased by the selective protein kinase A inhibitor Rp-8-CPT-cAMPS. The potentiating effect of rolipram is not due to changes in smooth muscle responsiveness since relaxations to cromakalim, an opener of ATP-dependent K+ channels, were unaffected. The cyclic AMP effector pathway may contribute to the levonorgestrel-induced relaxation by modulating Ca2+ entry, since cyclic AMP has been shown to decrease the activity of voltage-dependent Ca2+ channels in vascular smooth muscle cells (Wang et al., 1991; Xiong et al., 1994). In addition, a modulation of protein kinase C activity by the cyclic AMP pathway is rather unlikely since rolipram did not potentiate levonorgestrel-induced relaxations in PMA-constricted veins in the absence of extracellular Ca2+. Consistent with such an effect, maximal adenylyl cyclase activity is critically dependent on the presence of extracellular Ca2+ (Piascik et al., 1983), and rolipram evoked small relaxations of PMA-constricted veins in the presence but not in the absence of extracellular Ca2+ (Herkert, personal communication).
The progestin-induced reduction of venous tone observed in the present study may be of physiological relevance since this effect is observed at concentrations which are within the range of those found in the serum of women taking oral contraceptives. The peak serum concentrations of gestodene in women taking the combination of 30 μg ethinyloestradiol and 75 μg gestodene were approximately 0.1 μM (Kuhl et al., 1988a), those of 3-keto-desogestrel were approximately 0.01 μM after intake of the combination of 30 μg ethinyloestradiol and 150 μg desogestrel (Kuhl et al., 1988b), and those of levonorgestrel were approximately 0.01 μM after intake of 30 μg ethinyloestradiol and 150 μg levonorgestrel (Hümpel et al., 1978). Although the contribution of changes in venous tone to the development of thromboembolic events remains to be determined, one can speculate that progestins may intensify the vasodilatation caused by ethinyloestradiol in veins leading to a pronounced reduction of blood flow velocity which results in a closer contact of platelets with the vessel wall (Tangelder et al., 1988).
In conclusion, sex steroids used in oral contraceptives relaxed constricted jugular veins. Levonorgestrel similarly inhibited veins constricted with U46619, high K+, or PMA, suggesting that this compound controls a common crucial event in the cascade leading to activation of the contractile apparatus. The characterization of the response to levonorgestrel indicates that the relaxation was endothelium-independent, but appeared to be due to an inhibition of Ca2+ entry and activation of protein kinase C. The levonorgestrel-induced impairment of Ca2+ entry is likely to be mediated by the levonorgestrel-induced increase in cyclic AMP.
Abbreviations
- ATP
adenosine 5′-triphosphate
- Ac-MBP
acetylated myeline basic protein
- DMSO
dimethyl sulphoxide
- EC50
concentration of the drug evoking half-maximal constriction
- HEPES
4-(2-hydroxyethyl)-piperazine-1-ethanesulphonic acid
- IC50
concentration of the drug evoking half-maximal relaxation
- LNG
levonorgestrel
- L-NNA
NG-nitro-L-arginine
- PKC
protein kinase C
- PMA
phorbol-12-myristate-13-acetate
- TCA
trichloroacetic acid
- U46619
9,11-dideoxy-11α,9α-epoxymethano-prostaglandin F2α
References
- ARONICA S.M., KRAUS W.L., KATZENELLENBOGEN B.S. Estrogen action via the cAMP signaling pathway: Stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOEMENKAMP K.W.M., ROSENDAAL F.R., HEIMERHORST F.M., BÜLLER H.R., VANDENBROUCKE J.P. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet. 1995;346:1593–1596. doi: 10.1016/s0140-6736(95)91929-5. [DOI] [PubMed] [Google Scholar]
- BONNAR J. Coagulation effects of oral contraception. Am. J. Obstet. Gynecol. 1987;157:1042–1048. doi: 10.1016/s0002-9378(87)80129-1. [DOI] [PubMed] [Google Scholar]
- COLLINS P., SHAY J., JIANG C., MOSS J. Nitric oxide accounts for dose-dependent estrogen-mediated coronary relaxation after acute estrogen withdrawal. Circulation. 1994;90:1964–1968. doi: 10.1161/01.cir.90.4.1964. [DOI] [PubMed] [Google Scholar]
- CREWS J.K., KHALIL R.A. Antagonistic effects of 17β-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler. Thromb. Vasc. Biol. 1999;19:1034–1040. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- D'ORLEANS-JUSTE P., FINET M., DE NUCCI G., VANE J.R. Pharmacology of endothelin-1 in isolated vessels: effect of nicardipine, methylene blue, hemoglobin, and gossipol. J. Cardiovasc. Pharmacol. 1989;13:S19–S22. [PubMed] [Google Scholar]
- EDWARDS D.P., ALTMANN M., DEMARZO A., ZHANG Y., WEIGEL N.L., BECK C.A. Progesterone receptor and the mechanism of action of progesterone antagonists. J. Steroid. Biochem. Mol. Biol. 1995;53:449–458. doi: 10.1016/0960-0760(95)00091-d. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., WESTON A.H. Pharmacology of the potassium channel openers. Cardiovasc. Drugs Ther. 1995;9:185–193. doi: 10.1007/BF00878465. [DOI] [PubMed] [Google Scholar]
- GEVERS LEUVEN J.A., KLUFT C., BERTINA R.M., HESSEL W. Effects of two low-dose oral contraceptives on circulating components of the coagulation and fibrinolytic systems. J. Lab. Clin. Med. 1987;109:631–636. [PubMed] [Google Scholar]
- GILLIGAN D.M., BADAR D.M., PANZA J.A., QUYYUMI A.A., CANNON R.O. Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994a;90:786–791. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- GILLIGAN D.M., QUYYUMI A.A., CANNON R.O. Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994b;89:2545–2551. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- GJERTSEN B.T., MELLIGREN G., OTLEN A., MARONDE E., GENIESER H.-G., JASTORFF B., VINTERMYR O.K., MCKNIGHT G.S., DOSKELAND S.O. ‘Novel (Rp)-cAMPS analogs as tools for inhibition of cAMP-kinase in cell culture.'. J. Biol. Chem. 1995;270:20599–20607. doi: 10.1074/jbc.270.35.20599. [DOI] [PubMed] [Google Scholar]
- GLUSA E., GRÄSER T., WAGNER S., OETTEL M. Mechanisms of relaxation of rat aorta in response to progesterone and synthetic progestins. Maturitas. 1997;28:181–191. doi: 10.1016/s0378-5122(97)00057-1. [DOI] [PubMed] [Google Scholar]
- GODFRAIND T. Calcium exchange in vascular smooth muscle, action of noradrenaline and lanthanum. J. Physiol. 1976;260:21–35. doi: 10.1113/jphysiol.1976.sp011501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN S.-Z., KARAKI H., OUCHI Y., AKISHITA M., ORIMO H. 17β-estradiol inhibits Ca2+ influx and Ca2+ release induced by thromboxane A2 in porcine coronary artery. Circulation. 1995;91:2619–2626. doi: 10.1161/01.cir.91.10.2619. [DOI] [PubMed] [Google Scholar]
- HÜMPEL M., WENDT H., POMMERENKE G., WEISS C., SPECK U. Investigations of pharmacokinetics of levonorgestrel to specific consideration of a possible first-pass effect in women. Contraception. 1978;17:207–220. doi: 10.1016/0010-7824(78)90012-4. [DOI] [PubMed] [Google Scholar]
- JESPERERN J., PETERSEN K.R., SKOUBY S.O. Effects of newer oral contraceptives on the inhibition of coagulation and fibrinolysis in relation to dosage and type of steroid. Am. J. Obstet. Gynecol. 1990;163:396–403. doi: 10.1016/0002-9378(90)90590-4. [DOI] [PubMed] [Google Scholar]
- JIANG C., SARREL P.M., LINDSAY D.C., POOLE-WILSON P.A., COLLINS P. Endothelium-independent relaxation of rabbit coronary artery by 17β-oestradiol in vitro. Br. J. Pharmacol. 1991;104:1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG G., SARREL P.M., LINDSAY D.C., POOLE-WILSON P.A., COLLINS P. Progesterone induces endothelium-independent relaxation of rabbit coronary artery in vitro. Eur. J. Pharmacol. 1992;211:163–167. doi: 10.1016/0014-2999(92)90524-8. [DOI] [PubMed] [Google Scholar]
- JICK H., JICK S.S., GUREWICH V., MYERS M.W., VASILAKIS C. Risk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with different progestagen components. Lancet. 1995;346:1589–1593. doi: 10.1016/s0140-6736(95)91928-7. [DOI] [PubMed] [Google Scholar]
- KUHL H., JUNG-HOFFMANN C., HEIDT F. Alterations in serum levels of gestodene and SHBG during 12 cycles of treatment with 30 μg ethinylestradiol and 75 μg gestodene. Contraception. 1988a;38:477–486. doi: 10.1016/0010-7824(88)90088-1. [DOI] [PubMed] [Google Scholar]
- KUHL H., JUNG-HOFFMANN C., HEIDT F. Serum levels of 3-keto-desogestrel and SHBG during 12 cycles of treatment with 30 μg ethinylestradiol and 150 μg desogestrel. Contraception. 1988b;38:381–390. doi: 10.1016/0010-7824(88)90110-2. [DOI] [PubMed] [Google Scholar]
- LEATHARD H.L., ECCLES N.K. Does migraine result from a decrease in transmembrane potassium conductance Advances in Headache Research 1987John Libby & Co. Ltd., London; 35–40.Clifford Rose F ed. pp [Google Scholar]
- LUGNIER C., SCHOEFFTER P., LE BEC A., STROUTHOU E., STOCLET J.-C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem. Pharmacol. 1986;35:1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- MAGNESS R.R., ROSENFELD C.R. Local and systemic estradiol-17β: effects on uterine and systemic vasodilation. Am. J. Physiol. 1989;256:E536–E542. doi: 10.1152/ajpendo.1989.256.4.E536. [DOI] [PubMed] [Google Scholar]
- MCCAFFREY T.A., CZAJA J.A. Diverse effects of estradiol-17β: concurrent suppression of apetite, blood pressure and vascular reactivity in conscious, unrestrained animals. Physiol. Behav. 1989;45:649–657. doi: 10.1016/0031-9384(89)90086-3. [DOI] [PubMed] [Google Scholar]
- MCNEILL A.M., DUCKLES S.P., KRAUSE D.N. Relaxant effects of 17β-estradiol in the rat tail artery are greater in females than males. Eur. J. Pharmacol. 1996;308:305–309. doi: 10.1016/0014-2999(96)00374-3. [DOI] [PubMed] [Google Scholar]
- MILLER V.M., KOMORI K., BURNETT J.C., JR, VANHOUTTE P.M. Differential sensitivity to endothelin in canine arteries and veins. Am. J. Physiol. 1989;257:H1127–H1131. doi: 10.1152/ajpheart.1989.257.4.H1127. [DOI] [PubMed] [Google Scholar]
- MÜGGE A., RIEDEL M., BARTON M., KUHN M., LICHTLEN P.R. Endothelium independent relaxation of human coronary arteries by 17β-oestradiol in vitro. Cardiovasc. Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- OLESEN S.P., DREJER J., AXELSSON O., MOLDT P., BANG L., NIELSEN-KUDSK J.E., BUSSE R., MULSCH A. Characterization of NS2028 as a specific inhibitor of soluble guanylyl cyclase. Br. J. Pharmacol. 1998;123:299–309. doi: 10.1038/sj.bjp.0701603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMAR H.A., RAMIREZ X., GIBSON M. Properties of a progesterone-induced relaxation in human placental arteries and veins. J. Clin. Endocrinol. Metab. 1995;80:370–373. doi: 10.1210/jcem.80.2.7852492. [DOI] [PubMed] [Google Scholar]
- PETERS M., TEN CATE H., STURK A. Acquired protein S deficiency might be associated with a prethrombotic state during estrogen treatment for tall stature. Thromb. Haemostas. 1992;68:371–372. [PubMed] [Google Scholar]
- PIASCIK M.T., BABICH M., RUSH M.E. Calmodulin-stimulation and calcium regulation of smooth muscle adenylate cyclase activity. J. Biol. Chem. 1983;258:10913–10918. [PubMed] [Google Scholar]
- RAMIREZ R.J., GIBSON M., KALENIC J., EINYIG S., OMAR H.A. In vitro vascular relaxation to progesterone and its metabolites in human umbilical and placental blood vessels. J. Maternal-Fetal Invest. 1998;8:61–65. [PubMed] [Google Scholar]
- ROSENFELD C.R., COX B.E., ROY T., MAGNESS R.R. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J. Clin. Invest. 1996;98:2158–2166. doi: 10.1172/JCI119022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSING J., TANS G., NICOLAES G.A.F., THOMASSEN M.C.L.G.D., VAN OERLE R., VAN DER PLOEG P.M.E.N., HEIJNEN P., HAMULYAK K., HEMKER H.C. Oral contraceptives and venous thrombosis: different sensitivities to activated protein C in women using second- and third-generation oral contraceptives. Br. J. Hematol. 1997;97:233–238. doi: 10.1046/j.1365-2141.1997.192707.x. [DOI] [PubMed] [Google Scholar]
- SABRA A., BONNAR J. Hemostatic system changes induced by 50 μg and 30 μg estrogen/progestogen oral contraceptives. Modification of estrogen effects by levonorgestrel. J. Reproduc. Med. 1983;28:85–91. [PubMed] [Google Scholar]
- SARTWELL P.E., MASI A.T., ARHES F.D.G., GREEN G.R., SMITH H.E. Thromboembolism and oral contraceptives: an epidemiologic case-control study. Am. J. Epidemiol. 1969;90:365–380. doi: 10.1093/oxfordjournals.aje.a121082. [DOI] [PubMed] [Google Scholar]
- SHAN J., RESNICK L.M., LIU Q.-Y., WU X.-C., BARBAGALLO M., PANG P.K.T. Vascular effects of 17β-estradiol in male Sprague-Dawley rats. Am. J. Physiol. 1994;266:H967–H973. doi: 10.1152/ajpheart.1994.266.3.H967. [DOI] [PubMed] [Google Scholar]
- SPITZER W.O., LEWIS M.A., HEINEMANN L.A.J., THOROGOOD M., MACRAE K.D. Third generation oral contraceptives and risk of venous thromboembolic disorders: an international case-control study. B.M.J. 1996;312:83–88. doi: 10.1136/bmj.312.7023.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANGELDER G.J., SLAAF D.W., ARTS T., RENEMAN R.S. Wall shear rate in arterioles in vivo: least estimates from platelet velocity profiles. Am. J. Physiol. 1988;254:H1059–H1064. doi: 10.1152/ajpheart.1988.254.6.H1059. [DOI] [PubMed] [Google Scholar]
- VAN BUREN G.A., YANG D.-S., CLARK K.E. Estrogen-induced uterine vasodilatation is antagonized by L-nitroarginine methyl ester, an inhibitor of nitric oxide synthesis. Am. J. Obstet. Gynecol. 1992;167:828–833. doi: 10.1016/s0002-9378(11)91597-x. [DOI] [PubMed] [Google Scholar]
- VESSEY M.P., MANT D., SMITH A., YEATES D. Oral contraceptives and venous thromboembolism: findings in a large prospective study. B.M.J. 1986;292:526–529. doi: 10.1136/bmj.292.6519.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLTERRANI M., ROSANO G., COATS A., BEALE C., COLLINS P. Estrogen acutely increases peripheral blood flow in postmenopausal women. Am. J. Med. 1995;99:119–122. doi: 10.1016/s0002-9343(99)80130-2. [DOI] [PubMed] [Google Scholar]
- WANG R., WU L.Y., KARPINSKI E., PANG P.K. The effects of parathyroid hormone on L-type voltage-dependent calcium channel currents in vascular smooth muscle cells and ventricular myocytes are mediated by a cyclic AMP dependent mechanism. FEBS Lett. 1991;282:331–334. doi: 10.1016/0014-5793(91)80507-y. [DOI] [PubMed] [Google Scholar]
- WHITE R.E., DARKOW D.J., FALVO LANG J.L. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ. Res. 1995;77:936–942. doi: 10.1161/01.res.77.5.936. [DOI] [PubMed] [Google Scholar]
- WORLD HEALTH ORGANIZATION COLLABORATIVE STUDY OF CARDIOVASCULAR DISEASE AND STEROID HORMONE CONTRACEPTION Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. Lancet. 1995a;346:1575–1582. [PubMed] [Google Scholar]
- WORLD HEALTH ORGANIZATION COLLABORATIVE STUDY OF CARDIOVASCULAR DISEASE AND STEROID HORMONE CONTRACEPTION Effect of different progestagens in low oestrogen oral contraceptives on venous thromboembolic disease. Lancet. 1995b;346:1582–1588. [PubMed] [Google Scholar]
- XIONG Z., SPERELAKIS N., FENOGLIO-PREISER C. Regulation of L-type calcium channels by cyclic nucleotides and phosphorylation in smooth muscle cells from rabbit portal veins. J. Vasc. Res. 1994;31:271–279. doi: 10.1159/000159053. [DOI] [PubMed] [Google Scholar]


