Abstract
Prostaglandin (PG) E2 (PGE2) is a potent prostanoid derived from arachidonic which can interact with EP1, EP2, EP3 and EP4 prostanoid receptor subtypes.
Recombinant human EP4 receptors expressed in human embryonic kidney (HEK-293) cells were evaluated for their binding characteristics using [3H]-PGE2 and a broad panel of natural and synthetic prostanoids in order to define their pharmacological properties.
[3H]-PGE2 binding was optimal in 2-[N-Morpholino]ethanesulphonic acid (MES) buffer (pH 6.0) yielding 98±0.7% specific binding. The receptor displayed high affinity (Kd=0.72±0.12 nM; n=3) for [3H]-PGE2 and interacted with a saturable number of binding sites (Bmax=6.21±0.84 pmol mg−1 protein).
In competition studies, PGE2 (Ki=0.75±0.03 nM; n=12) and PGE1 (Ki=1.45±0.24 nM; n=3) displayed high affinities, as did two derivatives of PGE1, namely 11-deoxy-PGE1 (Ki=1.36±0.34 nM) and 13,14-dihydro-PGE1 (Ki=3.07±0.29 nM).
Interestingly, synthetic DP receptor-specific agonists such as BW245C (Ki=64.7±1.0 nM; n=3) and ZK118182 (Ki=425±42 nM; n=4), and the purported EP3 receptor-specific ligand enprostil (Ki=43.1±4.4 nM), also displayed high affinity for the EP4 receptor.
Two known EP4 receptor antagonists were weak inhibitors of [3H]-PGE2 binding akin to their known functional potencies, thus: AH23848 (Ki=2690±232 nM); AH22921 (Ki=31,800±4090 nM).
These studies have provided a detailed pharmacological characterization of the recombinant human EP4 receptor expressed in HEK-293 cells.
Keywords: EP4 receptor, prostanoids, cloned human EP4 receptor, ligand binding
Introduction
Prostanoids, including prostaglandins (PGs) and thromboxanes, exert diverse effects in biological systems, ranging from vasodilation and smooth muscle contraction or relaxation to platelet aggregation and immunoregulation (see Coleman et al., 1994b for review). This versatility is due, in part, to the number of prostanoid classes and their corresponding receptors (DP, EP, FP, IP, and TP) which are linked through G-proteins to different signalling pathways (cyclic AMP, phosphoinositide turnover, Ca2+ mobilization, etc.) (Coleman & Humphrey, 1993; Coleman et al., 1994b). Whilst prostanoids play important regulatory roles in foetal vascular development (Chemtob et al., 1996; Nguyen et al., 1997), maintenance of vascular tone (Schror, 1993), the immune response (Meja et al., 1997; Wise, 1998), and even neuroprotection (Akaike et al., 1994), they have also been implicated as possibly contributing to the pathogenesis of asthma (Wenzel, 1997), arthritis (Wittenburg et al., 1993), and immunosuppressive syndromes (Giacomini et al., 1998). Clinical interest in prostanoid agonists and antagonists has thus been stimulated due to their potential use as drugs to treat a variety of conditions. Recently, such compounds have also shown utility in reducing intraocular pressure (IOP), a risk factor associated with glaucoma (Bito et al., 1993). Studies on the distribution of prostanoid receptors in the eye have revealed receptors for EP, DP, and/or FP receptors in the ciliary muscle (Matsuo & Cynader, 1992; Sharif et al., 1999; Davis & Sharif, 1999) and trabecular meshwork (Anthony et al., 1998) structures which are key to the maintenance of IOP. As would be expected considering their repertoire of activities in other systems, different prostanoids serve to lower IOP by different mechanisms. Whereas PGF2α appears to increase outflow via the uveoscleral route by promoting remodelling of the extracellular matrix in the ciliary muscle (Gabelt & Kaufman, 1989; Lindsey et al., 1996), PGE1 may lower IOP by stimulating EP4 and/or EP2 receptors and promoting conventional outflow through the trabecular meshwork, though the exact mechanism is still unclear (Dijkstra et al., 1999). Even if limited to the E-series of prostanoids, a wide array of biological responses is still possible due to the presence of at least four subclasses of EP receptor (EP1, EP2, EP3, and EP4) (Coleman et al., 1994b) and splice variants within the EP3 class (e.g. EP3a, EP3b, etc.; Ichikawa et al., 1996). These EP receptor subtypes have been cloned from mouse, rat, and human cDNA libraries, and the mechanisms of signal transduction for each subclass delineated (Coleman et al., 1994b).
The EP4 subclass of EP receptor was first discovered in piglet saphenous vein (Coleman et al., 1994a,1994b) but has subsequently been detected in human leukocytes (Mori et al., 1996), human kidney (Morath et al., 1999), Chinese hamster ovary cells (Milne et al., 1994; Crider et al., 2000) and ocular tissues, namely the ciliary muscle and epithelium (Mukhopadhyay et al., 1997). Activation of the EP4 receptor by PGE2 increases intracellular cyclic AMP (Milne et al., 1994; Crider et al., 2000), and this leads to such responses as the relaxation of smooth muscle (Lydford et al., 1996). The initial characterization of the recombinant EP4 receptors from the rat (Boie et al., 1997), mouse (Kiriyama et al., 1997) and human (Marshall et al., 1997) has been described using a limited number of prostanoids and related compounds. In the current studies, we aimed to employ a battery of 37 natural and synthetic prostanoids to pharmacologically characterize the receptor binding of [3H]-PGE2 binding to membranes prepared from human embryonic kidney (HEK-293) cells transfected with the recombinant human EP4 receptor.
Methods
Recombinant human EP4 receptor preparation
Cell membranes from HEK-293 cells expressing the recombinant human EP4 receptor were obtained from Receptor Biology, Inc. (Beltsville, MD, U.S.A.). The membranes (Batch No. 1496, 3.8 pmole mg−1 protein) were stored in liquid nitrogen until use.
Radioligand
[3H]-PGE2 (NEN Life Science Products, Inc., Boston, MA, U.S.A.) was used in competitive binding studies. The radioligand (No. NET-428, lot 3281-134) had a specific activity of 200 Ci mmol−1 and was supplied at 0.1 mCi ml−1. This reagent was stored at −40°C prior to use.
Chemicals and prostanoid compounds
Ethylenediaminetetraacetic acid (EDTA), 2-[N-morpholino]ethanesulphonic acid (MES), polyethylenimine (PEI), and manganese chloride were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Potassium hydroxide (KOH, 45%) was purchased from EM Sciences (Gibbstown, NJ, U.S.A.). The native prostanoids PGE1, PGE2, PGF2α, PGI2, and PGD2 as well as the following prostanoid compounds were obtained from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.): latanoprost, cloprostenol, fluprostenol (racemic), iloprost, sulprostone, misoprostol, 17-phenyl-ω-trinor-PGE2, 11-deoxy-16,16-dimethyl- PGE2, 11-deoxy-PGE1, 13,14-dihydro-PGE1. An additional group of compounds was synthesized in-house at Alcon Research, Ltd. (Fort Worth, TX, U.S.A.): AL-5848 (+-isomer of fluprostenol), AL-6221 (isopropyl ester of AL-5848), PHXA85, enprostil, 16-R-Butaprost, BW245C, SQ27986, UFO-21, AL-6556 (13,14-dihydro-ZK118182) and AL-6598 (isopropyl ester of AL-6556) as well as the putative EP4 receptor ligands (Burk, 1997) AL-24615 (15-methoxy-17-(2-furanyl)-18,19,20-trinor-PGF2α) and AL-24620 (17-(5-methyl-2-furanyl)-18,19,20-trinor-PGF2α) (refer to Figure 1 for structures of these compounds). The compounds AH6809 and SQ29548 were obtained from Tocris Cookson, Inc. (Ballwin, MO, U.S.A.), and Research Biochemicals Inc (Natick, MA, U.S.A.), respectively. ZK110841 and ZK118182 were generous gifts from Schering AG (Berlin, Germany). In addition, S-1033, SC19920, and RS93520 were kind generous gifts from Shionogi (Osaka, Japan), G.D. Searle (Skokie, IL, U.S.A.), and Hoffman-La Roche (Basel, Switzerland), respectively. The compounds AH22921X, AH23848B and BWA868C were generous gifts from Glaxo Wellcome, Inc. (Stevenage, U.K.). Chemical structures and the names of the most commonly used prostanoids can be found in the publications of Coleman & Humphrey (1993) and Coleman et al. (1994b).
Figure 1.
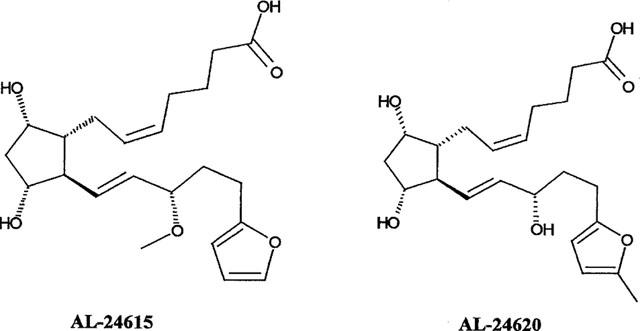
Chemical structures of two putative EP4 receptor ligands evaluated in this study. Chemical names for each are given in the Methods section.
Competitive binding assays
Assays were conducted in 10 mM 2-[N-Morpholino]ethanesulphonic acid (MES) buffer containing 1 mM EDTA, 10 mM MnCl2, adjusted to pH 6.0 with KOH. Initial tissue linearity studies were carried out using 0.5–160 μg of cell membranes containing the recombinant human EP4 receptor per 0.5 ml total volume in 96-well deep well assay blocks (Matrix Technologies Corp., Hudson, NH, U.S.A.), using 300 pM [3H]-PGE2 (final concentration). Membranes were thawed quickly, diluted to the desired concentration in the binding buffer (see above), and mixed to a homogeneous suspension prior to dispensation. After addition of the radioligand, the assay mixtures were incubated at 23°C for 90 min on a rotary shaker (60 r.p.m.). The assay was terminated by rapid vacuum filtration on Whatman GF/B glass fibre filter mats (previously soaked in 0.5% polyetheleneimine) using cold 10 mM MES, 1 mM EDTA (pH 6). The filter mats were dried in a microwave oven for 3 min prior to being sealed in a plastic bag with 30 ml Wallac Betaplate Scint scintillation fluid (Wallac Oy, Turku, Finland). Bound radioligand was then quantitated by liquid scintillation spectrometry at 50% efficiency. For routine assays, the recombinant human EP4 receptor preparation were used at 4 μg per 0.5 ml total volume. A series of competitive binding assays was carried out with unlabelled PGE2 to determine the dissociation constant (Kd) and maximal ligand binding (Bmax) values for the membrane preparation using the specific activity dilution methodology (McPherson, 1983). In these assays, unlabelled PGE2 was diluted in 10 half log steps and 50 μl of each dilution was added to the assay block in duplicate using a Biomek® 2000 automated laboratory workstation (Beckman Instruments, Inc., Fullerton, CA, U.S.A.). This was followed by the addition of 400 μl of membranes and 50 μl of [3H]-PGE2 (final concentration 200 pM). Other assay parameters were as detailed above. For determination of inhibition constant (Ki) values, prostanoid compounds were diluted in five log steps and assayed in duplicate as described above. Nonspecific binding (NSB) in both assay formats was determined with 10 μM PGE2 or 10 μM 11-deoxy-PGE1, both yielding very similar results In some instances, NSB was defined by the highest concentration of the test compound. The amount of specific binding obtained from these types of studies were very similar and the data were pooled. Multiple experiments were performed with each compound.
Data analysis
Resulting disintegrations per min (d.p.m.) values of bound [3H]-PGE2 from individual assays were analysed with a non-linear, iterative curve fitting computer program using logistic functions (Bowen & Jerman, 1995) to derive the inhibition constants (IC50s) for the competing compounds. For derivation of compound inhibition constant (Ki) values, the method of Cheng & Prusoff (1973) was employed using the following equation: Ki=IC50/(1+[L]/Kd), where IC50 is the compound concentration causing 50% inhibition of the binding, L is the radioligand concentration used in the competition experiments, and Kd the dissociation constant of the radioligand. The Kd and Bmax (apparent receptor density) values were computed from data obtained from additional 7-point competition curves of unlabelled PGE2 competing for [3H]-PGE2 (using the specific activity dilution technique) using the ‘KELL' (EBDA) software package (Biosoft, Cambridge, U.K.) as previously described (McPherson, 1983). All data were represented as the arithmetic mean±s.e.mean.
Statistical analyses, when comparing the Hill coefficients of the different compounds competing for specific [3H]-PGE2 binding, involved a two tailed unpaired t-test with Welch correction with unequal variances. The data were assumed to follow Gaussian distribution. A P value of at least 0.05 was considered statistically significant.
Results
Tissue linearity
Binding of [3H]-PGE2 to HEK-293 cell membranes containing the recombinant human EP4 receptor in the initial tissue linearity studies using the MES buffer is depicted in Figure 2. Specific binding was virtually indistinguishable from total binding with this membrane preparation, as the per cent specific binding values were 98.1±0.7% (n=12) across the entire titration range. In order to conserve membrane stocks and still preserve adequate signal-to-noise ratio, it was decided that 4 μg per 0.5 ml of the membrane preparation would be sufficient for subsequent assays. Krebs buffer was detrimental for specific [3H]-PGE2 binding (data not shown) and hence MES buffer was chosen for all subsequent studies.
Figure 2.
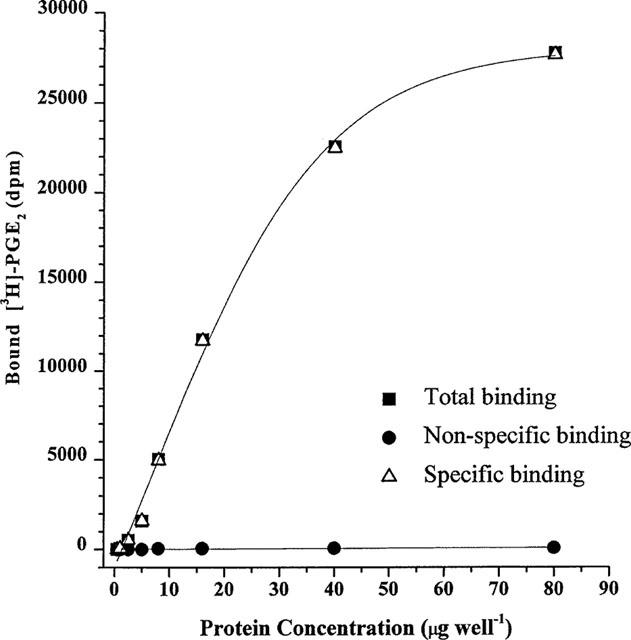
Tissue linearity of [3H]-PGE2 binding to HEK-293 cell membranes expressing the recombinant human EP4 receptor. Membranes were added in varying amounts to 0.2 nM [3H]-PGE2 in a final volume of 500 μl and the binding assay performed as described in the Methods. Data from a representative experiment is shown. Note the very high level of specific binding found in this system.
Determination of Kd and Bmax values
Unlabelled PGE2 (seven concentrations) competed for [3H]-PGE2 binding in a concentration-dependent manner and analysis of these data produced a linear Scatchard plot (Figure 3). Computer analyses of these competition binding data by the specific activity dilution technique yielded affinity parameters such as the apparent dissociation constant (Kd) and the apparent receptor density (Bmax) as follows: Kd=0.72± 0.12 nM and Bmax=6.21±0.84 pmol mg−1 protein (n=3).
Figure 3.
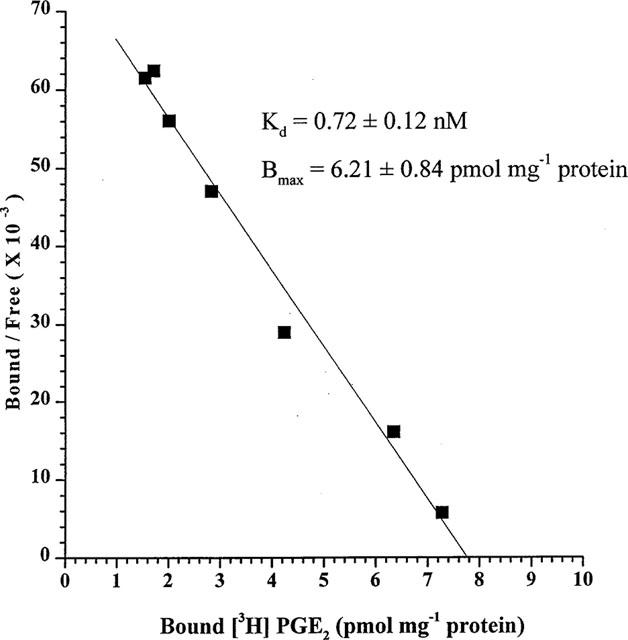
Scatchard analysis of [3H]-PGE2 binding to recombinant human EP4 receptors. Data from a representative experiment of three is shown. The composite Kd and Bmax values are shown.
Determination of Kivalues for prostanoid compounds
A broad range of prostanoid and related compounds with various prostanoid class specificities were allowed to compete against 0.2 nM [3H]-PGE2 to generate competition curves. Computer analyses of the binding data from these experiments produced inhibition constants (IC50) values for each compound which were then converted to Ki values as described in the Methods section. The compounds could be divided into groups representing either their source (natural prostanoids as opposed to synthetic) or their relative specificity/affinity for specific prostanoid receptor classes as described in the literature (e.g. Coleman et al., 1994b). Plots depicting such competition curves resulting from these experiments are presented in Figures 4 and 5, while summaries of the derived Ki data can be seen in Tables 1, 2, and 3.
Figure 4.
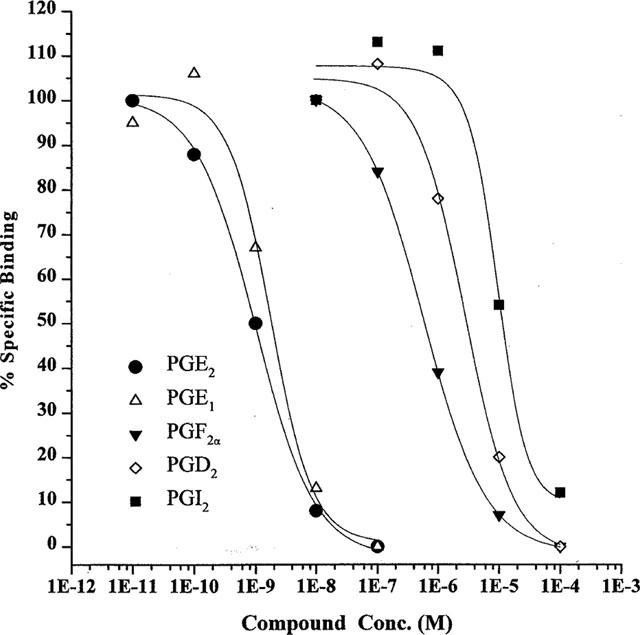
Competitive binding data for natural prostanoids at recombinant human EP4 receptors. Data are means from 3–12 experiments. Error bars are omitted for clarity.
Figure 5.
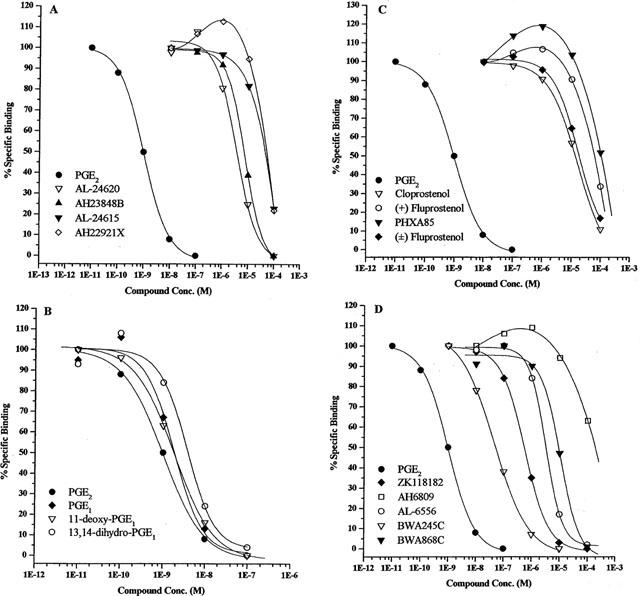
Competitive binding data for synthetic prostanoids, PGE1, PGE2 and putative EP4 receptor agonists and antagonists. (A) shows data for PGE2, two putative EP4 receptor agonists (AL-24620 and AL-24615) and two known EP4 receptor antagonists (AH23848B and AH22921X); (B) shows data for PGE2, PGE1 and its derivatives; (C) shows data for PGE2 and FP receptor agonists; (D) shows data for PGE2 and DP receptor ligands. Data are means from 3–12 experiments. Error bars are omitted for clarity.
Table 1.
Competitive binding data for natural prostanoids competing for [3H]-PGE2 binding to cloned human EP4 receptors
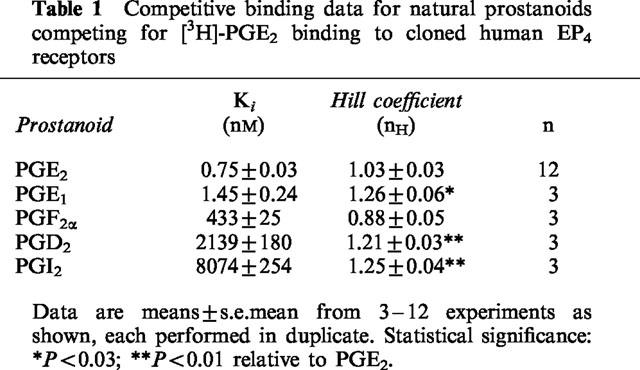
Table 2.
Ligand binding data for EP receptor-specific prostanoid compounds competing for [3H]-PGE2 binding to cloned human EP4 receptors
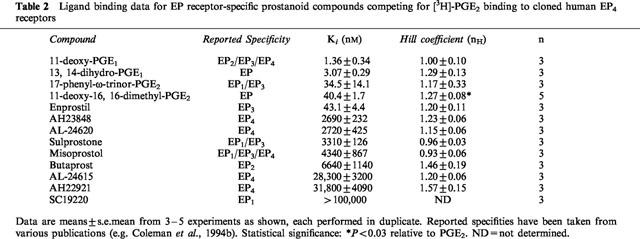
Table 3.
Ligand binding data for DP, FP, and IP receptor-specific compounds competing for [3H]-PGE2 binding to cloned human EP4 receptors
A correlation of −log Ki (pKi) values resulting from the current studies with those previously obtained by Boie et al. (1997) with recombinant rat EP4 receptors expressed in HEK-293 cells yielded a linear regression fit with a correlation coefficient of 0.84 (Figure 6). Similar comparisons to previously published binding data for mouse (Kiriyama et al., 1997) and human EP4 (Marshall et al., 1997) receptors and data from our current studies yielded correlation coefficients of 0.76 and 0.98, respectively (data not shown).
Figure 6.
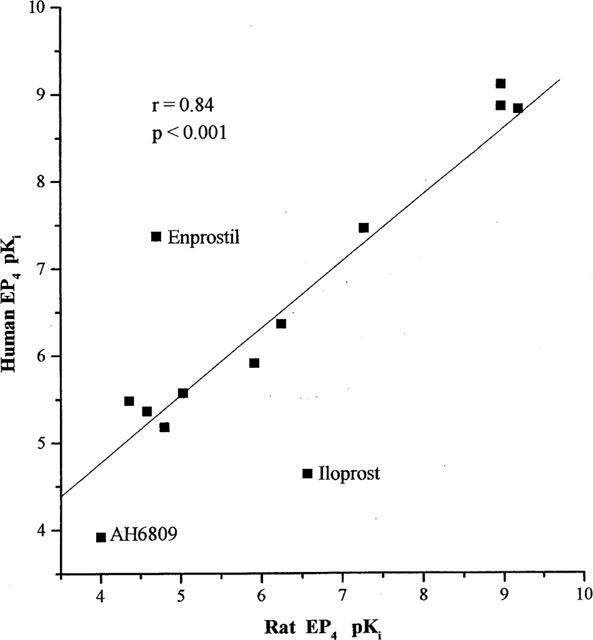
Correlation plot of recombinant human EP4 receptor binding data for various prostanoids from this study and those reported by Boie et al. (1997) for the recombinant rat EP4 receptor expressed in HEK-293 cells. Data points for compounds distinct from the regression line are individually labelled. Similar plots using data from mouse (Kiriyama et al., 1997) and human (Marshall et al., 1997) studies yielded correlation coefficients (r) of 0.76 and 0.98, respectively (data not shown). r=the correlation coefficient.
Discussion
The EP4 receptor represents one of the four major EP prostanoid receptors with which the endogenous prostanoid PGE2 can interact (Coleman et al., 1994a,1994b). Although EP4 and EP2 receptors are positively coupled to adenylyl cyclase and thus raise intracellular cyclic AMP levels (Ichikawa et al., 1996; Crider et al., 1998; 2000), there are distinct differences between the two subtypes. The EP4 receptor responds more quickly to agonist-induced desensitization than the EP2 receptor and the functional response to PGE2 is more rapidly attenuated as the agonist is metabolized (Nishigaki et al., 1996). Functional responses elicited by the EP4 receptor may be more finely regulated than those arising from EP2 receptor stimulation (Nishigaki et al., 1996). Whereas the EP2 receptor is apparently only incorporated into the cell surface membrane, the EP4 receptor has been detected in the nuclear envelope of porcine brain and rat liver endothelial cells in addition to the cell surface membrane (Bhattacharya et al., 1999), suggesting a role in transcription. However, to the best of our knowledge, all the ligand binding studies reported to-date in the literature, and the current studies, employed cell membranes for [3H]-PGE2 binding.
Comparisons can be made with the Kd and Bmax values generated in the present study to those generated in the three other studies utilizing recombinant human, mouse, or rat EP4 receptors. Overall, the Kd values compared well: in the current study we obtained a Kd value of 0.72±0.12 nM compared to 1.12±0.3 nM in the only other detailed study of the human EP4 receptor to-date (Marshall et al., 1997). The Bmax values in these two studies were also similar (6.21–0.84 pmol mg−1 protein here, versus 3.1±0.3 pmol mg−1 in the study by Marshall et al., 1997). The historical Kd values for the rat and mouse recombinant EP4 receptors are 1.1±0.6 nM (Boie et al., 1997) and 2.5±0.1 nM (Kiriyama et al., 1997), respectively. The Bmax values for the two rodent preparations (0.9±0.5 and 0.275±0.01 pmol mg−1 protein) are much lower than those obtained with the recombinant human receptor preparations.
Initial characterization of the EP4 receptor following its cloning from mouse (Kiriyama et al., 1997), rat (Boie et al., 1997), and human sources (Marshall et al., 1997) utilized only a small number of prostanoids and related compounds. To support future functional studies, we wished to define a prostanoid binding profile for the recombinant human EP4 receptor with a wide range of compounds (37 in total) that differ in their currently defined prostanoid receptor specificity. The receptor binding parameters and compound affinity data we obtained for the natural prostanoids compared very well with those generated by other investigators for the mouse, rat, and human EP4 receptors. Thus, we obtained a Ki value of 0.75 nM for PGE2, versus values of 1.9 nM (Kiriyama et al., 1997), 1.1 nM (Boie et al., 1997), and 2.51 nM (Marshall et al., 1997), respectively. Similar points of comparison could be made for PGE1 (Ki=1.45 nM, versus 2.1, 0.66, and 3.16 nM), and the other natural prostanoids (PGF2α, PGD2, and PGI2) tested in this study. In the case of the synthetic prostanoids, results were mixed. For the EP receptor specific compounds, some differences seemed to be species-dependent. In these cases, our results compared well with those generated by Boie et al. (1997) with the rat EP4 receptor (i.e. Ki for 11-deoxy-PGE1=1.45 nM at recombinant human EP4, versus 1.1 nM for the rat) but contrasted with those from the mouse EP4 receptor studies (Ki=23 nM) (Kiriyama et al., 1997). The same pattern is evident in the binding results for 17-phenyl-ω-trinor-PGE2 (Ki=23 nM at recombinant human EP4 here, versus 54 nM for the rat, and 1000 nM for the mouse receptor) (Boie et al., 1997; Kiriyama et al., 1997). However, there were instances in which the human and rat EP4 receptors yielded widely disparate results. This is most apparent in the data for sulprostone (Ki=3310 nM here at recombinant human EP4, versus 43,600 nM for the rat EP4) and misoprostol (Ki=4340 nM here at recombinant human EP4, versus 26,300 nM for the rat EP4). These comparisons can probably be ascribed to possible species differences for these particular compounds previously reported to have affinity for EP3 and EP1 receptors (Coleman et al., 1994b). However, it is worth noting that the EP4 receptor-specific antagonist AH23848 yielded similar results to other studies using human EP4 receptors (Ki=2690 nM here, versus 3981 nM from Marshall et al. (1997)) as well as in the rat (Ki=9380 nM; Boie et al., 1997). Furthermore, the recombinant human EP4 receptor binding affinities of both the EP4 receptor antagonists, AH22921 and AH23848, compared well with their functional antagonist potencies measured in the piglet saphenous vein (Kb=4–5 μM; Coleman et al., 1994a,1994b) and in the cyclic AMP production assay in wild-type CHO cells (Kb=25.1 μM and 5.01 μM, respectively; Crider et al., 2000). AL-24615 and AL-24620, which were reported to have IC25 values of 57 nM and 23 nM in the isolated rabbit jugular vein preparation (Burk, 1997), exhibited low micromolar affinities at the recombinant human EP4 receptor in our studies (Table 2). Functional studies with the recombinant human EP4 receptor with these compounds are necessary in order to determine whether these differences are species related.
Of the FP receptor-specific ligands tested in this study, only S-1033 yielded a Ki value less than 10 μM. This is somewhat surprising given the sub-micromolar affinity of PGF2α in this system. However, PGF2α is often regarded as a ‘promiscuous' ligand, well known for binding to other prostanoid receptors (Coleman et al., 1994b). Thus, the Ki for PGF2α at the recombinant human EP4 receptor in our studies is 400 nM, comparable to the values obtained at the rat EP4 receptor (570 nM; Boie et al., 1997) and the previous human EP4 receptor study (794 nM; Marshall et al., 1997). This value is also comparable to that of S-1033, although the most specific FP receptor agonists to-date, cloprostenol and fluprostenol (Sharif et al., 1998; 1999), displayed low affinities for the recombinant human EP4 receptor in our studies with Ki values greater than 10 μM.
Interestingly, some synthetic DP receptor-specific agonists showed a high affinity for the EP4 receptor in this study. Several of these compounds displayed affinities in the nanomolar range, with BW245C (Ki=64.7 nM) being the best example (Table 3). The affinity of PGD2 itself in this system is only approximately 2 μM. These results are similar to those previously reported in another study of the human EP4 receptor (Wright et al., 1998) with the exception that in our hands, ZK110841 yielded an approximate Ki of 845 nM, compared to the 41 nM value observed in their study. It is difficult to speculate on possible reasons for this difference, because Wright et al. (1998) provided virtually no details concerning their human EP4 receptor preparation other than the fact that the receptor was expressed in HEK-293 cells. In this study, the Ki values noted for the synthetic DP agonists are somewhat surprising considering the low affinity of PGD2 for recombinant human EP4. Agonist data generated using the recombinant human DP receptor indicate that the Ki values for PGD2, BW245C, and ZK110841 differ by only 2 fold or less (0.3–0.6 nM) (Wright et al., 1998). The DP antagonist BWA868C also demonstrated a high affinity in the above DP receptor preparation (Ki=2.3 nM). However, the same cannot be said for BWA868C in regards to this present study with recombinant human EP4 (Ki=8060 nM), yet the synthetic DP agonists display Ki values in the nanomolar range. Both BW245C and BWA868C have been demonstrated to function as agonists and antagonists, respectively, at the EP4 receptor in rabbit saphenous vein (Lydford et al., 1996). The ability of certain DP receptor-specific agonists to bind with relatively high affinity to the EP4 receptor and to function in isolated tissue preparations poses some intriguing possibilities for drug development, as both receptors increase intracellular cyclic AMP levels. While an extensive functional study of the recombinant human EP4 receptor remains to be done, it may be possible to develop bifunctional prostanoid compounds that elicit functional responses from both DP and EP4 receptors.
Some of the differences observed between our compound affinity data for recombinant human EP4 receptors expressed in HEK-293 cells and those from receptors expressed in CHO cells (Marshall et al., 1997; Kiriyama et al., 1997) may have resulted from the fact that since CHO cells express their own endogenous EP4 receptors (Milne et al., 1994; Crider et al., 2000) the data obtained by Marshall et al. (1997) and Kiriyama et al. (1997) may reflect a composite of the endogenous CHO EP4 receptors and the transfected human EP4 receptors. These issues, together with the low expression levels cited by Kiriyama et al. (1997), may account for the low the Hill slopes (nH) values reported in their study of the mouse EP4 receptor (Kiriyama et al. 1997), and may further explain the discrepancies noted above between the human, rat, and mouse binding data. When the nH values of different studies are compared, only Marshall et al. (1997) and Kiriyama et al. (1997) cited nH values for the limited number of compounds they evaluated on the cloned human and mouse EP4 receptors, respectively. Unfortunately, nH values for the rat EP4 receptor binding study (Boie et al., 1997) and another study employing human EP4 receptors (Wright et al., 1998) were not provided for comparison. Our results are generally in good agreement with those of Marshall et al. (1997) in that the majority of the few ligands they reported displayed nH values ⩾0.9. In the only two cases where they report nH values of 0.7, the Ki values are still in good agreement with those we obtained (for PGF2α, Ki=433 nM in this study, versus 794 nM; for AH23848, Ki=2690 nM here versus their Ki of 3981 nM).
In summary, a comprehensive evaluation of the binding characteristics of the recombinant human EP4 receptor has been undertaken using a total of 37 different natural and synthetic prostanoids possessing different prostanoid receptor specificities. Our results are generally comparable to previous limited studies of the human, rat, and mouse EP4 receptor, while providing additional data on a much greater number of compounds. Certain synthetic DP receptor agonists showed high affinity for the recombinant human EP4 receptor, but the true significance of these findings awaits the results of future functional studies that are currently in progress.
Acknowledgments
We thank Dr Mark Hellberg for generating Figure 1 and for helpful comments on the manuscript. The various companies that kindly provided gifts of their compounds are gratefully acknowledged.
Abbreviations
- HEK-293
human embryonic kidney cells
- IOP
intraocular pressure
- PG
prostaglandin
References
- AKAIKE A., KANEKO S., TAMURA Y., NAKATA N., SHIOMI H., USHIKUBI F., NARUMIYA S. Prostaglandin E2 protects cultured cortical neurons against N-methyl-D-aspartate receptor -mediated glutamate cytotoxicity. Brain Res. 1994;663:237–243. doi: 10.1016/0006-8993(94)91268-8. [DOI] [PubMed] [Google Scholar]
- ANTHONY T.L., PIERCE K.L., STAMER W.D., REGAN J.W. Prostaglandin F2α receptors in the human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 1998;39:315–321. [PubMed] [Google Scholar]
- BHATTACHARYA M., PERI K., RIBEIRO-DA-SILVA A., ALMAZAN G., SHICHI H., HOU X., VARMA D.R., CHEMTOB S. Localization of functional prostaglandin E2 receptors EP3 and EP4 in the nuclear envelope. J. Biol. Chem. 1999;274:15719–15724. doi: 10.1074/jbc.274.22.15719. [DOI] [PubMed] [Google Scholar]
- BITO L.Z., STJERNSCHANTZ J., RESUL B., MIRANDA O.C., BASU S. The ocular effects of prostaglandin and the therapeutic potential of a new PGF2α analog, PhXA41 (latanoprost ) for glaucoma management. J. Lipid Mediat. 1993;6:535–543. [PubMed] [Google Scholar]
- BOIE Y., STOCCO R., SAWYER N., SLIPETZ D.M., UNGRIN M., NEUSCHAFER-RUBE F., PUSCHEL G., METTERS K.M., ABRAMOVITZ M. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur. J. Pharmacol. 1997;340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- BOWEN W.P., JERMAN J. Nonlinear regression using spreadsheets. Trends. Pharmacol. Sci. 1995;16:413–417. doi: 10.1016/s0165-6147(00)89091-4. [DOI] [PubMed] [Google Scholar]
- BURK R.M. Cyclopentane heptan(ene)oic acid, 2-heteroarylalkenyl derivatives as therapeutic agents International Patent Application 1997. WO 97/31895
- CHEMTOB S., LI D.Y., ABRAN D., PERI K.G., VARMA D.R. Regulation of cerebrovascular prostaglandin E2 (PGE2) and PGF2α receptors and their functions during development. Semin. Perinatol. 1996;20:164–172. doi: 10.1016/s0146-0005(96)80045-0. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., GRIX S.P., HEAD S.A., LOUTTIT J.B., MALLETT A., SHELDRICK R.L. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostanoids. 1994a;47:151–168. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., HUMPHREY P.P.A. Prostanoid receptors: their function and classification. London: Edward Arnold; 1993. [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. VIII. International union of pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994b;46:205–229. [PubMed] [Google Scholar]
- CRIDER J.Y., GRIFFIN B.W., SHARIF N.A. Endogenous EP4 prostaglandin receptors coupled positively to adenylyl cyclase in Chinese hamster ovary cells: pharmacological characterization. Prostagland, Leukotri. Essent. Fatty Acids. 2000;62:21–26. doi: 10.1054/plef.1999.0120. [DOI] [PubMed] [Google Scholar]
- CRIDER J.Y., XU S.X., GRIFFIN B.W., SHARIF N.A. Use of a semi-automated, robotic radioimmunoassay to measure cAMP generated by activation of DP-, EP2- and IP-prostaglandin receptors in human ocular and other cell-types. Prostagland., Leukotri. Essent. Fatty Acids. 1998;59:77–82. doi: 10.1016/s0952-3278(98)90055-2. [DOI] [PubMed] [Google Scholar]
- DAVIS T.L., SHARIF N.A. Quantitative autoradiographic visualization and pharmacology of FP-prostaglandin receptors in human eyes using the novel phosphorimaging technology. J. Ocular Pharmacol. Ther. 1999;15:323–336. doi: 10.1089/jop.1999.15.323. [DOI] [PubMed] [Google Scholar]
- DIJKSTRA B.G., SCHEEMANN A., HOYNG P.F. Flow after prostaglandin E1 is mediated by receptor-coupled adenylyl cyclase in human anterior segments. Invest. Ophthalmol. Vis. Sci. 1999;40:2622–2626. [PubMed] [Google Scholar]
- GABELT B.T., KAUFMAN P.L. Prostaglandin F2α increases uveoscleral outflow in the cynomolgus monkey. Exp. Eye Res. 1989;49:389–402. doi: 10.1016/0014-4835(89)90049-3. [DOI] [PubMed] [Google Scholar]
- GIACOMINI E., GIORDANI L., DI MODUGNO F., CHERSI A., LUZZATI A.L. Increased PGE2 production meditates the in vitro inhibitory effect of human immunodeficiency virus P24 immunosuppressive heptapeptide Ch7. Scand. J. Immunol. 1998;48:248–253. doi: 10.1046/j.1365-3083.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- ICHIKAWA A., SUGIMOTO Y., NEGISHI M. Molecular aspects of the structures and functions of the prostanoid E receptors. J. Lipid Mediat. Cell Signal. 1996;14:83–87. doi: 10.1016/0929-7855(96)00512-3. [DOI] [PubMed] [Google Scholar]
- KIRIYAMA M., USHIKUBI F., KOBAYASHI T., HIRATA M., SUGIMOTO Y., NARUMIYA S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDSEY J.D., KASHIWAGI K., BOYLE D., KASHIWAGI F., FIRESTEIN G.S., WEINREB R.N. Prostaglandin increase proMMP-1 and proMMP-3 secretion by human ciliary smooth muscle cells. Curr. Eye Res. 1996;15:869–875. doi: 10.3109/02713689609017628. [DOI] [PubMed] [Google Scholar]
- LYDFORD S.J., MCKECHNIE K.C., DOUGALL I.G. Pharmacological studies on prostanoid receptors in the rabbit isolated saphenous vein: a comparison with rabbit isolated ear artery. Br. J. Pharmacol. 1996;117:13–20. doi: 10.1111/j.1476-5381.1996.tb15148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL F.H., PATEL K., LUNDSTROM K., CAMACHO J., FOORD S.M., LEE M.G. Characterization of [3H]-prostanoid E2 binding to prostanoid EP4 receptors expressed with Semliki Forest virus. Br. J. Pharmacol. 1997;121:1673–1678. doi: 10.1038/sj.bjp.0701332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUO T., CYNADER M.S. Localization of prostaglandin F2α and E2 binding sites in the human eye. Br. J. Opthalmol. 1992;76:210–213. doi: 10.1136/bjo.76.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPHERSON G.A. A practical computer based approach to the analysis of radioligand binding experiments. Computer Prog. Biomed. 1983;17:107–114. doi: 10.1016/0010-468x(83)90031-4. [DOI] [PubMed] [Google Scholar]
- MEJA K.K., BARNES P.J., GIEMBYCZ M.A. Characterization of the prostanoid receptor(s) on human blood monocytes at which prostanoid E2 inhibits lipopolysaccharide-induced tumour necrosis factor-alpha generation. Br. J. Pharmacol. 1997;122:149–157. doi: 10.1038/sj.bjp.0701360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNE S.A., LEE J., ARMSTRONG R.A., WOODWARD D.F. Human monocytes and cultured CHO cells both express EP4 receptors positively coupled to adenylate cyclase. Br. J. Pharmacol. 1994;113 suppl:8P. [Google Scholar]
- MORATH R., KLEIN T., SYBERTH H.W., NUSING R.M. Immunolocalization of the four prostaglandin E2 receptor proteins EP1, EP2, EP3, and EP4 in human kidney. J. Am. Soc. Nephrol. 1999;10:1851–1860. doi: 10.1681/ASN.V1091851. [DOI] [PubMed] [Google Scholar]
- MORI K., TANAKA I., KOTANI M., MIYAOKA F., SANDO T., MURO S., SASAKI Y., NAKAGAWA O., OGAWA Y., USUI T., OZAKI S., ICHIKAWA A., NAURMIYA S., NAKAO K. Gene expression of the human prostaglandin E receptor EP4 subtype: differential regulation in monocytoid and lymphoid lineage cells by phorbol ester. J. Mol. Med. 1996;74:333–336. doi: 10.1007/BF00207510. [DOI] [PubMed] [Google Scholar]
- MUKHOPADHYAY P., GEOGHEGAN T.E., PATIL R.V., BHATTACHERJEE P., PATERSON C.A. Detection of EP2, EP4, and FP receptors in human ciliary epithelial and ciliary muscle cells. Biochem. Pharmacol. 1997;53:1249–1255. doi: 10.1016/s0006-2952(97)00011-7. [DOI] [PubMed] [Google Scholar]
- NGUYEN M., CAMENISCH T., SNOUWAERT J.N., HICKS E., COFFMAN T.M., ANDERSON P.A.W., MALOUF N.N., KOLLER B.H. The prostanoid receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- NISHIGAKI N., NEGISHI M., ICHIKAWA A. Two G2-coupled prostaglandin E receptor subtypes, EP2 and EP4, differ in desensitization and sensitivity to the metabolic inactivation of the agonist. Mol. Pharmacol. 1996;50:1031–1037. [PubMed] [Google Scholar]
- SCHROR K. The effect of prostaglandin and thromboxane A2 on coronary vessel tone—mechanisms of action and therapeutic implications. Eur. Heart J. 1993;14 Suppl I:34–41. [PubMed] [Google Scholar]
- SHARIF N.A., DAVIS T.L., WILLIAMS G.W. [3H]AL-5848 ([3H]9-β-[+]fluprostenol): carboxylic acid of travoprost (AL-6221), a novel FP prostaglandin to study the pharmacology and autoradiographic localization of the FP receptor. J. Pharmac. Pharmacol. 1999;51:685–694. doi: 10.1211/0022357991772989. [DOI] [PubMed] [Google Scholar]
- SHARIF N.A, , XU S.X., WILLIAMS G.W., GRIFFIN B.W., CRIDER J.Y., DAVIS T.L. Pharmacology of [3H]Prostaglandin E1/[3H]Prostaglandin E2 and [3H]Prostaglandin F2α binding to EP3 and FP receptor binding sites in bovine corpus luteum: characterization and correlation with functional data. J. Pharmacol. Expt. Ther. 1998;286:1094–1102. [PubMed] [Google Scholar]
- WENZEL S.E. Arachidonic acid metabolites: mediators of inflammation in asthma. Pharmacotherapy. 1997;17:3S–12S. [PubMed] [Google Scholar]
- WISE H. Activation of the prostanoid EP4-receptor subtype is highly coupled to inhibition of N-formyl-methionyl-leucyl-phenylalanine- stimulated rat neutrophil aggregation. Prostanoid. Leukotri., Essent. Fatty Acids. 1998;58:77–84. doi: 10.1016/s0952-3278(98)90133-8. [DOI] [PubMed] [Google Scholar]
- WITTENBURG R.H., WILLBURGER R.E., KLEEMEYER K.S., PESKAR B.A. In vitro release of prostanoids and leukotrienes from synovial tissue, cartilage, and bone in degenerative joint diseases. Arthritis Rheum. 1993;36:1444–1450. doi: 10.1002/art.1780361017. [DOI] [PubMed] [Google Scholar]
- WRIGHT D.H., METTERS K.M., ABRAMOVITZ M., FORD-HUTCHINSON A.W. Characterization of the recombinant human prostanoid DP receptor and identification of L-644,698, a novel selective DP agonist. Br. J. Pharmacol. 1998;123:1317–1324. doi: 10.1038/sj.bjp.0701708. [DOI] [PMC free article] [PubMed] [Google Scholar]


