Abstract
This study examined whether activation of 5HT1B receptors in the rodent globus pallidus (GP) could reduce GABA release in vitro and reverse reserpine-induced akinesia in vivo.
Microdissected slices of GP from male Sprague Dawley rats (300–350 g) were preloaded with [3H]-GABA. During subsequent superfusion, 4 min fractions were collected for analysis of release. The effects of the 5HT1B receptor agonist, 3-(1,2,5,6-tetrahydropyrid-4-yl)pyrrolo[3,2-b]pyrid-5-one (CP-93129), on 25 mM KCl-evoked release were examined using a standard dual stimulation paradigm.
Male Sprague Dawley rats (270–290 g), stereotaxically cannulated above the GP, were rendered akinetic by injection of reserpine (5 mg kg−1 s.c.). Eighteen hours later, the rotational behaviour induced by unilateral injection of CP-93129 was examined.
CP-93129 (0.6–16.2 μM) produced a concentration-dependent inhibition of 25 mM KCl-evoked [3H]-GABA release reaching a maximum inhibition of 52.5±4.5%. The effect of a submaximal concentration of CP-93129 (5.4 μM) was fully inhibited by the 5HT1B receptor antagonist, isamoltane (10 μM).
Following intrapallidal injection, CP-93129 (30–330 nmol in 0.5 μl) produced a dose-dependent increase in net contraversive rotations reaching a maximum of 197±32 rotations in 240 min at 330 nmol. Pre-treatment with isamoltane (10 nmol in 1 μl) inhibited the effects of a submaximal dose of CP-93129 (220 nmol) by 84±6%.
These data suggest that at least some 5HT1B receptor function as heteroreceptors in the GP, reducing the release of GABA. Moreover, CP-93129-mediated activation of these receptors in the GP provides relief of akinesia in the reserpine-treated rat model of PD.
Keywords: Akinesia, CP-93129, GABA, globus pallidus, 5HT1B receptor, reserpine, Parkinson's disease
Introduction
Chronic use of dopaminergic treatments in Parkinson's disease (PD) precipitates debilitating dyskinetic side-effects, on–off fluctuations and reduced efficacy (Stocchi et al., 1997), clearly highlighting the need for alternative therapeutic approaches. The loss of striatal dopamine innervation in PD is believed to lead to subsequent overactivity of GABAergic striatopallidal projections, since increases in GABA release have been detected by microdialysis in the primate globus pallidus externus (GPe) (Robertson et al., 1991) and rodent globus pallidus (GP; rodent homologue of primate GPe) (Segovia et al., 1986) following lesion of the nigrostriatal tract.
The changes in pallidal GABA transmission are believed to contribute to the movement deficit since elevation of GABA levels in the GP induces akinesia (Pycock et al., 1976). Therefore, correcting this GABAergic imbalance in the GP may prove therapeutically useful in the treatment of PD. In support of this, previous studies have shown that direct blockade of GABAA receptors in the GP alleviates akinesia in the reserpine-treated rat model of PD (Maneuf et al., 1994). However, owing to the present difficulty of therapeutically targeting pallidal GABAA receptors (Chadha et al., 2000b), alternative ways of modulating GABA transmission in the GP are required.
Activation of Gi/o coupled 5HT1B receptors (Adham et al., 1992) has been shown in other brain regions to reduce neurotransmitter release in an auto- or hetero-receptor fashion (Davidson & Stamford, 1997; Boulenguez et al., 1997). The presence of presynaptic 5HT1B receptors in the rodent GP has recently been confirmed, using a combination of in situ hybridization, receptor autoradiography, immunocytochemistry and kainate lesioning of the striatopallidal pathway (Bruinvels et al., 1994; Sari et al., 1999; Riad et al., 2000). However, it is not known whether activation of 5HT1B receptors in the GP can reduce GABA release in this structure.
In light of this information, the present study set out to determine (i) whether 5HT1B receptor activation reduces GABA release in the rodent GP and (ii) whether direct activation of 5HT1B receptors in the GP can reverse akinesia in the reserpine-treated rat model of PD. Some of this work has been previously published in abstract form (Chadha et al., 2000a).
Methods
Animals
Male, Sprague Dawley rats (270–350 g) were housed in a temperature- and humidity-controlled environment with a 12 h light/dark cycle and free access to food and water. All procedures were performed in accordance with the U.K. Animals (Scientific Procedures) Act 1986, and all efforts were made to minimize animal suffering and the number of animals used.
[3H]-GABA release studies
Rats were killed by stunning and decapitation, and their brains rapidly removed. Pallidal slices (400 μm thick) were prepared on an ice-cold vibratome and briefly washed in modified artificial CSF (mM: NaCl, 134; KCl, 5; CaCl2, 1.3; MgSO4, 1; NaHCO3, 25; KH2PO4, 1.25; glucose, 10; β-alanine (glial uptake inhibitor), 10 μM) at pH 7.4. Slices were loaded with [3H]-GABA by incubating in aerated (95% O2 and 5% CO2) modified aCSF containing [3H]-GABA (specific activity 93.8 Ci mmol−1) for 40 min at 36°C. Slices were then superfused (0.25 ml min−1) with modified aCSF supplemented from this point onwards with 1 mM nipecotic acid and 10 μM amino-oxyacetic acid (to prevent GABA reuptake and inhibit GABA transaminase, respectively), constantly aerated and maintained at 36°C.
Following a 40 min equilibration period, collection of 4 min release fractions commenced. Basal [3H]-GABA release was determined over the first 12 min. This was followed by a 4 min stimulation (S1) with modified aCSF containing 25 mM KCl. A 28 min washout period was then followed by a second 25 mM KCl stimulation (S2). At the end of this time, both slices and 4 min release fractions were analysed for [3H]-GABA content by liquid scintillation spectroscopy. Fractional rate of [3H]-GABA release 4 min−1 was calculated as a percentage of total radioactivity present in the tissue at the beginning of that fraction. Basal release was calculated from the mean of basal release periods prior to S1 and S2, respectively. Total [3H]-GABA release at S1 and S2 was calculated by integrating the area under a time versus percentage release rate curve (comprising a 12 min period following stimulus application). The ratio of release for the two stimulation periods (S2/S1) was determined for each experiment.
The 5HT1B agonist, 3-(1,2,5,6-tetrahydropyrid-4-yl)pyrrolo[3,2-b]pyrid-5-one (CP-93129; Macor et al., 1990), (0.6, 1.8, 5.4 or 16.2 μM) or vehicle (aCSF) was included in the superfusate 16 min before, during and after S2 in order to examine its effects on release (n=8 animals per concentration). To confirm receptor specificity, isamoltane (10 μM), a relatively potent antagonist at 5HT1B receptors (Waldmeier et al., 1988), or vehicle (aCSF) was examined against a submaximal concentration of CP-93129 (5.4 μM) by inclusion in the superfusate 8 min prior to and during exposure to CP-93129 (n=8 animals per group).
Calcium dependency of the [3H]-GABA release was assessed in some slices by replacing the superfusate with Ca2+ free aCSF containing 5 mM EGTA between S1 and S2 (now 25 mM KCl in Ca2+ free aCSF).
Intrapallidal injections in reserpine-treated rats
Under halothane anaesthesia, rats were stereotaxically implanted with 23 gauge stainless steel guide cannulae positioned 2 mm above the GP (co-ordinates: 0.92 mm posterior to and 3.0 mm lateral to bregma and 5.75 mm below the skull, according to the rat brain atlas of Paxinos & Watson, (1986)). Following a minimum of 5 days recovery, animals were treated with reserpine (5 mg kg−1, s.c.). Eighteen hours later, when animals displayed a stable level of akinesia, the effects of the 5HT1B receptor agonist, CP-93129, were assessed on motor behaviours.
Animals were placed in 40 cm diameter, flat-bottomed hemispheric bowls for visual assessment. Following a 20 min acclimatization period, baseline activity was videotaped for 30 min. Animals then received a single, unilateral injection of CP-93129 (30, 110, 220 or 330 nmol) in 0.5 μl phosphate-buffered saline (PBS) (mM: NaCl, 137; KCl, 2.7; KH2PO4 1.8; Na2HPO4, 10; pH 7.4) or vehicle (0.5 μl PBS) into the GP (n=8–11 animals per dose). Injections were made over a 2 min period via 30-gauge stainless steel needles inserted through, and extending 2 mm below the tip of the guide cannulae and attached with flexible (Portex) tubing to a 5 μl Hamilton microsyringe. Animals were videotaped for a further 330 min. Net contraversive rotations (360°C) were assessed as an index of unilateral relief of akinesia (Dawson et al., 2000). These rotations were counted manually from the videotape recordings in 10 min time bins. To confirm the receptor specificity of CP-93129, the effects of isamoltane (10 nmol) were examined against a single effective dose of CP-93129 (220 nmol). In these experiments, 7 h after the initial injection of CP-93129 (220 nmol), rats were injected with either isamoltane (10 nmol in 1 μl, pH 7.0; given over 5 min), or vehicle (1 μl PBS) into the same site (n=6–7 animals per group). Rotational behaviour was videotaped throughout the 30 min equilibration period for isamoltane and for a further 330 min following a repeat dose of CP-93129 (220 nmol). At the end of each experiment, fast blue dye (0.2 μl of 1%, w v−1) was injected via the guide cannulae to allow histological verification of injection sites. Approximately 5 min after dye injection, animals were killed by halothane overdose followed by cervical dislocation. The brains were rapidly frozen in isopentane (cooled to −45°C with solid CO2) and stored desiccated at −70°C until subsequent cryostat sectioning (20 μm) and cresyl violet (0.1% w v−1) staining.
Data analysis
For the [3H]-GABA release studies, differences in the S2/S1 ratio between the various concentrations of CP-93129 in the presence and absence of isamoltane or vehicle were analysed using one-way Analysis of Variance (ANOVA) with a Student-Neuman-Keuls post-hoc test. For the behavioural studies, the number of rotations per 10 min were compared to baseline akinesia using a one-way ANOVA with a Dunnett's post-hoc test. The number of rotations evoked over 240 min by each dose of CP-93129 or vehicle were compared using a one-way ANOVA with a Student-Newman-Keuls post-hoc test. The effects of CP-93129 prior to and following isamoltane or vehicle treatment were compared using 2-tailed paired t-test. In all cases, P<0.05 was taken to represent a significant difference.
Drugs
Isamoltane hemifumarate and 3-(1,2,5,6-tetrahydropyrid-4-yl)pyrrolo[3,2-b]pyrid-5-one (CP-93129) were obtained from Tocris Cookson Ltd., U.K. Nipecotic acid was obtained from RBI, U.K. [3H]-GABA was obtained from NEN, U.K. Reserpine, β-alanine, amino-oxyacetic acid and all standard laboratory reagents were obtained from Sigma, U.K.
Results
Effects of the 5HT1B receptor agonist on [3H]-GABA release in the globus pallidus
Individual percentage release rate curves for [3H]-GABA release from single slices of GP are shown, for the three main experimental conditions, in Figure 1. Under control conditions (Figure 1a) basal [3H]-GABA release was approximately 0.2% per 4 min fraction (0.22±0.15% per 4 min fraction; mean±s.e.mean, n=8). [3H]-GABA release increased approximately 7 fold over basal levels following the first 25 mM KCl stimulus (S1) and approximately 6.5 fold following the second stimulus (S2). The resultant mean S2/S1 ratio in control slices was 0.89±0.1 (mean±s.e.mean, n=8). The 25 mM KCl-evoked [3H]-GABA release was significantly reduced in Ca2+ free conditions by 70.0±1.9% (mean±s.e.mean, n=6; data not shown).
Figure 1.
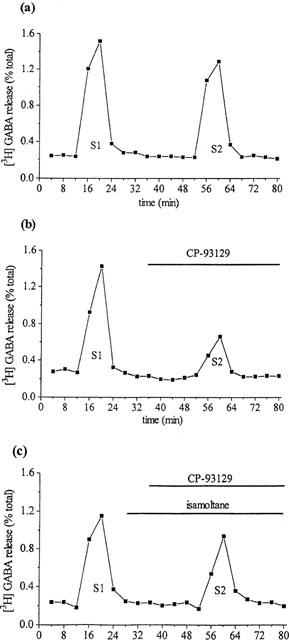
Percentage release rate of [3H]-GABA from single slices of rodent globus pallidus evoked by dual 25 mM KCl stimulation (S1 at 12 min and S2 at 52 min). (a) Release evoked under control conditions (b) effects of CP-93129 (5.4 μM) on S2 and (c) effects of CP-93129 (5.4 μM) on S2 in the presence of isamoltane (10 μM).
CP-93129 (5.4 μM) had no effect on basal [3H]-GABA release (P>0.05; n=8), but produced an approximate 45% reduction in [3H]-GABA release evoked at S2 (Figure 1b). Incubation with isamoltane (10 μM) similarly did not affect basal [3H]-GABA release (P>0.05; n=8) but reduced the inhibitory effects of CP-93129 on [3H]-GABA release evoked at S2 (Figure 1c).
The effects of increasing concentrations of CP-93129 on [3H]-GABA release ratios (S2/S1) are shown in Figure 2a (mean±s.e.mean; n=8). CP-93129 (0.6–16.2 μM) produced a significant concentration-dependent inhibition of 25 mM KCl-evoked [3H]-GABA release reaching a maximum inhibition of 52.5±4.5% with the highest concentration tested (16.2 μM) (see Figure 2a). Concentrations of CP-93129 below 0.6 μM failed to inhibit the release of [3H]-GABA (n=4; data not shown). Addition of isamoltane (10 μM) abolished the effect of 5.4 μM CP-93129 on [3H]-GABA release (P<0.05, n=8; Figure 2b).
Figure 2.
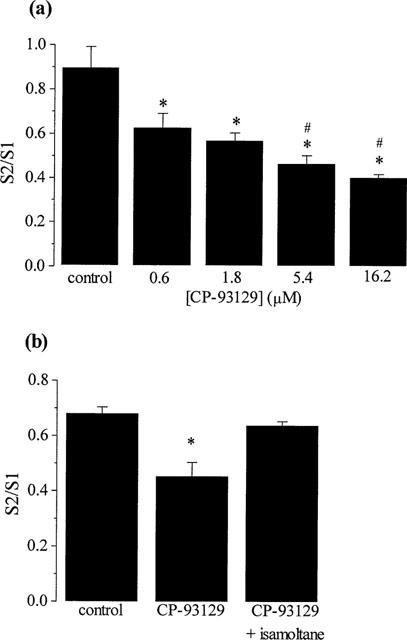
(a) Concentration-dependent effects of the 5HT1B receptor agonist, CP-93129 (0.6–16.2 μM) on 25 mM KCl-evoked [3H]-GABA release from slices of rodent globus pallidus and (b) effect of the 5HT1B receptor antagonist, isamoltane (10 μM) on the CP-93129 (5.4 μM)-mediated inhibition of 25 mM KCl-evoked [3H]-GABA release. In (b) isamoltane was present 8 min prior to and during exposure to CP-93129. Values represent mean±s.e.mean (n=8). *Indicates a significant difference compared to control; #indicates a significant difference compared to 0.6 μM (1-way ANOVA, P<0.05).
Effects of intrapallidal 5HT1B receptor agonist administration in the reserpine-treated rat
Post-mortem light microscopic examination confirmed the correct positioning of injection sites within the GP in approximately 80% of animals. Where aberrant injections were made adjacent to the GP, CP-93129 failed to induce any locomotor behaviour. Only data from those animals with correctly positioned cannulae were included in the analyses below.
During the baseline recordings, all reserpine-treated rats exhibited negligible locomotor activity (0 rotations 30 min−1) and were thus considered suitably akinetic for inclusion in the study. Unilateral injection of the 5HT1B receptor agonist, CP-93129 into the GP of reserpine-treated rats produced net contraversive rotations, the time-course for which is shown only for the maximum dose of CP-93129 (330 nmol) (Figure 3a). This rotational activity commenced immediately upon administration of CP-93129, reached a maximum rate of 10±2 turns 10 min−1 (n=8) and reversed back to baseline within 240 min. Subsequent quantification of locomotor activity induced by the full dose range of CP-93129 was thereafter made over 240 min (Figure 3b).
Figure 3.
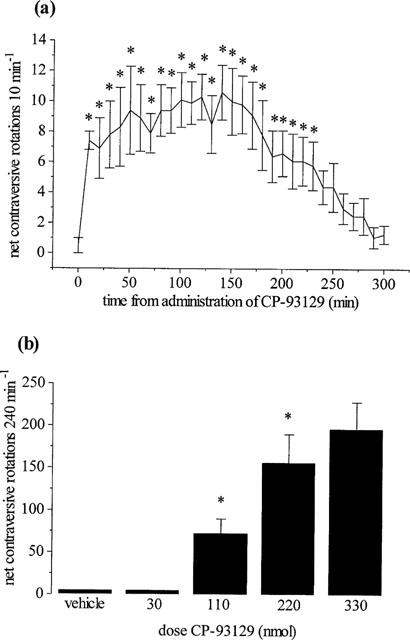
(a) Time-course of net contraversive rotations induced by the 5HT1B agonist, CP-93129 (330 nmol in 0.5 μl) and (b) dose-related effects of CP-93129 (30–330 nmol) or vehicle following unilateral intrapallidal injection in the reserpine-treated rat. Values represent mean±s.e.mean (n=8–9 animals per dose). *Indicates a significant difference compared to (a) baseline activity or (b) the previous dose (1-way ANOVA, P<0.05 in all cases).
Neither vehicle nor low dose CP-93129 (30 nmol) produced any significant net contraversive rotations over this period. In contrast, CP-93129 (110–330 nmol) produced a dose-dependent increase in net contraversive rotations 240 min−1. Three of the eleven animals tested with the highest dose (330 nmol) were excluded from the analysis since they produced central excitation in the form of wet dog shakes and intermittent barrel rolling.
Pre-treatment with isamoltane (10 nmol) significantly inhibited the CP-93129 (220 nmol)-induced net contraversive rotations 240 min−1 by 84±6% (mean±s.e.mean, n=7), reflecting a decrease in both peak response and duration. Pre-treatment with vehicle for isamoltane (PBS, pH 7.4) did not affect the subsequent response to CP-93129 (n=6). No locomotor activity was observed during the equilibration period with isamoltane alone.
Discussion
This study examined the hypothesis that activation of 5HT1B receptors may reduce GABA release in the GP and thereby provide a way of alleviating parkinsonian akinesia. The data presented indicate that (i) 5HT1B receptor activation with CP-93129 does inhibit the release of [3H]-GABA from pallidal slices and (ii) intrapallidal injection of CP-93129 alleviates akinesia in the reserpine-treated rat model of PD. Taken together these data suggest a role for 5HT1B receptors in the regulation of GABAergic function in the GP that may prove useful in the search for novel therapeutic approaches for the treatment of PD.
Heteroreceptor function of the 5HT1B receptor on GABA release in the globus pallidus
Depolarizing stimuli produced an approximate 7 fold increase in basal release of tritium from microdissected slices of rat GP. Since the GABA transaminase inhibitor, amino-oxyacetic acid, was present in the superfusate, the majority of this KCl-evoked tritium release is taken to represent [3H]-GABA and not its metabolites (Gardner & Richards, 1981). However, since no subsequent analytical examination of the release fraction was performed, the possibility of some contamination with metabolites cannot be ruled out. In agreement with previous studies on GABA release in the GP (Maneuf et al., 1994), we observed an approximate 70% reduction of release in the absence of extracellular Ca2+, indicating that the majority of the GABA released was likely to be neuronal in origin. Since the striatopallidal efferents comprise the major source of GABAergic input to the GP (Parent & Hazrati, 1995), these neurones are the most likely source of GABA release in the present study. The source of the remaining (∼30%) Ca2+-independent fraction of [3H]-GABA release is not known. However, since β-alanine and nipecotic acid were present throughout, it is unlikely to be either glial in origin or due to reverse GABA transport (Bernath & Zigmond, 1988).
Superfusion with CP-93129 (0.6–16.2 μM) resulted in a concentration-dependent inhibition of depolarization-evoked [3H]-GABA release from the GP. At the higher concentrations used, CP-93129 is not fully selective for 5HT1B receptors (Ki≈percnt;8 nM), but would also be expected to act on Gi/o coupled 5HT1A receptors (Ki≈percnt;2 μM). However, an action on 5HT1A receptors is unlikely to mediate this inhibition of [3H]-GABA release from the GP since 5HT1A receptors are largely absent from this region (Pompeiano et al., 1992; Kia et al., 1996). In contrast, 5HT1B receptors are densely expressed in the GP on preterminal axonal elements (Riad et al., 2000) that are presumed to be of striatal origin (Sari et al., 1999). These data suggest, therefore, that at least some 5HT1B receptors must be responsible for this CP-93129-induced inhibition of [3H]-GABA release from the GP via an heteroreceptor action on striatopallidal neurones. This is consistent with the previously reported auto- and hetero-receptor role of 5HT1B receptors in the brain (Davidson & Stamford, 1997; Boulenguez et al., 1997). The receptor selectivity of these effects of CP-93129 was examined further using the antagonist, isamoltane. Isamoltane shows an approximate 5 fold higher selectivity at β-adrenergic receptors (IC50=8.4 nM) compared to 5HT1B receptors (IC50=39 nM) (Waldmeier et al., 1988). However, the involvement of β-adrenergic receptors in mediating the present effects of CP-93129 is unlikely since the expression of β-adrenergic receptors in the GP is again very low (Wanaka et al., 1989). For this reason, the observed inhibitory effects of isamoltane are believed to represent inhibition of the 5HT1B-mediated effects of CP-93129.
Reversal of reserpine-induced akinesia by the 5HT1B agonist, CP-93129
Unilateral injection of CP-93129 into the GP of the reserpine-treated rat produced contraversive rotational behaviour indicative of a unilateral reversal of the reserpine-induced akinesia. Prior intrapallidal injection of isamoltane (10 nmol) inhibited the rotational response to CP-93129 by 84%, indicating that the rotational response is mediated primarily through activation of 5HT1B receptors in the GP. The dose-response relationship of this anti-akinetic effect of CP-93129 was narrow, with a threshold dose of 110 nmol and a maximally effective dose of 220 nmol. At the highest dose tested (330 nmol), CP-93129 produced central excitation, the cause of which is not known. However, this excitation is not thought to reflect the activation of adjacent non-pallidal 5HT1B receptors since aberrant injections of CP-93129 into regions adjacent to the GP did not produce any effect.
The CP-93129-mediated reduction in depolarization-evoked [3H]-GABA release from the GP, described above, is believed to be mediated via activation of 5HT1B heteroreceptors on the preterminal axons of striatopallidal neurones (Riad et al., 2000). Therefore, the most likely cellular mechanism underlying the anti-akinetic effects of intrapallidal CP-93129 in the reserpine-treated rat is inhibition of GABA release from overactive striatopallidal neurones.
Therapeutic implications for Parkinson's disease
In this study we have used the reserpine-treated rat model of PD to mimic the depletion of aminergic neurotransmitters and the akinetic symptomatology of the disease (Colpaert, 1987). These data reveal that activation of 5HT1B receptors in the GP may provide a useful means of reversing the parkinsonian akinesia. Although there is some 5-hydroxytryptaminergic neuronal degeneration in PD, the distribution and density of 5HT1B receptors appear to be preserved throughout the parkinsonian basal ganglia (Castro et al., 1998), thus providing a robust target for manipulation. However, 5HT1B receptors are also densely expressed preterminally on striatonigral neurones in the substantia nigra pars reticulata (Sari et al., 1999; Riad et al., 2000) where they appear to restrict GABA transmission (Stanford & Lacey, 1996). Studies using intraventricular or systemic administration of 5HT1B receptor agonists will help to clarify whether the in vivo activation of these additional receptors will influence the predicted beneficial effects seen following intrapallidal administration.
In conclusion, these data indicate that some 5HT1B receptors can function as heteroreceptors in the GP, reducing the release of GABA from striatopallidal neurones. Moreover, this cellular mechanism may underlie the anti-akinetic activity of CP-93129 seen in the reserpine-treated rat model of PD.
Acknowledgments
The authors would like to thank Miss E. Handford and Mr L. Dawson for their technical assistance. A. Chadha is a Medical Research Council student.
Abbreviations
- GP
globus pallidus
- PD
Parkinson's disease
- CP93129
3-(1,2,5,6-tetrahydropyrid-4-yl)pyrrolo [3,2-b] pyrid-5-one
- aCSF
artificial cerebrospinal fluid
- GABA
γ-aminobutyricacid
- 5HT
5-hdroxytryptamine
- GPe
globus pallidus externus
- EGTA
ethyleneglycol bis (B-aminoethylether) tetra-acetic acid
References
- ADHAM N., ROMANIEKO P., HARTIG P., WEINSHANK R.L., BRANCHEK T.A. The rat 5-hydroxytryptamine1B receptor is the species homologue of the human 5-hydroxytryptamine1Dα receptor. Mol. Pharmacol. 1992;41:1–7. [PubMed] [Google Scholar]
- BERNATH S., ZIGMOND M.J. Characterization of [3H]GABA release from striatal slices: evidence for a calcium independent process via the GABA uptake system. Neuroscience. 1988;27:563–570. doi: 10.1016/0306-4522(88)90289-8. [DOI] [PubMed] [Google Scholar]
- BOULENGUEZ P., RAWLINS J.N.P., CHAUVEAU J., JOSEPH M.H., MITCHELL S.N., GRAY J.A. Modulation of dopamine release in the nucleus accumbens by 5-HT1B agonists: involvement of the hippocampo-accumbens pathway. Neuropharmacol. 1997;35:1521–1529. doi: 10.1016/s0028-3908(96)00099-8. [DOI] [PubMed] [Google Scholar]
- BRUINVELS A.T., LANDWEHRMEYER B., GUSTAFSON E.L., DURKIN M.M, , MENGOD G., BRANCHEK T.A., HOYER D., PALACIOS J.M. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacol. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- CASTRO M.E., PASCUAL J., ROMON T., BERCIANO J., FIGOLS J., PAZOS A. 5-HT1B receptor binding in degenerative movement disorders. Brain Res. 1998;790:323–328. doi: 10.1016/s0006-8993(97)01566-7. [DOI] [PubMed] [Google Scholar]
- CHADHA A., ATACK J., SUR C., DUTY S. The 5-HT1B agonist, CP93129, inhibits GABA release from slices of rat globus pallidus and reverses akinesia following intrapallidal administration in the reserpine-treated rat. Br. J. Pharmacol. 2000a;129:61P. doi: 10.1038/sj.bjp.0703526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHADHA A., DAWSON L.G., JENNER P., DUTY S. Effect of unilateral 6-hydroxydopamine lesions of the nigrostriatal pathway on GABAA receptor subunit gene expression in the rodent basal ganglia and thalamus. Neuroscience. 2000b;95:119–126. doi: 10.1016/s0306-4522(99)00413-3. [DOI] [PubMed] [Google Scholar]
- COLPAERT F.C. Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rat. Neuropharmacol. 1987;26:1431–1440. doi: 10.1016/0028-3908(87)90110-9. [DOI] [PubMed] [Google Scholar]
- DAVIDSON C., STAMFORD J.A. Serotonin efflux in the rat ventral lateral geniculate nucleus assessed by fast cyclic voltammetry is modulated by 5-HT1B and 5-HT1D autoreceptors. Neuropharmacol. 1997;35:1627–1634. doi: 10.1016/s0028-3908(96)00081-0. [DOI] [PubMed] [Google Scholar]
- DAWSON L.G., CHADHA A., MEGALOU M., DUTY S. The group II metabotropic glutamate receptor agonist, DCG-IV, alleviates akinesia following intranigral or intraventricular administration in the reserpine-treated rat. Br. J. Pharmacol. 2000;129:541–546. doi: 10.1038/sj.bjp.0703105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDNER C.R., RICHARDS M.H. Presence of radiolabelled metabolites in release studies using [3H]gamma-aminobutyric acid. J. Neurochem. 1981;36:1590–1593. doi: 10.1111/j.1471-4159.1981.tb00604.x. [DOI] [PubMed] [Google Scholar]
- KIA H.K., MIQUEL M.C., BRISORGUEIL M.J., RIAD M., EL MESTIKAWY S., HAMON M., VERGE D. Immunocytochemical localization of serotonin-1A receptors in the rat central nervous system. J. Comp. Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- MACOR J.E., BURKHART C.A., HEYM J.H., IVES J.L., LEBEL L.A., NEWMAN M.E., NIELSEN J.A., RYAN K., SCHULZ D.W., TORGERSEN L.K. 3-(1,2,5,6-Tetrahydropyrid-4-yl)pyrrolo[3,2-b]pyrid-5-one: a potent and selective serotonin (5-HT1B) agonist and rotationally restricted phenolic analogue of 5-methoxy-3-(1,2,5,6-tetrahydropyrid-4-yl)indole. J. Med. Chem. 1990;33:2087–2093. doi: 10.1021/jm00170a007. [DOI] [PubMed] [Google Scholar]
- MANEUF Y., MITCHELL I.J., CROSSMAN A.R., BROTCHIE J.M. On the role of enkephalin cotransmission in the GABAergic striatal efferents to the globus pallidus. Exp. Neurol. 1994;125:65–71. doi: 10.1006/exnr.1994.1007. [DOI] [PubMed] [Google Scholar]
- PARENT A., HAZRATI L.N. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The rat brain in stereotaxic co-ordinates 1986U.K.: Academic Press; 2nd, edn [Google Scholar]
- POMPEIANO M., PALACIOS J.M., MENGOD G. Distribution and cellular localization of mRNA coding for the 5-HT1A receptor in rat brain: correlation with receptor binding. J. Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PYCOCK C., HORTON R.W., MARSDEN C.D. The behavioural effects of manipulating GABA function in the globus pallidus. Brain Res. 1976;116:353–359. doi: 10.1016/0006-8993(76)90916-1. [DOI] [PubMed] [Google Scholar]
- RIAD M., GARCIA S., WATKINS K.C., JODOIN N., DOUCET E., LANGLOIS X., EL MESTIKAWY S., HAMON M., DESCARRIES L. Somatodendritic localization of 5-HT1A and preterminal axomal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- ROBERTSON R.G., GRAHAM W.C., SAMBROOK M.A., CROSSMAN A.R. Further investigations into the pathophysiology of MPTP-induced parkinsonian in the primate: an intracerebral microdialysis study of γ-aminobutyric acid in the lateral segment of the globus pallidus. Brain Res. 1991;563:278–280. doi: 10.1016/0006-8993(91)91545-c. [DOI] [PubMed] [Google Scholar]
- SARI Y., MIQUEL M.-C., BRISORGUEIL M.-J., RUIZ G., DOUCET E., HAMON M., VERGE D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- SEGOVIA J., TOSSMAN U., HERRERA-MARSCHITZ M., GARCIA-MUNOZ M., UNGERSTEDT U. γ-aminobutyric acid release in the globus pallidus in vivo after a 6-hydroxydopamine lesion in the substantia nigra of the rat. Neurosci. Lett. 1986;70:364–368. doi: 10.1016/0304-3940(86)90580-x. [DOI] [PubMed] [Google Scholar]
- STANFORD I.M., LACEY M.G. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J. Neurosci. 1996;16:7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOCCHI F., NORDERA G., MARSDEN C.D. Strategies for treating patients with advanced Parkinson's disease with disastrous fluctuation and dyskinesias. Clin. Neuropharmacol. 1997;20:95–115. doi: 10.1097/00002826-199704000-00001. [DOI] [PubMed] [Google Scholar]
- WALDMEIER P.C., WILLIAMS M., BAUMANN P.A., BISCHOFF S., SILLS M.A., NEALE R.F. Interactions of isamoltane (CGP 361A), an anxiolytic phenoxypropanolamine derivative, with 5HT1 receptor subtypes in the rat brain. Naunyn Schmiedeberg's Arch. Pharmacol. 1988;337:609–620. doi: 10.1007/BF00175785. [DOI] [PubMed] [Google Scholar]
- WANAKA A., KIYAMA H., MURAKAMI T., MATSUMOTO M., KAMADA T., MALBON C.C., TOHYAMA M. Immunocytochemical localization of beta-adrenergic receptors in the rat brain. Brain Res. 1989;485:125–140. doi: 10.1016/0006-8993(89)90674-4. [DOI] [PubMed] [Google Scholar]


