Abstract
In the present study we tested the effects of the antihyperalgesic compound gabapentin on dorsal horn neurones in adult spinal cord. Slices were taken from control and hyperalgesic animals suffering from streptozocin-induced diabetic neuropathy. At concentrations up to 100 μM, bath application failed to affect the resting membrane properties of dorsal horn neurones taken from both groups of animal. In contrast, bath application of gabapentin dramatically reduced the magnitude of the excitatory postsynaptic current (EPSC) in neurones taken from hyperalgesic animals without altering the magnitude of the EPSC in control animals. Using a paired pulse stimulation protocol, together with analysis of miniature EPSC's, it was possible to demonstrate that gabapentin mediated these effects via a pre-synaptic site of action.
Keywords: Gabapentin, spinal cord, substantia gelatinosa, dorsal horn neurones, excitatory postsynaptic current, hyperalgesia, streptozocin
Introduction
Gabapentin (neurontin) is an antiepileptic agent currently used as an add-on therapy for patients suffering from partial seizures resistant to conventional therapies (Goa & Sorkin, 1993). Recent studies have also shown that gabapentin exhibits antihyperalgesic actions in a wide range of animal models of pain (Singh et al., 1996; Field et al., 1997) including neuropathic pain (Rosner et al., 1996; Field et al., 1999).
Despite its clinical effectiveness, the mechanisms of action of gabapentin remain unclear. Gabapentin has been shown to bind with high affinity to the alpha2-delta subunit of voltage gated calcium channels (Gee et al., 1996) but the functional significance of this interaction awaits clarification. For example, although gabapentin has been shown to inhibit voltage gated calcium channels in dissociated neocortical neurones (Stefani et al., 1998) others have failed to repeat these findings (Rock et al., 1993).
In view of these discrepancies, in the present study we have examined the effects of gabapentin on the electrophysiological properties of dorsal horn neurones in the adult spinal cord of normal and hyperalgesic rats suffering diabetic neuropathy.
Methods
Male Sprague-Dawley rats (250–350 g) were sacrificed by anaesthetic overdose and 150–300 μm longitudinal slices from the lumbar spinal cord prepared in physiological saline. Substantia gelatinosa neurones were visually selected within the translucent band of laminae II of the dorsal horn using infra-red video microscopy. An evoked synaptic response was obtained either by stimulation of the dorsal root by a suction electrode or via a bipolar stimulating electrode placed in the myelinated dorsal root entry zone adjacent to the translucent laminae II band and applying single shocks (2–12 V, 20–200 μs) at 30 s intervals throughout the course of the experiment. Slices were perfused (2.5 ml min−1; 30°C) with physiological saline containing (mM) NaCl 125.0, NaHCO3 25.0, glucose 10.0, KCl 2.5, NaH2PO4 1.25, CaCl2 2.0, MgCl2 1.0 and was bubbled with a 95%, 5% O2/CO2 gas mixture. The intracellular (pipette) solution comprised (mM) Kgluconate 120.0, NaCl 10.0, MgCl2 2.0, K2EGTA 0.5, HEPES 10.0, Na2ATP 4.0, Na2GTP 0.3, pH 7.2. Drugs were applied by superfusion and reached the recording chamber within 15 s of switching and a complete exchange of the slice chamber took less than 30 s.
To induce diabetic neuropathy, a single injection of 50 mg kg−1 streptozocin was administered i.p. 2–3 weeks before electrophysiological analysis. Control animals received a similar injection of isotonic saline. Hyperalgesia was assessed by measuring the degree of static allodynia present in the hind paw using Semmes-Weinstein von Frey hairs (Field et al., 1999). Data are presented as means±s.e.mean, and a students paired t-test used to determine significance. P<0.05 was taken to be statistically significant.
Drugs used were 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxalone (NBQX; Tocris Cookson, Bristol, U.K.); L-glutamic acid, bicuculline and strychnine, (Sigma, Poole, U.K.); gabapentin (Parke-Davis) Tetrodotoxin (TTX; Affiniti Research products Ltd.).
Results
This study was performed upon a total of 22 substantia gelatinosa neurones taken from control animals and 28 substantia gelatinosa neurones taken from animals suffering streptozocin-induced static allodynia (Field et al., 1999). Under control conditions, both groups of neurone exhibited similar resting membrane properties. Thus neurones taken from control animals had a resting membrane potential of −68.7±3.1 mV and had an input resistance of 318±59 MΩ (n=7). In comparison, neurones taken from hyperalgesic animals had a resting membrane potential of −63.9±1.6 mV and an input resistance of 392±47 MΩ (n=15). Under these resting conditions, bath application of gabapentin (1–100 μM) had no effect on the resting membrane potential or input resistance of these cells (n=8 for each, not shown).
Under voltage clamp conditions at −60 mV, electrical stimulation of either the dorsal root or the dorsal entry zone was found to evoke an inward synaptic current of similar magnitude in both sets of neurone (control, 173.8±35.4 pA (n=14); streptozocin 177.3±20.2 pA (n=20)). These currents appeared monosynaptic in nature, as judged by their constant latencies and absence of failures at high frequency repetitive stimulation (20 Hz), and were completely inhibited by bath application of 5 μM NBQX (n=4 for each, not shown) indicating there excitatory nature.
In control animals, bath application of gabapentin (100 μM) was found to have no significant effect on the magnitude of this excitatory postsynaptic current (EPSC) when applied both acutely (2 min) of for periods up to 20 min (Figure 1). In contrast, in neurones taken from neuropathic animals, bath application of 30–100 μM gabapentin caused a significant inhibition of the EPSC in 15 out of 20 neurones (Figure 2). This inhibition was slow to develop, taking between 7 and 12 min, but was fully reversed on washout. In the 15 neurones found to be sensitive to gabapentin, the magnitude of the EPSC was reduced by 75.2±6% (P<0.01; students paired t-test). These effects of gabapentin were maintained in the presence of 10 μM bicuculline and 50 μM strychnine (n=4, not shown).
Figure 1.
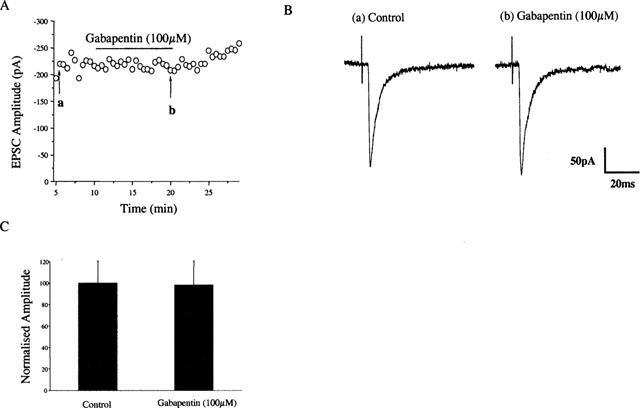
Application of gabapentin has no effect on the EPSC recorded from control animals. EPSCs were evoked every 30 s. Bath application of gabapentin (100 μM; 13 min) had no effect on the EPSC recorded from control animals (A). Representative traces taken at (a) control and (b) after 13 min of gabapentin exposure are shown in the middle panel (B). A histogram representing the mean change is shown in (C) (n=7).
Figure 2.
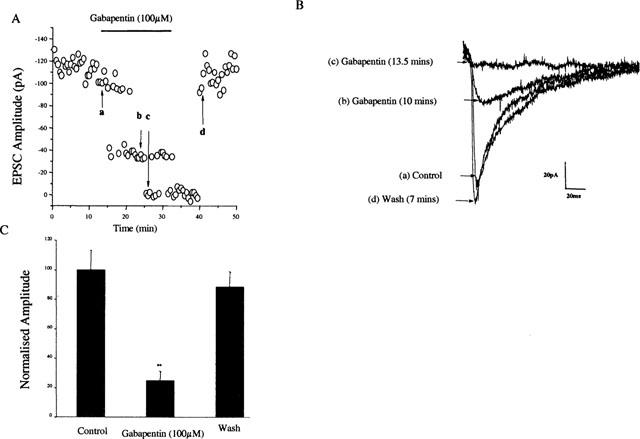
Application of gabapentin depresses the EPSC recorded from hyperalgesic animals. EPSCs were evoked every 30 s. Bath application of gabapentin (100 μM; 20 min) caused a reversible depression of the EPSC (A). In panel (B) traces representing (a) control, (b) and (c) after 10 and 13 min gabapentin perfusion respectively and (d) after 7 min washout are shown. (C) Histogram representing the mean inhibition of the EPSC in 15 out of 20 cells recorded.
To establish whether this depression of the EPSC induced by gabapentin was dependent on pre- or postsynaptic sites of action, the synaptic responses to a pair of stimuli was measured (Baskys & Malenka, 1991). In these experiments, the interstimulus interval was 30 ms. In control animals gabapentin had no effect on the paired pulse ratio (n=5), however, in 8 out of 12 cells recorded from hyperalgesic animals gabapentin decreased the amplitude of the first EPSC (EPSC1) and increased the paired pulse ratio (from 0.3±0.1 to 1.7±0.4, P<0.01; paired t-test: n=8; Figure 3).
Figure 3.
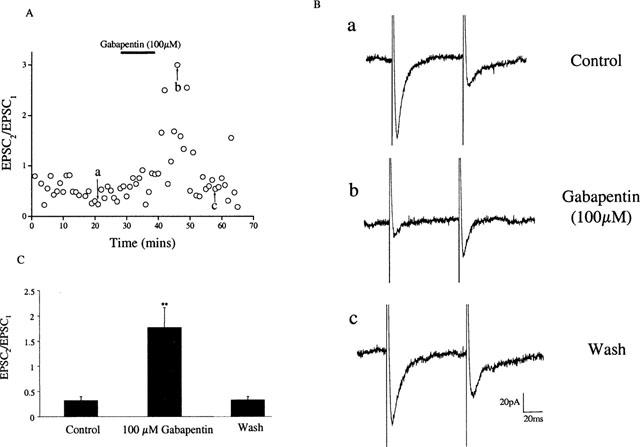
Application of gabapentin reduced paired pulse depression in hyperalgesic animals. Paired EPSCs (interpulse interval 30 ms) were evoked every 30 s. Bath application of gabapentin (100 μM) caused a reversible depression of the first EPSC (EPSC1) and an increase in the paired pulse ratio EPSC2/EPSC1) (A). Representative traces are shown in panel (B) where (a) control, (b) after 10 min gabapentin exposure and (c) after 16 min washout. (C) Histogram representing the mean increase in the paired pulse ratio in 8 out of 12 cells recorded.
In addition, miniature excitatory post synaptic current's (mEPSC's) were recorded in the presence of TTX (1 μM), bicuculline (10 μM) and strychnine (50 μM). Under these conditions mEPSC's had a mean frequency of 5.1±2.7 Hz (range 1.9–6.8 Hz; n=3) and a mean amplitude of 14.4±2.7 pA (range 11.6–17.3 pA; n=3). Gabapentin (100 μM) significantly decreased the frequency of the mEPSC's by 49.1±27% (P<0.05; paired t-test) with no effect on mEPSC amplitude. Taken together these data suggest a pre-synaptic site of action for gabapentin. This assumption is further supported by experiments performed with bath applied glutamate. Thus, in three neurones taken from hyperalgesic animals in which gabapentin reduced the magnitude of the evoked EPSC, gabapentin failed to affect the magnitude of the response to bath applied glutamate (control response to 1 mM glutamate, 112±32 pA; in the presence of 100 μM gabapentin 125±25 pA).
Discussion
Nociceptive transmission from the periphery is mediated by Aδ and C fibres that terminate predominantly on substantia gelatinosa neurones of laminae I and II of the dorsal horn (Light & Perl, 1979). In the present study we have shown for the first time that gabapentin is capable of modulating excitatory synaptic transmission within these neurones of neuropathic animals at therapeutically relevant concentrations (Goa & Sorkin, 1993). These findings extend those of Stanfa et al. (1997) who report similar differential effects in normal and carageenan treated animals.
The mechanism by which gabapentin achieves these effects appear to involve a presynaptic site of action. Although the exact nature of this presynaptic locus remains to be established, it is possible to speculate that presynaptic calcium channels are involved given that this compound binds to the alpha2-delta calcium channel subunit with high affinity (Gee et al., 1996). The inability of this compound to affect neuronal properties under normal conditions is remarkable and suggests that the profound changes which occur within the hyperalgesic spinal cord (Yaksh & Chaplan, 1997) leads to a change in some component of synaptic transmission which subsequently becomes gabapentin sensitive. In a recent study gabapentin has been shown to modulate synaptic transmission in the rat striatum albeit at elevated concentrations (Calabresi et al., 1999). It is not presently clear whether these effects arise from the same mechanism that in someway becomes sensitized on injury or whether this reflects some other non-specific interaction.
The inability of gabapentin to affect neurotransmission under control conditions is in keeping with clinical findings showing a low incidence of side effects and may explain in part why the in vitro actions of this drug have been difficult to obtain and reproduce.
In summary, in the present study we demonstrate that gabapentin is able to specifically modulate neurotransmission in the spinal cord of hyperalgesic animals suffering from diabetic neuropathy. As such this compound represents a new generation of well-tolerated analgesics which act exclusively as a consequence of the changes which accompany the onset of hyperalgesia in this disease and possibly other forms of persistant pain.
Abbreviations
- EPSC
excitatory postsynaptic current
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxalone
- TTX
tetrodotoxin
References
- BASKYS A., MALENKA R.C. Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J. Physiol. 1991;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALABRESI P., CENTONZE D., MARFIA G.A., PISANI A., BERNARDI G. An in vitro electrophysiological study on the effects of phenytoin, lamotrigine and gabapentin on striatal neurons. Br. J. Pharmacol. 1999;126:689–696. doi: 10.1038/sj.bjp.0702361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIELD M.J., MCCLEARY S., HUGHES J., SINGH L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999;80:391–398. doi: 10.1016/s0304-3959(98)00239-5. [DOI] [PubMed] [Google Scholar]
- FIELD M.J., OLES R.J., LEWIS A.S., MCCLEARY S., HUGHES J., SINGH L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br. J. Pharmacol. 1997;121:1513–1522. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEE N.S., BROWN J.P., DISSANAYAKE V.K., OFFORD J., THURLOW R., WOODRUFF G.N. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha 2 delta subunit of a calcium channel. J. Biol. Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- GOA K.L., SORKIN E.M. Gabapentin, A review of its pharmacological properties and clinical potency in epilepsy. Drugs. 1993;46:409–427. doi: 10.2165/00003495-199346030-00007. [DOI] [PubMed] [Google Scholar]
- LIGHT A.R., PERL E.R. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibres. J. Compar. Neurol. 1979;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- ROCK D.M., KELLY K.M., MACDONALD R.L. Gabapentin actions on ligand- and voltage-gated responses in cultured rodent neurons. Epilepsy Res. 1993;16:89–98. doi: 10.1016/0920-1211(93)90023-z. [DOI] [PubMed] [Google Scholar]
- ROSNER H., RUBIN L., KESTENBAUM A. Gabapentin adjunctive therapy in neuropathic pain states. Clin. J. Pain. 1996;12:56–58. doi: 10.1097/00002508-199603000-00010. [DOI] [PubMed] [Google Scholar]
- SINGH L., FIELD M.J., FERRIS P., HUNTER J.C., OLES R.J., WILLIAMS R.G, WOODRUFF G.N. The antiepileptic agent gabapentin (Neurontin) possesses anxiolytic-like and antinociceptive actions that are reversed by D-serine. Psychopharmacology. 1996;127:1–9. doi: 10.1007/BF02805968. [DOI] [PubMed] [Google Scholar]
- STANFA L.C., SINGH L., WILLIAMS R.G., DICKENSON A.H. Gabapentin (Neurontin), ineffective in normal animals, markedly reduces C-fibre evoked responses after inflammation. Neuroreport. 1997;8:587–590. doi: 10.1097/00001756-199702100-00002. [DOI] [PubMed] [Google Scholar]
- STEFANI A., SPADONI F., BERNARDI G. Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology. 1998;37:83–91. doi: 10.1016/s0028-3908(97)00189-5. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L., CHAPLAN S.R. Physiology and pharmacology of neuropathic pain. Anethesiol. Clin. North Am. 1997;15:335–352. [Google Scholar]


