Abstract
ADP, an important agonist in thrombosis and haemostasis, has been reported to activate platelets via three receptors, P2X1, P2Y1 and P2TAC. Given the low potency of ADP at P2X1 receptors and recognized contamination of commercial samples of adenosine nucleotides, we have re-examined the activation of P2X1 receptors by ADP following HPLC and enzymatic purification.
Native P2X1 receptor currents in megakaryocytes were activated by α,β-meATP (10 μM) and commercial samples of ADP (10 μM), but not by purified ADP (10–100 μM).
Purified ADP (up to 1 mM) was also inactive at recombinant human P2X1 receptors expressed in Xenopus oocytes. Purification did not modify the ability of ADP to activate P2Y receptors coupled to Ca2+ mobilization in rat megakaryocytes.
In human platelets, P2X1 and P2Y receptor-mediated [Ca2+]i responses were distinguished by their different kinetics at 13°C. In 1 mM Ca2+ saline, α,β-meATP (10 μM) and commercial ADP (40 μM) activated a rapid [Ca2+]i increase (lag time ⩽0.5 s) through the activation of P2X1 receptors. Hexokinase treatment of ADP shifted the lag time by ≈2 s, indicating loss of the P2X1 receptor-mediated response.
A revised scheme is proposed for physiological activation of P2 receptors in human platelets. ATP stimulates P2X1 receptors, whereas ADP is a selective agonist at metabotropic (P2Y1 and P2TAC) receptors.
Keywords: Platelets, ADP, P2 receptors, P2X1, purinoceptors, hexokinase, Ca2+
Introduction
P2 receptors for nucleotides are located on the extracellular surface of a variety of mammalian cell types including neurons, smooth muscle cells and a range of haematopoietic cells including megakaryocytes and platelets (Kunapuli & Daniel, 1998; Ralevic & Burnstock, 1998). They can be divided into two main classes according to their signalling mechanism (Fredholm et al., 1997); P2X receptors are ligand-gated cation channels, whereas metabotropic P2Y receptors are G-protein coupled receptors. Recombinant P2Y receptors couple through the activation of phospholipase-C. In addition, native P2Y receptors have been described that couple to adenylyl cyclase and ion channels (North & Barnard, 1997). To date, seven types of P2X receptor (P2X1–7) and at least five types of P2Y receptor (P2Y1,2,4,6,11) have been identified at the molecular level (Ralevic & Burnstock, 1998; Kunapuli & Daniel, 1998; Evans et al., 1999; Boeynaems et al., 2000). Frequently, multiple purinoceptor subtypes co-exist in the same cell, however their relative importance to physiological function is only just beginning to be elucidated (Boarder & Hourani, 1998).
P2X1 receptors are the major ionotropic purinoceptor in blood platelets and smooth muscle (Vulchanova et al., 1996; Mackenzie et al., 1996; Vial et al., 1997; Mulryan et al., 2000). They are characterized by rapid desensitization and a pharmacological profile of 2-methylthio-ATP⩾ATP >α,βMe-ATP>L-β,γ-me-ATP>ADP (Valera et al., 1994; Evans et al., 1995). ATP is co-stored and co-released with a number of ‘classical' neurotransmitters (e.g. noradrenaline and acetylcholine) and leads to the activation of P2X receptors during synaptic transmission (Burnstock, 1990). However, during haemostasis, both ADP and ATP are released at high concentrations from platelet dense granules (Holmsen & Weiss, 1979) and ADP levels may initially rise whilst ATP levels are falling due to the action of ectonucleotidases (Boeynaems & Pearson, 1990). Thus, following vascular damage, both ADP and ATP are potentially important stimuli of smooth muscle, endothelial and blood cell purinoceptors. ADP has long been recognized as an important activator of human platelets (Gaarder et al., 1961; Born, 1962; Kunapuli & Daniel, 1998), whereas ATP is normally regarded as an inhibitor of this cell type, via its antagonistic action at platelet metabotropic purinoceptors (Hourani & Hall, 1994; Kunapuli & Daniel, 1998). The discovery of P2X1 receptors on platelets has brought into question the purely antagonistic view of ATP during haemostasis, especially considering the large difference in EC50 values reported for activation of recombinant P2X1 receptors by ATP and ADP (0.8 and 34 μM, respectively; Evans et al., 1995). Furthermore, P2X1 receptors have recently been suggested to couple to a functional response in platelets (Rolf & Mahaut-Smith, 2000) and ATP will be the principal nucleotide directly released from the cytoplasm following injury. In view of the concerns that commercial samples of nucleotide diphosphates can be contaminated by nucleotide triphsophates (Nicholas et al., 1996; Hechler et al., 1998b), we have re-examined the relative activity of ADP and ATP on recombinant P2X1 receptors and native P2X1-like receptors in megakaryocytes and platelets. We find that purified ADP is completely inactive at P2X1 receptors and that previous reports of ADP-evoked activation of this receptor can be entirely accounted for by contamination of commercial samples by ATP. A revised scheme for activation of different platelet P2 receptor subtypes is proposed.
Methods
Materials
Fura-2AM was obtained from Molecular Probes, Europe (Leiden, The Netherlands). Hexokinase (EC 2.7.1.1, from yeast) was purchased from Boehringer Mannheim (Lewes, East Sussex, U.K.). ‘Commercial' ADP, type V or VII apyrase, α,β-meATP and all other chemicals were obtained from Sigma (Poole, Dorset, U.K.). HPLC-purified ADP was a kind gift of Prof M.R. Boarder, De Montfort University, Leicester.
Solutions
Two types of external or patch pipette salines were used during patch clamp and [Ca2+]i recordings, designated ‘A' or ‘B'. External solution ‘A' contained (in mM): NaCl 150, HEPES 10, KCl 2.5, MgCl2 1, pH 7.3 (NaOH), with or without 2.5 mM CaCl2. External solution ‘B' contained (in mM): NaCl 145, KCl 5, MgCl2 1, CaCl2 1, HEPES 10, glucose 10, pH 7.35 (NaOH). CaCl2 was replaced by 1 mM MgCl2 for Ca2+-free solution ‘B'. Patch pipette saline ‘A' contained (in mM): Kgluconate 140, NaCl 5, EGTA 9, HEPES 10, pH 7.3 (KOH). Patch pipette saline ‘B' contained (in mM): KCl 150, MgCl2, 2 EGTA 0.1, Na2EGTA 0.05, K5fura-2 0.05, HEPES 10, pH 7.2 (KOH). ‘A' salines were used for all recordings of P2X1 receptor currents in megakaryocytes; the external saline was Ca2+-free since the P2X1 response was more stable in the absence of Ca2+, as described in an earlier study by Kawa (1996). ‘B' salines were used for all [Ca2+]i recordings from rat megakaryocytes and human platelets, with or without external Ca2+ as indicated. Oocyte voltage clamp recordings used an extracellular solution of the following composition (mM): NaCl 96, KCl 2, HEPES 5, sodium pyruvate 5, MgCl2 1, BaCl2 1.8 and both electrodes were filled with 3 M KCl.
Enzymatic degradation of contaminating ATP within commercial ADP solutions was achieved by incubation of a stock of 10 mM ADP in Ca2+-containing external solution ‘A' at pH 8, with 22 mM glucose and 3 u ml−1 hexokinase for 1 h at 37°C. A similar procedure has been used to remove UTP contaminations from within commercial stocks of UDP (Nicholas et al., 1996).
Cell preparation
Megakaryocytes were prepared from the femoral and tibial marrow of MF1 mice or adult male Wistar rats using the protocol previously described for the rat (Mahaut-Smith et al., 1999). The saline used for the megakaryocyte isolation was either external saline ‘A' or ‘B' (see above) with Ca2+ and 0.16–0.32 u ml−1 apyrase. Human blood was drawn from the antecubetal vein of informed donors and platelets isolated and loaded with fura-2 as described previously (Mackenzie et al., 1996; Rolf & Mahaut-Smith, 2000). All platelets used in the present study were treated with aspirin (100 μM) and exposed to apyrase (0.16 or 0.32 u ml−1) in order to reduce the risk of spontaneous activation by endoperoxide metabolites and adenosine nucleotides, respectively.
Heterologous expression
Stage V oocytes from Xenopus laevis were isolated and human P2X1 receptor cRNA injected into each oocyte as described previously (Evans et al., 1995).
[Ca2+]i measurements
Cuvette fluorescence measurements of [Ca2+]i in fura-2-loaded human platelets were performed essentially as described previously (Mackenzie et al., 1996; Rolf & Mahaut-Smith, 2000). The cuvette temperature was lowered to 13°C using a combination of a reduced ambient temperature and Peltier effect devices (RS Components Ltd, Corby, U.K.). Standard methods to calibrate the fura-2 fluorescence were complicated by a temperature-dependent shift in the properties of the dye in the cytoplasm compared to free solution. The effect could not be accounted for by a simple shift in minimum and maximal ratio, as shown for viscosity, therefore background-corrected 340/380 nm ratios are presented as an indication of [Ca2+]i. Measurements of [Ca2+]i in single rat megakaryocytes were conducted following introduction of fura-2 free acid into the cell from a patch pipette under whole-cell patch clamp as described in detail elsewhere (Mahaut-Smith et al., 1999).
Electrophysiological recordings and agonist application
Conventional whole-cell patch clamp recordings from megakaryocytes were performed using an Axopatch 200B or 200A with CV-202 headstage (Axon Instruments, CA, U.S.A.). Correction for liquid : liquid junction potentials was applied a priori for experiments with pipette saline ‘A'. Two electrode voltage clamp recordings of Xenopus oocytes were conducted using a Geneclamp 500B amplifier (Axon Instruments). Membrane currents were acquired using a Digidata 1200 analogue to digital converter in combination with pClamp 7 acquisition software (Axon Instruments). For measurements of ionotropic purinoceptor currents, agonists were applied from a nearby U-tube perfusion system (Evans & Kennedy, 1994). Repeated agonist exposures were separated by 5 min in order to allow maximal recovery of P2X receptors from desensitization. During measurement of [Ca2+]i responses in rat megakaryocytes, agonists were applied through a gravity-driven perfusion system. For cuvette fluorimetric measurements of [Ca2+]i in human platelets; agonists were injected from Hamilton syringes inserted into a custom lid (Rolf & Mahaut-Smith, 2000). The timing of agonist application was recorded electronically to allow analysis of response times off-line. Lag times for agonist-evoked [Ca2+]i responses were measured as the first detectable increase above the resting level.
Statistical values are shown as the means±s.e.mean. Differences between means were tested by Student's unpaired t-test for significance at P<0.05 and P<0.01. Concentration response data were fitted with the equation response=α(A)H/([A]H+[A50]H) where a is the asymptote, H is the hill coefficient, A is the agonist concentration and A50 is the concentration of agonist evoking 50% of the maximum response.
Results
Patch clamp studies of megakaryocytes have shown that the initial rapid response to application of extracellular adenosine nucleotides is the activation of a transient inward cation current (Somasundaram & Mahaut-Smith, 1994; Kawa, 1996). The rapid onset kinetics, permeability to both Ca2+ and Na+ and sensitivity to α,β-meATP, together with molecular studies from platelets and megakaryocytic cell lines, indicate that the response is mediated by P2X1 receptors (Valera et al., 1994; Mackenzie et al., 1996; Vial et al., 1997). Using whole-cell patch clamped mouse megakaryocytes, we have compared P2X1 receptor currents activated by α,β-meATP, ADP from a commercial source and ADP immediately after purification by HPLC (Figure 1a). At a holding potential of −60 mV, 10 μM α,β-meATP rapidly activated an inward current with a peak of 531±112 pA (range 98–1119 pA, n=9) that started to desensitize during the 200 ms application. ADP from a commercial source evoked an inward current with similar kinetics but smaller amplitude (233.7±42 and 333.3±80 for 10 and 100 μM ADP respectively n=3 and 4). After HPLC purification, ADP (10 μM) had only a small effect (24.3±4.8 n=4) (Figure 1a). In these cells, current responses to ADP mediated via P2Y receptors (Uneyama et al., 1993; Somasundaram & Mahaut-Smith, 1994) were not observed due to the brief exposure to agonist, in addition to the Ca2+-free external saline and high concentration of EGTA in the pipette saline. Based on HPLC analysis ATP is the most likely contaminant of ADP responsible for P2X1 receptor activation, therefore we used hexokinase to convert ATP to ADP in the ‘commercial' stock (Nicholas et al., 1996; see Methods for conditions). Little activation of P2X1 receptors was observed with hexokinase-purified ADP up to concentrations of 100 μM (peak inward current 23.7±11.8 pA, n=4) (see Figure 1b). These data suggest that ATP contamination is responsible for the apparent activity of commercial samples of ADP at the megakaryocyte P2X1 receptor.
Figure 1.
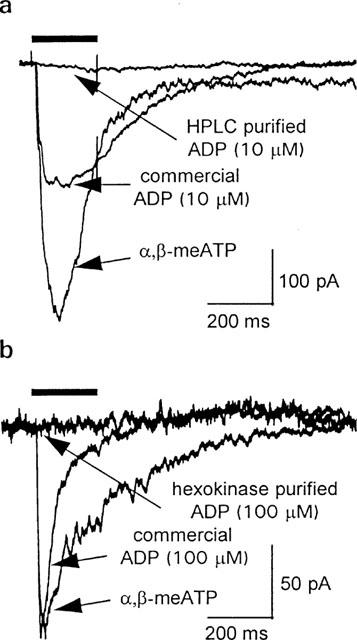
P2X1 receptor currents in mouse megakaryocytes: responses to commercial grade and purified ADP. Whole-cell currents at a holding potential of −60 mV. Agonist applications (bar) were separated by 5 min to allow recovery of P2X1 receptors from desensitization. (a) Representative traces during application of α,β-meATP, commercial ADP or HPLC-purified ADP, each at 10 μM. (b) Effect of commercially available and hexokinase-treated ADP (100 μM) compared to 10 μM α,β-meATP.
Commercial grade ADP also activated human P2X1 receptors expressed in Xenopus oocytes. Typical responses to 100 μM commercial ADP and 100 μM ATP are shown in Figure 2a,Figure 2b shows the concentration-response relationships for ATP and commercial ADP. The EC50 for ATP was ∼0.6 μM, and maximal responses were recorded at concentrations ⩾10 μM. Commercial ADP had an apparent EC50 value of ∼40 μM. HPLC-purified ADP (10 μM) and hexokinase-treated ADP (up to 1 mM) were completely ineffective at evoking P2X1 responses in oocytes (Figure 2a,b). This confirms the result obtained in the megakaryocytes (Figure 1) that contaminating ATP is most likely responsible for activation of P2X1 receptors by commercially available ADP.
Figure 2.
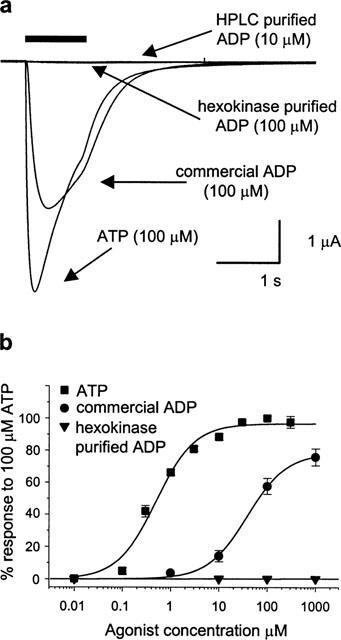
Human P2X1 receptors expressed in Xenopus oocytes: responses to ATP, commercial grade ADP and purified ADP. P2X1 receptor currents recorded by two-electrode voltage clamp at a holding potential of −60 mV. Agonist applications (bar) were separated by 5 min to allow recovery from desensitization. (a) Responses to ATP, commercial grade ADP, HPLC-purified ADP and hexokinase-treated ADP, each at 100 μM. (b) Average concentration:response relationships for ATP, commercial ADP and hexokinase-treated ADP, constructed from 4–6 oocytes.
To assess the possibility that the lack of P2X1 stimulation by HPLC-purified and hexokinase-treated ADP samples could result from reduced ADP activity, we conducted control experiments by measurement of [Ca2+]i responses mediated through P2Y receptors in rat megakaryocytes. ADP activates this receptor at concentrations almost 30 fold lower than ATP (Uneyama et al., 1993; Somasundaram & Mahaut-Smith, 1994). Figure 3 shows that hexokinase-treated ADP (1 μM) evoked a robust [Ca2+]i increase in the form of one or more spikes followed by a raised plateau level of [Ca2+]i, as reported previously (Mahaut-Smith et al., 1999). After wash and recovery of the [Ca2+]i to resting levels, 1 μM unpurified ADP stimulated a response of similar magnitude to that evoked by hexokinase-treated ADP. Owing to receptor desensitization and/or effects of whole-cell dialysis, repeated responses to the same agonist displayed variations in amplitude and pattern of oscillations, as demonstrated by the reduced response following a second exposure to hexokinase-treated ADP. However, in a total of nine cells, 1 μM unpurified (commercial) ADP, evoked a response which was qualitatively indistinguishable from that observed with hexokinase-treated ADP (Figure 3). Similar results were obtained with HPLC-purified ADP (1 μM) on the megakaryocyte P2Y receptor-mediated [Ca2+]i response (data not shown, n=6). These data confirm that the loss of activity at P2X1 receptors following HPLC purification or hexokinase treatment results from removal of a contaminant rather than a loss of ADP-dependent activity.
Figure 3.
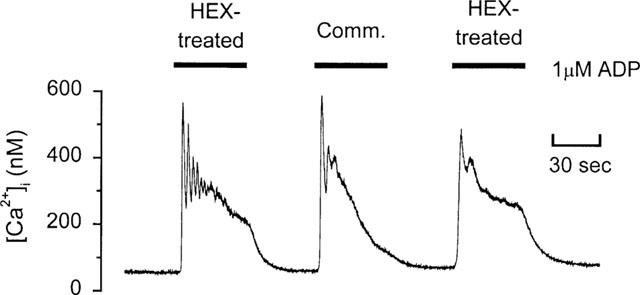
P2Y receptor-mediated [Ca2+]i responses to commercial grade ADP and hexokinase-treated ADP in a rat megakaryocyte. Ratiometric fluorescence measurements of fura-2 were used to monitor [Ca2+]i following introduction of the dye under whole-cell patch clamp conditions. Hexokinase-treated (Hex-treated) ADP or commercial (comm.) ADP were applied for the periods indicated by the bars. Holding potential was −75 mV.
The above data indicate that earlier reports of ADP-evoked currents through P2X1 receptors (Valera et al., 1994; Evans et al., 1995; Kawa, 1996) result from ATP contamination of commercial ADP samples. Studies on human platelets have also reported that ADP stimulates P2X1 receptors (Sage & Rink, 1987; Mahaut-Smith et al., 1990, 1992; Sage et al., 1991; Mackenzie et al., 1996). To investigate whether this response also results from ATP contamination, we conducted cuvette fluorescence measurements of [Ca2+]i from suspensions of fura-2-loaded platelets. A reduced temperature was used to slow the kinetics of the response so that P2X1 and metabotropic P2Y receptor-mediated rises in Ca2+ could be distinguished (Sage et al., 1990). Figure 4a compares the [Ca2+]i responses at 13°C following selective activation of either P2X1 or P2Y receptors in freshly isolated human platelets. At 13°C, the [Ca2+]i increase evoked by 40 μM ADP in Ca2+-free medium, which is due to activation of P2Y1 receptors (Hechler et al., 1998b; Daniel et al., 1998), showed a delay to initial increase of 2.52±0.06 s (n=15). On the other hand, the α,β-meATP-evoked Ca2+ influx was activated with faster kinetics. For 10 μM α,β-meATP, the time to initial [Ca2+]i increase was 0.30±0.02 s (n=19) and the response had significantly recovered prior to the start of the P2Y-mediated Ca2+ release. In Figure 4b, we compare [Ca2+]i responses to commercial and hexokinase-purified ADP in the presence and absence of external Ca2+. A sample trace for each condition is shown and the average lag times are shown in the bar chart in Figure 4c. In Ca2+-free medium, the [Ca2+]i responses to commercial and hexokinase-treated ADP had lag times of 2.52±0.06 s (n=15) and 2.71±0.06 s (n=15), respectively. This difference was significant at P<0.05 (Student's t-test), thus removal of ATP has only a small influence on the delay of the ADP-evoked Ca2+ release. In the presence of 1 mM external Ca2+, commercial grade ADP evoked a [Ca2+]i increase with a lag time of 0.50±0.03 s (n=16), which was only slightly slower than the response to α,β-meATP (see above), suggesting a significant contribution from P2X1 receptor-mediated Ca2+ influx. Hexokinase purification of the ADP resulted in a large shift in the lag time of the agonist-evoked [Ca2+]i increase in Ca2+-containing medium to 2.32±0.05 s (n=16), which can be explained by a loss of P2X1 receptor stimulation. The delay for hexokinase-treated ADP was slightly faster in the presence compared to the absence of external Ca2+ (2.32±0.05 s versus 2.71±0.06 s, respectively, significance at P<0.01). This small acceleration of the response by external Ca2+ has been reported previously for other agonists (Sage & Rink, 1987) and therefore could result from a non-specific effect of Ca2+ on the platelet membrane or on all metabotropic receptors rather than remaining receptor-operated Ca2+ influx. Taken together, these data indicate that removal of ATP contamination from ADP samples abolishes P2X1 receptor stimulation in human platelets, as described above for mouse megakaryocytes and human P2X1 receptors expressed in Xenopus oocytes.
Figure 4.
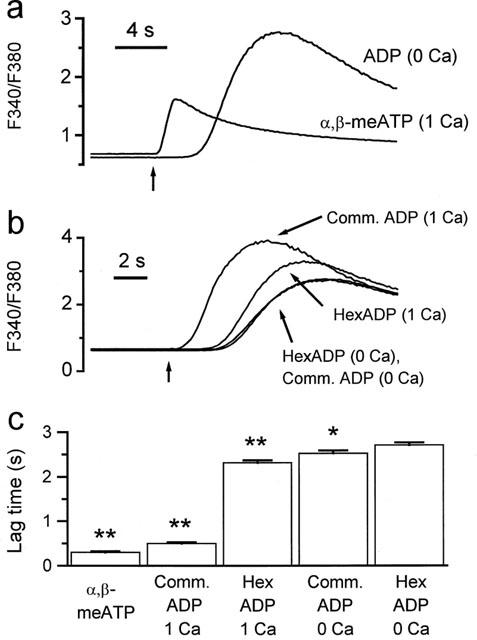
Effects of hexokinase purification on the ADP-evoked [Ca2+]i responses of human platelets. [Ca2+]i was monitored using the background-corrected 340/380 nm fluorescence ratio from stirred suspensions of fura-2-loaded human platelets at 13°C. The vertical arrow below the traces in (a) and (b), indicates injection of agonist. (a) Responses following selective activation of either P2X1 receptors (α,β-meATP in 1 mM Ca2+-containing saline) or P2Y receptors (ADP in nominally Ca2+-free saline). (b) Responses to commercial grade ADP (comm. ADP) and hexokinase-treated ADP (hexADP) in the presence or nominal absence of external Ca2+ (1 mM). (c) Average lag times from 15–19 experiments for each of the conditions in (a) and (b). *P<0.05, **P<0.01, (Student's t-test) when compared to hexokinase-treated ADP in Ca2+-free saline.
Discussion
Previous studies on ionotropic P2X1 receptors have reported EC50 values for activation by ATP and ADP of 0.8 μM and 34 μM, respectively (Valera et al., 1994; Evans et al., 1995). Despite the low estimated activity of ADP at P2X1 receptors, ADP and ATP are both released at high concentrations from platelet dense granules and the actions of nucleotidases can lead to a high ratio of ADP to ATP (Boeynaems & Pearson, 1990). However, we now show that apparent ADP activity at P2X1 receptors is completely lost following removal of contaminants by HPLC purification or incubation with hexokinase to convert any ATP to ADP. The contaminant in the commercial ADP activated P2X1 receptors expressed in Xenopus oocytes with an effective EC50 of ∼40 μM (Figure 2b), compared to 0.6 μM for ATP. Thus, ATP would only need to be present at 1–2% of the predicted ADP concentration in order to account for the actions of the contaminant. Indeed previous reports have indicated that commercial sources of nucleotide diphosphates are ⩽99% pure and are contaminated by nucleotide triphosphates (Nicholas et al., 1996; Hechler et al., 1998b). Thus, ATP contamination is likely to account entirely for previous reports that unpurified/commercially available ADP activates native and cloned P2X1 receptors (Valera et al., 1994; Evans et al., 1995). In addition, ATP rather than ADP accounts for the previous reports that ADP can activate rapid Ca2+ and Na+ influx via non-selective cation channels in platelets and megakaryocytes (Sage & Rink, 1987; Sage et al., 1991; Mahaut-Smith et al., 1990; 1992; Mackenzie et al., 1996; Kawa, 1996). Differing levels of ATP contamination of ADP stocks may account for apparent discrepancies in ADP sensitivity at megakaryocytes reported previously (Somasundaram & Mahaut-Smith, 1994; Kawa, 1996).
ADP has been reported to be a weak agonist at other P2X receptors, which should be re-examined in light of the present study. The EC50 for ADP-dependent activation of P2X receptors is typically 1–3% of that reported for ATP which can be entirely accounted for by ATP contamination (Evans et al., 1995; Chen et al., 1995; Lewis et al., 1995; Buell et al., 1996; Surprenant et al., 1996).
ADP is an important platelet agonist (Gaarder et al., 1961; Born, 1962; Kunapuli & Daniel, 1998). The current model for the action of ADP on platelets proposes the involvement of three purinoceptors; the ionotropic P2X1 receptor and two metabotropic purinoceptors, P2Y1 and P2TAC (Kunapuli & Daniel, 1998). The P2X1 receptor protein expressed by platelets is a ligand-gated ion channel and ATP binding opens the non-selective cation channel (Mackenzie et al., 1996; Vial et al., 1997). P2Y1 receptors are coupled to Ca2+ mobilization via the heterotrimeric G-protein Gq, and to Rho/Rho kinase via other G-proteins, most likely G12/13 (Leon et al., 1997; Jin et al., 1998; Paul et al., 1999). Selective activation of P2Y1 receptors can account for ADP-dependent shape change, however co-activation of P2Y1 and P2TAC is required for ADP-induced aggregation at all but high concentrations of ADP (Jin & Kunapuli, 1998; Hechler et al., 1998a; Savi et al., 1998; Fabre et al., 1999; Leon et al., 1999). The latter receptor has yet to be cloned and is termed P2TAC since it is coupled to inhibition of adenylyl cyclase via the heterotrimeric G-protein, Gi (Ohlmann et al. 1995). Whether the reduction in adenylyl cyclase activity is essential for the aggregation response is currently controversial (Daniel et al., 1999).
The findings of the present study that purified ADP is ineffective as an agonist at P2X1 receptors at concentrations up to 1 mM, requires a redefinition of the platelet ADP receptors. ADP should be considered to stimulate platelets only via two receptors, P2Y1 and P2TAC, whereas ATP is an agonist at P2X1 receptors and an antagonist at P2TAC receptors (Figure 5). ATP has low intrinsic efficacy at P2Y1 receptors (Palmer et al., 1998) and its actions are dependent on the level of receptor expression. ATP is an antagonist of the ADP response at low levels of receptor expression (Leon et al., 1997; Palmer et al., 1998) whereas at higher levels ATP is an agonist (30 fold less potent than ADP, Palmer et al., 1998). As ATP is an antagonist at platelet P2Y1 receptors this infers a low receptor density. The diadenosine polyphosphates, Ap4A, Ap5A and Ap6A, are also P2X1 receptor agonists (Sage et al., 1997; Wildman et al., 1999; Lewis et al., 2000) and are released from platelet dense granules (Flodgaard & Klenow, 1982; Jankowski et al., 1999), therefore may be important physiological activators of platelet ionotropic purinoceptors.
Figure 5.
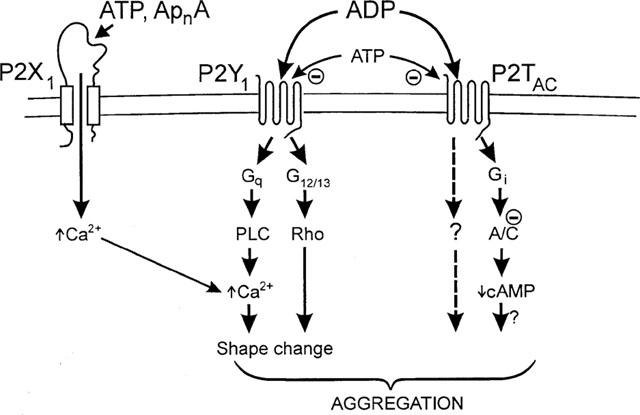
Model for activation of platelets via P2 receptors. A/C: adenylyl cyclase; PLC: phospholipase-C; G: G-protein. ApnA, diadenosine polyphosphates. See text for full explanation.
The role of P2X1 receptors during platelet activation following vascular injury is presently unclear. Some groups have reported that α,β-meATP fails to stimulate shape change or aggregation in platelet suspensions and also does not affect the ADP-evoked functional responses, when measured turbidimetrically (Savi et al., 1997; Jin et al., 1998). However, this may be a consequence of receptor desensitization since a recent study reports that P2X1-mediated shape changes are observed if steps are taken to reduce spontaneous activation during platelet isolation (Rolf & Mahaut-Smith, 2000). ATP is present in the cytoplasm at high concentrations (several mM) and will be one of the first agonists to stimulate platelets at the site of vascular injury. In support of this hypothesis, Born & Kratzer (1984) have shown that the initial increase in ATP levels detected at the site of vascular injury is due to release from damaged cells in the injured vessel wall. Activation of P2X1 receptors may also potentiate the action of agonists at metabotropic receptors, since the resultant Ca2+ influx occurs more rapidly than release from intracellular stores (reviewed in Sage et al., 1997). The rapid Ca2+ influx could accelerate phospholipase-C activity (Eberhard & Holz, 1988) or IP3-dependent Ca2+ release (Bezprozvanny & Ehrlich, 1995). Further studies are required to resolve the relative physiological and pathophysiological importance of ATP as a platelet agonist or antagonist.
In conclusion, the present study clearly demonstrates that ADP is not an agonist at P2X1 receptors as had been previously thought. Activation of P2X1 receptors during haemostasis depends upon ATP, released from damaged cells or ATP and diadenosine polyphosphates secreted by platelets and other cells.
Acknowledgments
This work, conducted in the laboratories of M.P. Mahaut-Smith and R.J. Evans, was supported by grants from the British Heart Foundation (BS/10, FS/97052 and PG/98128).
Abbreviations
- α,β-meATP
α,β-methyleneadenosine 5′-triphosphate
- ApnA
diadenosine polyphosphates
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- HEPES
N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]
- HPLC
high performance liquid chromatography
References
- BEZPROZVANNY I., EHRLICH B.E. The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Memb. Biol. 1995;145:205–216. doi: 10.1007/BF00232713. [DOI] [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M.O. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. TIPS. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BOEYNAEMS J.-M., COMMUNI D., SAVI P., HERBERT J.M. P2Y receptors: in the middle of the road. TIPS. 2000;21:1–3. doi: 10.1016/s0165-6147(99)01415-7. [DOI] [PubMed] [Google Scholar]
- BOEYNAEMS J.-M., PEARSON J.D. P2 purinoceptors on vascular endothelial cells: physiological significance and transduction mechanisms. TIPS. 1990;11:34–37. doi: 10.1016/0165-6147(90)90039-b. [DOI] [PubMed] [Google Scholar]
- BORN G.V.R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- BORN G.V.R., KRATZER M.A.A. Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits and man. J. Physiol. 1984;354:419–429. doi: 10.1113/jphysiol.1984.sp015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUELL G., LEWIS C., COLLO G., NORTH R.A., SURPRENANT A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. Overview. Purinergic Mechanisms. Ann. N.Y. Acad. Sci. 1990;603:1–17. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- CHEN C.-C., AKOPIAN A.N., SIVILOTTI L., COLQUHOUN D., BURNSTOCK G., WOOD J.N. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- DANIEL J.L., DANGELMAIER C., JIN J., ASHBY B., SMITH J.B., KUNAPULI S.P. Molecular basis for ADP-induced platelet activation. I. Evidence for three distinct ADP receptors on human platelets. J. Biol. Chem. 1998;273:2024–2029. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- DANIEL J.L., DANGELMAIER C., JIN J., KIM Y.B., KUNAPULI S.P. Role of intracellular signalling events in ADP-induced platelet aggregation. Thromb. Haemost. 1999;82:1322–1326. [PubMed] [Google Scholar]
- EBERHARD D.A., HOLZ R.W. Intracellular Ca2+ activates phospholipase C. TINS. 1988;11:517–520. doi: 10.1016/0166-2236(88)90174-9. [DOI] [PubMed] [Google Scholar]
- EVANS R.J., KENNEDY C. Characterization of P2-purinoceptors in the smooth muscle of the rat tail artery: a comparison between contractile and electrophysiological responses. Br. J. Pharmacol. 1994;113:853–860. doi: 10.1111/j.1476-5381.1994.tb17071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS R.J., LEWIS C., BUELL G., VALERA S., NORTH R.A., SURPRENANT A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2X purinoceptors) Mol. Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- EVANS R.J., SURPRENANT A., NORTH R.A.P2X receptors: cloned and expressed P2 Nucleotide Receptors 1999Totowa, N.J.: Humana Press Inc; 43–61.eds. Turner J.T., Weisman G.A., and Fedan. J.S. pp [Google Scholar]
- FABRE J.-E., NGUYEN M., LATOUR A., KEIFER J.A., AUDOLY L.P., COFFMAN T.M., KOLLER B.H. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nature Medicine. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- FLODGAARD H., KLENOW H. Abundant amounts of diadenosine 5′,5′′′-P1,P4-tetraphosphate are present and releasable, but metabolically inactive, in human platelets. Biochem. J. 1982;208:737–742. doi: 10.1042/bj2080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DUBYAK G.R., HARDEN T.K., JACOBSON K.A., SCHWABE U., WILLIAMS M. Towards a revised nomenclature for P1 and P2 receptors. TIPS. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAARDER A., JONSEN J., LALAND S., HELLEM A., OWREN P.A. Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature. 1961;192:531–532. doi: 10.1038/192531a0. [DOI] [PubMed] [Google Scholar]
- HECHLER B., LÉON C., VIAL C., VIGNE P., FRELIN C., CAZENAVE J.-P., GACHET C. The P2Y1 receptor is necessary for adenosine 5′-diphosphate-induced platelet aggregation. Blood. 1998a;92:152–159. [PubMed] [Google Scholar]
- HECHLER B., VIGNE P., LÉON C., BREITTMAYER J.-P., GACHET C., FRELIN C. ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol. Pharmacol. 1998b;53:727–733. [PubMed] [Google Scholar]
- HOLMSEN H., WEISS H.J. Secretable storage pools in platelets. Ann. Rev. Med. 1979;30:119–134. doi: 10.1146/annurev.me.30.020179.001003. [DOI] [PubMed] [Google Scholar]
- HOURANI S.M.O., HALL D.A. Receptors for ADP on human blood platelets. TIPS. 1994;15:103–108. doi: 10.1016/0165-6147(94)90045-0. [DOI] [PubMed] [Google Scholar]
- JANKOWSKI J., POTTHOFF W., VAN DER GIET M., TEPEL M., ZIDEK W., SCHLUTER H. High-performance liquid chromatographic assay of the diadenosine polyphosphates in human platelets. Anal. Biochem. 1999;269:72–78. doi: 10.1006/abio.1999.3097. [DOI] [PubMed] [Google Scholar]
- JIN J., DANIEL J.L., KUNAPULI S.P. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J. Biol. Chem. 1998;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- JIN J., KUNAPULI S.P. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci., U.S.A. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWA K. ADP-induced rapid inward currents through Ca2+-permeable cation channels in mouse, rat and guinea-pig megakaryocytes: a patch clamp study. J. Physiol. 1996;495:339–352. doi: 10.1113/jphysiol.1996.sp021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNAPULI S.P., DANIEL J.L. P2 receptor subtypes in the cardiovascular system. Biochem. J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEON C., HECHLER B., FREUND M., ECKLY A., VIAL C., OHLMANN P., DIERICH A., LEMEUR M., CAZENAVE J.-P., GACHET C. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor-null mice. J. Clin. Invest. 1999;104:1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEON C., HECHLER B., VIAL C., LERAY C., CAZENAVE J.-P., GACHET C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- LEWIS C., NELDHART S., HOLY C., NORTH R.A., BUELL G., SURPRENANT A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- LEWIS C.J., GITTERMAN D.P., SCHLUTER H., EVANS R.J. Effects of diadenosine polyphosphates (ApnAs) and adenosine polyphospho guanosines (ApnGs) on rat mesenteric artery P2X receptor ion channels. Br. J. Pharmacol. 2000;129:124–130. doi: 10.1038/sj.bjp.0702993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKENZIE A.B., MAHAUT-SMITH M.P., SAGE S.O. Activation of receptor-operated cation channels via P2X1 not P2T purinoceptors in human platelets. J. Biol. Chem. 1996;271:2879–2881. doi: 10.1074/jbc.271.6.2879. [DOI] [PubMed] [Google Scholar]
- MAHAUT-SMITH M.P., HUSSAIN J.F., MASON M.J. Depolarisation-evoked Ca2+ release in a non-excitable cell, the rat megakaryocyte. J. Physiol. 1999;515:385–390. doi: 10.1111/j.1469-7793.1999.385ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHAUT-SMITH M.P., SAGE S.O., RINK T.J. Receptor-activated single channels in intact human platelets. J. Biol. Chem. 1990;265:10479–10483. [PubMed] [Google Scholar]
- MAHAUT-SMITH M.P., SAGE S.O., RINK T.J. Rapid ADP-evoked currents in human platelets recorded with the nystatin permeabilized patch technique. J. Biol. Chem. 1992;267:3060–3064. [PubMed] [Google Scholar]
- MULRYAN K., GITTERMAN D.P., LEWIS C.J., VIAL C., LECKIE B.J., COBB A.L., BROWN J.E., CONLEY E.C., BUELL G., PRITCHARD C.A., EVANS R.J. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- NICHOLAS R.A., WATT W.C., LAZAROWSKI E.R., LI Q., HARDEN T.K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: Identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol. Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- NORTH R.A., BARNARD E.A. Nucleotide receptors. Curr. Opin. Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- OHLMANN P., LAUGWITZ K.L., NURNBERG B., SPICHER K., SCHULTZ G., CAZENAVE J.P., GACHET C. The human platelet ADP receptor activates Gi2 proteins. Biochem. J. 1995;312:775–779. doi: 10.1042/bj3120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER R.K., BOYER J.L., SCHACHTER J.B., NICHOLAS R.A., HARDEN T.K. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol. Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- PAUL B.Z.S., DANIEL J.L., KUNAPULI S.P. Platelet shape change is mediated by both calcium-dependent and -independent signaling pathways. J. Biol. Chem. 1999;274:28293–28300. doi: 10.1074/jbc.274.40.28293. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- ROLF M.G., MAHAUT-SMITH M.P. Functional roles of P2X1 purinoceptors in human platelets. Biophysical J. 2000;78:73A. [Google Scholar]
- SAGE S.O., MACKENZIE A.B., JENNER S., MAHAUT-SMITH M.P. Purinoceptor-evoked calcium signalling in human platelets. Prost. Leukot. Essent. Fatty Acids. 1997;57:435–438. doi: 10.1016/s0952-3278(97)90424-5. [DOI] [PubMed] [Google Scholar]
- SAGE S.O., REAST R., RINK T.J. ADP evokes biphasic Ca2+ influx in fura-2-loaded human platelets. Evidence for Ca2+ entry regulated by the intracellular Ca2+ store. Biochem. J. 1990;265:675–680. doi: 10.1042/bj2650675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGE S.O., RINK T.J. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J. Biol. Chem. 1987;262:16364–16369. [PubMed] [Google Scholar]
- SAGE S.O., RINK T.J., MAHAUT-SMITH M.P. Resting and ADP-evoked changes in cytosolic free sodium concentration in human platelets loaded with the indicator SBFI. J. Physiol. 1991;441:559–573. doi: 10.1113/jphysiol.1991.sp018767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVI P., BEAUVERGER P., LABOURET C., DELFAUD M., SALEL V., KAGHAD M., HERBERT J.M. Role of P2Y1 purinoceptor in ADP-induced platelet activation. FEBS Lett. 1998;422:291–295. doi: 10.1016/s0014-5793(98)00025-8. [DOI] [PubMed] [Google Scholar]
- SAVI P., BORNIA J., SALEL V., DELFAUD M., HERBERT J.M. Characterization of P2x1 purinoceptors on rat platelets: effect of clopidogrel. Br. J. Haematol. 1997;98:880–886. doi: 10.1046/j.1365-2141.1997.3133126.x. [DOI] [PubMed] [Google Scholar]
- SOMASUNDARAM B., MAHAUT-SMITH M.P. Three cation influx currents activated by purinergic receptor stimulation in rat megakaryocytes. J. Physiol. 1994;480:225–231. doi: 10.1113/jphysiol.1994.sp020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SURPRENANT A., RASSENDREN F., KAWASHIMA E., NORTH R.A., BUELL G. The cytolytic P2z receptor for extracellular ATP identified as a P2x receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- UNEYAMA C., UNEYAMA H., AKAIKE N. Cytoplasmic Ca2+ oscillation in rat megakaryocytes evoked by a novel type of purinoceptor. J. Physiol. 1993;470:731–749. doi: 10.1113/jphysiol.1993.sp019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALERA S., HUSSY N., EVANS R.J., ADAMI N., NORTH R.A., SURPRENANT A., BUELL G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- VIAL C., HECHLER B., LÉON C., CAZENAVE J.-P., GACHET C. Presence of P2X1 purinoceptors in human platelets and megakaryoblastic cell lines. Thromb. Haemost. 1997;78:1500–1504. [PubMed] [Google Scholar]
- VULCHANOVA L., ARVIDSSON U., RIEDL M., WANG J., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. Differential distribution of two ATP-gated ion channels (P2X receptors) determined by immunocytochemistry. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDMAN S.S., BROWN S.G., KING B.F., BURNSTOCK G. Selectivity of diadenosine polyphosphates for rat P2X receptor subunits. Eur. J. Pharmacol. 1999;367:119–123. doi: 10.1016/s0014-2999(98)00976-5. [DOI] [PubMed] [Google Scholar]


