Abstract
Previous studies have shown that β-adrenoceptor antagonists may be substrates of organic cation transporters in kidney and lung. In this study we examined the transport of the β-adrenoreceptor antagonists propranolol and metoprolol, in renal LLC-PK1 cell monolayers.
Experiments with BCECF (2′,7′-bis(2carboxyethyl)-5(6)-carboxyfluorescein) loaded LLC-PK1 cell monolayers demonstrated that metoprolol and propranolol flux across the basolateral membrane was consistent with non-ionic diffusion. Flux across the apical membrane consisted of both non-ionic diffusion and the uptake of the cationic form of the β-adrenoceptor antagonists.
Uptake of the cationic form of metoprolol across the apical membrane was Na+-independent, electrogenic and sensitive to external pH. Furthermore, uptake was sensitive to inhibition by Decynium-22 and the organic cations TEA (tetraethylammonium) and MPP+ (1-methyl 4-phenylpyridinium). These results, allied with the apical location of the uptake mechanism suggest that β-adrenoceptor antagonists may be substrates for the organic cation transporter, OCT2.
To confirm β-adrenoceptor antagonists as substrates for OCT2, we demonstrate, in cells transiently transfected with an epitope tagged version of hOCT2 (hOCT2-V5):(1) Decynium-22 sensitive [14C]-propranolol uptake, (2) cis-inhibition of OCT2 by a range of β-adrenoceptor antagonists and (3) metoprolol induced intracellular acidification.
Keywords: Organic cation transport, OCT2, LLC-PK1, β-adrenoceptor antagonist
Introduction
The kidney plays a central role in the elimination of a wide range of structurally divergent drug molecules from the body. Recently a number of the mechanisms have been identified at the molecular level and we are beginning to gain insight into how different cloned transporters integrate to provide effective routes for drug secretion. Renal excretion of cationic drug molecules can be considered a two step process consisting of uptake of organic cation across the basolateral membrane into the cell, followed by efflux across the apical membrane into the lumen of the nephron. The basolateral uptake step has been well characterized. Uptake of a range of organic cations across the basolateral membrane is electrogenic (Takano et al., 1984; Smith et al., 1988) and appears to be mediated by a single polyspecific transport system (Ullrich, 1994), the molecular identity of which is now thought to be the recently cloned OCT1 transport protein (Grundemann et al., 1994). The nature of the apical exit step is more complex, at least two distinct transport secretory mechanisms have been identified. Evidence suggests that permanently charged, relatively hydrophilic cations, e.g. quaternary ammonium compounds, are secreted across the apical membrane primarily by the actions of an organic cation proton antiporter (OC/H+) (Holohan & Ross, 1981; Takano et al., 1984; Hsyu & Giacomini, 1987). For more hydrophobic organic cations, P-glycoprotein located in the apical membrane of the proximal tubular epithelium (Thiebaut et al., 1987) is thought to be important (Nelson, 1988; Dellinger et al., 1992; Dutt et al., 1994). In addition to secretory fluxes of organic cations, there is also substantial evidence that a number of organic cations are reabsorbed across the proximal tubule (Ullrich, 1994; Ullrich & Rumrich, 1996). Indeed, recent molecular evidence implicates the cloned organic cation transporters OCT2 and OCTN2 in the reabsorption of organic cations (Gorboulev et al., 1997; Grundemann et al., 1997; Wu et al., 1999).
Many drug molecules are weak bases, and exist in both charged (organic cation) and uncharged (neutral weak base) forms. Recently we reported that measurement of intracellular pH (pHi) could be used as a powerful technique to differentiate between the transport of the charged organic cation and diffusion of the neutral weak base (Dudley & Brown, 1995; 1996). In this study, we have characterized the transport of a range of structurally related β-adrenoceptor antagonists across monolayers of LLC-PK1 cells, by measuring the effect of drug addition upon intracellular pH. Here we report that flux of metoprolol and propranolol comprises of the passive diffusion of the neutral weak base form across both the apical and basolateral membranes. In addition, we found clear evidence for the transport of the cationic form of these molecules across the apical membrane of LLC-PK1 cells. Analysis of the transport properties of this organic cation strongly suggests that the β-adrenoceptor antagonists metoprolol, propranolol and oxprenolol, are transported across the apical membrane by the organic cation transporter OCT2.
Methods
Materials
Cell culture media, supplements and tissue culture plastics were supplied by Life Technologies (Paisley, U.K.). Tissue culture filters were from Costar (High Wycombe, U.K.). LLC-PK1 cells were purchased from the European Tissue Culture Collection and mIMCD3 cells were a kind gift from Dr Bruce Stanton, Dartmouth Medical School, Hanover, U.S.A. BCECF-AM was from Calbiochem (Nottingham, U.K.). [3H]-Cimetidine and [14C]-propranolol were purchased from Amersham. [3H]-1-methyl-4-phenylpyridinium (MPP+) was from NEN. All other biochemicals were obtained from Sigma (Poole, U.K.) or BDH (Lutterworth, U.K.).
Cell culture
LLC-PK1 cells were cultured in Medium 199 (with 1.25 gl−1 NaHCO3) supplemented with 3% (v v−1) foetal calf serum (FCS), 2 mM L-glutamine and 50 μg ml−1 gentamycin. mIMCD3 cells (mouse inner medullary collecting duct cells) were grown in Hams F-12/DMEM supplemented with 10% (v v−1) FCS, 2 mM L-glutamine and 36 μg ml−1 gentamycin. Cells were maintained in culture at 37°C in a 5% CO2, 95% air atmosphere. The culture media was replaced every 2 days and cells were passaged only when confluent. For intracellular pH studies, LLC-PK1 cell monolayers were grown on 12 mm diameter Transwell polycarbonate filters (Costar), seeded at 4.4–5.0×105 cells cm−2, and monolayer confluence was estimated by microscopy and measurement of epithelial resistance (RT). Experiments were performed 5–7 days after seeding and 18–24 h after feeding. For mIMCD3 cell experiments, cells were plated at a density of 3.5×104 cells per well, into 12 well tissue culture plates.
Measurement of intracellular pH
Intracellular pH (pHi) was measured as previously described (Thwaites et al., 1993; Dudley & Brown, 1995). Briefly, cell monolayers grown on 12 mm diameter filter supports were loaded by incubation with BCECF-AM (5 μM) in both the apical and basolateral chambers for 40 min at 37°C in culture media. After loading, the filter insert was placed in a 24 mm perfusion chamber located on the stage of an inverted fluorescence microscope (Nikon Diaphot). The apical and basolateral surfaces were independently perfused using a compressed air driven system (flow rate 5 ml min−1) that allowed any combination of six apical and basolateral solutions. The volume of the apical chamber was 0.5 ml and that of the basolateral chamber 1 ml. All experiments were performed at 37°C. Intracellular H+ concentration was quantified by fluorescence (excitation at 440/490 nm, emission at 520 nm) from a group of 5–10 cells using a photon counting system (Newcastle Photometric Systems). This was converted to pHi by comparison of the 440/490 ratios with those derived from an intracellular calibration curve constructed at the end of each run using the nigericin/high K+ technique (Thomas et al., 1979). To measure intracellular pH in normal and transiently transfected Hela cells, cells plated on glass coverslips were mounted directly into the perfusion chamber and intracellular pH measured as described above.
Cloning of an epitope-tagged version of hOCT2
Poly-A+ RNA extracted from human kidney tissue (normal adult biopsy sample) was reversed transcribed using M-MLV (Moloney murine leukaemia virus) reverse transcriptase. The resulting cDNA was used in a PCR reaction using the Expand Long Template PCR System (Boehringer-Mannheim) with an annealing temperature of 56°C. Oligonucleotide primers were designed to amplify the full coding sequence of hOCT2 (Gorboulev et al., 1997, GenBank accession number X98333) with the stop codon (positions 1810–1812) excluded. The primer sequences were 5′-GCC CTC CTG CCT GCA GGA T-3′ (positions 125–143) and 5′-GTT CAA TGG AAT GTC TAG TTT CT-3′ (positions 1787–1809). The resulting 1684 bp PCR product was subcloned into the mammalian expression vector pcDNA3.1/V5/His, using the TOPO-TA cloning system (Invitrogen, The Netherlands). The resulting construct, hOCT2-V5 was sequenced using BigDye™ terminator chemistry and an ABI PRISM 377 DNA sequencer. Subsequent translation found this PCR product to be 100% identical at the amino acid level to the published sequence of hOCT2 and was in-frame with the V5 epitope tag on the expression vector.
Transient transfection of mIMCD3 and Hela cells
Twenty-four hours post-seeding, subconfluent layers of either mIMCD-3 or Hela cells were transiently transfected with hOCT2-V5 cDNA using the Effectene Transfection System (Qiagen, U.K.). The efficiency of the transfection protocol was assessed in parallel plates using a pcDNA3.1-Lac-Z construct, followed by a β-galactosidase staining assay (Invitrogen). A transfection efficiency of 30% was routinely achieved.
mIMCD3 cell transport measurements
The uptake of [3H]-cimetidine, [3H]-MPP+, and [14C]-propranolol was measured in mIMCD3 cells transfected with hOCT2-V5, 48 h post-transfection. Subconfluent cell monolayers were gently washed (3×) in a modified Krebs' buffer (composition detailed below). To begin the uptake measurement, the Krebs' buffer was aspirated and replaced with 1 ml of Krebs' buffer (pH 7.4) containing either [3H]-cimetidine, [3H]-MPP+ or [14C]-propranolol (0.5 μCi ml−1) with the addition of the appropriate test compounds as detailed in the results section. After a 10 min uptake period, the uptake buffer was removed and the cells washed five times with ice-cold Krebs' buffer. Cells were then permeabilized with 1 ml of 2% (v v−1) Triton X-100, samples removed and [3H] or [14C] activity determined by liquid scintillation counting. Control experiments were carried out using native mIMCD3 cells, after first establishing that the expression vector (pcDNA3.1) alone did not mediate substrate uptake. hOCT2-V5 mediated uptake is defined as the difference between uptake into transfected cells and native cells.
Solutions
Monolayers were perfused with a modified Krebs' solution (mmol l−1): NaCl 140, KCl 5.4, MgSO4 1.2, KH2PO4 0.3, NaH2 PO4 0.3, CaCl2 2, Glucose 10 and HEPES 10 (buffered to the desired pH at 37°C with Tris base) or a Na+-free Krebs' solution (composition as above, except that choline Cl replaced NaCl and KH2PO4 replaced NaH2PO4). To study the effect of membrane potential, a high K+ buffer was used. The high K+ buffer consisted of (mmol l−1): NaCl 100, KCl 45, MgSO4 1.2, KH2PO4 0.3, NaH2PO4 0.3, CaCl2 2, Glucose 10 and HEPES 10 (buffered to the desired pH with Tris).
Statistical analysis
Data are expressed as mean±s.e.mean. Statistical comparison of mean values was made using a Student's t-test (2-tailed solution) for paired or unpaired data as appropriate. For multiple comparisons, an analysis of variance (ANOVA) test was employed and significance assigned using a Dunnett post-test.
Results
Effect of metoprolol upon intracellular pH
Figure 1 shows the effect of metoprolol (pKa 9.6) upon pHi in monolayers of LLC-PK1 cells loaded with BCECF. Addition of metoprolol (10 mM) to the basolateral perfusate in the presence of the outwardly directed H+ gradient (basolateral perfusate pH 8.4) resulted in a significant rapid intracellular alkalinization from pH 7.43±0.04 to a new steady state pH of 7.72±0.04 (n=8, P<0.001). This was maintained as long as metoprolol was present in the basolateral perfusate (Figure 1a). Removal of metoprolol and reperfusion with pH 7.4 Kreb's resulted in a rapid recovery of pHi to pre-stimulation values. Imposition of outwardly directed H+ gradient alone was without effect upon pHi (mean 7.43±0.04, n=8, P>0.05).
Figure 1.
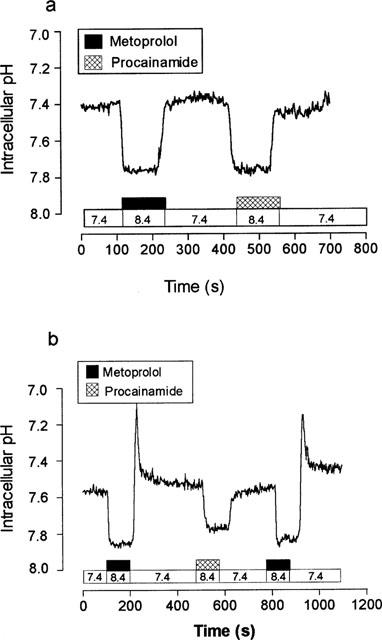
The effect of 10 mM metoprolol upon intracellular pH. Intracellular pH was measured in monolayers of LLC-PK1 cells loaded with BCECF. (a) The effect of basolateral addition of metoprolol at a basolateral perfusion pH of 8.4, apical perfusion pH was constant at pH 7.4. A single trace representative of eight separate experiments. (b) The effect of apical addition of metoprolol at an apical perfusion pH of 8.4, basolateral perfusion pH was constant at pH 7.4. A single trace representative of eight separate experiments.
Addition of metoprolol (Figure 1b) to the apical perfusate resulted in a more complicated response. At pH 8.4, this resulted in a similar, rapid intracellular alkalinization from pH 7.50±0.04 to a new steady state pHi of 7.86±0.01 (n=8, P<0.001). However, in contrast to the almost ‘square-wave' return to pre-challenge pHi seen at the basolateral membrane, removal of metoprolol from the apical perfusate resulted in a biphasic change in intracellular pH. Immediately upon the removal of metoprolol from the apical perfusate there was a rapid, transient, intracellular acidification (pH 7.27±0.05, n=8, P<0.001) followed by a slow recovery of intracellular pH to initial values (7.48±0.03, n=8). This biphasic response of pHi to metoprolol is consistent with the entry of both uncharged (B) and protonated (BH+) forms of metoprolol across the apical membrane.
The effect of structurally related β-adrenoceptor blockers upon intracellular pH in LLC-PK1 cells
Figure 2a compares the effects of apical addition of a range of structurally related β-adrenoceptor antagonists upon pHi in LLC-PK1 cells. Similar to metoprolol, apical challenge with either propranolol (1 mM) or oxprenolol (10 mM) resulted in a significant intracellular alkalinization upon drug addition (P<0.01, n=6) and a significant intracellular acidification upon removal of the drug from the perfusate (P<0.01, n=6). In contrast, we found only a ‘square wave' response of pHi to apical addition of 10 mM pindolol, with a significant intracellular alkalinization of 0.18±0.03 pH units upon addition (n=6, P<0.001) but no significant acidification upon removal (P>0.5). Apical challenge with atenolol (Figure 2) was completely without effect upon pHi (n=6, P>0.5). These results suggest that in addition to non-ionic diffusion, metoprolol, propranolol and oxprenolol may share a common transport mechanism at the apical membrane of LLC-PK1 cells.
Figure 2.
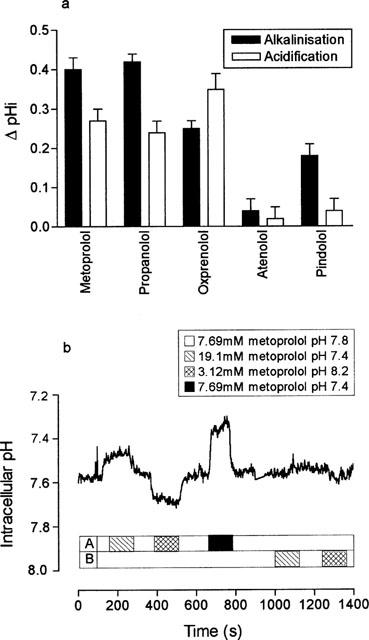
The effect of a range of β-adrenoceptor antagonists upon intracellular pH or upon hOCT2-V5 mediated cimetidine uptake. β-adrenoceptor antagonists (all 10 mM except propranolol (1 mM)) were added to the apical membrane at a perfusate pH of 8.4. The basolateral pH was held constant at pH 7.4. Results are the mean±s.e.mean of four separate determinations. (b) To test the relative permeabilities of neutral weak base (B) and the conjugate weak acid (BH+) forms of metoprolol across the apical and basolateral membranes of LLC-PK1 cells, cell monolayers were loaded with metoprolol (7.69 mM) at pH 7.8 for 40 min. Intracellular pH was measured under four conditions; (1) in the absence of an imposed pH or metoprolol gradient (pH 7.8, 7.69 mM metoprolol), (2) in the presence of an inwardly directed [BH+] gradient (pH 7.4 19.1 mM metoprolol), (3) an outwardly directed [BH+] gradient (pH 8.2, 3.12 mM metoprolol) and (4) an outwardly directed [B] gradient (pH 7.4, 7.69 mM metoprolol). A single trace representative of three separate experiments.
Is the ionization state of metoprolol important for transport?
To understand better the mechanism by which metoprolol, propranolol and oxprenolol influence intracellular pH from the apical perfusate, we investigated whether the apical membrane was permeable to both the protonated (BH+) and neutral (B) forms of these agents. To do this, LLC-PK1 cell monolayers were first loaded with BCECF-AM (5 μM) and metoprolol (7.69 mM) at pH 7.8 for 40 min, then by manipulating extracellular pH and metoprolol concentration in the appropriate way we imposed a range of neutral or protonated metoprolol gradients across the apical membrane. The results of these manipulations are shown in Figure 2b. In the continued presence of metoprolol in the both apical and basolateral perfusates, the intracellular pH of a typical monolayer was pH 7.59 (mean 7.60±0.03, n=3) at a perfusate pH of 7.8. Under these conditions, assuming [Bi]=[Bo], steady-state [BH+]i was calculated to be 12.02 mM.
Evidence in favour of a significant apical permeability to the protonated form of metoprolol (BH+) came from the marked intracellular acidification (P<0.05) found upon imposition of an inwardly-directed BH+ gradient across the apical membrane (Figure 2b). Equally, a significant intracellular alkalinization (P<0.05) was found in the presence of an outwardly-directed BH+ gradient across the apical membrane. In both cases, pHi recovered to pre-stimulation levels upon re-addition of metoprolol to the apical perfusate at pH 7.8.
Evidence in favour of a significant (B) permeability came from the observation that imposition of an outwardly-directed neutral weak base gradient across the apical membrane resulted in a significant intracellular acidification (pH 7.58±0.03 to 7.33±0.02 n=3, P<0.01).
In contrast to the marked changes in pHi at the apical membrane, imposition of identical BH+ gradients across the basolateral membrane of LLC-PK1 cells had no effect upon pHi (Figure 2b). Taken together, these results suggest that the apical membrane of LLC-PK1 cells has a significant permeability to both the charged (BH+) and uncharged (B) forms of metoprolol. In contrast, the basolateral membrane appears to have a significant permeability only to the uncharged form of metoprolol.
Characterization of protonated metoprolol transport across the apical membrane
Protonated metoprolol uptake is Na+ independent
To investigate whether metoprolol permeation across the apical membrane was a Na+-dependent process, we measured the effect of replacing Na+ in the apical perfusate with choline. Figure 3a shows that the entry of protonated metoprolol into the cell is a sodium independent process. Replacement of sodium by choline had no significant effect upon the magnitude of either the intracellular alkalinization (P>0.05) or intracellular acidification (P>0.05). In contrast however, recovery from the intracellular proton load induced by the metoprolol challenge was dependent upon the presence of sodium in the apical perfusate. It is likely that extrusion of protons is mediated by Na+/H+ exchange, located in the apical membrane.
Figure 3.
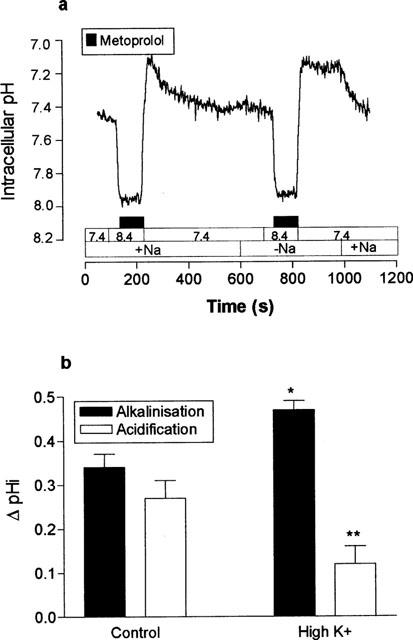
Metoprolol uptake is Na+ dependent. The effect of apical addition of 10 mM metoprolol at an apical perfusate pH 8.4 upon intracellular pH, was measured in the presence and absence of Na+ in both perfusates. The basolateral perfusate pH was constant at pH 7.4. A single trace representative of three separate experiments. (b) Metoprolol uptake is electrogenic. The effect of apical challenge with 10 mM metoprolol at pH 8.4 was measured under control (4.5 mM) or high K+ (40 mM) conditions. [K+] was changed in both apical and basolateral perfusates simultaneously. The basolateral perfusate pH was constant at pH 7.4. The data are the mean±s.e.mean of four separate determinations. *P<0.05, **P<0.01, compared to control.
Protonated metoprolol uptake is electrogenic
In the next series of experiments, we investigated whether the uptake of protonated metoprolol across the apical membrane was an electrogenic process. To do this, the effects of metoprolol upon pHi were measured under control conditions and whilst the transmembrane potential difference was depolarized (Figure 3b). At depolarizing membrane potentials, the metoprolol induced alkalinization at pH 8.4 was significantly larger (ΔpH: 0.47±0.02 vs 0.34±0.03 units, n=4, P<0.05) and the transient acidification, significantly smaller (ΔpH: 0.12±0.04 vs 0.27±0.04 units, n=4, P<0.01) compared to control conditions.
Protonated metoprolol uptake is inhibited by Decynium-22
Figure 4a shows that the magnitude of both the initial alkalinization and the subsequent acidification upon metoprolol removal are affected by 1,1′-Diethyl-2,2′-cyanine (Decynium-22). The initial alkalinization induced by metoprolol addition was significantly larger in the presence of Decynium-22 (2 μM) (ΔpH 0.44±0.03 vs 0.35±0.02 units, n=4, P<0.05) than in its absence. Furthermore, the transient intracellular acidification normally found upon metoprolol removal was almost completely abolished by pre-exposure to Decynium-22 (ΔpH 0.04±0.03 vs 0.28±0.02 units, n=4, P<0.001). Both the increased magnitude of alkalinization and the significant reduction in acidification upon metoprolol removal, reflect the inhibition of protonated metoprolol entry and cellular accumulation by Decynium-22. In contrast, Decynium-22 had no significant effect upon magnitude of either the alkalinization or the transient acidification generated in response to a 20 mM ammonium pulse across the apical membrane (Figure 4a). These observations suggest that NH4+ and protonated metoprolol do not share a common transport mechanism. Similarly Decynium-22 had no effect on the response of pHi to basolateral metoprolol challenge (mean alkalinization 0.28±0.03 vs 0.25±0.05 units, n=4, P>0.05).
Figure 4.
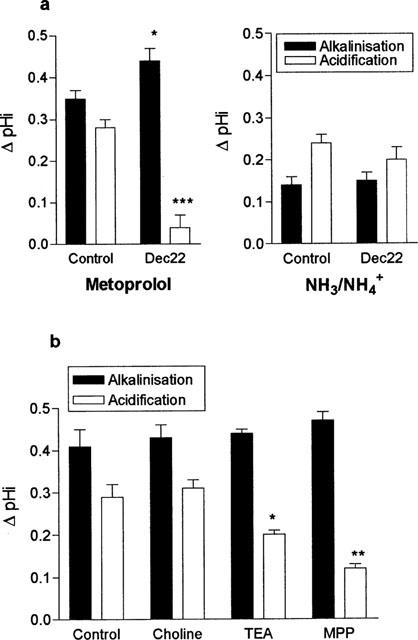
Pharmacological characterization of metoprolol uptake. (a) The effect of the organic cation transport inhibitor Decynium-22 (2 μM) was tested upon the pH response to either 10 mM metoprolol or 20 mM NH4Cl. Both compounds were added to the apical perfusate at pH 8.4. The basolateral pH was held constant at pH 7.4. Results are the mean±s.e.mean of four separate determinations. *P<0.05, ***P<0.001 compared to control condition. (b) The effect of apical addition of various organic cations (20 mM) upon the pH response to a 10 mM metoprolol challenge (pH 8.4). The basolateral pH was held constant at pH 7.4. Results are the mean±s.e.mean of four separate determinations, *P<0.05, **P<0.01 compared to control.
Protonated metoprolol uptake is modified by a range of organic cations
To determine the nature of protonated metoprolol uptake across the apical membrane of LLC-PK1 cells, the effect upon metoprolol uptake of a range of organic cations was assessed (Figure 4b). The addition of 20 mM TEA resulted in an increased intracellular alkalinization and significantly reduced the magnitude of intracellular acidification induced by a 5 mM pulse of metoprolol. Similarly, the addition of 20 mM MPP+ resulted in both an increased alkalinization and a very significant inhibition of metoprolol induced acidification. In contrast, 20 mM choline appeared to have no significant effect upon metoprolol induced acidification.
Protonated metoprolol uptake is pH dependent
Figure 5 shows that the magnitude of the response to apical addition of metoprolol was dependent upon the extracellular pH. Compared with pH of 7.4, we found a significantly larger intracellular alkalinization when metoprolol challenge was repeated at pH 8.4 (ΔpH=0.35±0.01 units vs 0.27±0.03 units at pH 7.4, n=13, P<0.01). Furthermore, this was accompanied by a significantly larger transient intracellular acidification upon removal of metoprolol (0.30±0.02 pH units) than found after metoprolol challenge at pH 7.4 (0.12±0.04 pH units, n=13, P<0.001).
Figure 5.
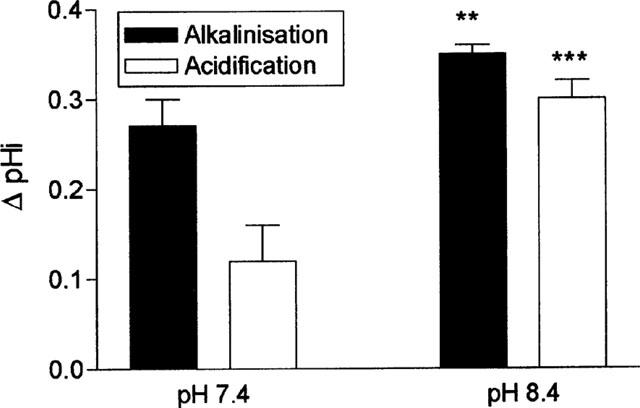
The effect of metoprolol upon intracellular pH was dependent upon extracellular pH. The effect of a 10 mM apical metoprolol challenge was assayed at either an apical pH of 7.4 or 8.4. The basolateral pH was held constant at 7.4. Results are the mean±s.e.mean of four separate determinations, **P<0.01, ***P<0.001 compared to pH 7.4.
β-adrenoceptor antagonists cis-inhibit hOCT2 mediated cimetidine uptake
To assess whether β-adrenoceptor antagonists may be substrates for the organic cation transporter OCT2, found on the apical membrane of LLC-PK1 cells, we measured the ability of metoprolol to cis-inhibit the uptake of [3H]-cimetidine in cultured mouse inner medullary collecting duct cells (mIMCD3) transiently transfected with an epitope tagged version of the human organic cation transporter hOCT2. Addition of the epitope tag, designed to allow localization of expression of hOCT2 (Bleasby et al., 1999) had no effect upon the function of hOCT2. For example, the Km for the transport of MPP+ by the epitope tagged version of hOCT2, hOCT2-V5, was 22±1.8 μM (Figure 6a), a value similar to that recently reported for native hOCT2 (Gorboulev et al., 1997). Similar to rOCT2 (Grundemann et al., 1999) we recently reported cimetidine to be a high affinity substrate for hOCT2-V5 with an apparent Km of 99±14 μM (Bleasby et al., 2000). In addition, hOCT2-V5 mediated MPP+ uptake was 53% inhibited (P<0.001) in the presence of 500 nM Decynium-22 (Figure 6b).
Figure 6.
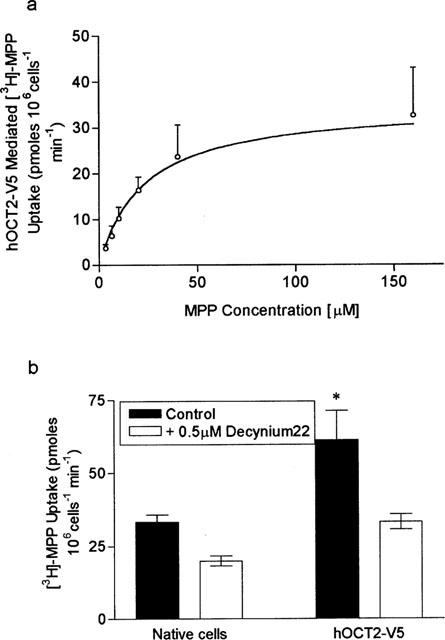
MPP+ uptake into hOCT2-V5 transfected mIMCD3 cells. (a) The dose response relationship between hOCT2-V5 mediated MPP+ uptake and MPP+ concentration, analysis of the data gave an apparent Km of 22.2±1.8 μM. The results are the mean±s.e.mean of six separate determinations. (b) Initial rates of MPP+ uptake (25 μM) into native and hOCT2-V5 transfected cells measured in the presence and absence of 500 nM Decynium-22. The results are the mean±s.e.mean of nine separate determinations, *P<0.05 compared to native cells in the control condition.
Under control conditions, the uptake of 100 μM cimetidine was 2 fold greater in mIMCD3 cells transfected with hOCT2-V5, compared to control cells (Figure 7a). Addition of 1 mM metoprolol to the uptake buffer significantly inhibited the hOCT2-V5 mediated component of cimetidine uptake into transfected cells from 37.5±2.8 to 10.1±1.0 pmoles 106 cells−1 min−1 (n=7, P<0.05). In contrast, the presence of metoprolol had no significant effect upon the magnitude of cimetidine uptake in control cells (P>0.5, n=7).
Figure 7.
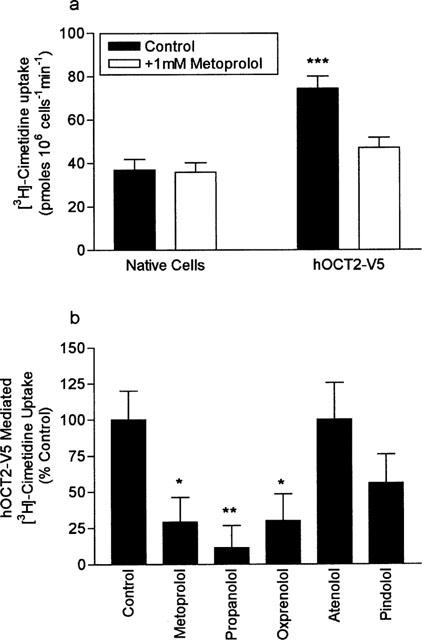
The effect of metoprolol upon [3H]-cimetidine uptake into native and hOCT2-V5 transfected mIMCD3 cells. Initial rates of cimetidine uptake (100 μM) were measured at pH 7.4 in the absence and presence of 1 mM metoprolol. Results are the mean±s.e.mean of 12 separate determinations, ***P<0.001 compared to native cells in the control condition. (b) The effect of a range of β-adrenoceptor antagonists (all at 1 mM) upon hOCT2-V5 mediated [3H]-cimetidine uptake (100 μM) measured at pH 7.4. Uptake is expressed as per cent of the control condition. Results are the mean±s.e.mean of eight separate determinations, *P<0.05, **P<0.01 compared to control cells.
In Figure 7b we extend this data set and compare the effects of a range of β-blockers (1 mM) upon hOCT2-V5 mediated cimetidine uptake into mIMCD3 cells. The results clearly show that metoprolol (P<0.05), propranolol (P<0.01) and oxprenolol (P<0.05) all significantly cis-inhibit hOCT2-V5 mediated cimetidine uptake. In contrast, atenolol and pindolol were without effect upon cimetidine uptake (P<0.05).
Propranolol and metoprolol are substrates for hOCT2
To demonstrate a direct interaction of metoprolol and propranolol with hOCT2 we measured (i) the uptake of propranolol, a commercially available radiolabelled β-adrenoceptor antagonist, into mIMCD3 cells transfected with hOCT2-V5 and (ii) the effect of metoprolol upon intracellular pH in hOCT2-V5-transfected and mock-transfected Hela cells. Figure 8a shows that the uptake of 75 μM propranolol was significantly greater (P<0.001) in cells transfected with hOCT2-V5 compared with control cells. Furthermore the hOCT2-V5-mediated component of propranolol uptake was completely abolished in the presence of 500 nM Decynium-22.
Figure 8.
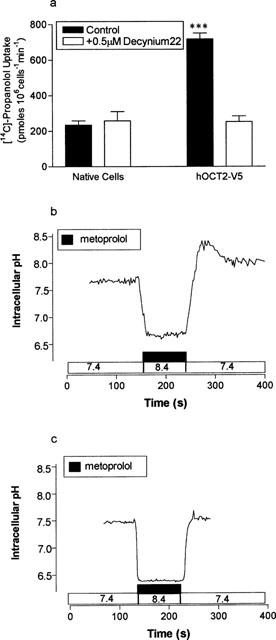
The uptake of propranolol (75 μM) into native and hOCT2-V5 transfected cells. Initial rates of propranolol uptake were measured at pH 7.4 in the absence and presence of 500 nM Decynium-22. Results are the mean±s.e.mean of nine separate determinations, ***P<0.001 compared to native cells in the control condition. To investigate the effect of 10 mM metoprolol upon intracellular pH in Hela cells transiently transfected with hOCT2-V5 intracellular pH was measured in subconfluent monolayers of cells loaded with BCECF. (b) The effect of metoprolol challenge upon Hela cells transfected with hOCT2-V5 at a perfusion pH of 8.4 and subsequent recovery at pH 7.4. A single trace representative of five separate experiments. (c) The effect of an identical metoprolol challenge upon intracellular pH in mock-transfected Hela cells. A single trace representative of five separate experiments.
Figure 8b shows the effect of a 10 mM metoprolol challenge upon intracellular pH in Hela cells transiently transfected with hOCT2-V5. Addition of metoprolol to the perfusate resulted in a rapid intracellular alkalinization (ΔpH 0.52±0.05, n=5). Release of the metoprolol challenge and re-perfusion with Krebs' buffer at pH 7.4 generated a significant, transient, intracellular acidification (ΔpH 0.42±0.04, n=5). In contrast, in mock-transfected Hela cells (Figure 8c) an identical metoprolol challenge elicited a significantly greater intracellular alkalinization (ΔpH 0.68±0.04, P<0.05, n=5) but upon metoprolol release pHi returned to pre-challenge pH values with no evidence of the intracellular acidification (ΔpH 0.06±0.03, n=5) seen in hOCT2-V5 transfected Hela cells.
Discussion
In a previous study, we demonstrated the potential of measurement of intracellular pH as a tool to characterize the relative contribution of charged and uncharged forms of a weak base to its overall transport (Dudley & Brown, 1995; 1996). In this study, we set out to characterize the handling of the weak base metoprolol and related β-adrenoceptor antagonists at the apical and basolateral membranes of epithelial monolayers of cultured renal LLC-PK1 cells.
For many organic cations, uptake across the basolateral membrane can be accounted for by the actions of a single electrogenic transporter (Ullrich, 1994). Expression cloning studies in rat identified this polyspecific transporter at the molecular level as rOCT1. Functional studies clearly show that rOCT1 transports a range of organic cations including TEA, is Na+-independent, electrogenic and is extremely sensitive to inhibition by Decynium-22 (Grundemann et al., 1994; Urakami et al., 1998). To date, there has been no definitive evidence to suggest that OCT1 is expressed in LLC-PK1 cells. In this study, we found that the uptake of metoprolol across the basolateral membrane was not sensitive to inhibition by Decynium-22 or competition by TEA. In conjunction with the apparent lack of transport of the cationic form of metoprolol, these results argue against a significant role for OCT1 in the accumulation of metoprolol across the basolateral membrane of LLC-PK1 cells. Since metoprolol is a relatively lipophilic molecule, it is likely that in LLC-PK1 cells, the contribution of passive diffusion to metoprolol uptake across the basolateral membrane is functionally more important than the contribution of OCT1.
Apical handling of metoprolol appears to be more complex than uptake across the basolateral membrane. As at the basolateral membrane, transport of metoprolol across the apical membrane involves the passive flux of uncharged metoprolol. In addition, there is also a significant transport of protonated metoprolol across the apical membrane. Direct evidence in support of this comes from: the significant changes in pHi found in response to inwardly, or outwardly directed gradients of protonated metoprolol (Figure 2) and from the transient intracellular acidification observed upon metoprolol removal from the apical perfusate (Figure 1b). Thus, at the apical membrane, the magnitude of the initial alkalinization reflects the balance between the influx of uncharged metoprolol (which would raise pHi as it consumes protons to become protonated) and the influx of protonated metoprolol (which would tend to lower pH as it dissociated to uncharged metoprolol and a proton). Equally the transient acidification upon metoprolol removal reflects the balance between the relative rates of uncharged and protonated metoprolol exit across the apical membrane. At 10 mM concentration of metoprolol, the estimated contribution of protonated metoprolol uptake to the total metoprolol uptake is of the order of 10–20%.
The transient intracellular acidification indicates that overall the exit of metoprolol across the apical membrane must release more protons than its entry consumes i.e. metoprolol which enters the cell in the protonated form predominately exits the cell as uncharged metoprolol leaving behind a proton. Given the inside-negative membrane potential, the exit of positively charged metoprolol across the apical membrane would be less energetically favoured than exit of uncharged metoprolol.
The increase in alkalization and subsequent inhibition of acidification by Decynium-22, MPP+ and TEA point towards a mediated transport of protonated metoprolol rather than passive diffusion of the charged form. As these agents tend to both increase intracellular pH and inhibit acidification, it is likely that they cis-inhibit protonated metoprolol uptake rather than trans-stimulate protonated metoprolol exit from the cell. This is certainly the case for Decynium-22 since inhibition of an efflux mechanism for protonated metoprolol by Decynium-22 would tend to increase intracellular acidification. The effect of membrane potential upon intracellular pH in response to metoprolol challenge is also consistent with inhibition of electrogenic metoprolol uptake.
What mechanism underlies the transport of protonated metoprolol across the apical membrane? It has been reported that propranolol inhibits TEA-sensitive guanidine uptake into rabbit alveolar epithelial cells (Shen et al., 1999). Equally, organic cation uptake into rat proximal and distal tubules is sensitive to inhibition by β-adrenoceptor antagonists (Stupack et al., 1999). These data suggest that organic cation transport plays an important role in modulating the transport of β-adrenoceptor antagonists across epithelia.
Two Na+ independent, electrogenic, organic cation transporters have been identified in kidney: OCT1 and OCT2 (Okuda et al., 1996; Gorboulev et al., 1997; Grundemann et al., 1997). OCT1 and OCT2 share a ∼70% identity at the amino acid level and have similar pharmacology and substrate specificities (Grundemann et al., 1999; Sweet & Pritchard, 1999a). Whilst it is universally accepted that OCT1 is responsible for the uptake of organic cations across the basolateral membrane, the cellular location and function of OCT2 remain less clear. Urakami et al. (1998) provided strong functional and immunological evidence to suggest that rOCT2 is expressed solely at the basolateral membrane of MDCK cells transfected with rOCT2, although more recent studies show localization of rOCT2 at both membranes of MDCK cells (Sweet & Pritchard, 1999c). In contrast, Gorboulev et al. (1997) concluded, on the basis of immunohistochemical evidence, that hOCT2 is expressed on the apical membrane of distal tubules. In agreement with this, Grundemann et al. (1997) demonstrated that pOCT2, cloned from LLC-PK1 cells, is functionally expressed at the apical membrane of epithelial monolayers of LLC-PK1 cells. hOCT2 and pOCT2 share 85% identity at the amino acid level over the full-length sequence (Sweet & Pritchard, 1999a). Although not yet conclusive, it would appear that the bulk of the evidence supports an apical location for OCT2, particularly since questions have been raised over the fidelity of membrane targeting mechanisms in transfected cells (Sweet & Pritchard, 1999b,1999c).
The presence of OCT2 in the apical membrane of LLC-PK1 cells, coupled with the demonstration that β-adrenoceptor antagonists are substrates for organic cation transporters raises the possibility that metoprolol uptake across the apical membrane of LLC-PK1 cells is mediated by OCT2. In this study we have presented evidence to show that metoprolol uptake is: (i) Na+-independent; (ii) electrogenic; (iii) sensitive to inhibition by Decynium-22 in the sub-micromolar range (iv) moderately sensitive to pH. These results, coupled with the observation of inhibition of metoprolol uptake by the organic cations MPP+ and TEA, are entirely consistent with the actions of OCT2 (Okuda et al., 1996; 1999; Gorboulev et al., 1997; Grundemann et al., 1999). Interestingly, we found that choline at concentrations of up to 137 mM had no effect upon the transport of protonated metoprolol in LLC-PK1 cells. Since choline has been reported to be a substrate for hOCT2 (Gorboulev et al., 1997) but not a substrate for rOCT2 (Grundemann et al., 1999), the lack of effect of choline may simply reflect that it is not a substrate for pOCT2. Taken together, the characteristics of protonated metoprolol uptake, allied with the apical location of the transport event, strongly implicate OCT2 as the molecular identity of the transporter responsible for a range of uptake across the apical membrane of LLC-PK1 cells.
To confirm the involvement of OCT2 in the transport of β-adrenoceptor antagonists, we designed a series of experiments in which we measured the effect of the same series of β-adrenoceptor antagonists in cells transiently transfected with the human isoform of OCT2; hOCT2. In this series of experiments, firstly, we demonstrated a significant Decynium-22 sensitive propranolol uptake specific to mIMCD3 cells transfected with hOCT2-V5. Secondly we extended these observations, to show a near identical match between the ability of a series of β-adrenoceptor antagonists to generate an intracellular acidification in LLC-PK1 cells and to cis-inhibit hOCT2-mediated cimetidine uptake in transiently transfected mIMCD3 cells. Finally, we provide direct evidence for the interaction of metoprolol with OCT2 by demonstrating a metoprolol induced intracellular acidification in Hela cells transiently transfected with hOCT2-V5. A result in marked contrast to the ‘square-wave' response of intracellular pH to metoprolol challenge found in mock-transfected Hela cells.
In summary, we have investigated the transport of metoprolol and related β-adrenoceptor antagonists in epithelial monolayers of renal LLC-PK1 cells. The data suggest that transport of metoprolol, propranolol and oxprenolol is mediated by the passive diffusion of the uncharged form of these drugs across both membranes. In addition, we found clear evidence for the uptake of protonated metoprolol, propranolol and oxprenolol at the apical membrane. We provide strong evidence to suggest that the molecular identity of the uptake mechanism at the apical membrane of LLC-PK1 cells is consistent with the properties of the organic cation transporter OCT2.
Acknowledgments
This work was supported by the National Kidney Research Fund (R35/1/96). K. Bleasby holds a National Kidney Research Fund Studentship.
Abbreviations
- BCECF
2′,7′-bis(2carboxyethyl)-5(6)-carboxyfluorescein
- bp
base pair
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid
- MPP+
1-methyl-4-phenylpyridinium
- OCT
organic cation transporter
- PCR
polymerase chain reaction
- TEA
tetraethylammonium
- Tris
2-amino-2-hydroxymethyl-1,3-propanediol
References
- BLEASBY K., CLARK M.A., BROWN C.D.A. Functional expression of an epitope tagged version of the human organic cation transporter hOCT2 in mIMCD3 cells. J. Physiol. 1999;520P:S41. [Google Scholar]
- BLEASBY K., LAUFFART B., CLARK M.A., BROWN C.D.A. DIDS sensitive uptake of cimetidine mediated by an epitope tagged version of the human organic cation transporter hOCT2. FASEB J. 2000;14:A353. [Google Scholar]
- DELLINGER M., PRESSMAN B.C., CALDERON-HIGGINSON C., SAVARAJ N., TAPIERO H., KOLONIAS D., LAMPIDIS T.J. Structural requirements of simple organic cations for recognition by multidrug-resistant cells. Cancer Res. 1992;52:6385–6389. [PubMed] [Google Scholar]
- DUDLEY A.J., BROWN C.D.A. pH-dependent transport of procainamide in cultured renal epithelial monolayers of OK cells: consistent with non-ionic diffusion. Br. J. Pharmacol. 1995;116:1685–1691. doi: 10.1111/j.1476-5381.1995.tb16392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDLEY A.J., BROWN C.D.A. Mediation of cimetidine secretion by P-glycoprotein and a novel H+ coupled mechanism in cultured renal epithelial monolayers of LLC-PK1 cells. Br. J. Pharmacol. 1996;117:1139–1144. doi: 10.1111/j.1476-5381.1996.tb16708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUTT A., HEATH L.A., NELSON J.A. P-glycoprotein an organic cation secretion by the mammalian kidney. J. Pharmacol. Exp. Ther. 1994;269:1254–1260. [PubMed] [Google Scholar]
- GORBOULEV V., ULZHEIMER J.C., AKHOUNDOVA A., ULZHEIMER-TEUBER I., KARBACH U., QUESTER S., BAUMANN C., LANG F., BUSCH A.E., KOEPSELL H. Cloning and characterisation of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16:871–881. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- GRUNDEMANN D., BABIN EBELL J., MARTEL F., ORDING N., SCHMIDT, SCHOMIG E. Primary structure and functional expression of the apical organic cation transporter from kidney epithelial LLC-PK1 cells. J. Biol. Chem. 1997;272:10408–10413. doi: 10.1074/jbc.272.16.10408. [DOI] [PubMed] [Google Scholar]
- GRUNDEMANN D., GORBOULEV V., GAMBARYAN S., VEYHL M., KOEPSELL H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994;372:549–555. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- GRUNDEMANN D., LIEBICH G., KIEFER N., KOSTER S., SCHOMIG E. Selective substrates for non-neuronal monoamine transporters. Mol. Pharmacol. 1999;56:1–10. doi: 10.1124/mol.56.1.1. [DOI] [PubMed] [Google Scholar]
- HOLOHAN P.D., ROSS C.R. Mechanisms of organic cation transport in kidney plasma membranes: 2; pH studies. J. Pharmacol. Exp. Ther. 1981;216:294–298. [PubMed] [Google Scholar]
- HSYU P.H., GIACOMINI K.M. The pH gradient dependent transport of organic cations in the renal brush border. J. Biol. Chem. 1987;262:3964–3968. [PubMed] [Google Scholar]
- NELSON J.A. A physiological function for multidrug resistant glycoproteins: A hypothesis regarding the renal organic cation secretory system. Cancer Chemother. Pharmacol. 1988;22:92–93. doi: 10.1007/BF00254191. [DOI] [PubMed] [Google Scholar]
- OKUDA M., SAITO H., URAKAMI Y., TAKANO M., INUI K.-I. cDNA cloning and function of a novel rat kidney organic cation transporter, OCT2. Biochem. Biophys. Res. Comm. 1996;224:500–507. doi: 10.1006/bbrc.1996.1056. [DOI] [PubMed] [Google Scholar]
- OKUDA M., URAKAMI Y., SAITO H., INUI K.-I. Molecular mechanisms of organic cation transport in OCT2-expressing Xenopus oocytes. Biochem. Biophys. Acta. 1999;1471:224–231. doi: 10.1016/s0005-2736(99)00005-x. [DOI] [PubMed] [Google Scholar]
- SHEN J., ELBERT K.J., YAMASHITA F., LEHR C.M., KIM K.J., LEE V.H. Organic cation transport in rabbit alveolar epithelial cell monolayers. Pharm. Res. 1999;16:1280–1287. doi: 10.1023/a:1014814017316. [DOI] [PubMed] [Google Scholar]
- SMITH P.M., PRITCHARD J.B., MILLER D.S. Membrane potential drives organic cation transport in teleost proximal tubules. Am. J. Physiol. 1988;255:R492–R499. doi: 10.1152/ajpregu.1988.255.3.R492. [DOI] [PubMed] [Google Scholar]
- STUPACK D.G., ESCOBAR M.R., CARLISLE DAVIE T., SITAR D.S. Heterogeneity among beta-adrenoceptor blockers in the modulation of energy-dependent uptake of the organic cation amantidine by rat renal tubules. Can. J. Physiol. Pharmacol. 1999;77:407–413. [PubMed] [Google Scholar]
- SWEET D.H., PRITCHARD J.B. The molecular biology of renal organic anion and organic cation transporters. Cell Biochem. Biophys. 1999a;31:89–118. doi: 10.1007/BF02738157. [DOI] [PubMed] [Google Scholar]
- SWEET D.H., PRITCHARD J.B. Localization of an organic anion transporter-GFP fusion construct (rROAT1-GFP) in intact proximal tubules. Am. J. Physiol. 1999b;276:F863–F864. doi: 10.1152/ajprenal.1999.276.6.F864. [DOI] [PubMed] [Google Scholar]
- SWEET D.H., PRITCHARD J.B. rOCT2 is a basolateral potential-driven carrier, not an organic cation/proton exchanger. Am. J. Physiol. 1999c;277:F890–F898. doi: 10.1152/ajprenal.1999.277.6.F890. [DOI] [PubMed] [Google Scholar]
- TAKANO M., INUI K., OKANO T., SAITO H., HORI R. Carrier mediated transport systems of tetraethylammonium in rat renal brush border and basolateral membrane vesicles. Biochim. Biophys. Acta. 1984;773:113–124. doi: 10.1016/0005-2736(84)90556-x. [DOI] [PubMed] [Google Scholar]
- THIEBAUT F., TSURUO T., HAMADA H., GOTTESMAN M.M., PASTAN I., WILLINGHAM M.C. Cellular localisation of the multidrug-resistance gene product P-glycoprotein in normal tissues. Proc. Natl. Acad. Sci., U.S.A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS J.A., BUSCHBAUM R.N., ZIMNIAK A., RACKER E. Intracellular pH measurements in Erlich Ascites tumour cells utilising spectroscopic probes generated in situ. Biochemistry. 1979;18:2230–2238. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., BROWN C.D.A., HIRST B.H., SIMMONS N.L. Transepithelial glycylsarcosine transport in intestinal Caco-2 cells; mediated by expression of H+ coupled carriers at both apical and basal membranes. J. Biol. Chem. 1993;268:7640–7642. [PubMed] [Google Scholar]
- ULLRICH K.J. Specificity of transporters for ‘organic anions' and ‘organic cations' in the kidney. Biochim. et Biophys. Acta. 1994;1197:45–62. doi: 10.1016/0304-4157(94)90018-3. [DOI] [PubMed] [Google Scholar]
- ULLRICH K.J., RUMRICH G. Luminal transport system for choline in relation to the other organic cation transport systems in the rat proximal tubule. Pflügers Arch. 1996;432:471–485. doi: 10.1007/s004240050159. [DOI] [PubMed] [Google Scholar]
- URAKAMI Y., OKUDA M., MASUDA S., SAITO H., INUI K.-I. Functional characteristics and membrane localisation of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J. Pharmacol. Exp. Ther. 1998;287:800–805. [PubMed] [Google Scholar]
- WU X., HUANG W., PRASAD P.D., SETH P., RAJAN D.P., LEIBACH F.H., CHEN J., CONWAY S.J., GANAPATHY V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. J. Pharmacol. Exp. Ther. 1999;290:1482–1492. [PubMed] [Google Scholar]


