Abstract
The influence of the cannabinoids anandamide, methanandamide and WIN 55212-2 on the delayed rectifier K+ current (IK(V)) in rat arterial myocytes was investigated.
Anandamide caused a concentration-dependent reduction of total peak and late K+ current (IK). The maximal effect (about 50% inhibition of IK) was reached with 3 μM, and half-maximal current block was observed at 0.6 μM. Blockade was voltage-independent. Inhibition of IK by the cannabinoid was associated with a characteristic increase in the rate of current relaxation.
Methanandamide (10 μM), a metabolically more stable analogue of anandamide, decreased IK with a similar time course. Current traces in the presence of the drug also showed an acceleration of inactivation.
The presence of TEA did not impair the inhibition by anandamide or methanandamide, but inhibition was prevented by pre-exposure to 4-AP, showing that both cannabinoids inhibited IK(V) while having no influence on Ca2+-dependent K+ current (IK(Ca)).
The CB1 receptor antagonist SR141716A (10 μM) did not influence the action of anandamide or methanandamide.
Arachidonic acid (1 μM) increased IK considerably. However, in the presence of TEA it caused a decrease of IK(V) with a characteristic increase in the rate of current relaxation.
WIN 55212-2 (20 μM) caused similar inhibition of IK.
Internally applied anandamide (10 μM) or methanandamide (10 μM) was ineffective at influencing IK. In the dialyzed cells, the additional external application of a cannabinoid promptly initiated inhibition.
The results show that anandamide, methanandamide and WIN 55212-2 affect IK(V) in a cannabinoid receptor-independent way similar to that of arachidonic acid, which, unlike the cannabinoids, additionally increases a Ca2+-activated K+ current. It is suggested that cannabinoids might bind to an external site on or near the Kv channel of the vascular smooth muscle cells.
Keywords: Anandamide, methanandamide, cannabinoids, delayed rectifier K+ current, arachidonic acid, vascular smooth muscle cells, potassium channels, endothelium-dependent hyperpolarization, membrane potential, vasodilation
Introduction
Arachidonylethanolamide (anandamide) has been identified as an endogenous counterpart of Δ9-tetrahydrocannabinol, the psychoactive component of marijuana. Anandamide is synthesized by cleavage of the lipid precursor arachidonyl phosphatidylethanolamine, and is metabolized relatively quickly to arachidonic acid and ethanolamine by an amidohydrolase enzyme (Di Marzo et al., 1994). The central and peripheral actions of cannabinoids have been shown to be mediated by G protein-coupled cannabinoid receptors, of which CB1, CB1A and CB2-subtypes have been cloned (Pertwee, 1997). The CB1 and CB1A subtypes are generally considered to be central receptors, whereas CB2 receptors have been localized principally in peripheral tissues, in particular immune tissue. More recently, however, CB1 receptors have also been reported in endothelial cells (Deutsch et al., 1997; Bilfinger et al., 1998) and vascular smooth muscle cells (Gebremedhin et al., 1999).
The potent vasodilatory influence of anandamide has been shown in a variety of isolated vascular preparations. Its mechanism of action, however, is complex and the subject of intense investigation. In rabbit pial arterioles and mesenteric arteries, the anandamide-induced dilation was blocked by indomethacin and diclofenac, respectively, suggesting it was mediated by generation of vasodilator eicosanoids (Ellis et al., 1995; Fleming et al., 1999). Similarly, in isolated bovine coronary arteries, the vasorelaxant effect of anandamide has been suggested to be due to hydrolysis of this cannabinoid to arachidonic acid followed by conversion to vasodilatory eicosanoids (Pratt et al., 1998). In rat renal arteries and cultured endothelial cells, anandamide stimulated the release of nitric oxide via an activation of CB1 receptors, and vasodilation could be blocked by inhibition of NO-synthase (Deutsch et al., 1997). Also in human vascular endothelium, anandamide stimulated CB1 receptor-mediated NO release (Bilfinger et al., 1998). However, in several vascular tissues anandamide was reported to directly induce vasorelaxation, unrelated to its metabolism or to NO-release. Indeed, exogenous application of the cannabinoid caused vasodilation of the mesenteric (Randall et al., 1996) and coronary (Randall & Kendall, 1997) vascular bed, also after removal of the endothelium, a relaxing influence which was inhibited by SR141716A, a selective CB1 receptor antagonist (Rinaldi-Carmona et al., 1994). Since this antagonist also inhibited the endothelium-dependent, NO- and prostanoid-independent dilation induced by carbachol, generally attributed to an endothelium derived hyperpolarizing factor (EDHF), anandamide was even proposed as EDHF (Randall et al., 1996). Moreover, it should be noted that anandamide can have actions unrelated to cannabinoid receptor stimulation, such as decreasing gap junctional permeability in astrocytes, an effect which is not induced by other cannabinoid receptor agonists and which is insensitive to SR141716A (Venance et al., 1995).
The cellular effects of cannabinoids were mostly investigated in neural cells. These include inhibition of adenylyl cyclase (Felder & Glass, 1998), inhibition of Ca2+-current through N-type (Mackie & Hille, 1992; Twitchell et al., 1997), and P/Q-type (Mackie et al., 1995; Twitchell et al., 1997) Ca2+ channels, activation of inward rectifier K+ current (Henry & Chavkin, 1995; Mackie et al., 1995) and of voltage-dependent A-type K+ current (Deadwyler et al., 1995). In vascular smooth muscle cells, modulation of K+ channel activity plays an essential role in regulating membrane potential and, thereby, the open probability of voltage-operated Ca2+ channels, contractile tone, and blood flow in general (Nelson & Quayle, 1995). Little is known, however, about the action of cannabinoids on isolated vascular smooth muscle cells. In myocytes of the rat hepatic artery, anandamide caused an increase of spontaneous transient outward currents and of caffein-induced outward currents, and an inhibition of the delayed rectifier K+ current (Zygmunt et al., 1997). In smooth muscle cells of the cat cerebral artery, cannabinoids caused a decrease of L-type Ca2+-currents which was completely antagonized by SR141716A (Gebremedhin et al., 1999).
In the present experiments, we used smooth muscle cells freshly isolated from the rat aorta to investigate the influence of anandamide and of its amidohydrolase-resistant analogue methanandamide on whole cell K+ currents. In addition, the influence of another cannabinoid receptor agonist, WIN 55212-2, and of arachidonic acid was investigated. Furthermore, the influence of SR141716A on anandamide- and methanandamide-induced changes in K+ currents was assessed. Part of these results were communicated in abstract form (Van den Bossche & Vanheel, 1999).
Methods
Cell isolation
Aortic smooth muscle cells were freshly dissociated on the day of the experiments. The thoracic aorta from 2–3-month-old female Wistar rats was excised and placed in a chilled solution containing (mM): NaCl, 135; KCl, 5; NaHCO3, 20; CaCl2, 2.5; MgSO4.7H2O, 1.3; KH2PO4, 1.2; EDTA, 0.026; glucose, 10, which was continuously bubbled with 95% O2/5% CO2. The aorta was carefully cleared of adherent fat and connective tissue. Before the vessel was opened along its longitudinal axis, the lumen was rinsed with this solution using a polyethylene tubing, thereby deliberately damaging the endothelium. The tissue was then cut into small pieces and the aortic strips were directly transferred to cold (4°C) low calcium medium containing (mM): NaCl, 110; KCl, 5; CaCl2, 0.16; MgCl2, 2; HEPES, 10; NaHCO3, 10; KH2PO4, 0.5; NaH2PO4, 0.5; glucose, 10; phenol red, 0.04; EDTA, 0.49; taurine, 10 titrated to pH 7.0. The strips were allowed to rest in this medium for 30 min at 4°C. The tissue was then transferred to the enzyme solution, containing (mM): KOH,130; CaCl2, 0.05; taurine, 20, Na-pyruvate, 5; creatine, 5; HEPES, 10, buffered with methanesulphonic acid to pH 7.4 and supplemented with collagenase (Type VIII, 1 mg ml−1), pronase (0.2 mg ml−1) and albumin (1 mg ml−1). The mixture was warmed to 37°C for 37 or 40 min. The partially digested tissue was then transferred to KB medium (Klöckner & Isenberg, 1985) containing (mM): KCl, 85; KH2PO4, 30; MgSO4, 5; Na2ATP, 5; K-pyruvate, 5; creatine, 5; taurine, 20; Na-β-hydroxy-butyrate, 5, titrated with KOH to pH 7.2 and supplemented with albumin (1 mg ml−1). A suspension of single cells was obtained by gently triturating the tissue with a wide bore pipette and cells were allowed to rest in KB for at least 30 min at 4°C.
Electrophysiological recordings
Electrophysiological recordings were performed as previously described (Vanheel et al., 1999). Briefly, part of the cell suspension was pipetted into a perfusion chamber on the stage of an inverted microscope (Nikon, Diaphot). After 10 min, allowing cells to adhere to the glass bottom of the chamber, they were superfused with a normal calcium containing solution containing (mM): NaCl, 112; KCl, 5; CaCl2, 1.8; MgCl2, 1; HEPES, 5; NaHCO3, 15; KH2PO4, 0.5; NaH2PO4, 0.5; glucose, 11; phenol red, 0.04, titrated to pH 7.3 and continuously gassed with 95% O2/5% CO2. Whole-cell membrane currents were recorded at room temperature using the conventional and the perforated patch-clamp technique. For membrane potential recordings only perforated patches were used. Pipettes were pulled from borosilicate capillaries (Hilgenberg, Malsfeld, Germany) and attached to the headstage of an EPC-9 patch clamp amplifier (Heka, Lambrecht/Pfalz, Germany). Pipette resistance ranged from 2–5 MΩ after fire-polishing. The composition of the pipette solution for conventional whole-cell recording was (mM): KCl, 130; MgCl2, 1; EGTA, 1; HEPES, 20, NaGTP, 1, pH 7.2 (KOH). The intracellular pipette solution for perforated patches contained 145 mM KCl instead of 130 mM. In these experiments, amphotericin B was used to gain access to the cell. A fresh amphotericin stock solution (30 mg ml−1) was made daily by dissolving this agent in dimethylsulphoxide (DMSO). Immediately before experimentation an amphotericin-containing pipette solution (100 μl stock solution ml−1 pipette solution) was made and used for up to 90 min. Pipettes were briefly dipped into normal pipette solution and then backfilled with the amphotericin-containing solution. A similar backfilling procedure was used for anandamide when investigating the influence of internal application of the cannabinoid, whereas normal filling could be used when investigating the influence of intracellular application of methanadamide. Pipettes for conventional patch-clamp were coated with beeswax. After breaking in, cell capacitance was measured from the capacitive transient using the automatic procedures of the EPC-9 amplifier. Series resistance was compensated by 2–30%. Membrane currents were evoked by stepping the membrane potential from a holding potential of −70 mV for 200 ms to various test potentials between −60 and +60 mV, in 10 mV increments. Currents were filtered at 2.3 kHz and followed on the computer monitor. Membrane currents were sampled at 5 kHz, recorded on hard disk and analysed using the Review program of the EPC-9 system. Currents in this paper are shown as recorded, i.e. without capacitance compensation and leak correction. Current values given in the Results represent the steady state current, measured as the average current during the terminal 50 ms portion of the voltage step.
Drugs
Anandamide (N-arachidonylethanolamide), collagenase and albumin were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). R(+)-methanandamide [(R+) - arachidonyl -1′-hydroxy -2′- propylamide)], R (+) -WIN55212-2 [R (+) - [2,3-dihydro-5-methyl -3- [(morpholinyl) methyl] pyrrolo [1,2,3-de] -1,4-benzoxazin-yl] -(1-naphthalenyl) methanone mesylate] and SR141716A [N- (piperidin -1- yl) -5- (4-chlorophenyl) -1-(2,4- dichloro- phenyl) -4- methyl-1H-pyrazole-3-carboxamide.HCl] were obtained from RBI (Natick, MA, U.S.A.). Arachidonic acid was purchased from Fluka Chemie AG (Switzerland). Pronase and amphothericin B were from Calbiochem (La Jolla, CA, U.S.A.).
The influence of a drug was investigated by adding the appropriate amount from a stock solution to a reservoir containing equilibrated extracellular solution a few seconds before use. Stock solutions of anandamide and methanandamide were made in anhydrous ethanol, while SR141716A and WIN 55212-2 were dissolved in DMSO. The concentration of solvent never exceeded 0.033% (v v−1) (EtOH) or 0.05% (DMSO). In preliminary experiments, these solvent concentrations were found not to affect whole cell K+ current of the aortic myocytes. In some experiments, 4-AP (10 mM) and TEA-Cl (3 mM) were added as a solid to the external medium and pH was corrected to 7.4. All concentrations are expressed as final molar concentrations in the superfusate.
Statistics
Results are presented as means±s.e.mean. Statistically significant differences between means were evaluated using Student's t-test for paired or unpaired observations, as appropriate, and n indicates the number of cells.
Results
Rat aortic smooth muscle cells were clamped at a holding potential of −70 mV. Depolarizing voltage steps of increasing amplitude resulted in the activation of a family of outward currents which displayed graded voltage-dependent activation kinetics, and showed little inactivation during the 200 ms test pulse (Figure 1a). Activation threshold for the current was observed at −20 mV. Mean current amplitude at +60 mV, measured as the average current during the last 50 ms of the voltage step, was 586±29 pA (n=75). After replacing Cs+ for K+ in the pipette solution, the mean current amplitude was 14±2 pA (n=5, not shown), indicating that K+ ions are the main charge carriers of the current (IK) in the present control conditions.
Figure 1.
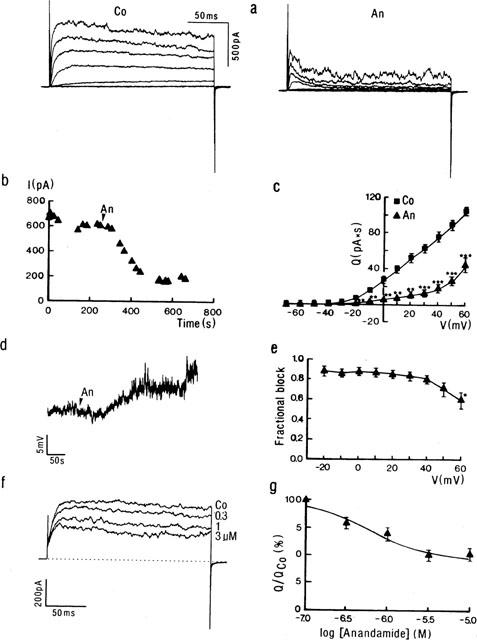
Influence of anandamide on IK (a–c, e–f) and membrane potential (d) of rat aortic smooth muscle cells. (a) Representative current traces obtained from a myocyte in control conditions (Co) and at steady state in the presence of anandamide (An, 10 μM). The membrane potential was stepped for 200 ms from a Vhold of −70 mV to various test potentials between −60 and +60 mV (20 mV increments). (b) Time course of the decrease in IK caused by application of anandamide (An, 10 μM) in the superfusate (arrow). Currents were measured as the average current amplitude during the last 50 ms of the voltage step to +60 mV. (c) Mean charge (Q)-voltage (V) plot calculated from recordings as shown in (a) and showing the steady influence of anandamide (10 μM). Q values represent the integrated current flowing during the whole 200 ms of the depolarization step. Each point represents the mean±s.e.mean (n=5). ** and *** mean significantly different from control value at P<0.01 and P<0.001, respectively. (d) Influence of application (arrow) of anandamide (An, 10 μM) on the membrane potential recorded in current clamp mode. (e) Fractional block by anandamide calculated from the Q-values as shown in (c) and plotted as a function of voltage. Each value represents the mean±s.e.mean (n=5). (f) Current traces showing concentration-dependent inhibition of IK. Currents were obtained in control conditions (Co) and at steady state in the presence of 0.3, 1, and 3 μM anandamide. Each trace is the digital average of five consecutive currents measured after depolarizing the membrane potential from a Vhold of −70 mV to +60 mV. Dotted line indicates zero current level. (g) Dose-response relationship of the effect of anandamide on the potassium current. Q-values were calculated from traces as shown in (f) and were normalized to the charge quantity of each cell in control conditions (QCo). Values are means±s.e.mean from 4–12 cells. Calculated EC50 was 0.51 μM.
Influence of anandamide on IK
Extracellular application of anandamide (10 μM) substantially decreased IK (Figure 1a). Inhibition reached a steady state about 4 min (n=7) after addition of the cannabinoid to the superfusate (Figure 1b). At steady state, currents in the presence of anandamide displayed a rather characteristic time course in most (eight out of 11) of the cells, activating quickly after the onset but decreasing towards the end of the command step (cf. Figure 1b). In order to obtain a more accurate quantitative estimate of the inhibition by anandamide, therefore, currents were measured as the charge quantity (Q) flowing during the whole 200 ms voltage steps. Figure 1c shows the average Q/V plot calculated from five experiments in which the complete step protocol was performed. The inhibitory influence of anandamide was statistically significant (P<0.05) in the range of test potentials between −20 and +60 mV. In addition, zero current potential was shifted from −45±5 mV to −29±3 mV. This depolarization was confirmed in separate membrane potential measurements. In three cells current clamped at 0 pA, 10 μM anandamide depolarized the membrane potential from a mean resting level of −42±13 mV by 7±3 mV (Figure 1d). In voltage clamp mode, the anandamide-sensitive (difference) current reversed at −73 mV (not shown), close to the calculated K+ equilibrium potential in the present conditions (−81 mV), indicating that anandamide inhibited a K+ current. The block of whole cell IK by anandamide was essentially voltage-independent (P>0.1) between −20 and +50 mV (Figure 1e). At the more positive potential, the percentage of block of total IK was significantly (P<0.05) decreased.
Inhibition of IK by anandamide was concentration-dependent (Figure 1f). In the presence of 0.3, 1, 3 and 10 μM of the cannabinoid, mean current amplitude measured at the terminal portion of the step to +60 mV was decreased in steady state by 176±49 (n=7), 240±53 (n=6), 336±73 (n=4) and 286±29 pA (n=12), respectively, representing 29, 39, 55 and 47% of the control current flowing at this potential. The current decay during the voltage step increased with larger anandamide concentrations. pD2, calculated using the fractional decrease in charge transfer, was 6.2±0.2 (Figure 1g).
Influence of methanandamide on IK
The more stable analogue of anandamide, methanandamide (10 μM), significantly decreased IK. The current block by methanadamide reached a steady state approximately 5 min (n=7) after its addition to the superfusate (not shown). Representative traces in steady state conditions are shown in Figure 2. In seven experiments, the mean current amplitude measured at the end of the voltage step to +60 mV was diminished by 215±29 pA, which represents 44±7% of the current in control conditions. The I-V plot reveals a statistically significant inhibition (P<0.01) in the potential range of −20 to +60 mV, and a shift in zero current potential from −37±4 to −28±3 mV. As was observed with anandamide, the inhibitory influence of methanandamide was larger toward the end of the voltage step. Methanandamide block was voltage-independent between −10 and +50 mV (data not shown).
Figure 2.
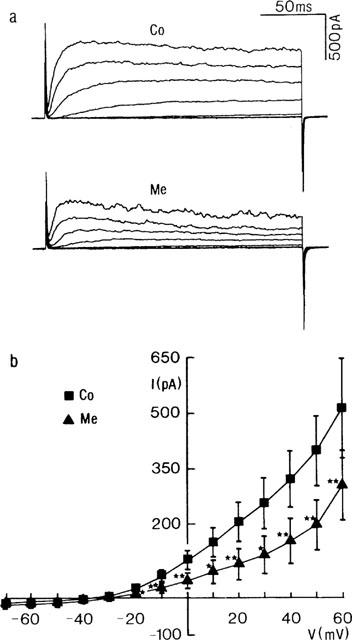
Influence of methanandamide on IK. (a) Representative current traces from a myocyte in control conditions (Co) and at steady state in the presence of methanandamide (Me, 10 μM). The membrane potential was stepped for 200 ms from a Vhold to various test potentials between −60 and +60 mV (20 mV increments). (b) Mean current (I)-Voltage (V) plot showing the influence of methanandamide (Me, 10 μM) on the steady state current. Each point represents the mean±s.e.mean from seven cells. * and ** denote statistically significant from control value at P<0.05 and P<0.01, respectively.
Similar results were obtained with perforated patches. In three experiments, methanandamide decreased (P<0.01) mean IK at+60 mV from 685±47 to 394±43 pA, i.e. to 58±6% of the control current.
Influence on IBK(Ca) and IK(V)
In seven cells, voltage-dependent K+ currents (IK(V)) were inhibited by pre-exposure to 10 mM 4-AP (Nelson & Quale, 1995) before examining the influence of the cannabinoids. In these cells, the application of 4-AP decreased the mean IK at +60 mV from 475±70 to 220±34 pA (Figure 3a). In the continuous presence of the K+ channel inhibitor, residual currents were not significantly affected by 10 μM methanandamide (n=4). Similar observations were made with anandamide (n=3, data not shown). In another series of experiments, cells were pre-exposed to 3 mM TEA, which at this concentration mainly inhibits current through large conductance Ca2+-dependent K+ channels (IBK(Ca)) (Beech & Bolton, 1989; Langton et al., 1991). This is also reflected by the smoother appearance of the currents in the presence of TEA (Figure 3b). In the presence of TEA, the application of 10 μM methanandamide significantly reduced the amplitude of the residual current (mainly delayed rectifier). At +60 mV, the mean current at the end of the voltage step decreased from 62±5 to 30±4% of the control current (P<0.05). Current traces in the presence of methanandamide showed the usual increase in current relaxation (Figure 3b). In five other cells the influence of anandamide on the TEA-insensitive current was tested. In this group, the endocannabinoid significantly (P<0.01) decreased mean IK(V) from 60±6% of the control current amplitude to 30±7% (data not shown).
Figure 3.
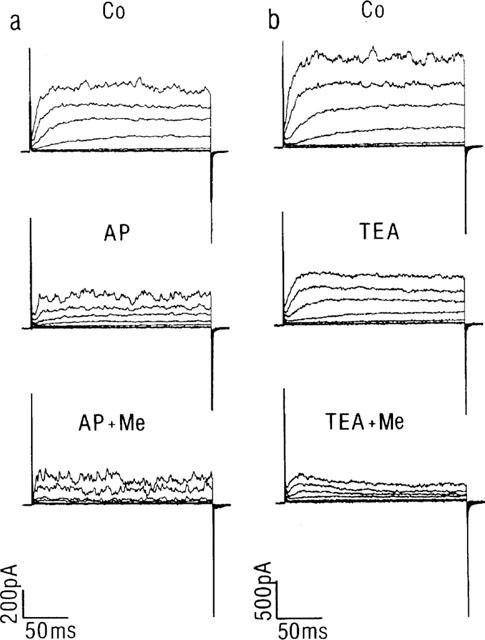
Influence of methanandamide on IBK(Ca) and IK(V). Representative current traces from two cells in which methanandamide (Me, 10 μM) was applied in the continuous presence of 4-AP (a) or TEA (b). The membrane potential was stepped for 200 ms from a Vhold of −70 mV to various test potentials between −60 and +60 mV (20 mV increments). Co represents currents from the cell in control conditions.
Influence of pre-exposure to SR141716A
The antagonist of the cannabinoid receptor, SR141716A (10 μM) caused a slight decrease of IK. Figure 4a shows representative traces. At the end of the voltage step to +60 mV, IK was significantly (P<0.01) decreased by 185±48 pA (n=8). Peak currents, however, were less affected (cf. Figure 4a). Pre-exposure to SR141716A did not prevent the inhibitory action of anandamide on IK (Figure 4a). Currents were significantly reduced in the −10 to+60 mV potential range (Figure 4b). At +60 mV, the mean current amplitude was diminished by 208±21 pA (n=3). Similar observations were made with methanandamide (n=3, data not shown).
Figure 4.
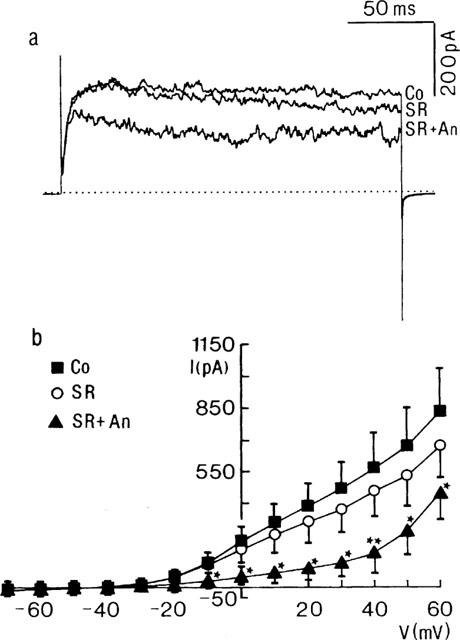
Influence of anandamide on IK of cells pre-exposed to SR141716A. (a) Current traces obtained from a myocyte in control conditions (Co), in the presence of SR141716A (SR, 10 μM), and after the additional application of anandamide (SR+An). The membrane potential was stepped for 200 ms to 600 mV from a Vhold of −70 mV. Dotted line indicates zero current level. Each trace is the digital average from three individual current traces. (b) Current (I)-voltage (V) plot showing the steady state influence of SR141716A (10 μM) and of anandamide (10 μM) in the continuous presence of SR141716A on the steady state current. Each point represents the mean±s.e.mean from three cells. * and ** mean statistically significant from control value at P<0.05 and P<0.01, respectively.
Influence of WIN 55212-2
In five experiments, the influence of another cannabinoid receptor agonist, WIN 55212-2, was investigated. Addition of WIN 55212-2 (20 μM) substantially reduced IK (Figure 5). In steady state, the effect of WIN 55212-2 was statistically significant (P<0.05) at test potentials ranging from 0 to +60 mV (Figure 5b). At the most positive potential, mean IK was reduced by 325±70 pA, representing 65±9% of the control current. WIN 55212-2 shifted the zero current level from −30±4 to −26±2 mV. Block was voltage-independent between −10 and+50 mV (not shown).
Figure 5.
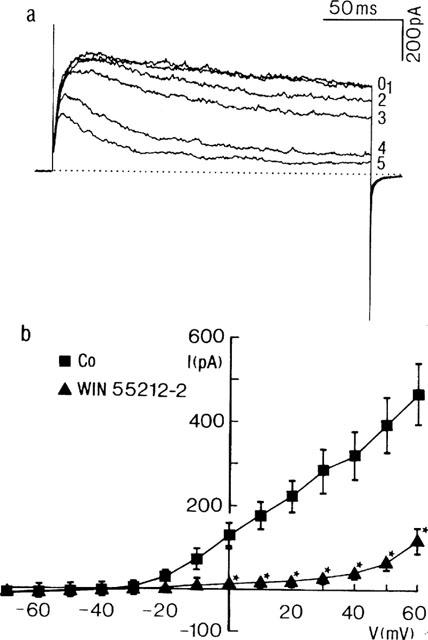
Influence of WIN 55212-2 on IK. (a) Current traces obtained from a myocyte in control conditions (time zero) and 1, 2, 3, 4 and 5 min after application of WIN 55212-2 (20 μM). The membrane potential was stepped from a Vhold of −70 mV for 200 ms to +60 mV. Dotted line indicates zero current. (b) Current (I)-voltage (V) plot showing the steady state influence of WIN 55212-2 on the current during the terminal portion of the voltage step. Each point represents the mean±s.e.mean from three cells. *Denotes statistically significant from control current at P<0.05.
As for the other cannabinoids, the inhibitory influence of WIN 55212-2 was more expressed towards the end of the voltage step (Figure 5a), resulting in an increased rate of current inactivation.
Influence of arachidonic acid
Arachidonic acid (1 μM) reversibly increased total outward current (Figure 6a). At +60 mV, mean current amplitude was significantly (P<0.01) enhanced by 707±196 pA (n=8). Since the observed increase in current noise (cf. Figure 6a) was suggestive of increased activity of BK(Ca) channels, additional experiments were performed in the continuous presence of TEA. In six out of eight cells in this series, the presence of 3 mM TEA was able to prevent the rise in IK induced by arachidonic acid. Moreover, in five of these cells, a decrease of IK(V) was noted (Figure 6b). At steady state (5 min after application of the fatty acid), the mean IK(V) measured during the last portion of the voltage step to +60 mV was reduced to 62±11% of its control amplitude (n=6, P<0.05). As was observed with the cannabinoids, the inhibition of IK(V) was associated with an increase in current relaxation (Figure 6b).
Figure 6.
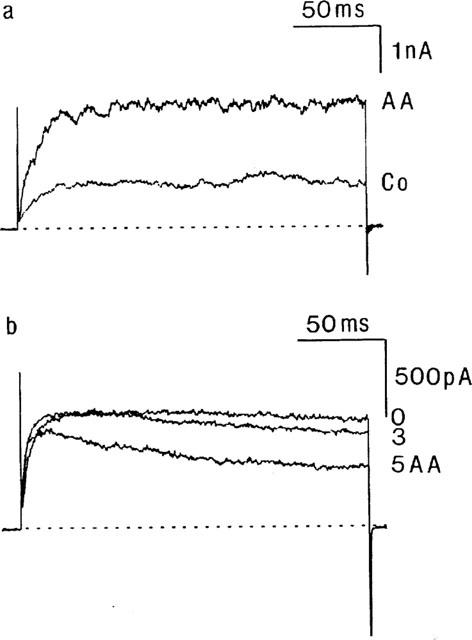
Influence of arachidonic acid on IK and IK(V). (a) Current traces obtained on depolarizing the membrane potential for 200 ms from a Vhold of −70 mV to +60 mV in control conditions (Co) and in the presence of arachidonic acid (AA, 1 μM). (b) Current traces from another myocyte on depolarizing the membrane potential for 200 ms from a Vhold of −70 mV to +60 mV in the presence of TEA (time zero) and 3 and 5 min after additional application of arachidonic acid (AA). Dotted lines indicate zero current level.
Cannabinoids bind to an external site
Inclusion of 10 μM anandamide in the patch pipette solution did not significantly change IK during a 6 min period after rupturing the patch and establishing intracellular contact. In four myocytes, the mean IK as measured during the terminal portion of the voltage step to +60 mV was slightly but not significantly (P=0.74) increased to 113±10% of the control current (measured immediately after breaking in the cell). In cells internally dialyzed with anandamide for this time period, the additional external application of the cannabinoid (10 μM) decreased IK with similar time course and magnitude as above described. After 5 min of extracellular superfusion, the mean IK was reduced (P<0.05) to 66±10% of the amplitude of the control current. In another series of experiments (n=3), intracellular application of methanandamide (10 μM) did not notibly modify IK for 6 min after breaking in (113±12% of the control current amplitude), while subsequent bath application of the cannabinoid promptly initiated inhibition in these cells. Five minutes after additional extracellular superfusion with methanandamide, the mean IK was reduced significantly (P<0.05) to 63±10% of the control current amplitude (n=3, data not shown).
Discussion
Anandamide (3–10 μM) decreased whole cell IK of the aortic myocytes to about half its original magnitude. In the continuous presence of TEA this inhibition was still observed, whereas after pre-exposure to 4-AP no significant further decrease of IK was noted. These experiments clearly show that anandamide drastically decreases IK(V), which makes an important contribution to the total IK, while having no significant influence on IBK(Ca). A similar conclusion was reached by Zygmunt et al. (1997), who found that anandamide (10 μM) essentially abolished IK(V) and did not affect IBK(Ca) activated by the K+ channel opener NS1619 in smooth muscle cells of the rat hepatic artery.
From the rather slowly developing influence of anandamide, as observed in the present experiments, it might be argued that the substance acts indirect, after conversion to arachidonic acid or a further metabolite of the endocannabinoid. However, the amidohydrolase resistant analogue methanandamide induced similar (although somewhat weaker) inhibition, an influence occurring with a comparable time course. Moreover, direct application of arachidonic acid caused the opposite change in total IK. These findings suggest that the observed decrease by the cannabinoids is due to the action of the added, native compounds.
Arachidonic acid has been shown to induce the opening of BK(Ca) channels in rat and rabbit vascular smooth muscle cells (Kirber et al., 1992; Dopico et al., 1994). In addition, the fatty acid has been described to inhibit IK(V) in freshly isolated rat pulmonary artery myocytes (Smirnov & Aaronson, 1996). In this latter study, it was shown that this effect was independent of arachidonic acid metabolism, since it was mimicked by ETYA, the acetylenic analogue of arachidonic acid that inhibits its cyclo-oxygenase, lipoxygenase and cytochrome P450 dependent metabolism. Moreover, inhibition was also exerted by a number of fatty acids of which not the chain length but the degree of unsaturation was proportional to inhibitory potency. Our results are fully in agreement with these reports. In the aortic myocytes, arachidonic acid caused an increase of IK which is presumably due to activation of BK(Ca) current, as judged from both its sensitivity to TEA and the increase in current noise. In those cells where the continuous presence of 3 mM TEA was able to fully prevent this increase, a decrease of the TEA-insensitive current (IK(V)) was observed.
Cannabinoid receptors are Gi/Go protein-coupled to the inhibition of adenylyl cyclase. In cultured hippocampal neurones, cannabinoids increase the voltage-dependent A current in a pertussis toxin sensitive way, via inhibition of adenylyl cyclase (Deadwyler et al., 1995). The inhibition of the voltage-dependent K+ current of aortic smooth muscle cells by anandamide, as observed in the present experiments, was concentration-dependent. Half-maximal inhibition occurred at 600 nM, which is much higher than the Ki (39 nM) determined for anandamide binding to the CB1 receptor. In addition, methanadamide, which displays somewhat higher specificity for the CB1 receptor (Ki=20 nM), was somewhat less efficient at inhibiting IK(V). Moreover, the selective inhibitor of CB1 receptors, SR141716A, did not prevent the influence of either anandamide or methanandamide. Furthermore, cannabinoid receptors show a high degree of specificity for anandamide versus arachidonic acid (Schuel et al., 1994), for which in the present study a similar inhibition of IK(V) was unmasked after pre-exposure to TEA. These findings strongly suggest that the observed inhibitory action of the cannabinoids is unrelated to CB1 receptor stimulation. In support of this conclusion is the fact that most recordings were performed with ATP-free pipette solution. Using perforated patches, however, a similar inhibition was observed.
In most of the cells, extracellular anandamide changed the shape of the current traces from nearly non-inactivating to inactivating. The fact that this was not apparent in all myocytes might be due to variations in relative proportion of the inhibited Kv versus the unaffected BK(Ca) currents among cells. The block by anandamide was essentially voltage-independent between −20 and +50 mV. At the most positive potential, however, a decrease of the fractional block was observed, which might be explained by the proportional increase of the outward rectifying IBK(Ca) to the total IK at more positive potentials. The increase in inactivation rate induced by the cannabinoids resembles the observed influence of arachidonic acid on IK(V) in myocytes of the rat pulmonary artery (Smirnov & Aaronson, 1996) and aorta (present study). An inhibition with similar increase in rate of decay of voltage-dependent K+ current by micromolar concentrations of arachidonic acid has been described for the cloned Kv1.1 (Gubitosi-Klug et al., 1995), Kv1.2 (McEvoy et al., 1996), Kv1.5 (Honoré et al., 1994) and Kv4.2 channels (Villaroel & Schwartz, 1996). In these studies, a direct interaction of the fatty acid with the Kv channel protein or a closely associated component has been proposed, most probably by binding to an external site (HONORÉ et al., 1994; McEvoy et al., 1996). Poling et al. (1996) described a similar block by anandamide and Δ9-tetrahydrocannabinol of Kv1.2 K+ currents, although with a larger IC50 value (2.7 μM for anandamide) than observed in the present study. In the cited study, an SR141716A-insensitive, pertussis toxin-insensitive direct inhibition was shown, anandamide acting only from the outside of the membrane (Poling et al., 1996). The molecular nature of the native Kv channels expressed in rat aortic smooth muscle cells is at present unknown. However, our results for the first time show that anandamide inhibits the native delayed rectifier of vascular smooth muscle cells in a similar way, the substance most probably exerting its influence from the extracellular side. Moreover, with methanandamide and WIN 55212-2, and to some small extent also with SR141716A, an inhibition with comparable characteristics was observed. We therefore speculate than an external site at or close to the native Kv channel recognizes cannabinoid-like molecules.
Interference of cannabinoids with other membrane proteins has been described in other systems. Both anandamide and WIN 55212-2 have been shown to inhibit the 5-HT-induced currents in rat nodose ganglion neurons (Fan, 1995) by binding to the 5-HT3 receptors. This inhibition was slowly developing and membrane potential independent. Similarly, the activity of the NMDA receptor, which has a putative fatty acid binding domain as determined on the basis of sequence homology and cytosolic fatty acid binding proteins (Petrou et al., 1993), and which response is potentiated by arachidonic acid, is modulated by anandamide in rat brain slices (Hampson et al., 1998) and by the nonpsychotropic cannabinoid HU-211 in rat brain cortical membranes (Feigenbaum et al., 1989). More recently, activation by anandamide (but not by arachidonic acid) of the VR1 vanilloid receptor has been shown (Zygmunt et al., 1999).
Inhibition of IK by the cannabinoids was accompanied by a shift in zero current potential towards more positive values, suggesting depolarization of the smooth muscle cells. This was confirmed by direct measurements of the resting membrane potential in current clamp mode. These findings rule out the possibility that endogenous anandamide acts as an endothelium-derived hyperpolarizing factor in the rat aorta. Depolarization is expected to contract aortic smooth muscle. The reported direct vasorelaxating influence of anandamide, therefore, has to be due to mechanisms other than activation of a K+ conductance or influencing the cell membrane potential. Possibilities include interference with intracellular calcium handling (Zygmunt et al., 1997) or inhibition of the voltage-dependent Ca2+-current (Gebremedhin et al., 1999).
In conclusion, we found that anandamide and two other cannabinoids, methanadamide and WIN 55212-2, and to some minor extent also SR141716A, decrease current through the native Kv channels of aortic smooth muscle cells with a characteristic influence on the currents time course. This effect is similar to that described for polyunsaturated fatty acids, and is presumably caused by direct binding to an external site on the channel protein or a closely associated structure. In arterial smooth muscle cells this effect of cannabinoids is more selective towards Kv channels than is the influence of arachidonic acid, which additionally increases the activity of TEA-sensitive channels. Although the inhibitory influence has been described in one study for anandamide and Δ9-tetrahydrocannabinol on the cloned Kv1.2 channel (Poling et al., 1996), and other precedents for non-specific cannabinoid binding can be found in the literature, this is the first description for direct interference with native vascular Kv channels of the cannabinoids methanandamide and WIN 55212-2. Therefore, some of the reported inhibitory effects of exogenous cannabinoids–or even of the CB1 receptor antagonist SR141716A–might be found to be caused by their inhibitory influence of IK(V) instead of being mediated by activation–or inhibition, respectively,–of cannabinoid receptors.
Acknowledgments
This work was supported by a grant from the Fund for Scientific Research–The Flanders (FWO-Vlaanderen, Belgium). B. Vanheel is a senior research associate of the FWO. We are grateful to Eliane De Wulf, Dirk De Gruytere, and Cyriel Mabilde for unfailing technical assistance and to Marc Gillis for the artwork.
Abbreviations
- 4-AP
4-aminopyridine
- BK(Ca)
large conductance calcium-activated potassium channels
- CB
cannabinoid receptor
- EDHF
endothelium-derived hyperpolarizing factor
- IBK(Ca)
current through large conductance calcium-activated potassium channels
- IK
total potassium current
- IK(Ca)
current through calcium-activated potassium channels
- IK(V)
current through voltage dependent potassium channels
- KCa
calcium-activated potassium channels
- Kv
voltage dependent potassium channels
- NO
nitric oxide
- SR141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-1H-pyrazole-3-carboxamide.HCl
- TEA
tetra-ethylammonium chloride
- WIN 55212-2
R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-yl]-(1-naphthalenyl)methanone mesylate
References
- BEECH D.J., BOLTON T.B. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J. Physiol. 1989;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILFINGER T.V., SALZET L., FIMIANI C., DEUTSCH D.G., TRAMU G., STEFANO G.B. Pharmacological evidence for anandamide amidase in human cardiac and vascular tissues. Int. J. Cardiol. 1998;61 Suppl. 1:S15–S22. doi: 10.1016/s0167-5273(98)00031-x. [DOI] [PubMed] [Google Scholar]
- DEADWYLER S.A., HAMPSON R.E., MU J., WHYTE A., CHILDERS S. Cannabinoids modulate voltage sensitive potassium A-current in hippocampal neurons via a cAMP-dependent process. J. Pharmacol. Exp. Ther. 1995;273:734–743. [PubMed] [Google Scholar]
- DEUTSCH D.G., GOLIGORSKY M.S., SCHMID P.C., KREBSBACH R.J., SCHMID H.O., DAS D.K., DEY S.K., ARREAZA G., THORUP C., STEFANO G., MOORE L.C. Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI MARZO V., FONTANA A., CADAS H., SCHINELLI S., CIMINO G., SCHWARTZ J.-C., PIOMELLI D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- DOPICO A.M., KIRBER M.T., SINGER J.J., WALSH J.V. Membrane stretch directly activates large conductance Ca2+-activated K+ channels in mesenteric artery smooth muscle cells. Am. J. Hypertens. 1994;7:82–89. doi: 10.1093/ajh/7.1.82. [DOI] [PubMed] [Google Scholar]
- ELLIS E.F., MOORE S.F., WILLOUGHBY K.A. Anandamide and Δ9-THC dilation of cerebral arterioles is blocked by indomethacin. Am. J. Physiol. 1995;269:H1859–H1864. doi: 10.1152/ajpheart.1995.269.6.H1859. [DOI] [PubMed] [Google Scholar]
- FAN P. Cannabinoid agonists inhibit the activation of 5-HT3 receptors in rat nodose ganglion neurons. J. Neurophysiol. 1995;73:907–910. doi: 10.1152/jn.1995.73.2.907. [DOI] [PubMed] [Google Scholar]
- FEIGENBAUM J.J., BERGMANN F., RICHMOND S.A., MECHOULAM R., NADLER V., KLOOG Y., SOKOLOVSKY M. Nonpsychotropic cannabinoid acts as a functional N-methyl-D-aspartate receptor blocker. Proc. Natl. Acad. Sci. U.S.A. 1989;86:9584–9587. doi: 10.1073/pnas.86.23.9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDER C.C., GLASS M. Cannabinoid receptors and their endogenous agonists. Ann. Rev. Pharmacol. Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- FLEMING I., SCHERMER B., POPP R., BUSSE R. Inhibition of the production of endothelium-dependent hyperpolarizing factor by cannabinoid receptor agonists. Br. J. Pharmacol. 1999;126:949–960. doi: 10.1038/sj.bjp.0702381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEBREMEDHIN D., LANGE A.R., CAMPBELL W.B., HILLARD C.J., HARDER D.R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 1999;276:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- GUBITOSI-KLUG R., YU S.P., CHOI D.W., GROSS R.W. Concomitant acceleration of the activation and inactivation kinetics of the human delayed rectifier K+ channel (Kv1.1) by Ca2+-independent phospholipase A2. J. Biol. Chem. 1995;270:2885–2888. doi: 10.1074/jbc.270.7.2885. [DOI] [PubMed] [Google Scholar]
- HAMPSON A.J., BORNHEIM L.M., SCANZIANI M., YOST C.S., GRAY A.T., HANSEN B.M., LEONOUDAKIS D.J., BICKLER P.E. Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J. Neurochem. 1998;70:671–676. doi: 10.1046/j.1471-4159.1998.70020671.x. [DOI] [PubMed] [Google Scholar]
- HENRY D.J., CHAVKIN C. Activation of inwardly rectifying potassium channels (GIRK 1) by co-expressed rat brain cannabinoid receptors in Xenopus oocytes. Neurosci. Lett. 1995;186:91–94. doi: 10.1016/0304-3940(95)11289-9. [DOI] [PubMed] [Google Scholar]
- HONORÉ E., BARHANIN J., ATTALI B., LESAGE F., LAZDUNSKI M. External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1937–1944. doi: 10.1073/pnas.91.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBER M.T., ORDWAY R.W., CLAPP L.H., WALSH J.V., JR, SINGER J.J. Both membrane stretch and fatty acids directly activate large conductance Ca2+-activated K+ channels in vascular smooth muscle cells. FEBS Lett. 1992;297:24–28. doi: 10.1016/0014-5793(92)80319-c. [DOI] [PubMed] [Google Scholar]
- KLÖCKNER U., ISENBERG G. Action potential and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig) Pflügers Arch. 1985;405:329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- LANGTON P.D., NELSON M.T., HUANG Y., STANDEN N.B. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am. J. Physiol. 1991;260:H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- MACKIE K., HILLE B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKIE K., LAI Y., WESTENBROEK R., MITCHELL R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J. Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCEVOY M., OWEN D.G., GARRATT J.C. Effects of arachidonic acid on the cloned potassium channel mKv1.2 in CHO cells. (abstract) J. Physiol. 1996;487:193P–194P. [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- PETROU S., ORDWAY R.W., SINGER J.J., WALSH J.V. A putative fatty acid-binding domain of the NMDA receptor. Trends Biochem. Sci. 1993;18:41–42. doi: 10.1016/0968-0004(93)90050-w. [DOI] [PubMed] [Google Scholar]
- POLING J.S., ROGAWSKI M.A., SALEM N., JR, VICINI S. Anandamide, an endogenous cannabinoid, inhibits Shaker-related voltage-gated K+ channels. Neuropharmacology. 1996;35:983–991. doi: 10.1016/0028-3908(96)00130-x. [DOI] [PubMed] [Google Scholar]
- PRATT P.F., HILLARD C.J., EDGEMOND W.S., CAMPBELL W.B. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am. J. Physiol. 1998;274:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., ALEXANDER S.P.H., BENNETT T., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., KENDALL D.A. Involvement of a cannabinoid in endothelium-derived hyperpolarizing factor-mediated coronary vasorelaxation. Eur. J. Pharmacol. 1997;335:205–209. doi: 10.1016/s0014-2999(97)01237-5. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HÉAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NÉLIAT G., CAPUT D., FERRARA P., SOUBRIE P., BRELIÈRE J.C., LE FUR G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- SCHUEL H., GOLDSTEIN E., MECHOULAM R., ZIMMERMAN A.M., ZIMMERMAN S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc. Natl. Acad. Sci, U.S.A. 1994;91:7678–7682. doi: 10.1073/pnas.91.16.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMIRNOV S.V., AARONSON P.I. Modulatory effects of arachidonic acid on the delayed rectifier K+ current in rat pulmonary arterial myocytes. Circ. Res. 1996;79:20–31. doi: 10.1161/01.res.79.1.20. [DOI] [PubMed] [Google Scholar]
- TWITCHELL W., BROWN S., MACKIE K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J. Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- VAN DEN BOSSCHE I., VANHEEL B. Electrophysiological actions of the cannabinoid receptor agonists anandamide and R(+)-methanandamide in freshly dissociated vascular smooth muscle cells. Physiol. Res. 1999;48 Suppl. 1:S133. [Google Scholar]
- VANHEEL B., CALDERS P., VAN DEN BOSSCHE I., VAN DE VOORDE J. Influence of some phospholipase A2 and cytochrome P450 inhibitors on rat arterial smooth muscle K+ currents. Can. J. Physiol. Pharmacol. 1999;77:481–489. [PubMed] [Google Scholar]
- VENANCE L., PIOMELLI D., GLOWINSKI J., GIAUME C. Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature. 1995;376:590–594. doi: 10.1038/376590a0. [DOI] [PubMed] [Google Scholar]
- VILLAROEL A., SCHWARZ T.L. Inhibition of the Kv4 (Shal) family of transient K+ currents by arachidonic acid. J. Neurosci. 1996;16:2522–2531. doi: 10.1523/JNEUROSCI.16-08-02522.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., HÖGESTÄTT E.D., WALDECK K., EDWARDS G., KIRKUP A.J., WESTON A.H. Studies on the effects of anandamide in rat hepatic artery. Br. J. Pharmacol. 1997;122:1679–1686. doi: 10.1038/sj.bjp.0701601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HÖGESTÄTT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


