Abstract
Cell surface A2A adenosine receptor (A2AR) mediated signalling affects a variety of important processes and adenosine analogues possess promising pharmacological properties.
Demonstrating the receptor specificity of potentially lymphotoxic adenosine-based drugs facilitates their development for clinical applications.
To distinguish between the receptor-dependent and -independent lymphotoxicity and apoptotic activity of adenosine and its analogues we used lymphocytes from A2AR-deficient mice.
Comparison of A2AR-expressing (+/+) and A2AR-deficient (−/−) cells in cyclic AMP accumulation assays confirmed that the A2AR agonist CGS 21680 is indeed selective for A2A receptors in T-lymphocytes.
Incubation of A2AR-expressing thymocytes with extracellular adenosine or CGS 21680 in vitro results in the death of about 7–15% of thymocytes. In contrast, no death was induced in parallel assays in cells from A2AR-deficient mice, providing genetic evidence that CGS 21680 does not display adenosine receptor-independent intracellular cytotoxicity.
The A2A receptor-specific lymphotoxicity of CGS 21680 is also demonstrated in a long-term (6-day) in vitro model of thymocyte positive selection where addition of A2AR antagonist ZM 241,385 did block the effects of CGS 21680, allowing the survival of T cells.
The use of cells from adenosine receptor-deficient animals is proposed as a part of the screening process for potential adenosine-based drugs for their receptor-independent cytotoxicity and lymphotoxicity.
Keywords: Adenosine, purinergic receptors, T-lymphocytes, cytotoxicity
Introduction
Adenosine and adenosine analogues have attracted significant interest as potential pharmacological agents, based on studies implicating adenosine-triggered signalling through A1, A2A, A2B and A3 subtypes of adenosine receptors in a variety of normal and pathological processes, including cardioprotection, and neuro- and immunomodulation (Jacobson et al., 1996; 1999; van der Ploeg et al., 1996; Olsson, 1996; Apasov et al., 1995; 1997). Adenosine and adenosine agonists were shown to protect brain and heart tissues during ischaemia, and these effects of adenosine were explained by functional antagonism with other transmitters (Rudolphi & Schubert, 1996; Olsson & Pearson, 1990; Abbracchio et al., 1997; Brambilla et al., 1998; Abbracchio, 1996; Kohno et al., 1996). Agonists of A2A receptors were also found to accelerate wound healing (Montesinos et al., 1997).
Adenosine A2A receptors are considered to be involved in the regulation of normal immune responses (Koshiba et al., 1997; Apasov et al., 1995; 1997), and these observations point to A2A receptors as attractive molecular targets for immunomodulation and to adenosine receptor agonists and antagonists as potential immunomodulators. However, consideration of adenosine receptor agents as drugs, in general, is complicated by the fact that adenosine and adenosine analogues may cause such diverse and opposing effects as cytoprotection and cell death (Jacobson et al., 1999).
The lymphotoxic properties of adenosine attracted significant attention several decades ago in studies of an inherited disease, severe combined immunodeficiency (SCID), in adenosine deaminase (ADA)-deficient patients (reviewed in Hershfield & Mitchell, 1995; Hirschhorn, 1995). The pathogenesis of this disease was explained by both intracellular (Hershfield & Mitchell, 1995; Hirschhorn, 1995) and extracellular mechanisms (Apasov et al., 1997; Huang et al., 1997), since adenosine was shown to induce cyclic AMP accumulation (Hirschhorn et al., 1970; Kizaki et al., 1990; McConkey et al., 1990; Szondy, 1994; Apasov et al., 1997; Huang et al., 1997).
It was suggested that the extracellular adenosine may cause T-cell depletion (and therefore the immunodeficiency) by A2A receptor-mediated inhibition of TCR signalling, and thus by preventing TCR-dependent positive and negative selection of thymocytes (Apasov et al., 1997; Huang et al., 1997; Apasov & Sitkovsky, 1999). Immunodeficiency could also be due to inhibition of important TCR-triggered effector functions of T cells, including T-cell expansion, T-cell-mediated cytotoxicity and lymphokine production (Koshiba et al., 1997; 1999).
More recently, the ability of adenosine analogues to cause necrotic or apoptotic cell death was documented (Abbracchio, 1996; Kohno et al., 1996; Sei et al., 1997; Barbieri et al., 1998), raising the issue of undesirable side effects of adenosine receptor agonists due to their general cytotoxicity. Especially worrisome are observations of receptor-independent intracellular toxicity of adenosine receptor agonists and antagonists (Barbieri et al., 1998; Abbracchio et al., 1995). Indeed, it has been shown in several cellular systems including embryonic sympathetic neurons (Wakade et al., 1995), HL-60 cells (Tanaka et al., 1994), leukaemia cells (Ruchaud et al., 1995; Hoffmann et al., 1996), and thymocytes (Apasov et al., 1995; Szondy, 1995) that adenosine analogues kill non-specifically following their intracellular uptake and without the involvement of adenosine receptors. It is believed that intracellular toxicity of adenosine analogues could involve caspases (Porter et al., 1997) and that intracellular phosphorylation of nucleosides is required for cell death (reviewed in Jacobson et al., 1999).
Overall, these observations suggest that the cytotoxic effect of these agents occurs independently of adenosine receptor recognition, a concept that has hindered the development of clinical applications for adenosinergic agents. However, this issue remains debatable and the ability of selective adenosine receptor agonists to cause receptor-independent cell death should be carefully evaluated.
The development of specific agonists and antagonists of different adenosine receptors (mostly for A1, A2A, and A3 subtypes) (Jacobson & van Rhee, 1997) has been instrumental in assessing the potential effects of adenosine. One of the most selective agonists of A2A receptors is the 2-substituted adenosine derivative 2-[4-[(2-carboxyethyl)-phenyl]ethylamino]-5′-N-ethylcarboxamidoadenosine, also known as CGS 21680; while 8-chlorostyrylcaffeine (CSC) and 4-(2-[7-amino-2-(2-furyl) [1,2,4]triazolo[2,3a] [1,3,5]triazinyl-amino]ethyl)-phenol (ZM 241385) are among the most selective A2A receptor antagonists.
Here we utilize thymocytes from A2AR-deficient mice to determine whether the A2AR-specific agonist CGS 21680 is able to cause thymocyte death in the absence of functional adenosine receptors.
Methods
Animals
Mice were maintained in a pathogen-free environment at NIH animal care facilities. Mice were 6–10 weeks old, and two to three animals were used in each experiment. A2AR gene-mutant mice (−/−) were generated by gene targeting, and lacked functional A2A receptors as described by Chen et al. (1999). Littermates were genotyped for wild type (+/+), heterozygous (+/−) and homozygous mutant (−/−) A2AR alleles by Southern blot analysis as shown in Figure 1.
Figure 1.
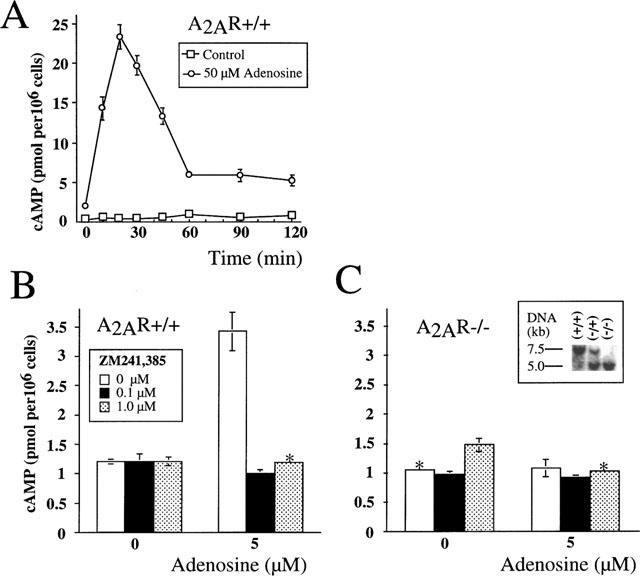
Measurement of cyclic AMP accumulation in murine thymocytes after incubation with adenosine. Results are representative of four independent experiments with P<0.01. (*indicates that standard deviations are not visible on the graph). Ex vivo thymocytes from A2AR wild type (+/+) (A,B) and from homozygous (−/−) A2AR-deficient ‘knock-out' mice (C) were incubated with adenosine in the presence or absence of A2AR antagonist ZM 241385 and cyclic AMP accumulation was measured as described in Methods. (A) Transient accumulation of cyclic AMP in thymocytes from A2AR wild type (+/+) mice during 2 h incubation with 50 μM adenosine. Thymocytes from A2AR wild type (+/+) mice (B) or A2AR (−/−) mice (C) were incubated 30 min with 5 μM adenosine in the presence or absence ZM 241385 at indicated concentrations. Inset illustrates Southern blot screening procedure for identification of wild type (+/+), heterozygous (+/−) and homozygous (−/−) mice.
Cells and medium
Thymocytes were isolated from adult thymus ex vivo and incubated in RPMI-1640 medium (Biofluids, Rockville, MD, U.S.A.) supplemented with 5% dialyzed foetal calf serum (heat-inactivated), or in AIM-V serum free medium (Life Technology, Grand Island, NY, U.S.A.) and 100 u ml−1 penicillin, 100 mg ml−1 streptomycin, 1 mM sodium pyruvate, 1 mM HEPES, non-essential amino acids, and 5×10−5 M 2-mercaptoethanol.
Reagents
Adenosine and adenosine analogues were prepared freshly as 20–100 mM stock solution with pH adjusted to 7.1 and were purchased either from Sigma ImmunoChemicals (St. Louis, MO, U.S.A.) or from RBI (Natick, MA, U.S.A.). MAb were purchased from PharMingen (San Diego, CA, U.S.A.)
Analysis of thymocytes
A single-cell suspension of murine thymocytes was isolated by standard procedures. Cells were washed and incubated at 37°C in a 5% CO2 incubator. Cells (0.5–106 ml−1 were cultured in a total of 0.2 ml of medium in 96-well plates. Control incubation was done in parallel at 4°C at the same cell concentration. Adenosine and adenosine analogues were added at various concentrations as indicated in figure legends. After incubation for 16–18 h, or as indicated (4 or 6 days in the thymocyte survival assay), cells were harvested and analysed by flow cytometry.
Flow cytometry quantitation of live, apoptotic, and dead cells was done according to a modified flow cytometry procedure (Darzynkiewicz et al., 1992). This assay allowed the quantitation of spontaneous thymocyte death and the determination of the proportion of cells that were killed due to adenosine- or CGS 21680-induced cytotoxicity. Briefly, cells from the short-term cultures were gently pipetted and transferred from 96-well plates (200 ml) directly into polystyrene tubes (12×75 mm; Falcon, Becton Dickinson Labware, Lincoln Park, NJ, U.S.A.), and 200 ml of FACS buffer (PBS with 2% foetal calf serum and 0.05% sodium azide) was added to each sample. Each sample had equal volume and was analysed at the same flow rate in duplicate or triplicate. Propidium iodide solution (1 μg ml−1 final concentration) was added to each tube for 10 s prior to FACS analysis. Live, dead, and apoptotic cells were estimated from 2×105 cells in each sample by counting cell numbers in appropriate gates using a forward/side scatter dot plot in linear scale and PI staining in log scale (Apasov et al., 1997). Data are presented as percentage of surviving cells from total cell input. Statistical analysis of triplicate sample measurements was calculated as described below and as previously reported (Apasov et al., 1997). Standard deviations of triplicate measurements within the same experiment were typically lower than 1%.
Fluorimetric measurements of apoptosis in cell culture were also done using the annexin V binding assay as described (Martin et al., 1995). Briefly, 0.5–1×106 cells from 96-well plate were resuspended in 100 μl of buffer containing (mM): HEPES 10 pH 7.3, NaCl 150, KCl 5, MgCl2 1, CaCl2 1.8 and incubated with 0.3–1 μg/ml of FITC-conjugated annexin V and with 1–5 μg ml−1 propidium iodide for 5 min. After incubation samples were diluted four times with buffer containing 1.8 mM CaCl2 and analysed by Flow cytometry. Annexin V-FITC was purchased from Trevingen (Gaithersburg, MD, U.S.A.) and BioWhitaker (Walkersville, MD, U.S.A.).
Statistical analysis
Statistical analysis of percentage of live, dead and apoptotic cells and flow cytometry data acquisition and analysis were done on FACScan using FACScan research software and CellQuest programs (Becton-Dickinson, San José, CA, U.S.A.) after acquisition of 20,000 cells in each triplicate or duplicate sample. Standard deviations in estimations of percentage of dead, survived cells were calculated using Stat-View analysis program (Abacus Concept Inc., Berkeley, CA, U.S.A.).
Measurements of cyclic AMP
DBA-2 thymuses were harvested, thymocytes isolated and resuspended at 4×106 cells ml−1 in the culture media (RPMI-1640) at 4°C. Incubations of cells (4×105 thymocytes per assay) with various agents were performed in 1.5 ml Eppendorf tubes in a final volume 200 μl containing media alone, or adenosine (0–125 μM), CGS 21680 (0–10 μM), and/or ZM 241,385 (0–1 μM). Controls such as the complete reaction mix at time zero or no cell mixture were used with every experimental set. The reactions were initiated by the addition of adenosine or adenosine analogues and incubation lasted from 0–120 min at 37°C in an Eppendorf Thermomixer Model 5436. Thymocyte reaction mixtures were gently resuspended every 5 min and reactions were terminated by the addition of 25 μl of 1N HCl, followed by freezing of samples on dry ice. The cyclic AMP levels were determined using cyclic AMP enzyme immunoassay (EIA) kit from Amersham according to manufacturer's instructions.
Cell incubations and extractions were performed in the absence of cyclic AMP phosphodiesterase inhibitors IBMX to avoid complications with interpretation of results due to the possibility of their effects on adenosine receptors (Beavo & Refsnyder, 1990).
Results
In our earlier studies (Huang et al., 1997; Koshiba et al., 1997; 1999) we established that A2A adenosine receptors are mostly responsible for adenosine-induced cyclic AMP accumulation in peripheral T-lymphocytes. Figures 1 and 2 demonstrate that the same is true for thymocytes upon exposure to adenosine or CGS 21680. As shown, both CGS 21680 (Figure 2A) and extracellular adenosine (Figure 1A) trigger transient accumulation of cyclic AMP in thymocytes that peaks at 15 or 30 min, followed by a rapid decline, which most likely reflects the desensitization of Gs-coupled A2a-receptors on thymocytes. This ability of the selective A2AR agonist CGS 21680 to trigger cyclic AMP accumulation suggests that signalling through A2A receptors is responsible for the observed effects in thymocytes. This conclusion is supported by the ability of the selective A2A receptor antagonist ZM 241385 to completely inhibit both adenosine- or CGS 21680-induced accumulation of cyclic AMP in thymocytes (Figures 1B and 2B, respectively). Similar results were obtained after thymocytes were incubated with 10, 25 or 50 μM adenosine (data not shown).
Figure 2.
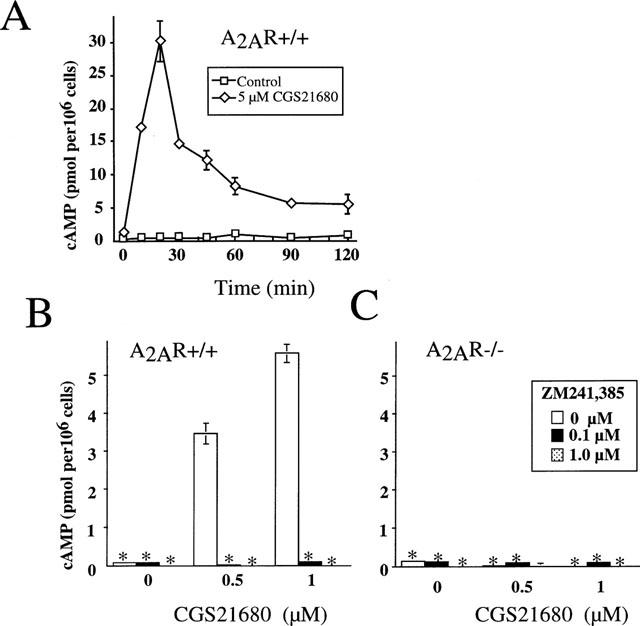
Measurements of cyclic AMP accumulation in murine thymocytes after incubation with adenosine analogue CGS 21680. Results are representative of four independent experiments with P<0.01. (*Indicates that standard deviations are not visible on the graph). Ex vivo thymocytes from A2AR wild type (+/+) (A,B) and from homozygous (−/−) A2AR-deficient ‘knock out' mice (C) were incubated with CGS 21680 in the presence or absence of A2AR antagonist ZM 241385 and cyclic AMP accumulation was measured as described in Methods. (A) transient accumulation of cyclic AMP in thymocytes from A2AR wild type (+/+) mice during 2 h incubation with 5 μM CGS 21680. Thymocytes from A2AR wild type (+/+) mice (B) or A2AR-deficient (−/−) mice (C) were incubated 30 min with 0.5 μM or 1 μM CGS 21680 in the presence or absence ZM 241385 at indicated concentrations.
The definitive genetic evidence for the selectivity of CGS 21680 for A2ARs was provided by experiments using cells from A2AR-deficient mice (Figures 1C and 2C). It is shown that while both extracellular adenosine and CGS 21680 were able to trigger cyclic AMP accumulation in cells from wild type mice, A2AR-deficient cells were refractory to both adenosine or CGS 21680 (up to 100 μM, data not shown). Moreover, the inability of adenosine or CGS 21680 to trigger cyclic AMP accumulation in A2AR-deficient thymocytes even at high concentrations also indicates the lack of compensation by other adenosine receptors.
The above experiments allowed us to address the issue of whether CGS 21680 exerts A2AR-independent lymphotoxicity in long-term functional assays. This was done by culturing thymocytes in the presence or absence of adenosine or CGS 21680 and estimating the proportion and number of surviving thymocytes using several independent assays. Flow cytometry analysis allowed us to detect proportions of live, apoptotic, and dead thymocytes following exposure to adenosine or CGS 21680 in vitro. Figure 3 shows that both adenosine and CGS 21680 produce a moderate decrease in thymocyte survival after 16 h cultures. Corresponding results were obtained by evaluating cell morphology as indicated by the forward (size) and side (granularity) scatter profiles (Figure 3A) and by specific Annexin-V/PI staining in separate experiments (Figure 3B). While CGS 21680 and adenosine both caused lymphotoxicity, a much higher concentration of adenosine (∼400 fold) was required to achieve similar proportions of cell death. The effects of CGS 21680 were observed at a concentration as low as 100 nM (data not shown) with maximum effect around 1 μM.
Figure 3.
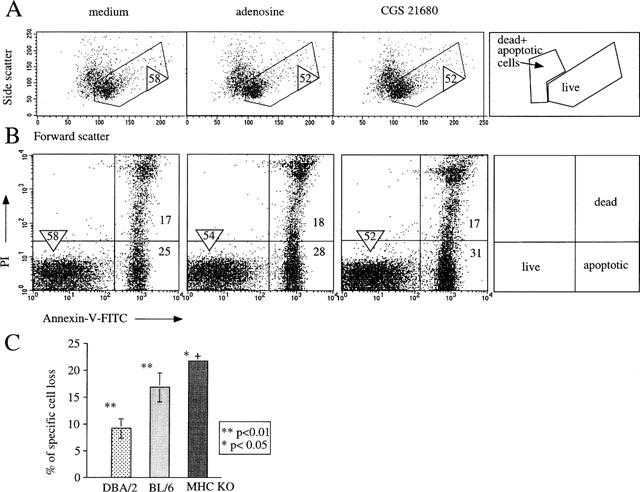
Extracellular adenosine and CGS 21680 induce hymocytes death. Ex vivo thymocytes from DBA/2, wild type (A2AR+/+) mice were incubated with media alone (control) and with adenosine (125 μM) or CGS 21680 (300 nM, (A,B) or 1 μM, (C) for 16 h in parallel assays. The survival of thymocytes after 16 h of incubation was measured by flow cytometry assays using Side scatter vs Forward scatter analysis (A) or Propidium Iodide (PI) staining vs Annexin V (B) to discriminate between live, dead and apoptotic cells as described in Methods and illustrated on cartoons. The percentage of survived, live cells (gated) is indicated by a circled number. The data are representative of eight separate experiments. The selection of gates was based on results of multiple earlier experiments where flow cytometry parameters were correlated with other methods of determination of cell death, including trypan blue and DNA fragmentation. (C) CGS 21680 induces death in a small proportion of thymocytes. Demonstration of variability in susceptibility of thymocytes from different strains of mice. DBA/2 (three experiments); BL/6 (three experiments) and double MHC class I and II gene deficient mice (two experiments). *Indicates P<0.05; **indicates P<0.01; +indicates that standard deviation is not visible on the graph.
The maximum number of dead cells following incubation with CGS 21680 varied between 7–15% among different mouse strains (Figure 3C). Interestingly, thymocyte from MHC classI/II deficient mice exhibited the highest susceptibility to the effect of CGS 21680 (Figure 3C) presumably due to the higher proportions of CD4/CD8 double positive thymocytes, which are more prone to the CGS 21680-induced cell death (not shown, manuscript in preparation). Evidently, CGS 21680 is much more potent than adenosine in the induction of both signalling and death of thymocytes.
To determine whether signalling through A2AR was indeed responsible for adenosine-induced thymocyte death, we used a sensitive Annexin V assay to detect changes in dying cells that are characteristic of apoptosis (Figure 4). We hypothesized that if CGS 21680-induced cell death was receptor-independent, then the same degree of cell death should be observed after incubation of thymocytes from wild type and from A2AR deficient mice in parallel experiments. If, however, A2AR was required, we would expect to see a higher proportion of apoptotic cells in wild type cultures than A2AR-deficient cultures. Indeed, 22AR-deficient thymocytes were resistant to the apoptosis-inducing affects of CGS 21680. Figure 4 shows that thymocytes from A2AR-deficient mice survived while 12±0.2% of A2AR+/+ thymocytes were killed following CGS 21680 (Figure 4A) treatment. In general, CGS 21680 was able to reproducibly induce cell death in about 8–15% of total thymocytes from wild type A2AR+/+ mice and no increases in the proportion of dead A2A R+/+ thymocytes were observed after incubation of thymocytes with increasing concentrations of CGS 21680 (up to 50 μM; data not shown), suggesting that this number correctly reflects the proportion of thymocytes that express apoptotic signal-transducing A2A receptors among total thymocytes. Furthermore, the ability of A2AR antagonist ZM 241385 to prevent the CGS 21680-induced thymocyte death (Figure 4A) provides pharmacological evidence that the effect of CGS 21680 is, in fact, mediated by signalling through A2A receptors. Overall, these results prove that the lymphotoxic effects of CGS 21680 are A2AR-dependent.
Figure 4.
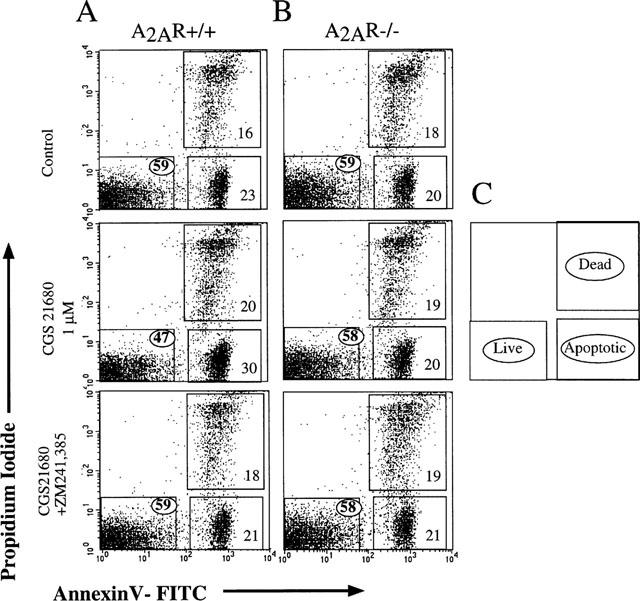
Extracellular adenosine and CGS 21680 are not lymphotoxic with A2AR deficient thymocytes. Ex vivo thymocytes from wild type (A, +/+) and from A2AR−/−) mice (B) were incubated in parallel assays with media alone (control) and with adenosine or CGS 21680. The survival of thymocytes after 16 h of incubation was measured by flow cytometry using Propidium Iodide vs Annexin V staining analysis to discriminate between live, dead and apoptotic cells as described in Methods and illustrated in C. Results are representative of four independent experiments with an analysis of 20×103 cells in each sample. The percentage of survived, live cells (gated) is indicated by a circled number.
Figure 5 summarizes the results of three separate experiments in which CGS 21680-induced A2AR+/+ thymocyte death in the presence or absence of ZM 241385 was assessed by PI versus Annexin V assays. These data confirm that a small, but significant, proportion of thymocytes can be targeted by CGS 21680. In general, the number of thymocytes in the murine thymus that are susceptible to extracellular adenosine-induced death ranged from 7–15% (based on 10 independent experiments) depending on the age and sex of the animal. These thymocytes are identified as part of the larger thymocyte subset of double positive CD4+/CD8+ cells (data not shown).
Figure 5.
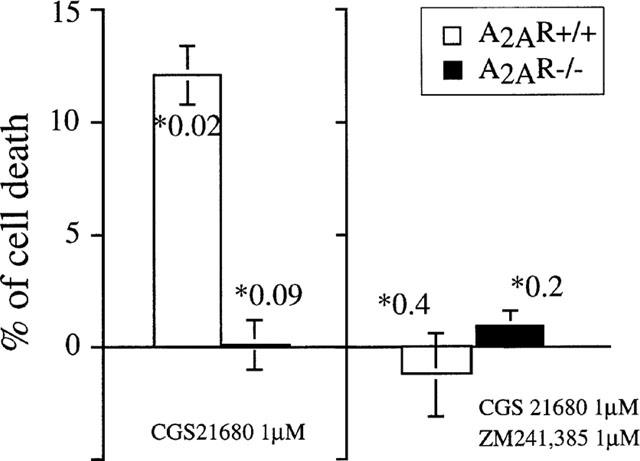
CGS 21680 does not possess A2AR unrelated lymphotoxicity. Results of three parallel independent cell death assays are summarized to demonstrate the presence of a thymocyte subset which is susceptible to CGS 21680-induced death in A2AR+/+ but not in A2AR−/− mice. Concentrations of CGS 21680 and ZM 241385 are indicated on the graph. *Number indicates P value for each point.
Additional evidence for the absence of intracellular lymphotoxicity by CGS 21680 was obtained in long-term (6 day) in vitro assays of thymocytes. The design of the experiments in Figure 6 was based on the ability of anti-TCR/CD3 mAb (to the antigen receptor complex) to rescue thymocytes from spontaneous death in which thymocytes die in the absence of activating stimuli unless selected by appropriate levels of TCR-mediated signalling (Surch & Sprent, 1994). Accordingly, we incubated thymocytes ex vivo with immobilized mAb to TCR/CD3 complex in the presence or absence of CGS 21680 and/or ZM 241385 and evaluated cell survival after 6 days by flow cytometry (Figure 6). It is shown that there were virtually no surviving thymocytes in the absence of anti-CD3 mAb (medium alone). However, up to 15% of thymocytes could be rescued from death by anti-CD3 mAb. As expected, and in agreement with our earlier observation (Huang et al., 1997; Apasov & Sitkovsky, 1999), the addition of CGS 21680 abolished signalling through the TCR and prevented the rescue of anti-TCR mAb-treated thymocytes. As expected, the A2AR antagonist ZM 241385 prevented the effects of CGS 21680, and the combined addition of both CGS 21680 and of ZM 241385 allowed the survival of the same number of thymocytes on the immobilized anti-CD3 mAb. According to these results, the combined presence of CGS 21680 and ZM 241385 was not lymphotoxic, but together with anti-TCR/CD3 complex mAb, allowed the survival of thymocytes which would otherwise have died. These observations demonstrate that even after 6 days the survival of thymocytes was not affected by the presence of CGS 21680 and ZM 241385, even though control experiments confirm that these drugs were signalling under these conditions, as evidenced by the ability of CGS 21680 to prevent anti-CD3 mAb induced survival and the abrogation of such event by ZM 241285 (Figure 6).
Figure 6.
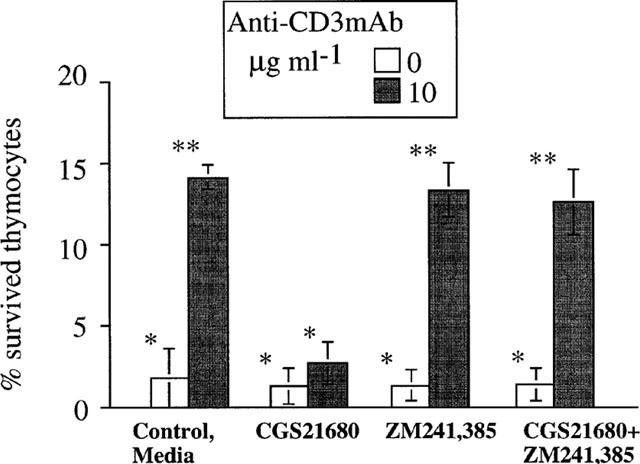
Selectivity of CGS 21680 and ZM 24385 mediated effects on thymocytes. CGS 212680 and ZM 241385 are not lymphotoxic in long thymocyte survival assay. Ex vivo thymocytes from wild type (A2AR+/+) DBA/2 mice were incubated in parallel assays alone or with thymocyte-activating anti-CD3/TCR mAb (10 μg ml−1) in the presence of media (control) or CGS 21680 (1 μM), ZM 241385 (1 μM) alone or both ZM 241385 and CGS 21680 as indicated on the graph. The survival of thymocytes after 5–6 days of incubation was measured by flow cytometry as described in Methods. The results represent a summary of five independent experiments. *Indicates P<0.4; **indicates P<0.07.
The experiments shown here may provide the best means to evaluate the absence of lymphotoxicity by these compounds, since the readout entails the enumeration of live rather than dead cells. Taken together, these data support the conclusion that adenosine receptor agonist CGS 21680 and antagonist ZM 241385 are not lymphotoxic in long-term in vitro assays.
Discussion
The issue of non-specific or receptor-independent cytotoxicity by pharmacological agents and, particularly, of adenosine receptor agonists (reviewed in Jacobson et al., 1999), is the subject of significant interest due to the need to minimize drug side effects. The rationale for considering the use of adenosine and adenosine analogues as agonists of distinct adenosine receptors to modulate in vivo physiology has been weakened by concerns over the well-known lymphotoxic effects of adenosine. These effects include inhibition of pyrimidine synthesis, inhibition of transmethylation, inhibition of ribonucleotide reductase, DNA nicking and apoptosis (Hershfield & Mitchell, 1995; Hirschhorn, 1995; Liu et al., 1996).
The hope that so-called slow hydrolyzable adenosine analogues and agonists of adenosine receptors may not have intracellular cytotoxic effects was dispelled by studies in different cellular systems (Abbracchio, 1996; Kohno et al., 1996; Sei et al., 1997; Barbieri et al., 1998; Abbracchio et al., 1995; Wakade et al., 1995; Tanaka et al., 1994; Ruchaud et al., 1995; Hoffmann et al., 1996; Apasov et al., 1995; Szondy, 1995; Porter et al., 1997; reviewed in Jacobson et al., 1999). Thus, there is a need to evaluate every promising adenosine receptor agonist for its ability to induce non-receptor mediated intracellular toxicity. However, due to the intrinsic pharmacological limitations of earlier experiments, it was not possible to conclusively discriminate adenosine receptor-mediated toxicity from intracellular toxicity of adenosine or adenosine analogues.
The ability to definitively address receptor specificity in this case has now become possible with the development of A2AR-deficient mice (Ledent et al, 1997; Chen et al., 1999) and with a better understanding of thymocyte differentiation and the signals that drive T-cell development in long-term differentiation in vitro assays (Cibotti et al., 1997). The use of cells from A2AR-deficient mice in cyclic AMP accumulation assays confirms the pharmacological selectivity of the A2AR agonist CGS 21680 (Figures 1 and 2), and provides a convenient system with which to detect adenosine-independent lymphotoxic effects.
We show here that adenosine and CGS 21680 cause cell death in a relatively small proportion of thymocytes after 16 h incubation. Moreover, only A2AR-expressing thymocytes are susceptible to these direct cytotoxic effects (Figures 3 and 4). Differences in susceptibility of thymocytes to CGS 21680-induced death were found between DBA-2, C57BL/6, and genetically engineered MHC class I and II deficient mice (Figure 3C). It is noteworthy that the larger proportion of thymocytes killed by CGS 21680 was in MHC class I and II double knock-out mice, which predominantly express double positive CD4+CD8+ thymocytes (data not shown, manuscript in progress). This is consistent with our observation that CD4+CD8+ double positive T cells (precursors of single positive CD4+ and CD8+ T cells) are the main targets of A2A receptor-mediated effects. An important goal of our ongoing studies is to identify and understand the functional role of A2AR on thymocyte development and T-cell differentiation.
Taken together, the observation that A2AR+/+, but not A2AR−/−, thymocytes are susceptible to cell death by CGS 21680 shows that the lymphotoxic effects of such pharmacological adenosine analogues is a receptor-dependent process. Furthermore, the fact that the A2A receptor-specific antagonist ZM 241385 could abrogate the CGS 21680-induced death indicates that A2A receptors are specifically involved in this process. Overall, these data support a model in which A2A adenosine receptors are directly involved in CGS 21680-mediated lymphotoxicity.
Acknowledgments
We would like to thank Dr Gregorio Gomez for discussions, Brenda Marshall for editorial assistance and Shirley Starnes for helping in the preparation of this manuscript. Supported by NIH Grant DA07496 and the grants from NARSAD and Scottish-Rite Foundation to J.F. Chen.
Abbreviations
- ADA
adenosine deaminase activity
- Ado
adenosine
- A2AAR
A2A adenosine receptor
- CGS 21680
A2AR agonist
- CTL
cytotoxic T-lymphocytes
- extAdo
extracellular adenosine
- FCS
foetal calf serum
- PI
propidium iodide
- SCID
severe combined immunodeficiency
- TCR
T-cell antigen receptor
- ZM 241,385
A2AR antagonist
References
- ABBRACCHIO M.P. P1 and P2 receptors in cell growth and differentiation. Drug Devel. Res. 1996;39:393–406. [Google Scholar]
- ABBRACCHIO M.P., CERUTI S., BARBIERI D., FRANCESCHI C., MALORNI W., BIONDI L., BURNSTOCK G., CATTABENI F. A novel action for adenosine: apoptosis of astroglial cells in rat brain primary cultures. Biochem. Biophys. Res. Commun. 1995;213:908–915. doi: 10.1006/bbrc.1995.2215. [DOI] [PubMed] [Google Scholar]
- ABBRACCHIO M.P., RAINALDI G., GIAMMARIOLI A.M., CERUTI S., BRAMBILLA R., CATTABENI F., BARBIERI D., FRANCESCHI C., JACOBSON K.A., MALORNI W. The A3 adenosine receptor mediates cell spreading, reorganization of actin cytoskeleton, and distribution of Bcl-XL: studies in human astroglioma cells. Biochem. Biophys. Res. Commun. 1997;241:297–304. doi: 10.1006/bbrc.1997.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APASOV S., KOSHIBA M., REDEGELD F., SITKOVSKY M. Role of extracellular ATP and P1 and P2 classes of purinergic receptors in T-cell development and cytotoxic T lymphocyte effector functions. Immunol. Rev. 1995;146:5–19. doi: 10.1111/j.1600-065x.1995.tb00680.x. [DOI] [PubMed] [Google Scholar]
- APASOV S., SITKOVSKY M. The extracellular versus intracellular mechanism of inhibition of TCR-triggered activation in thymocytes by adenosine under conditions of inhibited adenosine deaminase. Int. Immunol. 1999;11:179–189. doi: 10.1093/intimm/11.2.179. [DOI] [PubMed] [Google Scholar]
- APASOV S.G., KOSHIBA M., CHUSED T.M., SITKOVSKY M.V. Effects of extracellular ATP and adenosine on different thymocyte subsets: possible role of ATP-gated channels and G protein-coupled purinergic receptor. J. Immunol. 1997;158:5095–5105. [PubMed] [Google Scholar]
- BARBIERI D., ABBRACCHIO M.P., SALVIOLI S., MONTI D., COSSARIZZA A., CERUTI S., BRAMBILLA R., CATTABENI F., JACOBSON K.A., FRANCESCHI C. Apoptosis by 2-chloro-e′-deoxy-adenosine and 2-chloro-adenosine in human peripheral blood mononuclear cells. Neurochem. Int. 1998;32:493–504. doi: 10.1016/s0197-0186(97)00129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVO J.A., REIFSNYDER D.H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol. Sci. 1990;11:150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- BRAMBILLA R., CATTABENI F., CERUTI S. Activation of the human A3 adenosine receptor in CHO transfected cells results in cytosolic acidification and block of cells at the S phase. Drug Devel. Res. 1998;43:13. [Google Scholar]
- CHEN J.F., HUANG Z., MA J., ZHU J.-M., MORATALLA R., STANDAERT D., MOSKOWITZ M.A., FINK J.S., SCHWARZSCHILD M.A. Deficiency in A2a adenosine receptors attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIBOTTI R., PUNT J.A., DASH K.S., SHARROW S., SINGER A. Surface molecules that drive T cell development in vitro in the absence of thymic epithelium and in the absence of lineage-specific signals. Immunity. 1997;6:245–255. doi: 10.1016/s1074-7613(00)80327-1. [DOI] [PubMed] [Google Scholar]
- DARZYNKIEWICZ Z., BRUNO S., DEL BINO G., GORCZYCA W., HOTZ M.A., LASSOTA P., TRAGANOS F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- HERSHFIELD M.S., MITCHELL B.S. New York: McGraw-Hill; 1995. Immunodeficiency Diseases Caused by Adenosine Deaminase Deficiency and Purine Nucleoside Phosphorylase Deficiency. [Google Scholar]
- HIRSCHHORN R. Adenosine deaminase deficiency: molecular basis and recent developments. Clin. Immunol. Immunopathol. 1995;76:S219–S227. doi: 10.1016/s0090-1229(95)90288-0. [DOI] [PubMed] [Google Scholar]
- HIRSCHHORN R., GROSSMAN J., WEISSMANN G. Effect of cyclic 3′-5′ adenosine monophosphate and theophylline on lymphocyte transformation. Proc. Soc. Exp. Biol. Med. 1970;133:1361–1365. doi: 10.3181/00379727-133-34690. [DOI] [PubMed] [Google Scholar]
- HOFFMANN C., RAFFEL S., RUCHAUD S. Chloro-substituted cAMP analogues and their adenosine metabolites induce apoptosis of the human promelocytic leukemia cell line NB4: molecular basis for cell type selectivity. Cell. Pharmacol. 1996;3:417–427. [Google Scholar]
- HUANG S., KOSHIBA M., APASOV S., SITKOVSKY M. Role of A2a adenosine receptor-mediated signalling in inhibition of T cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- JACOBSON K.A., VAN RHEE A.M.Development of selective purinoceptor agonists and antagonists Purinergic Approaches in Experimental Therapeutics 1997New York: Wiley; 101–128.ed. Jacobson, K.A. & Jarvis, M.F., pp [Google Scholar]
- JACOBSON K.A., HOFFMANN C., CATTABENI F., ABBRACCHIO M.P. Adenosine-induced cell death: evidence for receptor-mediated signalling apoptosis. Apoptosis. 1999;4:197–211. doi: 10.1023/a:1009666707307. [DOI] [PubMed] [Google Scholar]
- JACOBSON K.A., VON LUBITZ D.K., DALY J.W., FREDHOLM B.B. Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol. Sci. 1996;17:108–113. doi: 10.1016/0165-6147(96)10002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIZAKI H., SUZUKI K., TADAKUMA T., ISHIMURA Y. Adenosine receptor-mediated accumulation of cyclic AMP-induced T lymphocyte death through internucleosomal DNA cleavage. J. Biol. Chem. 1990;265:5280–5284. [PubMed] [Google Scholar]
- KOHNO Y., SEI Y., YOSHIBA M., KIM H.O., JACOBSON K.A. Induction of apoptosis in HL-60 human promyelocytic leukemia cells by selective adenosine A3 receptor agonists. Biochem. Biophys. Res. Commun. 1996;219:904–910. doi: 10.1006/bbrc.1996.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSHIBA M., KOJIMA H., HUANG S., APASOV S., SITKOVSKY M.V. Memory of extracellular adenosine/A2a purinergic receptor-mediated signalling in murine T cells. J. Biol. Chem. 1997;272:25881. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- KOSHIBA M., ROSIN D.L., HAYASHI N., LINDEN J., SITKOVSKY M.V. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Molec. Pharmacol. 1999;55:614–624. [PubMed] [Google Scholar]
- LEDENT C., VAUGEOIS J.M., SCHIFFMANN S.N., PEDRAZZINI T., EL YACOUBI M., VANDERHAEGHEN J.J., COSTENTIN J., HEATH J.K., VASSART G., PARMENTIER M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- LIU X., KIM C.N., YANG J., JEMMERSON R., WANG X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- MARTIN S.J., REUTELINGSPERGER C.P.M., MCGAHON A.J., RADER J.A., VAN SHIE R.C.A.A., LAFACE D.M., GREEN D.R. Early redistribution of plasma membrane phosphatidilserine is a general feature of apoptosis regardless of initiating stimulus: Inhibition by overexpression of bcl-2 and abl. J. Exp. Med. 1995;182:1545. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCONKEY D.J., ORRENIUS S., JONDAL M. Agents that elevate cAMP stimulate DNA fragmentation in thymocytes. J. Immunol. 1990;145:1227–1230. [PubMed] [Google Scholar]
- MONTESINOS M.C., GADANGI P., LONGAKER M., SUNG J., LEVINE J., NILSEN D., REIBMAN J., LI M., JIANG C.K., HIRSCHHORN R., RECHT P.A. , OSTAD E., LEVIN R.I., CRONSTEIN B.N. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J. Exp. Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSSON R.A. Adenosine receptors in the cardiovascular system. Drug Devel. Res. 1996;39:301–307. [Google Scholar]
- OLSSON R.A., PEARSON J.D. Cardiovascular purinoceptors. Physiol. Rev. 1990;70:761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- PORTER A.G., NG P., JANICKE R.U. Death substrates come alive. BioAssay. 1997;19:501–507. doi: 10.1002/bies.950190609. [DOI] [PubMed] [Google Scholar]
- RUCHAUD S., ZORN M., DAVILARD-VILLAR E. Evidence for several pathways of biological response to hydrolysable cAMP analogues using a model system of apoptosis in IPC-81 leukaemia cells. Cell. Pharmacol. 1995;2:127–140. [Google Scholar]
- RUDOLPHI K.A., SCHUBERT P.Purinergic interventions in traumatic and ischemic injury Novel Therapies for CNS Injuries. Rationales and Results 1996Boca Raton: CRS PRESS; 327–346.ed. Peterson, P.L. & Phillis, J.W. pp [Google Scholar]
- SEI Y., VON LUBITZ D.K., ABBRACCHIO M.P., JI X.D., JACOBSON K.A. Adenosine A3 receptor agonist-induced neurotoxicity in rat cerebellar granule neurons. Drug Devel. Res. 1997;40:267–273. [Google Scholar]
- SURCH C.D., SPRENT J. T-cell apoptosis detected in situ during positive and negative selection in thymus. Nature. 1994;372:100–103. [PubMed] [Google Scholar]
- SZONDY Z. The 2-chlorodeoxyadenosine-induced cell death signalling pathway in human thymocytes is different from that induced by 2-chloroadenosine. Biochem. J. 1995;311:585–588. doi: 10.1042/bj3110585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZONDY Z. Adenosine stimulates DNA fragmentation in human thymocytes by Ca2+-mediated mechanisms. Biochem. J. 1994;304:877–885. doi: 10.1042/bj3040877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA Y., TOSHIHARA K., TSUYUKI M., KAMIYA T. Apoptosis induced by adenosine in human leukemia HL-60 cells. Exp. Cell Res. 1994;213:242–252. doi: 10.1006/excr.1994.1196. [DOI] [PubMed] [Google Scholar]
- VAN DER PLOEG I., AHLBERG S., PARKINSON FE., OLSSON R.A., FREDHOLM B.B. Functional characterization of adenosine A2 receptors in Jurkat cells and PC12 cells using adenosine receptor agonists. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:250. doi: 10.1007/BF00168626. [DOI] [PubMed] [Google Scholar]
- WAKADE T.D., PALMER K.C., MCCAULEY R., PRZYWARA D.A., WAKADE A.R. Adenosine-induced apoptosis in chick embryonic sympathetic neurons: a new physiological role for adenosine. J. Physiol. 1995;488:123–128. doi: 10.1113/jphysiol.1995.sp020951. [DOI] [PMC free article] [PubMed] [Google Scholar]


