Abstract
We have examined the effects of Y-27632, a specific inhibitor of Rho-activated kinases (ROCK I and ROCK II) upon sustained hypoxic pulmonary vasoconstriction (HPV) in both rat isolated small intrapulmonary arteries (IPA) and perfused rat lungs in situ. Y-27632 (100 nM–3 μM) was found to cause a concentration-dependent inhibition of acute sustained HPV in rat IPA. Application of Y-27632 (10–600 nM) in perfused rat lungs caused no change in basal perfusion pressure, but was found to inhibit HPV in a concentration-dependent manner, resulting in complete ablation of the pressor response to hypoxia at a concentration of 600 nM. Furthermore, addition of Y-27632 at any point during hypoxia caused a reversal of HPV in perfused rat lungs. These results suggest that activation of Rho-associated kinase may be a pivotal step in the generation of sustained HPV.
Keywords: Hypoxic pulmonary vasoconstriction, Rho-associated kinases
Introduction
Hypoxic pulmonary vasoconstriction (HPV) is a unique, and vital homeostatic mechanism resident within the lung (Von Euler & Lijestrand, 1946). The mechanisms underlying HPV remain the subject of much controversy despite extensive research. It is apparent however that HPV is associated with a rise in intracellular Ca2+ concentration ([Ca2+]i) (Robertson et al., 1995) which has been proposed to result from membrane depolarization (Post et al., 1992; Osipenko et al., 1997), Ca2+ influx (Cornfield et al., 1994), and/or the release of intracellular Ca2+ stores (Jabr et al., 1997). HPV in rat isolated intrapulmonary arteries (IPA) typically consists of two phases (e.g. Robertson et al., 1995), both of which are strongly potentiated if a small degree of preconstriction is present (e.g. Leach et al., 1994). The first phase is transient in nature, reaching a peak approximately 5 min after the induction of hypoxia (phase 1), and is superimposed on a more slowly developing, but sustained, endothelium-dependent constriction (phase 2). We have previously reported that the transient force development observed during phase 1 is accompanied by a transient rise in [Ca2+]i (Robertson et al., 1995). However, during phase 2 [Ca2+]i remains elevated, but does not rise in parallel with tension. We therefore proposed that the rise in tension during phase 2 is produced via sensitization of the myofilaments to Ca2+ (Robertson et al., 1995).
Ca2+-sensitization refers to the ability of agonists to enhance smooth muscle force development at a given level of [Ca2+]i (Nishimura et al., 1989). Recently interest has focused upon the role of RhoA, and its effector Rho-associated kinase (ROCK I or ROCK II) in agonist-induced Ca2+-sensitization. In the present study we have used Y-27632, an inhibitor of Rho-associated kinase (Uehata et al., 1997) to investigate the possible involvement of Rho-associated kinase in the generation of sustained HPV in rat isolated IPA, and in the perfused rat lung in situ.
Methods
IPA mounting and hypoxic protocol
Male Wistar rats (∼300 g) were anaesthetized with sodium pentobarbitone (55 mg kg−1 i.p.) and killed by cervical dislocation. The lungs were excised and placed in a physiological salt solution (PSS) containing (in mM): NaCl 118, NaHCO3 24, MgSO4 1, NaH2PO4 0.44, glucose 5.56, Na-pyruvate 5, CaCl2 1.8, and KCl 4. Small IPA (150–500 μm internal diameter) were mounted in a small vessel myograph (Cambustion AM10, Cambustion, Cambridge, U.K.) as previously described (Leach et al., 1992) and gassed with 95% air/5% CO2.
IPA were subjected to an equilibration procedure of four exposures to 80 mM KCl-PSS (KPSS, 2 min duration, isotonic replacement of NaCl). The presence of a functioning endothelium was determined by ACh (1 μM) following PGF2α (10 μM) induced contraction. Since a small degree of agonist-induced tone is required to facilitate HPV in rat IPA (e.g. Leach et al., 1994), vessels were exposed to 3 μM PGF2α for 20 min prior to, and during, the hypoxic challenge (1% O2/5% CO2/balance N2 for 45 min) after which the vessels were reoxygenated for 20 min, and washed with PSS. PO2 was monitored via a dissolved oxygen meter (control PO2: 135–145 mmHg, hypoxic PO2 15–18 mmHg, Diamond General electrode, Michigan, U.S.A.; Strathkelvin oxygen meter, Glasgow, U.K.).
Preliminary experiments demonstrated that an incubation time of 15 min was sufficient for Y-27632 to reduce agonist-induced constrictions in rat IPA. Cumulative concentration-response curves were constructed to PGF2α and to KPSS in either the absence of Y-27632, or after 15 min incubation with varying concentrations of the latter. HPV is reproducible in rat IPA providing 1 h is left between hypoxic challenges (e.g. Robertson et al., 1995). Two hypoxic challenges were therefore performed Y-27632 being added 15 min prior to the second.
Perfused lung
Male Wistar rats (250–350 g) were anaesthetized by 4% enflurane, administered 1000 i.u. of heparin intravenously, and then killed by exsanguination. A catheter was inserted into the pulmonary artery, via the right ventricle and secured with surgical thread. The carcass was then placed on a perspex sheet over a water-bath heated to 40°C. The lung was perfused at 30 ml min−1 with 10 ml of perfusate (composition in mM): NaCl 118, KCl 4, NaH2PO4 1.2, MgSO4 1, NaHCO3 24, CaCl2 2, glucose 5.56, Ficoll 4 g 100 ml−1 directed into the pulmonary arterial catheter, a side arm of which was connected to a blood pressure transducer to give a direct index of pulmonary vascular resistance. Venous outflow was drained from the left atrium into a reservoir from which the perfusate was then re-circulated. The lungs were inflated (via tracheotomy) 30 times per min with 5% CO2/20% O2/75% N2 (maximum ventilation pressure <15 cm H2O). Hypoxia was induced by switching to 5% CO2/2% O2/93% N2 for 40 min. After a recovery period of 1 h, a second hypoxic exposure was performed, Y-27632 being added to the perfusate 15 min prior to the secondary exposure in some experiments.
Tension in isolated arteries is presented as percentage of maximum tension (TK) obtained to the final exposure to KPSS during the equilibration procedure. Results are expressed as mean±s.e.mean and means were compared using ANOVA for repeated measures, or paired or unpaired Student's t-test as appropriate (SigmaStat, Jandel Inc., U.S.A.). A difference was deemed significant if P<0.05.
Results
Effect of Y-27632 upon agonist and K+-induced contractile responses in isolated IPA
Figure 1 shows the effect of pre-incubation with Y-27632 upon contractile responses elicited by increasing concentrations of either PGF2α or KPSS. Y-27632 was found to significantly attenuate constrictions induced by either KPSS (Figure 1A) or PGF2α (Figure 1B) in a concentration-dependent manner. However, Y-27632 was relatively selective for agonist (PGF2α) over depolarization-induced responses. For example 10 μM Y-27632 inhibited the contractile response to 100 μM PGF2α by approximately 95%, whereas the response to 70 mM KPSS was depressed by approximately 35% (Figure 1).
Figure 1.
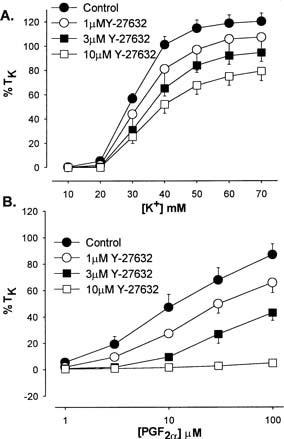
Effect of Y-27632 (1, 3 and 10 μM) pre-incubation upon KPSS (A) and PGF2α (B) induced vasoconstriction in isolated rat IPA. Each point is the mean of 4–6 experiments.
Effect of Y-27632 upon HPV in isolated IPA
As we have previously reported, hypoxia induced a biphasic contractile response in the presence of a small degree of agonist induced pretone (3 μM PGF2α, which elicited tension of 12.5±1.4% TK) in rat isolated IPA. It is well established that the degree of pretone employed prior to the induction of hypoxia can have profound effects upon the magnitude of HPV (e.g. Robertson et al., 2000). Since Y-27632 attenuated contractile responses to PGF2α, the concentration of the latter was therefore titrated upwards until an equivalent degree of pretone to that prior to the control hypoxic challenge was elicited (Control, 100, 300 nM Y-27632, pretone induced by 3 μM PGF2α, 1 μM and 3 μM Y-27632, pretone induced by 5–7 and 50–70 μM PGF2α respectively). Figure 2 shows the effect of Y-27632 upon HPV in isolated rat IPA. Y-27632 was found to inhibit phase 2 of HPV in concentration-dependent manner with an IC50 of approximately 300 nM. The inhibitory effect upon HPV was relatively selective for phase 2 over phase 1 (Figure 2). For example 300 nM Y-27632 inhibited the sustained phase 2 rise in tension by approximately 44%, whereas the transient phase 1 response was inhibited by approximately 5%. (Figure 2). Phase 2, measured immediately prior to reoxygenation, was significantly inhibited at all concentrations of Y-27632 used; phase 2 responses: control=29.7±2.1% TK, n=25; 100 nM=22.3±1.7% TK, P<0.01, n=9; 300 nM =16.7±1.5% TK, P<0.001, n=8; 1 μM=13.2% TK, P<0.001, n=9; and 3 μM=7.7±1.3% TK, P<0.001, n=7. Phase 1 was only significantly reduced and 1 and 3 μM Y-27632; phase 1 peak responses; control=68.4±2.3% TK, n=25, 100 nM =60.5±3.4% TK, P=0.08, n=9; 300 nM=64.7± 5.2% TK, P=0.5, n=8; 1 μM=51.2±3.1% TK, P<0.001, n=9; 3 μM =41.4±9.2, P<0.05, n=7). Unfortunately a maximum effect of Y-27632 against HPV could not be ascertained in this model, since concentrations above 3 μM prevented the necessary induction of pretone by PGF2α.
Figure 2.
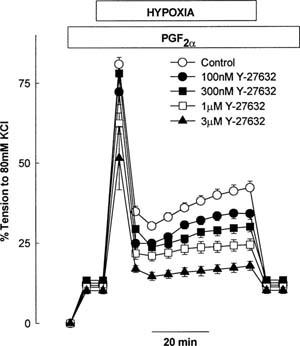
Effect of Y-27632 upon HPV in rat isolated IPA. Each point is the mean of 7–25 experiments.
Effect of Y-27632 upon HPV in perfused lungs
Figure 3 shows the effects of Y-27632 upon HPV in the perfused and ventilated rat lung in situ. (A) shows the increase in perfusion pressure to alveolar hypoxia in the absence of Y-27632. Two hypoxic exposures were given, 1 h apart. The hypoxic pressor response was found to be sustained and reproducible (basal perfusion pressure: 8.0±0.2 mmHg, peak increase in perfusion pressure during hypoxia: 9.5±0.2 mmHg, n=30). Y-27632 was found to inhibit HPV in a concentration-dependent manner reducing the hypoxic pressure response from 9.1±0.5 to 5.5±0.7 mmHg at 30 nM (P<0.01, n=6), 10.1±0.6 to 5.3±0.2 mmHg at 60 nM (P<0.01, n=6), and from 9.9±0.4 to 0.4±0.1 mmHg at 600 nM (P<0.01, n=6). (B and C) show the effects of 60 and 600 nM Y-27632 respectively upon HPV. Pre-incubation with 60 nM Y-27632 reduced the maximum pressor response to hypoxia by approximately 50% (Figure 3B). When the concentration of Y-27632 was raised to 600 nM HPV was completely abolished (Figure 3C). Application of this compound at any point during the hypoxic response resulted in a reversal of the pressor response. Figure 3D shows the effects of cumulative addition of 60 nM, and subsequently 600 nM Y-27632 to the perfusate after the increase in perfusion pressure to hypoxia.
Figure 3.
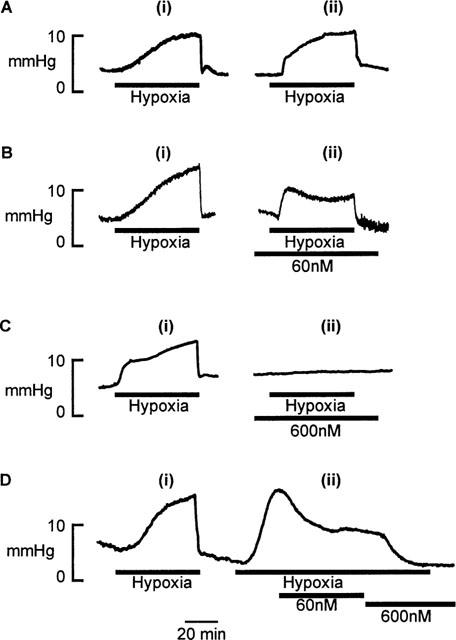
Effect of Y-27632 upon HPV in perfused rat lung in situ. (A) Two hypoxic challenges, 1 h apart. (B) Effect of pre-incubation with 60 nM Y-27632. (C) Effect of 600 nM Y-27632. (D) Effect of addition of Y-27632 (60 and 600 nM) after the establishment of the pressor response to hypoxia. Data are presented as perfusion pressure in mmHg.
Figure 4 shows the inhibitory effect Y-27632 upon HPV in perfused rat lungs, phase 1 and phase 2 of HPV in rat IPA, and upon PGF2α-induced and KPSS-induced constriction of rat IPA. Y-27632 was found to be significantly more potent against HPV in the perfused rat lung than against phase 2 in rat isolated IPA. In turn, Y-27632 was more effective in inhibiting phase 2 than phase 1 of HPV, PGF2α-induced, or depolarization-induced constriction in rat isolated IPA.
Figure 4.
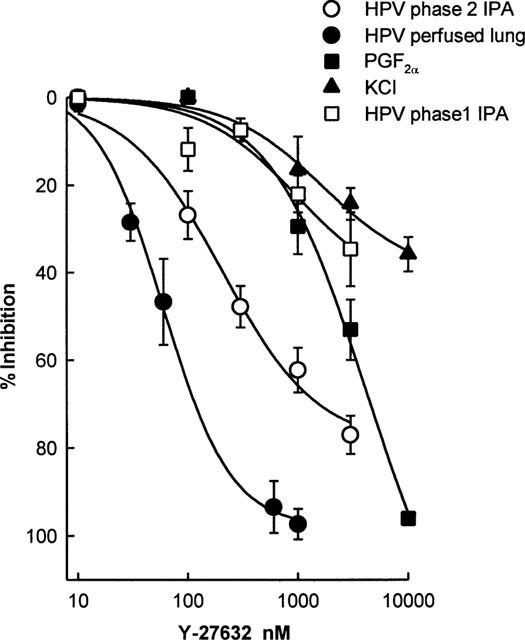
Effect of pre-incubation with Y-27632 upon the maximum responses to hypoxia in the perfused lung in situ, phase 2 and phase 1 of HPV in isolated rat IPA, 100 μM PGF2a and 70 mM KPSS in isolated rat IPA. Data are presented as per cent inhibition of the control response, and each point is the mean of 4–9 experiments. Concentration-response curves were fitted using non-linear least-squares regression (SigmaPlot, Jandel Scientific, U.S.A.).
Discussion
HPV is a vital homeostatic process within the lung, the mechanisms underlying which remain elusive despite extensive research. In the present study we have provided evidence which suggests that the activation of a Rho-associated kinase may play a pivotal role in the generation of sustained HPV in both rat isolated IPA, and in the perfused rat lung in situ.
In the presence of a small amount of agonist-induced pretone the hypoxic response in isolated rat IPA is biphasic (Leach et al., 1994), consisting of a rapid, and transient, response (phase 1), superimposed upon a more slowly developing constriction (phase 2). We have previously reported that the sustained constrictor response in rat IPA develops whilst cytoplasmic Ca2+ appears to remain constant (Robertson et al., 1995), implying that phase 2 results from an increase in myofilament sensitivity to Ca2+. Increased myofilament Ca2+-sensitivity can be invoked by a number of differing pathways, including activation of protein kinase C (Lee et al., 1994), protein tyrosine kinases (Steusloff et al., 1995), and RhoA (e.g. Kimura et al., 1996).
Recently, attention has focused upon RhoA, and its effector Rho-associated kinase, and their role in agonist-induced myofilament Ca2+-sensitization. RhoA is a member of the Ras superfamily of monomeric G proteins. In its active form, when bound to GTP, RhoA translocates to the plasmalemma (Gong et al., 1997; Wang et al., 1998), activating a Rho-associated kinase, which is thought to effect Ca2+-sensitization via an inhibition of myosin phosphatase, thus increasing overall myosin light chain phosphorylation (Kimura et al., 1996).
Studies into the physiological role of Rho-associated kinases have been aided by the development of the potent inhibitor Y-27632 (Uehata et al., 1997). Evidence for the selectivity of Y-27632 is drawn from observations that Y-27632 appears to have a single binding site within smooth muscle cells (namely a Rho-associated kinase), and that Y-27632 was found to be relatively selective for agonist-induced over depolarization-induced vasoconstriction (Uehata et al., 1997). The latter observation is in accordance with our present finding that Y-27632 was more effective in preventing agonist (PGF2α)-induced constriction than that induced by KPSS (Figure 1). This selectivity is presumably due to the important, but not sole, involvement of Ca2+-sensitization in agonist-induced vasoconstriction (Nishimura et al., 1989). Since Y-27632 was more potent against sustained HPV than against PGF2α (Figure 4) it would appear that the development of sustained HPV, at least in the preparations used, is biased towards pathways which induce constriction via myofilament Ca2+-sensitization.
Application of Y-27632 to isolated rat IPA in the present study produced a concentration-dependent inhibition of both phase 1 and phase 2 of HPV. However, the inhibitory effect of Y-27632 was relatively selective for the sustained phase 2 constriction (Figures 2 and 4). We have previously suggested that phase 2 in rat isolated IPA may contribute most to HPV in vitro, since it is sustained (Ward & Robertson, 1995). Our current findings would appear to support this view. This is evident from the fact that Y-27632 is a potent inhibitor of HPV in the perfused rat lung in situ, and of phase 2 in the isolated artery (Figures 2, 3 and 4) whereas Y-27632 was far less effective versus phase 1 and depolarization-induced vasoconstriction (Figures 1, 2 and 4). In addition, the apparent reduction in phase 1 may be partly due to the reduction in phase 2 upon which it is superimposed. It would therefore appear that phase 1, much like KPSS-induced constriction, is primarily dependent upon Ca2+ and that myofilament sensitization plays little part in its underlying mechanism. This concept is consistent with recent evidence that phase 1 in rat IPA is mediated by a rise in [Ca2+]i which results from the release of intracellular Ca2+ stores and activation of capacitative Ca2+ entry (Robertson et al., 2000).
In the present study Y-27632 was found to be approximately 5 fold more potent against HPV in the perfused lung than against phase 2 in isolated IPA (IC50's of approximately 60 and 300 nM respectively, Figure 4). The isolated lung has several advantages over the isolated artery model when studying HPV. The most pertinent, with respect to the present study, is that HPV can be elicited in the absence of pre-stimulation with an agonist. It is possible that the difference in IC50's against HPV observed between the two models could be due to the presence in the isolated arteries of PGF2α which has been shown to elevate [Ca2+]i (Robertson et al., 1995). This might reduce the effectiveness of Y-27632 by increasing the contribution to HPV of Ca2+-dependent mechanisms, since it is clear that Y-27632 is less effective at inhibiting predominantly Ca2+-dependent constrictions than it is at inhibiting constrictions that are associated with increased myofilament Ca2+-sensitivity (Uehata et al., 1997; see also Figure 1).
Upon addition of Y-27632 to the perfusate, no effect on baseline perfusion pressure was observed in the perfused lung in situ (Figure 3), whereas HPV was concentration-dependently inhibited. This apparent selective inhibition of vascular tone in the ‘activated' preparation has parallels with the systemic effects of Y-27632 described by Uehata et al. (1997). In this initial study Y-27632 was found to be without effect upon resting systemic blood pressure in normotensive rats, whereas an identical dose normalized the elevated high blood pressure in spontaneously hypertensive rats. This would appear to be consistent with the hypothesis that activation of a Rho-associated kinase may play some role within the development and/or maintenance of hypertensive states (Uehata et al., 1997). Since the current study would imply that Rho-associated kinase activation may be a pivotal step in the generation of HPV, at least in the rat, it is also possible that a similar process may promote the pulmonary hypertensive state associated with hypoxia-associated lung diseases. It is tempting to speculate that if indeed this is the case, this pathway may provide a novel and selective target for the treatment of pulmonary hypertensive disorders.
In summary we have found that Y-27632 is effective in both preventing, and reversing sustained HPV in isolated rat IPA and perfused rat lung in situ. This would be consistent with the activation of RhoA-associated kinases being involved in the mechanism underlying the generation of sustained HPV.
Acknowledgments
The authors would like to thank Ms A. Yoshimura and Mr Miura of Yoshitomi Pharmaceuticals for the generous gift of Y-27632, and Ms H. Hill for technical assistance. This work was supported by the British Heart Foundation (PG/96044), and by The Wellcome Trust. Dr A. Mark Evans is a Welcome Trust Career Development Research Fellow.
Abbreviations
- [Ca2+]i
intracellular Ca2+ concentration
- HPV
hypoxic pulmonary vasoconstriction
- KPSS
80 mM KCl physiological salt solution
- PSS
physiological salt solution
- TK
maximum tension generated to 80 mM potassium
- Y-27632
(+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexane carboxamide dihydrochloride, monohydrate
References
- CORNFIELD D.N., STEVENS T., MCMURTRY I.F., ABMAN S.H., RODMAN D.M. Acute hypoxia causes membrane depolarization and calcium influx in fetal pulmonary artery smooth muscle cells. Am. J. Physiol. 1994;266:L469–L475. doi: 10.1152/ajplung.1994.266.4.L469. [DOI] [PubMed] [Google Scholar]
- GONG M.C., FUJIHARA H., SOMLYO A.V., SOMLYO A.P. Translocation of RhoA associated with Ca2+-sensitization of smooth muscle. J. Biol. Chem. 1997;272:10704–10709. doi: 10.1074/jbc.272.16.10704. [DOI] [PubMed] [Google Scholar]
- JABR R.I., TOLAND H., GELBAND C.H., WANG X.X., HUME J.R. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br. J. Pharmacol. 1997;122:21–30. doi: 10.1038/sj.bjp.0701326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMURA K., ITO M., AMANO M., CHIHARA K., FUKATA Y., NAKAFUKU M., YAMAMORI B., FENG J., NAKANO T., OKAWA K., IWAMATSU A., KAIBUCHI K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) [see comments] Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- LEACH R.M., ROBERTSON T.P., TWORT C.H.C., WARD J.P.T. Hypoxic vasoconstriction in rat pulmonary and mesenteric arteries. Am. J. Physiol. 1994;266:L223–L231. doi: 10.1152/ajplung.1994.266.3.L223. [DOI] [PubMed] [Google Scholar]
- LEACH R.M., TWORT C.H.C., CAMERON I.R., WARD J.P.T. A comparison of the pharmacological and mechanical properties in vitro of large and small pulmonary arteries of the rat. Clin. Sci. 1992;82:55–62. doi: 10.1042/cs0820055. [DOI] [PubMed] [Google Scholar]
- LEE M.W., SEVERSON D.L. Signal transduction in vascular smooth muscle: diacylglycerol second messengers and PKC activation. Am. J. Physiol. 1994;267:C659–C678. doi: 10.1152/ajpcell.1994.267.3.C659. [DOI] [PubMed] [Google Scholar]
- NISHIMURA J., KHALIL R.A., VAN BREEMAN C. Agonist-induced vascular tone. Hypertension. 1989;13:835–844. doi: 10.1161/01.hyp.13.6.835. [DOI] [PubMed] [Google Scholar]
- OSIPENKO O.N., EVANS A.M., GURNEY A.M.Regulation of the resting potential of rabbit pulmonary artery myocytes by a low threshold, O2-sensing potassium current Br. J. Pharmacol. 199719971461–1470.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST J.M., HUME J.R., ARCHER S.L., WEIR E.K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am. J. Physiol. 1992;262:C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- ROBERTSON T.P., AARONSON P.I., WARD J.P.T. Hypoxic vasoconstriction and intracellular Ca2+ in pulmonary arteries: Evidence for PKC-independent Ca2+-sensitization. Am. J. Physiol. 1995;268:H301–H307. doi: 10.1152/ajpheart.1995.268.1.H301. [DOI] [PubMed] [Google Scholar]
- ROBERTSON T.P., HAGUE D., AARONSON P.I., WARD J.P.T. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J. Physiol. 2000;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEUSLOFF A., PAUL E., SEMENCHUK L.A., DI SALVO J., PFITZER G. Modulation of Ca2+-sensitivity in smooth muscle by genistein and protein tyrosine phosphorylation. Arch. Biochem. Biophys. 1995;320:236–242. doi: 10.1016/0003-9861(95)90005-5. [DOI] [PubMed] [Google Scholar]
- UEHATA M., ISHIZAKI T., SATOH H., ONO T., KAWAHARA T., MORISHITA T., TAMAKAWA, YAMAGAMI K., INUI J., MAEKAWA M., NARUMIYA S. Ca2+-sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- VON EULER U.S., LILJESTRAND G. Observations on the pulmonary arterial blood pressure in the cat. Acta. Physiol. Scand. 1946;12:301–320. [Google Scholar]
- WANG P., BITAR K.N. Rho A regulates sustained smooth muscle contraction through cytoskeletal reorganization of HSP27. Am. J. Physiol. 1998;275:G1454–G1462. doi: 10.1152/ajpgi.1998.275.6.G1454. [DOI] [PubMed] [Google Scholar]
- WARD J.P.T., ROBERTSON T.P. The role of the endothelium in hypoxic pulmonary vasoconstriction. Exp. Physiol. 1995;80:793–801. doi: 10.1113/expphysiol.1995.sp003887. [DOI] [PubMed] [Google Scholar]


