Abstract
Histamine, vascular endothelial growth factor, acetylcholine, oestrogen as well as fluid shear stress activates a mechanism that recruits heat shock protein 90 to the endothelial nitric oxide synthase. The interaction between Hsp90 and eNOS enhances the activation of the enzyme in cells and in intact blood vessels leading to NO production.
Intraplantar administration of carrageenan (50 μl paw−1) to mice causes an oedema lasting 72 h. Geldanamycin (0.1, 0.3, 1 mg kg−1), a specific inhibitor of Hsp-90, that inhibits endothelium-dependent relaxations of the rat aorta, mesentery and middle artery inhibits carrageenan-induced mouse paw oedema in a dose dependent manner.
Co-administration to mice of dexamethasone (1 mg kg−1) with geldanamycin (0.3 mg kg−1) at anti-inflammatory dose causes a loss of the total anti-inflammatory effect of each agent alone.
RU 486 (10 mg kg−1), a well known glucocorticoid receptorial antagonist, does not inhibit oedema formation but prevents the anti-inflammatory action of dexamethasone (1 mg kg−1). Similarly, RU 486 prevents the anti-inflammatory action of geldanamycin (0.3 mg kg−1).
In conclusion we have described for the first time that geldanamycin, an inhibitor of Hsp90 dependent signal transduction, is anti-inflammatory in vivo implying that Hsp90 is critical for pathways involved in carrageenan-induced paw oedema. In addition the ability of GA to block NO release and reduce oedema formation suggests a therapeutic rationale for specific inhibitors of Hsp90 as potential anti-inflammatory drugs.
Keywords: Geldanamycin, Hsp90, inflammation, glucocorticoid receptor, dexamethasone, RU 486
Introduction
Recently, it has been shown that agonists that stimulates NO production such as histamine, vascular endothelial growth factor (VEGF), acetylcholine, oestrogen as well as fluid shear stress activate a mechanism that recruits heat shock protein 90 (Hsp90) to the endothelial nitric oxide synthase (eNOS). Interaction between Hsp90 and eNOS enhances the activation of the enzyme in cells and in intact blood vessels leading to NO production (Garcia-Cardena et al., 1998; Shah et al., 1999; Viswanathan et al., 1999; Russell et al., 2000). Hsp90 is a member of the heat shock family of proteins involved in restoring cellular homeostasis, and subcellular trafficking and folding of several signalling proteins (for review see Pearl & Prodromou, 2000). Recent studies have also shown that geldanamycin (GA), a specific inhibitor of Hsp-90, inhibits endothelium-dependent relaxations of the rat aorta, mesentery and middle artery (Garcia-Cardena et al., 1998; Shah et al., 1999; Viswanathan et al., 1999). In cultured endothelial cells, GA reduces histamine, VEGF and oestrogen-stimulated cyclic GMP production (Garcia-Cardena et al., 1998; Russell et al., 2000). GA and a related analogue herbamycin A are members of the benzoquinone ansamicyn antibiotic that specifically bind to the unique ATP binding pocket of Hsp90 in a stable and pharmacologically specific fashion (Whitesell & Cook, 1994).
Steroid hormone receptors are members of a growing family of nuclear receptors (Mangelsdorf et al., 1995; Gronemeyer & Laudet, 1995) assembled into a multiprotein complex containing p23, an immunophillin, and heat shock proteins, mostly Hsp90, and some Hsp70. Genetic analysis in yeast (Picard et al., 1990; Kimura et al., 1995) and biochemical evidence in mammalian cells (Bresnick et al., 1989; Dalman et al., 1989) indicates that Hsp90 is necessary to maintain the glucocorticoid receptor (GR) in a conformation able to bind hormone. When hormone binding occurs, the interaction between Hsp90 and GR is disrupted and the receptor then translocates to the nucleus to activate the transcription of target genes. GA has also been widely used to study the glucocorticoid receptor and it has been shown to inhibit its function (Whitesell & Cook, 1996; Segnitz & Gehring, 1990). Thus, Hsp90 plays an important role in regulating eNOS and GR signalling. Since NO, VEGF and histamine are all mediators involved in inflammation we have sought to investigate if GA has in vivo anti-inflammatory effects in carrageenan-induced paw oedema. In addition, we have evaluated the possible interaction between Hsp90, GR and GA in an in vivo context.
Methods
Induction of oedema in the mouse paw
Male Swiss mice weighing 25–30 g were divided in groups (n=7) and lightly anaesthetized with enflurane. Each group of animals received subplantar administration of 50 μl of carrageenan 1% w v−1 (Henriques et al. 1987; Calhoun et al., 1987). Paw volume was measured using a hydroplethismometer specially modified for small volumes (Ugo Basile, Milan, Italy) immediately before the subplantar injection and 24, 48 and 72 h thereafter. The increase in paw volume was evaluated as difference between the paw volume at each time-point and the basal paw volume.
Drug treatment protocols
Geldanamycin was administered i.p. and its effect was tested using different regimen protocols: (i) 0.1, 0.3 and 1 mg kg−1 0, 24 and 48 h after oedema induction; (ii) 0.3 and 1 mg kg−1 administered at time 0 and 24 h after oedema induction; (iii) 1 mg kg−1 administered only 24 h after oedema induction. Dexamethasone (DEX) at the dose of 0.1 and 1 mg kg−1 was administered s.c. to mice at the same time (time 0), 24 and 48 h after oedema induction. The control group was treated with vehicle (PEG/distilled water 1 : 1) at the same time-points. All the administrations were i.p. and the final volume injected was always 100 μl.
In vivo interaction between dexamethasone and geldanamycin
To study the interaction between dexamethasone and geldanamycin in a first set of experiments mice received both drugs at doses that are anti-inflammatory e.g. dexamethasone 0.1 mg kg−1 s.c. together with geldanamycin 0.3 mg kg−1 i.p. at the time of oedema induction (time 0), 24 and 48 h. In a second set of experiments a low dose of dexamethasone 0.1 mg kg−1 s.c. was still used together, this time, with a higher dose of geldanamycin 1 mg kg−1 i.p.
Effect of RU 486 on dexamethasone and geldanamycin treatments
Groups of mice (n=8 each group) were treated with RU 486 10 mg kg−1 i.p. (Peers et al., 1988; Perretti et al., 1994) and either with dexamethasone 1 mg kg−1 s.c. or with geldanamycin 0.3 mg kg−1 i.p. All the administrations were performed at the time of oedema induction (time 0), 24 and 48 h after oedema induction and the final volume injected was 100 μl for each drug.
Drugs
RU 486 (Mifepristone), λ carrageenan, dexamethasone phosphate, and polyethylene glycol 400 were purchased from Sigma Chemical Co (Milan, Italy). Geldanamycin was a generous gift of the Drug Synthesis & Chemistry Branch, Development Therapeutics Program, Division of Cancer treatment at the National Cancer Institute (Bethesda, MD, U.S.A.).
Statistical analysis
All data are expressed as mean±s.e.mean and analysed by using ANOVA for multiple comparisons followed by Dunnett's test. Statistical significance is set at P<0.05 (n=7–8).
Results
Effect of geldanamycin and dexamethasone on mouse paw oedema
Geldanamycin 0.1, 0.3 and 1 mg kg−1 administered at time 0, and at 24 and 48 h after oedema induction, caused a significant time and dose dependent inhibition of oedema formation (Figure 1a). While the dose of 0.3 mg kg−1 and 1 mg kg−1 caused a significant inhibition at all time points the dose of 0.1 mg kg−1 caused a significant inhibition at 48 and 72 h only. The dose of 1 mg kg−1, administered at time 0 and 24 h after oedema induction, but not at 48 h, was still able to significantly inhibit oedema formation at 24 and 48 h (data not shown). All others dosage regimens did not significantly inhibit oedema development (data not shown).
Figure 1.
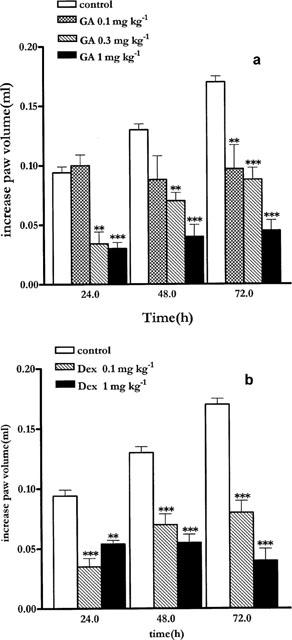
(a) Systemic administration of geldanamycin at 0, 24 and 48 h at the dose of 0.1, 0.3 and 1 mg Kg−1 ip inhibits mouse paw oedema development. (b) Dexamethasone at the dose of 0.1 and 1 mg kg sc−1 administered at 0, 24 and 48 h inhibits mouse paw oedema. Basal paw volume was 0.2±0.025 ml. Data are expressed as mean±s.e.mean (n=7) of the increase in paw volume calculates as described in the Methods section. **P<0.01; ***P<0.001 versus control.
Treatment with DEX 0.1 and 1 mg kg−1 s.c. significantly inhibited oedema development at each time-point (Figure 1b).
In vivo interaction between dexamethasone and geldanamycin
Co-administration of dexamethasone (0.1 mg kg−1) and GA (0.3 mg kg−1) reduced the anti-inflammatory effect of each drug given alone (Figure 2a). In particular, at 24 h the anti-inflammatory effect was abrogated. At 48 and 72 h there was a reduction of the anti-inflammatory effect given by each drug alone (Figure 2a). However, when GA was administered at the dose of 1 mg kg−1 together with dexamethasone, at 0.1 mg kg−1 changing the ratio DEX:GA from 1 : 3 to 1 : 10, the anti-inflammatory effect of GA was predominant (Figure 2b).
Figure 2.
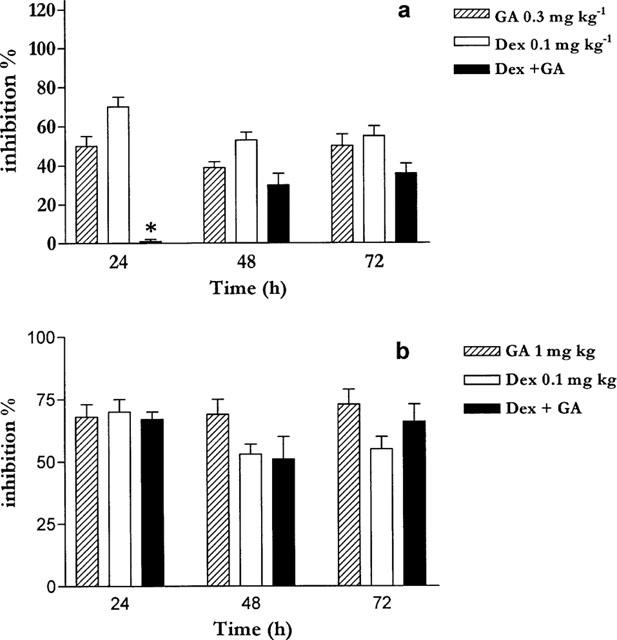
(a) Simultaneous administration of geldanamycin (0.3 mg kg−1 ip) and dexamethasone (0.1 mg kg−1) reduces the total anti-inflammatory effect. (b) Simultaneous administration of an higher dose of GA (1 mg kg−1) and dexamethasone (0.1 mg kg−1) does not reduce the total anti-inflammatory effect. *P<0.01.
RU 486 blocks the inhibitory action of GA on paw oedema
To further evaluate the interaction between dexamethasone and GA, the GR antagonist RU 486 was used. Co-treatment of mice with RU 486 at the dose of 10 mg kg−1 i.p. and dexamethasone 1 mg kg−1 abolished the anti-inflammatory effect of dexamethasone confirming the ability of RU 486 to antagonize the interaction of dexamethasone with glucocorticoid receptor in vivo (Figure 3a). Treatment of mice with RU 486 (10 mg kg−1) and GA (0.3 mg kg−1) caused loss of the anti-inflammatory effect obtained with GA alone (Figure 3b). RU 486 had no effect on oedema formation at the dose used (Figure 3a).
Figure 3.
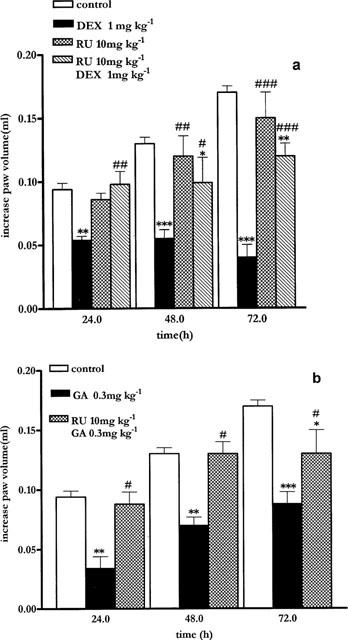
(a) The GR antagonist RU 486 10 mg kg−1 ip reverts dexamethasone (1 mg kg−1 sc) anti-inflammatory effect. (b) The GR antagonist RU 486 10 mg kg−1 ip reverts geldanamycin (0.3 mg kg−1) anti-inflammatory effect. **P<0.01; ***P<0.001 versus control; #P<0.05; ##P<0.01; ###P<0.001 versus DEX treatment (a) or versus GA treatment (b).
Discussion
The idea of studying GA as an anti-inflammatory drug was driven by our previous finding that GA inhibited eNOS through a specific Hsp90 dependent mechanism (Garcia-Cardena et al., 1998). Since nitric oxide has been shown to be involved in the increased vascular permeability in inflammatory experimental model, we sought to investigate whether GA had any possible anti-inflammatory activity (Marczin et al., 1993). Administration of GA to mice caused a dose dependent inhibition of the oedema which may be ascribed to a reduction of NOS activity. To demonstrate that this in vivo action was linked, in part, to this mechanism we studied the effect of GA administered with the potent anti-inflammatory steriod dexamethasone. Indeed, GA has been widely used in vitro to study the glucocorticoid receptor and it has been shown to interfere with the steroid hormone effect by affecting the binding of glucocorticoid to its specific receptor (Segnitz & Gehring, 1990) through a Hsp90-dependent mechanism.
In the absence of ligand binding, the glucocorticoid receptor (GR) exists as an 8-9S multiprotein cytosolic complex that contains, among other components, two molecules of Hsp90. Genetic analysis and biochemical studies have shown that Hsp90 is required to maintain the GR complex in a conformation that can bind steroid hormone. In addition, geldanamycin by binding to Hsp90 inhibits dexamethasone dependent translocation from the cytoplasm to the nucleus and this binding in intact cells is stable and specific (Whitesell & Cook, 1996). Since both GA and GR have been shown to bind to Hsp90 in vitro we examined if GA could interfere with the anti-inflammatory actions of dexamethasone (via the GR) in our in vivo system. Our hypothesis is supported by the observation that co-treatment of mice with dexamethasone and GA, at anti-inflammatory doses, causes a loss of the total anti-inflammatory effect. In addition it appears clear that the inhibitory effect given by either GA or DEX alone is reduced by co-administration. Indeed, a total reversion of the anti-inflammatory effect is clearly present at the 24 h point when DEX and GA are administered in a ratio 1 : 3. At the 48 and 72 h point instead the anti-inflammatory effect is present but is well below the inhibition given by dexamethasone and GA alone. This result fits well with in vitro data obtained from other laboratories, showing an interaction between GR and Hsp90. When GA binds to Hsp90 complexed to the GR, the glucocorticoid can no longer bind to its receptor (Whitesell & Cook, 1996). In this regard our data imply that Hsp90 is important for steroid action in vivo as well in vitro. In addition, this effect is clearly present only in a certain dose range and exactly in the ratio DEX:GA 1 : 3. Indeed, if a higher dose of GA is used (ratio DEX:GA 1 : 10), the anti-inflammatory effect of GA will predominate and its ability to antagonize the actions of dexamethasone is abrogated. However, since these results could also be interpreted in terms of pharmacokinetic interference to further confirm that the interaction between GA and dexamethasone occurs in vivo at GR level, we performed experiments by using a well characterized glucocorticoid receptor antagonist RU 486. Simply stated, we hypothesized that if the GA-dexamethasone interaction is at level of the GR receptor via the interaction of GR with Hsp90, then RU 486 should be able to interfere with the anti-inflammatory actions of GA. RU 486 does not reduce inflammation but binding to the GR blocks dexamethasone action. As seen in Figure 3, RU 486 antagonized the anti-inflammatory actions of both dexamethasone and GA in vivo suggesting that these two drugs have overlapping mechanisms of action.
In summary, our data are the first to describe GA, an inhibitor of Hsp90 dependent signal transduction, as an anti-inflammatory drug in vivo. More importantly, the anti-inflammatory actions of GA can be neutralized by co-administration of RU 486 and the simultaneous administration of dexamethasone causes a loss of the total anti-inflammatory effect. Thus, our results suggest that Hsp90 is critical for pathways involved in carrageenan induced oedema. Furthermore, the ability of GA to block NO release and reduce oedema formation suggests a therapeutic rationale for specific inhibitors of Hsp90 as potential anti-inflammatory drugs.
Abbreviations
- DEX
dexamethasone
- eNOS
endothelial nitric oxide synthase
- GELDA
geldanamycin
- GR
glucocorticoid receptor
- Hsp90
heat shock protein 90
- PEG
polyethylene glycol 400
References
- BRESNICK E.H., DALMAN F.C., SANCHEZ E.R., PRATT W.B. Evidence that the 90 kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J. Biol. Chem. 1989;264:4992–4997. [PubMed] [Google Scholar]
- CALHOUN W., CHANG J., CARLSON R.P. Effect of selected antiinflammatory agents and other drugs on zymosan, arachidonic acid, PAF and carrageenan induced paw edema in the mouse. Agent Actions. 1987;21:306–309. doi: 10.1007/BF01966499. [DOI] [PubMed] [Google Scholar]
- DALMAN F.C., BRESNICK E.H., PATEL P.D., PERDEW G.H., WATSON S.J.J., PRATT W.B. Direct evidence that the glucocorticoid receptor binds to Hsp90 at or near the termination of receptor translation in vitro. J. Biol. Chem. 1989;264:19815–19821. [PubMed] [Google Scholar]
- GARCIA-CARDEÑA G., FAN R., SHAH V., SORRENTINO R., CIRINO G., PAPAPETROPOULOS A., SESSA W.C. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- GRONEMEYER H., LAUDET V. Transcription factors 3: nuclear receptors. Protein Profile. 1995;2:1173–1308. [PubMed] [Google Scholar]
- HENRIQUES M.G.M.O., SILVA P.M.R., MARTIUS M.A., FLORES C.A., CUNHA F.Q., ASSRENY-FILHO J., CORDEIRO R.S.B. Mouse paw oedema, a novel model for inflammation. Brazilian J. Med. Biol. Res. 1987;20:243–249. [PubMed] [Google Scholar]
- KIMURA Y., YAHARA I., LINDQUIST S. Role of the protein chaperone YDJ1 in establishing Hsp90-mediated signal transduction pathways. Science. 1995;268:1362–1365. doi: 10.1126/science.7761857. [DOI] [PubMed] [Google Scholar]
- MANGELSDORF D.J., THUMMEL C., BEATO M., HERRLICH P., SCHUTZ G., UMESONO K., BLUMBERG B., KASTNER P., MARK M., CHAMBON P., EVANS R.M. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCZIN N., PAPAPETROPOULOS A., CATRAVAS J.D. Tyrosine kinase inhibitors suppress endotoxin-and IL-1 beta-induced NO synthesis in aortic smooth muscle cells. Am. J. Physiol. 1993;265:H1014–H1018. doi: 10.1152/ajpheart.1993.265.3.H1014. [DOI] [PubMed] [Google Scholar]
- PEARL L.H., PRODROMOU C. Structure and in vivo function of HSP90. Current Opinions in Structural Biology. 2000;10:46–51. doi: 10.1016/s0959-440x(99)00047-0. [DOI] [PubMed] [Google Scholar]
- PEERS S.H., MOON D., FLOWER R.J. Reversal of the anti-inflammatory effect of dexamethasone by the glucocorticoid antagonist RU 38486. Biochem. Pharmacol. 1988;37:556–557. doi: 10.1016/0006-2952(88)90230-4. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., HARRIS G.H., FLOWER R.J. A role for endogenous histamine in interleukin-8 induced neutrophil infiltration into mouse air pouch: investigation of the modulatory action of systemic and local dexamethasone. Br. J. Pharmacol. 1994;112:801–808. doi: 10.1111/j.1476-5381.1994.tb13150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICARD D., KHURSHEED B., GARABEDIAN M.J., FORITN M.G., LINDQUIST S., YAMAMOTO K.R. Reduced levels of Hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- RUSSELL K.S., HAYNES M.P., CAULIN-GLASER T., ROSNECK J., SESSA W.C., BENDER J.R. Estrogen stimulates heat shock protein 90 binding to endothelial nitric oxide synthase in human vascular endothelial cells. Effects on calcium sensitivity and NO release. J. Biol. Chem. 2000;275:5026–5030. doi: 10.1074/jbc.275.7.5026. [DOI] [PubMed] [Google Scholar]
- SEGNITZ B., GEHRING U. Mechanism of action of a steroidal antiglucocorticoid in lymphoid cells. J. Biol. Chem. 1990;265:2789–2796. [PubMed] [Google Scholar]
- SHAH V., WIEST R., GARCIA-CARDENA G., CADELINA G., ROBERTO J., GROSZMANN R.J., SESSA W.C. Hsp90 regulation of endothelial nitric oxide synthase contributes to vascular control in portal hypertension. Am. J. Physiol. 1999;277:G463–G468. doi: 10.1152/ajpgi.1999.277.2.G463. [DOI] [PubMed] [Google Scholar]
- VISWANATHAN M., RIVERAB O., SHORTA B.L. Heat Shock Protein 90 is involved in pulsatile flow-induced dilation of rat middle cerebral artery. J. Vasc. Res. 1999;36:524–527. doi: 10.1159/000025696. [DOI] [PubMed] [Google Scholar]
- WHITESELL L., COOK P. Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Mol. Endocrinol. 1996;10:705–712. doi: 10.1210/mend.10.6.8776730. [DOI] [PubMed] [Google Scholar]


