Abstract
Interleukin-1 (IL-1) is an important mediator of immunoinflammatory responses in the brain. In the present study, we examined whether prostaglandin E2 (PGE2) production after IL-1β stimulation is dependent upon activation of protein kinases in astroglial cells.
Astrocyte cultures stimulated with IL-1β or the phorbol ester, PMA significantly increased PGE2 secretion. The stimulatory action of IL-1β on PGE2 production was totally abolished by NS-398, a specific inhibitor of cyclo-oxygenase-2 activity, as well as by the protein synthesis inhibitor cycloheximide, and the glucocorticoid dexamethasone. Furthermore, IL-1β induced the expression of COX-2 mRNA. This occurred early at 2 h, with a maximum at 4 h and declined at 12 h. IL-1 β treatment also induced the expression of COX-2 protein as determined by immunoblot analysis. In that case the expression of the protein remained high at least up to 12 h.
Treatment of cells with protein kinase C inhibitors (H-7, bisindolylmaleimide and calphostin C) inhibited IL-1β stimulation of PGE2. In addition, PKC-depleted astrocyte cultures by overnight treatment with PMA no longer responded to PMA or IL-1. The ablation of the effects of PMA and IL-1β on PGE2 production, likely results from down-regulation of phorbol ester sensitive-PKC isoenzymes. Immunoblot analysis demonstrated the translocation of the conventional isoform cPKC-α from cytosol to membrane following treatment with IL-1β.
In addition, IL-1β treatment led to activation of extracellular signal-regulated kinase (ERK1/2) and p38 subgroups of MAP kinases in astroglial cells. Interestingly, the inhibition of ERK kinase with PD 98059, as well as the inhibition of p38 MAPK with SB 203580, prevented IL-1β-induced PGE2 release.
ERK1/2 activation by IL-1β was sensitive to inhibition by the PKC inhibitor bisindolylmaleimide suggesting that ERK phosphorylation is a downstream signal of PKC activation.
These results suggest key roles for PKC as well as for ERK1/2 and p38 MAP kinase cascades in the biosynthesis of PGE2, likely by regulating the induction of cyclo-oxygenase-2, in IL-1β-stimulated astroglial cells.
Keywords: Interleukin-1β, prostaglandin E2, protein kinase C, mitogen activated protein kinases, cyclo-oxygenase-2, murine astrocytes
Introduction
The pro-inflammatory cytokine, interleukin-1 (IL-1) is secreted by a variety of cells in response to infection, activated lymphocyte products, microbial toxins, and inflammatory stimuli (Dinarello, 1996). Besides the classical role of IL-1 as immune mediator, this cytokine has numerous effects in the central nervous system (CNS) (Berkenbosh et al., 1987; Hansen & Krueger, 1997; Rothwell, 1991; Hopkins & Rothwell, 1995). IL-1β has been shown to be produced in the CNS in response to a number of stimuli, such as peripheral lipopolysaccharide administration (Layé et al., 1994), traumatic brain injury (Fan et al., 1995) and brain viral infection (Marquette et al., 1996; Lledó et al., 1999). Under pathological situations involving CNS inflammation, IL-1 is mainly secreted by activated microglia/macrophages (Giulian et al., 1986; Benveniste, 1992), while astrocytes appear to be its main target as suggested by the presence of IL-1 receptors in their surface (Ban et al., 1993). In astroglial cells, IL-1 induces the expression of other cytokines, such as interleukin-6 and tumour necrosis factor-α, as well as other inflammatory mediators that may be implicated in the CNS response to injury (Merrill & Benveniste, 1996).
Arachidonic acid metabolites, such as prostaglandins are closely involved in the inflammatory responses following brain injury and bacterial or viral infections. High levels of prostaglandins have been measured in multiple sclerosis, AIDS-associated dementia, and other neurodegenerative disorders (Bolton et al., 1986; Fretland et al., 1992; Griffin et al., 1994). Cyclo-oxygenase is the rate-limiting enzyme in prostanoids biosynthesis and exists as a widely constitutive isoform (COX-1), responsible for physiological levels of prostaglandins and as an inducible COX-2 encoded by an immediate early gene, rapidly induced after pro-inflammatory stimuli. COX-2 is expressed by inflammatory cells and is responsible for the high levels of prostaglandins present in acute and chronic inflammation, being inhibited by glucocorticoid hormones (O'Banion et al., 1992; Herschman 1996). Interestingly, systemic LPS and IL-1β injections have been shown to stimulate production of COX-2 within the mouse (Breder & Saper, 1996) and rat (Cao et al., 1996; Lacroix & Rivest, 1998) brain microvaculature. In addition, COX-2 expression is increased in frontal cortex of Alzheimer's disease brains (Pasinetti & Aisen, 1998). During the last years, the physiological role of COX-2 has been a topic of much interest (Vane et al., 1998).
In vitro studies have revealed the capacity of astrocytes to release prostaglandins and express mRNA COX-2 in response to IL-1β (Hartung et al., 1989; Katsuura et al., 1989; O'Banion et al., 1996). However, the intracellular signalling mechanisms triggered by IL-1β and responsible for prostaglandin production are not completely defined. Protein kinase C (PKC) represents a family of closely related serine/threonine kinases that play a key role in different cellular signal transduction pathways (Nishizuka, 1986; 1992). A number of studies have demonstrated that phorbol esters, direct activators of PKC, stimulate the synthesis of prostaglandins (Crofford et al., 1994). However, in mesangial cells, prostaglandin biosynthesis induced by IL-1β seems to be independent on PKC activation (Conquer et al., 1992). Activation of mitogen-activated protein kinases (MAPK) cascade is one of the most rapid cellular responses to different stimuli, including IL-1 (Wilmer et al., 1997; O Neill & Greene 1998). Moreover, the cascade of MAPKs represent a point of convergence in intracellular signalling activated by protein Tyr kinases, by G-protein coupled receptors and by PKC. Several studies have linked prostaglandin biosynthetic pathway which mediate inflammatory responses, with activation of MAPK signalling cascade (Lin et al., 1993; Sanghera et al., 1996; Hambleton et al., 1996; Niiro et al., 1998). In mammalian cells, three subgroups of MAPKs have been detected and included the extracellular signal-regulated kinase, p42/44 (ERK1/2), the C-jun amino terminal kinases (JNKs) and p38 MAPKs (Cano & Mahadevan, 1995). These distinct sets of MAPKs can be activated by a variety of extracellular stimuli, and individual MAPK kinases may be implicated in the expression of a number of pro-inflammatory molecules depending on the cell type (Pouliot et al., 1997).
In the current study, we described that IL-1β induced the expression of mRNA COX-2 and the protein in astrocytes. In addition, we analysed pharmacologically the possible participation of PKC activation and MAPKs cascade in IL-1β-induced PGE2 production by mouse astrocyte cultures.
Methods
The following reagents were obtained from the indicated suppliers: Dulbecco's modified Eagle medium, foetal calf serum (FCS), penicillin/streptomycin mix from Gibco-BRL (Barcelona, Spain); recombinant mouse interleukin-1β (IL-1β) from Genzyme (Cambridge, MA, U.S.A.); monoclonal anti-GFAP, lipopolysaccharide (LPS) (from E. Coli 026:B6), H-7 1-(5-isoquinolinylsulphonyl)-2-)-2-methylpiperazine), 12-O-tetradecanoylphorbol 13 acetate (TPA); 4-α phorbol 12-myristate 13 acetate (4-αPMA), actinomycine D and cycloheximide from Sigma (St. Louis, MO, U.S.A.); bisindolylmaleimide I, NS-398, calphostin-C, PD 98059, SB 203580, from Calbiochem (La Jolla, CA, U.S.A.); PGE2 enzymeimmunoassay system BIOTRAK, Hybond ECL-nitrocellulose membrane and ECL Western blotting detection reagents from Amersham Pharmacia Biotech (London, U.K.); culture flasks and dishes were from Falcon (Franklin Lakes, NJ, U.S.A.); Affinity-purified rabbit anti-phospho p42/44 and anti-phospho p38 were from New England Biolabs (Beverly, MA, U.S.A.); rabbit polyclonal anti-PKC-α was from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.); COX-2 antibody from Cayman Chemicals (MI), Mac-1 antibody from Serotec (Oxford, U.K.) and the secondary antibody peroxidase-conjugated goat anti-rabbit IgG was from Jackson Immuno Research Laboratories (West Grove, PA, U.S.A.). Secondary antibodies for immuno-fluorescence were from Southern Biotechnology (Birmingham, AL, U.S.A.). All other reagents were obtained from standard suppliers.
Astrocyte cultures
Primary astrocyte cultures were generated from the cerebral cortex of 1-day-old neonatal mice (Balb/c Cajal Institute, Madrid, Spain) as described by McCarthy & de Vellis (1980) with our modifications (Molina-Holgado et al., 1995). Cells were plated on 75 cm2 flasks in Dulbecco's modified Eagle medium (DMEM) containing 10% heat-inactivated foetal calf serum plus 50 units ml−1 penicillin and 50 μg ml−1 streptomycin (growth medium). After 10 days, the cultures were shaken for 18 h at 240 r.p.m. at 37°C to remove microglia and oligodendrocyte progenitors. The astrocyte monolayers were then trypsinized and plated at an approximate density of 30,000 cells cm−2 (6 or 24 cm well dishes and 90 cm2 culture plates) and subcultured for 3–4 days before experiments. The cultures were immunocytochemically characterized by specific markers for different cell types. More than 95% of the cells reacted positively with the monoclonal anti Glial Fibrilar Acidic Protein (GFAP), a marker of astrocytes and less than 5% were A2B5 oligodendrocyte progenitors or Mac-1 positive microglia. Cultures with more than 5% of cells positive for A2B5 or Mac-1 were discarded.
Astrocyte stimulation
Cells were stimulated in 1 ml of growth media containing mouse recombinant IL-1β, LPS or TPA for different periods of time up to 24 h, and supernatants collected for PGE2 determination. The protein synthesis inhibitor, cycloheximide, the COX-2 blocker NS-398 and the protein kinase inhibitors used were prepared as 1000 times stock solutions in DMSO and added 60 min before stimulation to the wells. Control cells were only exposed to equivalent amounts of DMSO.
For PKC or MAPK immunoblotting cells were serum starved for 24 h and then stimulated for the indicated times with mouse recombinant IL-1β or pharmacological agents as described in the Figure legends.
Western blot analysis
After culture in serum-free medium for 24 h, quiescent astrocytes were stimulated with test agents for the indicated times. Cells were washed with ice-cold PBS and lysed in 150 μl of TBS, pH 7.6, containing 10% glycerol, 1% NP-40, 0.5 mM EDTA, 1 mM PMSF Western blot, 50 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, 5 mM benzamidine, 100 mM sodium ortovanate, 2 mM NaF and 5 mM DTT. Lysates were added 5× Laemmli sample buffer before boiling for 4 min. Then, equal amounts of protein (20 μg) were resolved on 10% SDS-polyacrylamide gel electrophoresis and electroblotted at 4°C for 1 h to ECL nitrocellulose. The membranes were blocked for 1 h at room temperature in 5% (w v−1) dry skim milk (Sveltese, Nestlé) in TBS (pH 7.6) plus 0.1% Tween-20 (TTBS) and then blots rinsed in TTBS. The membranes were incubated overnight with activation state-specific antibodies for the MAPKs (rabbit anti-phospho p42/44 and p38, New England BioLabs) at 1 : 1000 dilution or specific COX-2 antibody (Cayman Chemicals, MI, U.S.A.) at 1 : 2000 dilution. After extensive washing in 5% milk TTBS solution, blots were incubated with peroxidase-conjugated goat anti-rabbit IgG1 at 1 : 10,000 dilution. Blots were washed again before developing with an enhanced chemiluminiscence (ECL) kit.
To determine changes in the subcellular distribution of PKC isoenzymes, the cells were fractionated into cytosol and membrane fractions according to the following protocol. Astrocytes grown into 100 cm2 were stimulated with IL-1β for different times and harvested in 1 ml of ice-cold homogenization buffer (20 mM K-HEPES, pH 7.5, 5 mM EDTA, 5 mM EGTA, 1 mM PMSF, 50 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, 5 mM benzamidine, 100 mM sodium ortovanate, 2 mM NaF and 5 mM DTT) and disrupted with a teflon homogenizer. The cytosolic fraction was obtained as supernatant after centrifugation at 100,000×g for 60 min. To prepare a membrane fraction, the pellets were resuspended in 400 μl of the same buffer plus 1% Triton X-100 and collected after centrifugation at 100,000×g for 30 min. Lysates (20 μg) were resolved on 10% SDS–PAGE and immunoblotted with rabbit polyclonal anti-PKC-α (1 : 6000) overnight at 4°C as described above.
RT–PCR analysis of COX-2
Astrocytes were plated in 35 mm culture dishes and stimulated with or without IL-1β (10 ng ml−1) for different time periods. The cells were washed with PBS, and total RNA was isolated by the guanidinium isothiocyanate/phenol/chloroform method (Chomczynski & Sacchi, 1987). RNA concentration was quantified spectrophotometrically and the isolated RNA was treated with DNase to digest any contaminant genomic DNA. RT–PCR was performed in one step using Titan™ one tube RT–PCR system according to the manufacturer's instructions (Roche Molecular Biochemicals). RT–PCR amplification was carried out with 2 μg of RNA using the primer pair 5′-CCATGTCAAAACCGTGGTGAATG-3′ and 5′-ATGGGAGTTGGGCAGTCATAG-3′ (Nogawa et al., 1997). The steps of amplification were 94°C for 30 s, 55°C for 45 s, 68°C for 45 s for 30 cycles. PCR products were resolved on 2% agarose gel with 0.5 μg ml−1 of ethidium bromide. Only one PCR product of the expected size was obtained and COX-2 transcript was identified as a 374 bp band. The mouse glyceraldehyde 3 phosphate dehydrogenase (GAPDH) gene (a houskeeping gene) was used as an internal standard gene.
Measurement of PGE2
The cell-free supernatants were collected and stored at −70°C until measurement of PGE2. The PGE2 concentration in the culture medium was determined by a PGE2 enzyme-immunoassay system (BIOTRAK, Amersham Pharmacia Biotech, U.K.). PGE2 standard curve range from 2.5 to 320 pg well−1. The sensitivity of the assay was 2 pg well−1. The cross-reactivity with PGE1 is 7.0%, with PGF2α 0.04% and 6-Keto-PGF1 α <0.1 and with Arachidonic acid <0.001%. The within assay precision was <7.6% and between assay was <14%.
The evaluation of the protein content in the cultures was determined by a BCA protein assay reagent (Pierce, IL, U.S.A.) with bovine serum albumin as the standard.
Statistical analysis
The data are presented as mean±s.e.mean values of n independent determinations, and were triplicated within each experiment. Comparisons were analysed by using one-way analysis of variance (ANOVA) followed by the posteriori Student-Newman-Keuls' t-test. A value of P<0.05 was considered significant.
Results
IL-1β stimulates prostaglandin biosynthesis and induces the expression of COX-2 mRNA and protein in mouse cultured astrocytes
Treatment of astrocyte cultures with IL-1β (10 ng ml−1) caused a time-dependent increase in PGE2 release, that was nearly 2 fold and more than 3 fold increased in comparison to control cultures at 8 and 12 h respectively (Figure 1). Exposure of the cells to IL-1β also induced the expression of mRNA COX-2 as well as COX-2 protein (Figure 2A–C). The kinetic profile of the expression of COX-2 mRNA by RT–PCR analysis revealed a time-dependent induction of COX-2 mRNA by IL-1β. COX-2 mRNA was detected as early as 2 h, peaked at 4 h and then, started to decline by 12 h (Figure 2A). The earliest induction of COX-2 protein occurred at 2 h and remained elevated up to 12 h (Figure 2B).
Figure 1.

Time course of IL-1β (10 ng ml−1) induced release of PGE2 from cultured mouse astrocytes in the presence or not of dexamethasone (1 μM). Supernatants were collected at the indicated times. Levels of PGE2 in the medium were determined by enzyme-immunoassay system. Data are mean±s.e.mean (bars) values for three to four independent experiments in triplicate. Statistical significance: *P<0.001 compared with non-treated and dexamethasone treated cells after the corresponding incubation period.
Figure 2.

(A) Time-course of COX-2 mRNA expression in IL-1β (10 ng ml−1)-cultured astrocytes. Total RNA was extracted as described in Methods and levels of mRNA for COX-2 and GAPDH were determined by RT–PCR. Representative results of three independent experiments. (B) Time course of COX-2 protein expression in IL-1β (10 ng ml−1)-stimulated astrocytes. Cell lysates were used to determine COX-2 expression by Western blot analysis. Proteins were analysed by SDS-polyacrylamide gel electrophoresis and immunoblotting techniques using specific COX-2 antibody. Equal amount of proteins (20 μg) were loaded in all lanes. Bands were visualized by the ECL method. Representative results of three independent experiments. (C) Integrated band densities were obtained by scanning using a densitometer. Plotted means±s.e.mean of three independents experiments.
Dexamethasone (1 μM) prevented IL-1β-induced PGE2 release (Figure 1), while both PMA (100 nM) and LPS (1 μg ml−1) increased PGE2 secretion after 24 h treatment (Figure 3). As expected, the inactive phorbol 4-αPMA (100 nM) failed to stimulate PGE2 release. Figure 3 also shows that 2 μM of NS-398, a specific inhibitor of COX-2, (Futaki et al., 1993), totally abolished the stimulatory action of IL-1β on PGE2 secretion, clearly indicating that prostaglandin production is due to COX-2 activity. In addition, PGE2 production was completely prevented by the protein synthesis inhibitor cycloheximide (10 μg ml−1), as well as by the transcription inhibitor actinomycine D (1 μg ml−1) confirming the requirement for the de novo protein synthesis.
Figure 3.
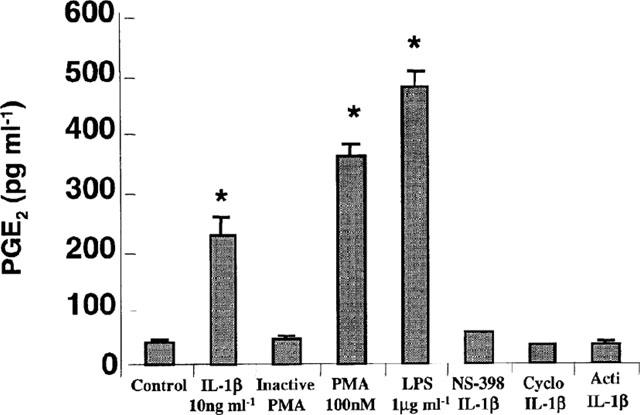
Interleukin-1β (10 ng ml−1), PMA (100 nM) and LPS (1 μg ml−1) stimulate the production of PGE2 in murine astrocytes. Supernatants were collected after 24 h stimulation. Pre-treatment (60 min before) with the COX-2 inhibitor, NS-398 (2 μM), or the inhibitor of the synthesis protein, cycloheximide (Cyclo) (10 μg ml−1) or the transcription inhibitor actinomycine-D (1 μg ml−1) abrogates the stimulatory effect of IL-1β on PGE2 secretion. The data are presented as the mean±s.e.mean (vertical lines) of four independent determinations in triplicate. Statistical significance: *P<0.0001 vs control; NS-398+IL-1β, Cyclo+IL-1β and Acti+IL-1β.
Signal transduction pathways involved in IL-1β-increased prostaglandin production
Involvement of PKC and MAP kinases
The ability of PMA to increase PGE2 production in astrocytes, suggests that PKC may be involved in the action of IL-1β. To test this hypothesis, astrocytes were pre-treated with various kinase inhibitors at the adequate doses prior to stimulation with IL-1β (10 ng ml−1). As shown in Figure 4, the compound, H7 (10 μM), or bisindolylmaleimide (2 μM), inhibitors of PKA and PKC suppressed PGE2 accumulation by 52.4 and 51.8%, respectively. The more specific PKC inhibitor, calphostin C (1 μM), also blocked (64.5%) IL-1β-stimulated PGE2 production. Since MAP kinase pathways have been shown to be activated by IL-1β treatment in a number of cells, we also used specific inhibitors of p38 and p42/44 (ERK1/2) MAP kinase cascade. As shown in Figure 4, PGE2 production was inhibited by 77% in IL-1β-stimulated astrocytes exposed to the specific inhibitor of ERK kinase (MEK), PD 98059 at the dose of 15 μM, known to inhibit ERK phosphorylation (Alessi et al., 1995). This dose of PD 98059 did not induce cell death during the 24 h treatment, as examined by the MTT reduction assay (data not shown). In addition, IL-1β-induced-PGE2 release was blunted by 15 μM SB 203580 (79.4%), a specific inhibitor of p38 MAP kinase (Badger et al., 1996), without affecting cellular viability. These results indicate the involvement of both, PKC and MAPKs in IL-1β-induced PGE2 biosynthesis by astroglial cells.
Figure 4.
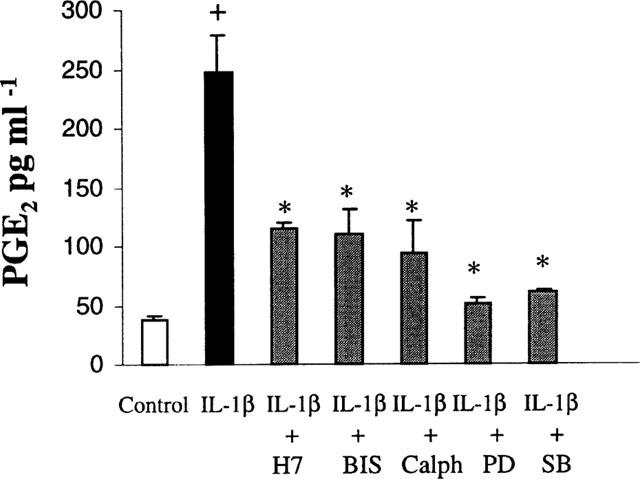
The protein kinase C inhibitors (10 μM H-7, 2 μM BIS and 1 μM Caphostin C) as well as MAPKinase inhibitors (15 μM PD 98059, 15 μM SB 203580) blocked IL-1β-induced PGE2 production. Inhibitors were added 60 min before IL-1β (10 ng ml−1) stimulation and supernatants were collected 24 h after for PGE2 determination by enzyme-immunoassay. Values are the means±s.e.mean (vertical bars) of four independent experiments in triplicate. Statistical significance: *P<0.0001 vs IL-1β alone; +P<0.0001 vs control.
To further confirm a role of PKC on PGE2 biosynthesis, astrocyte cultures were depleted of PKC by overnight treatment with PMA (100 nM), then IL-1β (10 ng ml−1) or PMA (10 nM) were added for 4 h and the amount of PGE2 in the culture supernatants measured. As shown in Figure 5, PKC-depleted cells no longer responded to PMA or IL-1β, suggesting the requirement for a conventional PKC isoform. A potential candidate is cPKC-α, which is present in astrocytes at high level. Therefore, astrocyte cultures were treated with IL-1β for different periods of time and cell extracts were analysed for cPKC-β activation. Immunoblot analysis (Figure 6) showed that IL-1β (10 ng ml−1) caused translocation of cPKC-α from cytosol to the membrane fraction. This effect occurred rapidly, after 5 min, was maximal after 30 min, and declined after 6 h treatment. As expected, TPA treatment also caused the translocation of PKC-α from cytosol to the membrane fraction (data not shown).
Figure 5.

Depletion of PKC by overnight PMA pre-treatment prevents PGE2 secretion by IL-1β or PMA. Astrocytes were treated with 100 nM PMA overnight and then were stimulated with IL-1β (10 ng ml−1) or PMA (10 nM) for 4 h to collect supernatants for PGE2 determination by enzyme-immunoassay. Results are means ±s.e.mean of four independent experiments. *P<0.001 vs control.
Figure 6.

Subcellular distribution of cPKC-α in response to activation with IL-1β. Cultured astrocytes were stimulated with 10 ng ml−1 IL-1β, for 0, 5, 30 min, 1 h, or 6 h, and fractionated into soluble cytosolic or membrane fractions as described in Methods. Then, cytosolic and membrane fractions were immunoblotted with antibody against PKC-α. The experiments were repeated at least four times with similar results. Bands were visualized by the ECL method. Densitometric values were plotted, and values represent the means±s.e.mean of four experiments. •P<0.01; ••P<0.001 vs basal.
MAPKs activation in astrocyte cultures by IL-1β was assessed by Western blot analysis, using specific antibodies for the phosphorylated forms of p38 and ERK1/2. Figure 7 shows the time course of IL-1β activation of p38 MAPK. IL-1β (10 ng ml−1) elicited a rapid (detectable at 5 min) Thr/Tyr phosphorylation of p38 MAPK that increased with time, reaching a maximum at 30 min–1 h, followed by a gradual decline reaching basal levels by 5 h. Next, we examined whether the p42/44 MAPK pathway was also involved in IL-1β signalling in astrocytes. Figure 8 shows the time course activation of p42 and p44 MAPKs in response to IL-1β (10 ng ml−1). IL-1β induced a rapid (5 min) and prolonged p42/44 MAPKs activation (over 5 h in the case of p44 MAPK), peaking at 30 min. Besides, IL-1β-induced phosphorylation of p42/44 MAPK was significantly inhibited by prior treatment (30 min) with the PKC inhibitor, bisindolylmaleimide (Figure 9), suggesting that IL-1β induced p42/44 MAPK activation may be the downstream signal of PKC activation. Collectively, these results suggest that both, activation of PKC and then, the phosphorylation of p42/44 and p38 MAPKs are required for the production of PGE2 by IL-1β in murine astrocytes.
Figure 7.

Time-dependent activation of p38 MAPK by IL-1β in astrocytes. Astrocyte cultures were treated with 10 ng ml−1 IL-1β for various time periods. Whole-cell lysates were prepared and subjected to immunoblot analysis using antibodies specific for the activated (phosphorylated) forms of p38 MAPK as described in Methods. Parallel blots were assayed using the antibodies recognizing the total p38 MAPK protein. The experiments were repeated at least four times with similar results. Bands were visualized by the ECL Method. Densitometric values were plotted, and values represent the means ±s.e.mean of four experiments. •P<0.01; ••P<0.001 vs basal.
Figure 8.

Time-dependent activation of p42/44 MAPKs by IL-1β in astrocytes. Astrocyte cultures were treated with 10 ng ml−1 IL-1β for various time periods. Whole-cell lysates were prepared and subjected to immunoblot analysis using antibodies specific for the activated (phosphorylated) forms of p42/44 MAPKs as described in Methods. Parallel blots were assayed using the antibodies recognizing the total p42/44 MAPK proteins. The experiments were repeated at least four times with similar results. Bands were visualized by the ECL method. Densitometric values were plotted, and values represent the means ±s.e.mean of four experiments. •P<0.05; ••P<0.001 vs corresponding basal. *P<0.001 vs corresponding basal.
Figure 9.

The inhibition of PKC activity by bisindolylmaleimide (2 μM) reduced the phosphorylation of p42/44 MAPK induced by IL-1β (10 ng ml−1). Cells were treated with bisindolylmaleimide (Bis) 30 min prior to stimulation with IL-1β. Whole cell lysates were prepared and subjected to immunoblot analysis using a specific antibody for the phosphorylated form of p42/44 MAPK. Representative results of three independent experiments with similar results. Bands were visualized by the ECL method. Densitometric values were plotted, and values represent the means±s.e.mean of three experiments. p42 MAPK: •P<0.05 vs corresponding basal; ○○P<0.001 vs corresponding IL-1β. p44 MAPK: *P<0.001 vs corresponding basal; ⋆P<0.001 vs corresponding IL-1β.
Discussion
The pro-inflammatory cytokine IL-1β enhanced PGE2 production in mouse astrocyte cultures, in agreement with previous studies (Hartung et al., 1989; Katsuura et al., 1989; Gerbicke-Haerter, et al., 1991). Our study demonstrated that IL-1β-mediated PGE2 release is a consequence of COX-2 induction, which is expressed by inflammatory agents and down-regulated by glucocorticoid hormones (O'Banion et al., 1992; Herschman, 1996). Thus, IL-1β treatment induced COX-2 mRNA expression as measured by RT–PCR, and COX-2 protein as shown by Western blot analysis; while the stimulatory action of IL-1β in PGE2 release was abolished by the COX-2 selective inhibitor, NS-398, and by the glucocorticoid, dexamethasone. Furthermore, IL-1β-induced PGE2 release was found to depend on the de novo transcription and protein synthesis since both actinomycine D and cycloheximide blocked the response.
However, the signalling pathways implicated in IL-1β-induced COX-2 expression and, thus, PGE2 biosynthesis are not well understood. Cellular responses to IL-1β include a cascade of protein phosphorylation which transmits signals from cell surface to the nucleus and that ultimately regulates gene expression (O'Neill & Greene, 1998). The PKC family of serine/threonine kinases have been implicated in the signalling pathway of cell surface receptors, including those used by IL-1β in several cell types. Consistent with this, activation of PKC has been previously involved in IL-1-regulated astrocyte function (Aloisi et al., 1994; Norris et al., 1994; Ballestas & Benveniste, 1995). We observed a stimulatory effect of PMA on PGE2 production, suggesting a positive role of PKC, in agreement with other studies (Hartung et al., 1989). Further evidence for the involvement of PKC was obtained using H-7 and bisindolylmaleimide, since these protein kinases inhibitors significantly reduced PGE2 production in response to IL-1β. In addition, a more specific PKC inhibitor, calphostin C, at the dose of 1 μM almost completely blocked PGE2 responses to IL-1β. Since calphostin C interacts with the regulatory domain of PKC and competes with the DAG phorbol ester binding site located in the C1 domain (Hug & Sarre, 1993; Hofman, 1997), our results suggest the possible involvement of a conventional PKC isoform in the signal transduction leading to the increased PGE2 release by IL-1β. Consistent with this, down-regulation of PKC abolished the stimulatory effect of PMA, but also that of IL-1β. Moreover, Western blot analysis showed that IL-1β induced translocation of the conventional isoform, PKC-α, from cytosol to the membrane fraction indicating its activation. Nevertheless, because of lack of using selective inhibitors for PKC-α (Hofmann, 1997) in the present work, the possibility of involvement of other PKC isoenzymes in COX-2 expression after IL-1β treatment cannot be discounted.
Cellular prostaglandin production depends on cyclo-oxygenase activity as well as the availability of arachidonic acid substrate, which is controlled by enzymes such as phospholipase A2 (PLA2). Previous studies (Oka & Arita, 1991) have shown that enhanced PGE2 production by IL-1β is subsequent to PLA2 activation in rat astrocytes. Furthermore, activation of PKC stimulates the release of arachidonic acid due to increased PLA2 activity which also implicated MAPK activation (Nam et al., 1995). Thus, it is possible that enhanced PGE2 release in PMA-stimulated astrocytes also involved PLA2 activation.
Recent work has linked activation of the prostaglandin generative pathway with the MAPK pathway (Guan et al., 1998; Niiro et al., 1998). Moreover, one of the signalling mechanisms triggered by IL-1β includes the activation of MAPKs, a family of serine/threonine kinases activated by dual phosphorylation of Thr and Tyr within a Thr-X-Tyr motif (Kyriakis & Avruch, 1996; Guan et al., 1997). The kinase cascade involving MAPKs appears to have a fundamental role in integrating multiple intracellular signals activated by Tyr kinases, but also by PKC (Blumer & Johnson 1994). Therefore, we investigated whether IL-1β stimulation was able to activate the ERK1/2 and p38 subgroups of MAPKs and whether such activation could be related to the ability of IL-1β to activate PKC and induce the biosynthesis of PGE2. Our results indicate for the first time that IL-1β activates ERK1/2 and p38 MAPK in astroglial cells. The activation occurs rapidly at 5 min and with maximum at 30 min, indicating that the activation of MAPK cascade is an early event in astrocytes in response to IL-1β stimulation. An important question was therefore whether IL-1β induced activation of MAPKs was related to PGE2 production. To this end, treatment of astrocytes with the specific inhibitors of MEK and p38 MAPK (PD 98059 or SB 203580) dramatically decreased PGE2 release by IL-1β, suggesting key roles for the kinases in this event. Support for this notion was provided by Guan et al. (1997) showing that IL-1β-mediates PGE2 production and COX-2 expression with concomitant activation of p38 MAPK-mediated signalling pathways in human mesangial cells. Our study presents further evidence that the intracellular-signalling pathway activated by IL-1β involves p42/44 MAPK and requires PKC activation.
In conclusion, we propose that the phosphorylation of MAPKs might constitute the signalling pathway through which an activation of PKC by IL-1β leads to increased PGE2 biosynthesis in astroglial cells. This pathway in astrocytes provides further evidence for the role of the MAPKs cascade in the mediation of prostaglandin biosynthesis and as a potential target for modulation of the inflammatory response.
Acknowledgments
The present studies were supported by grants from the Ministry of Education and Culture, (DGICYT: PM 98/014), and EC BIOMED 2 Program (CT 97-2492). We thank Le Minh Qujnh Uyen for her excellent technical assistance and Dr G. Almazán for critical reading of the manuscript and helpful advice. We also thank C. Torres for her technical assistance in cell cultures.
Abbreviations
- CNS
central nervous system
- DMEM
Dulbecco's modified Eagle Medium
- DMSO
dimethyl sulphoxide
- DTT
dithiothreitol
- ERK
extracellular signal regulated kinase
- FCS
foetal calf serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFPA
glial fibrilar protein acidic
- H-7
1-(5-isoquinolinyl-sulphonyl)-2-methylpiperazine
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid
- IL-1
interleukin-1
- LPS
lipopolysacharide
- MAPK
mitogen activated protein kinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
- PAGE
polyacrylamide gel electrophoresis
- PGE2
prostaglandin E2
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- 4-α PMA
4-α phorbol 12-myristate 13 acetate
- PCR
polymerase chain reaction
- RT
reverse transcription
- SDS
sodium dodecyl sulphate
- Thr
threonine
- TPA
12-O-tetradecanoylphorbol 13 acetate
- Tyr
tyrosine
References
- ALESSI D.R. , CUENDA A., COHEN P., DUDLEY D. T., SALTIEL A.R. PD 98059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase, in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- ALOISI F., ROSA S, TESTA U., BONSI P., RUSSO G., PESCHL EC., LEVI G. Regulation of leukemia inhibitory factor synthesis in culture human astrocytes. J. Immunol. 1994;152:5022–5031. [PubMed] [Google Scholar]
- BADGER A.M., BRADBEER J.N., VOTTA B., LEE J.C., ADAMS J.L., GRISWOLD D.E. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J. Pharmacol. Exp. Ther. 1996;279:1453–1461. [PubMed] [Google Scholar]
- BALLESTAS M.E., BENVENISTE E.N. Interleukin-1β and tumor necrosis factor α mediated regulation of ICAM-1 gene expression in astrocytes requires protein kinase C activity. GLIA. 1995;14:267–278. doi: 10.1002/glia.440140404. [DOI] [PubMed] [Google Scholar]
- BAN E.M., SARLIEVE L.L., HAOUR F.G. Interleukin-1 binding sites on astrocytes. Neuroscience. 1993;52:725–733. doi: 10.1016/0306-4522(93)90421-b. [DOI] [PubMed] [Google Scholar]
- BENVENISTE E.N. Inflammatory cytokines within the central nervous system: sources, function and mechanism of action. Am. J. Physiol. 1992;263:C1–C16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- BERKENBOSCH F., VAN OERS J., DEL REY A., TILDERS F., BESEDOVSKY H. Corticotropin-releasing-factor producing neurons in the rat activated by interleukin-1. Science. 1987;238:519–521. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- BLUMER K.J., JOHNSON G.L. Diversity in function and regulation of MAP kinase pathways. Trends Biochem. Sci. 1994;19:236–241. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- BOLTON C., PARKER D., MCLEOD J., TURK J.L. A longitudinal study of the prostaglandin content of central nervous system from guinea pigs with acute experimental allergic encephalomyelitis. J. Neuroimmunol. 1986;6:151–156. [Google Scholar]
- BREDER C.D., SAPER C.B. Expression of inducible cyclooxygenase mRNA in the mouse brain after systemic administration of bacterial lipopolyssacharide. Brain Res. 1996;713:64–69. doi: 10.1016/0006-8993(95)01474-8. [DOI] [PubMed] [Google Scholar]
- CANO E., MAHADEVAN L.C. Parallel signal processing among mammalian MAPKs. Trends Biochem. Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- CAO C.Y., MATSUMURA K., YAMAGATA K., WATANABE Y. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 messenger RNA in response to systemic interleukin-1: a possible site of prostaglandin synthesis responsible for fever. Brain Res. 1996;733:263–272. doi: 10.1016/0006-8993(96)00575-6. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:1556–1559. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CONQUER J.A., KANDEL R.A., CRUZ T.F. Interleukin-1 and phorbol 12-myristate 13-acetate induce collagenase and PGE2 production through a protein kinase C independent mechanisms in chondrocytes. Biochem Biophys. Acta. 1992;1134:1–10. doi: 10.1016/0167-4889(92)90021-3. [DOI] [PubMed] [Google Scholar]
- CROFFORD L.J., WILDER R.L., RISTIMÄKI A.P., SANO H,. , REMMERS E.F. , EPPS H.R., HLA T. Cyclooxygenase 1 and 2 expression in rheumatoid synovial tissues: effects of interleukin-1β, phorbol ester, and corticosteroids. J. Clin. Invest. 1994;93:1095–1099. doi: 10.1172/JCI117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINARELLO C.A. Biologic basis for interleukin-1 disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- FAN L., YOUNG P.R., BARONE F., FEUERSTEIN G.Z., SMITH D. H., MCINTOSH K. Experimental brain injury induces expression of interleukin-1β mRNA in the rat brain. Mol. Brain Res. 1995;30:125–130. doi: 10.1016/0169-328x(94)00287-o. [DOI] [PubMed] [Google Scholar]
- FRETLAND D.J. Potential role of prostaglandins and leukotrienes in multiple sclerosis and experimental allergic encephalomyelitis. Prostaglandin s Leukot. Essent. Fatty Acids. 1992;45:149–257. doi: 10.1016/0952-3278(92)90080-3. [DOI] [PubMed] [Google Scholar]
- FUTAKI N., TAKAHASHI S., YOKOYAMA M., ARAI I., IHIGUCHI S., IZUKA H., OTOMO S. Selective inhibition of NS-398 on prostanoid production in inflammed tissue in rat carrageenan-air-pouch inflammation. J. Pharm. Pharmacol. 1993;45:753–755. doi: 10.1111/j.2042-7158.1993.tb07103.x. [DOI] [PubMed] [Google Scholar]
- GERBICKE-HAERTER P.J., SCHOBERT A., HERRTING G. Pertussis and cholera toxins inhibit prostaglandin synthesis in rat astrocyte cultures at distinct metabolic steps. Biochem. Pharmacol. 1991;42:1267–1271. doi: 10.1016/0006-2952(91)90264-6. [DOI] [PubMed] [Google Scholar]
- GIULIAN D., BAKER T.J., SHICH L.H., LACHMAN L.B. Interleukin-1 of the central nervous system is produced by ameboid microglia. J. Exp. Med. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN D.E., WESSELINGH S.L., MCARTHUR J.C. Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann. Neurol. 1994;35:592–597. doi: 10.1002/ana.410350513. [DOI] [PubMed] [Google Scholar]
- GUAN Z., BAIER L.D., MORRISON A.R. p38 Mitogen-activated protein kinase down-regulates nitric oxide and up-regulates prostaglandin E2 biosynthesis stimulated by interleukin-1β. J. Biol. Chem. 1997;272:3296–3301. doi: 10.1074/jbc.272.12.8083. [DOI] [PubMed] [Google Scholar]
- GUAN Z., BUCKMAN S.Y., PENTLAND A.P., TEMPLETON D.J., MORRISON A.R. Induction of cyclooxygenase-2 by the activated MEKK1, SEK1/MKK4 p38 mitogen activated protein kinase pathway. J. Biol. Chem. 1998;273:12901–12908. doi: 10.1074/jbc.273.21.12901. [DOI] [PubMed] [Google Scholar]
- HAMBLETON J., WEINSTEIN S:L., LEM L., DEFRANCO A.L. Activation of C-Jun N-terminal kinase in bacterial lipopolyssacharide-stimulated macrophages. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSEN M.K., KRUEGER J.M. Subdiaphragmatic vagotomy blocks the sleep- and fever-promoting effects of interleukin-1β. Am. J. Physiol. 1997;273:1246–1253. doi: 10.1152/ajpregu.1997.273.4.R1246. [DOI] [PubMed] [Google Scholar]
- HARTUNG H.P., SCHÄFFER B., HEININGER K., TOYKA K.V. Recombinant interleukin-1β stimulates eicosanoid production in rat primary culture astrocytes. Brain Res. 1989;489:113–119. doi: 10.1016/0006-8993(89)90013-9. [DOI] [PubMed] [Google Scholar]
- HERSCHMAN H.R. Prostaglandin synthase 2. Biochim. Biophis. Acta. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- HOFMANN J. The potential for isoenzyme-selective modulation of protein kinase C. FASEB J. 1997;11:649–669. doi: 10.1096/fasebj.11.8.9240967. [DOI] [PubMed] [Google Scholar]
- HOPKINS S.J., ROTHWELL N.J. Cytokines in the nervous system I. Expression and recognition. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- HUG H., SARRE T.F. Protein kinase C isoenzymes: Divergence in signal transduction. Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATSUURA G., GOTTSCHALL P.E., DAHL R. R., ARIMURA A. Interleukin-1β increases PGE2 in rat astrocyte cultures: modulatory effects of neuropetides. Endocrinology. 1989;124:3125–3127. doi: 10.1210/endo-124-6-3125. [DOI] [PubMed] [Google Scholar]
- KYRIAKIS J.M., AVRUCH J. Sounding the alarm: protein kinases cascades activated by stress and inflammation. J. Biol. Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- LACROIX S., RIVEST S. Effect of acute systemic inflammatory response and cytokines on the transcription of genes encoding cyclooxygenase enzymes COX-1 and COX-2 in the rat brain. J. Neurochem. 1998;70:452–466. doi: 10.1046/j.1471-4159.1998.70020452.x. [DOI] [PubMed] [Google Scholar]
- LAYE S., PARNET P., GOUJON E., DANTZER R. Peripheral administration of lipopolyssacharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Mol. Brain. Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- LIN L. L., WARTMANN M., LIN A.Y., KNOPF J.L., SETH A., DAVIS R. J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- LLEDÖ A., BORRELL J., GUAZA C. Dexamethasone regulation of interleukin-1 receptors in the hippocampus of Theiler's virus infected mice: effects on virus-mediated demyelination. Eur. J. Pharmacol. 1999;372:75–83. doi: 10.1016/s0014-2999(99)00187-9. [DOI] [PubMed] [Google Scholar]
- MARQUETTE C., VAN DAM A.M., CECCALDI P.E., WEBER P., HAOUR F., TSIANG T. Induction of immunoreactive interleukin-1β and tumor necrosis factor-α in the brains of rabies-virus-infected rats. J. Neuroimmunol. 1996;68:45–51. doi: 10.1016/0165-5728(96)00056-2. [DOI] [PubMed] [Google Scholar]
- MCCARTHY K.D., DE VELLIS J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRILL J., BENVENISTE E.N. Cytokines in inflammatory brain lesions. Trends Neurosci. 1996;19:331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- MOLINA-HOLGADO F., LLEDÓ A., GUAZA C. Evidence for cyclooxygenase activation by nitric oxide in astrocytes. GLIA. 1995;15:167–172. doi: 10.1002/glia.440150209. [DOI] [PubMed] [Google Scholar]
- NAM M.J., THORE C., BUSIJA D. Rapid induction of prostaglandin synthesis in piglet astroglial cells by interleukin-1-α. Brain Res. Bull. 1995;3:215–218. doi: 10.1016/0361-9230(94)00187-6. [DOI] [PubMed] [Google Scholar]
- NIIRO H., OTSUKA T., OGAMI E., YAMAOKA K., NAGANO S., AKAHOSHI M., NAKASHIMA H., ARINOBU Y., IZUHARA K., NIHO Y. MAP kinase pathways as a route for regulatory mechanisms of IL-10 and IL-4 which inhibit COX-2 expression in human monocytes. Biochem. Biophys. Res. Commun. 1998;250:200–205. doi: 10.1006/bbrc.1998.9287. [DOI] [PubMed] [Google Scholar]
- NISHIZUKA Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- NISHIZUKA Y. Intracellular signalling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- NOGAWA S., ZHANG F., ROSS M.E., IADECOLA C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J. Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORRIS J.G., TANG L.P., SPARACIO S.M., BENVENISTE E.N. Signal transduction pathways mediating IL-6 induction by IL-1β and tumor necrosis factor α. J. Immunol. 1994;152:841–850. [PubMed] [Google Scholar]
- O'BANION M.K., MILLER J.C., CHANG J.W., KAPLAN M.D., COLEMAN P.D. Interleukin-1β induces prostaglandin G/H synthase-2 (cyclooxygenase-2) in primary murine astrocyte cultures. J. Neurochem. 1996;66:2532–2540. doi: 10.1046/j.1471-4159.1996.66062532.x. [DOI] [PubMed] [Google Scholar]
- O'BANION M.K., WINN V.D., YOUNG D.A. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKA S., ARITA H. Inflammatory factors stimulate expression of group II phospholipase A2 in rat cultured astrocytes. J .Biol. Chem. 1991;266:9956–9960. [PubMed] [Google Scholar]
- O'NEILL L.A.J., GREENE C. Signal transduction pathways activated by the IL-1 receptor family: ancient signalling machinary in mammals, insect and plants. J. Leukoc. Biol. 1998;63:650–657. [PubMed] [Google Scholar]
- PASINETTI G.M., AISEN P.S. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer's disease brain. Neuroscience. 1998;87:319–324. doi: 10.1016/s0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- POULIOT M., BAILLARGEON J., LEE J.C., CLELAND L.G., JAMES M.J. Inhibition of prostaglandin endoperoxide synthase-2 expression in stimulated human monocytes by inhibitors of p38 mitogen-activated protein kinase. J. Immunol. 1997;158:4930–4937. [PubMed] [Google Scholar]
- ROTHWELL N.J. Functions and mechanisms of action of interleukin-1 in the brain. Trends Pharmacol. Sci. 1991;12:430–436. doi: 10.1016/0165-6147(91)90623-z. [DOI] [PubMed] [Google Scholar]
- SANGHERA J.S., WEINSTEIN S.L., ALUWALIA M., GIRN J., PELECHJ S.L. Activation of multiple proline-directed kinases by bacterial lipopolyssacharide in murine macrophages. J Immunol. 1996;156:4457–4465. [PubMed] [Google Scholar]
- VANE J.R., BAKHLE Y.S., BOTTING R.M. Cyclooxygenases 1 and 2. Annu. Rev Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- WILMER W.A., TAN L.C., DICKERSON J.A., DANNE M., ROVIN B.D. Interleukin-1β induction of mitogen-activated protein kinases in human mesangial cells. J. Biol Chem. 1997;272:10877–10881. doi: 10.1074/jbc.272.16.10877. [DOI] [PubMed] [Google Scholar]


