Abstract
The objective of this study was to examine the effect of dexamethasone on tumour necrosis factor-α (TNF-α)-induced expression and function of macrophage inflammatory protein-2 (MIP-2) and neutrophil recruitment. For this purpose, we used air pouches raised on the dorsal skin of C57/B16 mice.
Initially, we examined the dose-response (0.01–0.5 μg ml−1) and kinetics (0–24 h) of TNF-α-induced leukocyte accumulation. The cellular response was maximal at 0.1 μg ml−1 of TNF-α and 4 h after challenge and comprised more than 90% neutrophils.
Intraperitoneal (i.p.) pretreatment with 10 mg kg−1 of dexamethasone for 2 h, but not 1 mg kg−1, reduced TNF-α-induced recruitment of neutrophils by 87%. Administration of dexamethasone had no effect on the expression of CD18 on neutrophils.
TNF-α (0.1 μg ml−1) markedly increased the levels of MIP-2 in the air pouches 1 h after challenge and after 4 h the MIP-2 values returned to baseline. Notably, 2 h pretreatment with dexamethasone (10 mg kg−1, i.p.) reduced MIP-2 expression by 65% in response to TNF-α (0.1 μg ml−1). On the other hand, dexamethasone treatment did not change the levels of interleukin-10 (IL-10) in the pouch exudate.
Administration of recombinant MIP-2 increased neutrophil accumulation at 0.5 and 1.0 μg ml−1 after 4 h of challenge. Dexamethasone pretreatment for 2 h (10 mg kg−1, i.p.) abolished the MIP-2-induced recruitment of neutrophils.
Taken together, our data demonstrate that dexamethasone may downregulate TNF-α-induced neutrophil recruitment by inhibiting both the expression and function of MIP-2 in vivo.
Keywords: Adhesion, CD18, dexamethasone, IL-10, leukocyte, MIP-2, TNF-α
Introduction
Leukocyte recruitment to the extravascular space is a key feature in inflammatory reactions. The extravasation process of leukocytes is a multistep process, in which a rolling adhesive interaction is a precondition for the subsequent adhesion and transendothelial migration (Carlos & Harlan, 1994). TNF-α is considered to play a central role in several conditions, including inflammatory bowel disease and rheumatic arthritis (Deventer, 1997; Ksontini et al., 1998). Activation and firm adhesion of leukocytes to microvascular endothelium is predominately mediated by β2-integrins (CD11/CD18), which is a group of adhesion molecules expressed on the surface of leukocytes. These molecules consist of a common β-subunit designated CD18 and the functional significance of CD18 is illustrated by the leukocyte adhesion deficiency-1 (LAD-1) syndrome in which a mutation in the gene encoding for the common CD18 subunit results in the absence of β2-integrins (Anderson et al., 1985; Harlan, 1993). This autosomal recessive disorder is characterized by an inability to recruit neutrophils and an increased susceptibility to recurrent bacterial infections (Harlan 1993; Bowen et al., 1982). Migration and activation of leukocytes in TNF-α-stimulated tissues is mainly mediated indirectly through the induction and secretion of chemokines (Smart & Casale, 1994: Tessier et al., 1997; McColl & Clark-Lewis, 1999; Liu et al., 2000). The chemokine family includes small peptides that are known to be key regulators of leukocyte adhesion and tissue accumulation (Rollins, 1997; Bacon & Oppenheim, 1998; Zlotnik et al., 1999). The members of this family are subdivided into two main groups (C-C and C-X-C) based on structural properties. The C-C chemokines which comprise macrophage inflammatory protein-1α and β (Sherry et al., 1988) and JE (monocyte chemoattractant protein-1) (Cochran et al., 1983; Rollins et al., 1988) are mainly chemotactic for mononuclear leukocytes. In the mouse, the C-X-C chemokine family includes macrophage inflammatory protein-2 (MIP-2) (Tekamp-Olson et al., 1990) and KC (Rollins et al., 1988; Oquendo et al., 1989). MIP-2 and KC are considered to be murine homologues of human GRO chemokines (Tekamp-Olson et al., 1990; Oquendo et al., 1989). The C-X-C chemokines are considered to predominately attract neutrophils (Rollins. 1997; Bacon & Oppenheim, 1998; Zlotnik et al., 1999). MIP-2 has been implicated as an important mediator of several important conditions, such as endotoxaemia-induced lung injury (Schmal et al., 1996), glomerulonephritis (Feng et al., 1995) and bacterial meningitis (Diab et al., 1999). Thus, it is important to study the role and expression of MIP-2 in acute models of inflammation.
Glucocorticoids constitute a common therapy in inflammatory conditions. These potent anti-inflammatory agents, including dexamethasone, have been shown to effectively inhibit leukocyte recruitment (Schleimer, 1993; Goulding et al., 1998). In general, the main target of dexamethasone in the leukocyte extravasation process is firm adhesion and transmigration whereas leukocyte rolling is not affected (Schneider et al., 1997; Tailor et al., 1997; Manusco et al., 1995). In spite of the fact that glucocorticoids are known to attenuate leukocyte recruitment, the literature on the detailed mechanisms by which glucocorticoids inhibit tissue accumulation of leukocytes remain complex and partly contradictory. For example, some reports have suggested that dexamethasone decrease the surface density of CD18 (Burton et al., 1995; Filep et al., 1997; Davenpeck et al., 1998) whereas others have reported that CD18 expression is insensitive to glucocorticoid treatment (Roth et al., 1994; Trowald-Wigh et al., 1998). Considering the fact that neutrophils are the main target of C-X-C chemokines, it is interesting to note that KC is apparently negatively regulated by dexamethasone (Deng et al., 1994; Tailor et al., 1999). In contrast, some authors have suggested that MIP-2 expression is insensitive to glucocorticoid treatment in the lung (O'Leary et al., 1997) while others can not confirm such findings (Haddad et al., 1995; Yi et al., 1996). However, it is not known if glucocorticoids mainly inhibit the expression of chemokines in cytokine-activated tissues or whether the function of these molecules may also be impaired by these anti-inflammatory agents. Moreover, some studies have reported that dexamethasone may upregulate counter-regulatory cytokines with anti-inflammatory properties, such as IL-10 (Tabardel et al., 1996; Dandona et al., 1999), which could help explain the mechanism behind the reduced leukocyte infiltration into glucocorticoid-treated tissues.
The objective of this study was to examine the effect of dexamethasone on the expression of MIP-2, IL-10 and CD18 in TNF-α-induced leukocyte recruitment in vivo. Additionally, we wanted to determine the impact of dexamethasone on leukocyte influx provoked by MIP-2. For this purpose, we used air pouches raised on the dorsal skin of mice treated with TNF-α.
Methods
Animals
Male C57/B16 mice were maintained on 12-h light/dark cycles and given food and water ad libitum. Mice weighing ∼25–30 g were anaesthetized by intraperitoneal administration of 7.5 mg ketamine hydrochloride and 2.5 mg xylazine per 100 g body weight. Blood samples were taken from the tail artery after the experiments for analysis of systemic leukocyte and differential counts. The local ethics committee approved all the experiments of this study.
Air pouches
Two-and-a-half ml sterile air was inflated on day 0 and 3 in the dorsal skin. On day 6, TNF-α or MIP-2 solved in 1 ml PBS was administered into the pouch at different doses and time-points before the exudate was harvested by injecting and aspirating ice-cold PBS (1-2-2 ml) containing 3 mM EDTA. Samples were centrifuged and the number and differentials of leukocytes were determined using a haematocytometer.
Flow cytometry
Blood was collected by cardiac puncture in EDTA tubes. Then 50 μl of blood samples were stained using a FITC-labelled rat (Lewis) anti-mouse CD18 antibody (C71/16) as well as a FITC-labelled isotype-matched control antibody (RB40.34) from Pharmingen (San Diego, CA, U.S.A.). After antibody incubation for 30 min at 4°C, the red blood cells were lyzed (1 ml of NH4Cl-EDTA for 5 min) and washed (1500 r.p.m.) for 5 min. The cell pellet was resuspended with 0.5 ml PBS and put on ice until analysis, which was performed within 45 min. Neutrophils were gated based on forward and side scatter characteristics.
ELISA
Exudate fluid harvested form the air pouches were centrifugated and the levels of immunoreactive murine MIP-2 and IL-10 protein in the supernatant were determined using double-antibody specific Quantikine ELISA kit using recombinant murine MIP-2 and IL-10 as standards (R&D Systems Europe, Ltd., Abingdon, Oxon, U.K.). The minimum detectable concentrations of MIP-2 and IL-10 are in these assays less than 1.5 pg ml−1.
Materials
FITC-labelled anti-CD18 antibody (C71/16) and anti-P-selectin antibody (RB40.34) were from Pharmingen (San Diego, CA, U.S.A.). Dexamethasone (Decadron) was from Merck Sharp & Dohme, B.V., Haarlem, Netherlands. Recombinant MIP-2 and TNF-α and ELISA for MIP-2 and IL-10 were from R&D Systems Europe, Ltd., Abingdon, Oxon, U.K. Ketamine was from Hoffman-La Roche, Basel, Switzerland. Xylazine/rompum was from Janssen Pharmaceutica, Beerse, Belgium.
Statistics
Statistical evaluations were performed using Kruskal-Wallis one-way analysis of variance for unpaired samples. The results are presented as mean values±s.e.mean. Unless stated otherwise, n represents number of animals.
Results
TNF-α-induced neutrophil recruitment
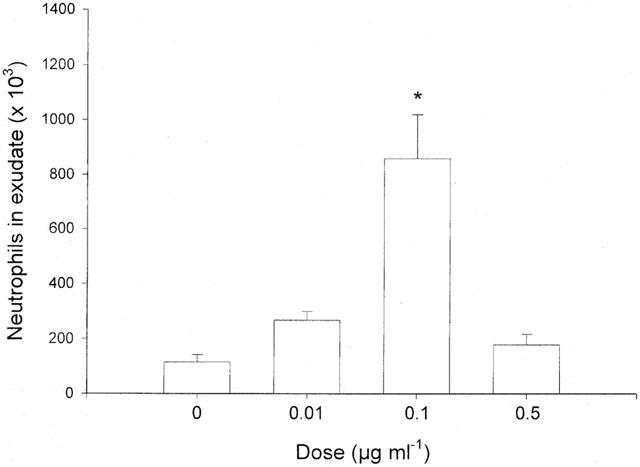
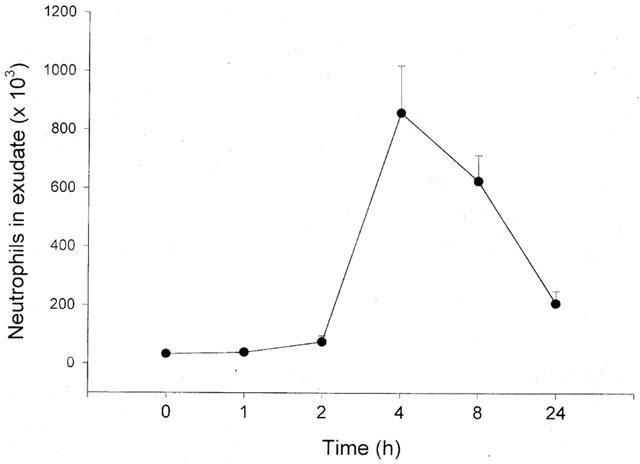
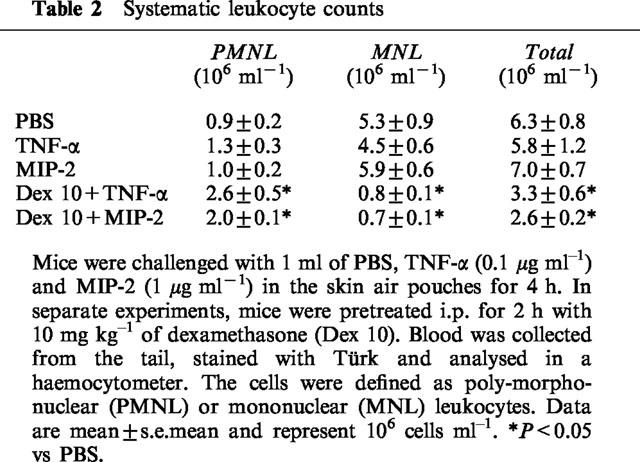
Administration of TNF-α increased leukocyte accumulation in the air pouches in a dose- and time-dependent manner. Differential analysis revealed the leukocyte infiltrate comprised 92% neutrophils while mononuclear leukocytes were rarely found (Table 1). Maximal neutrophil recruitment was observed at 0.1 μg ml−1 of TNF-α whereas the cellular response at a higher dose (0.5 μg ml−1) was not different from control treatment (Figure 1). Kinetic experiments on 0.1 μg ml−1 of TNF-α disclosed that neutrophil infiltration was highest, i.e. 859×103 cells pouch−1, at 4 h of challenge (Figure 2). The cellular response returned back to baseline values after 24 h of TNF-α treatment (Figure 2). In separate experiments, we found that dexamethasone dose-dependently reduced TNF-α-induced neutrophil accumulation. In fact, i.p. administration of 10 mg kg−1 of dexamethasone 2 h prior to TNF-α challenge (0.1 μg ml−1, 4 h, n=8) reduced the number of pouch neutrophils by 87% (Figure 3, P<0.05 vs TNF-α alone, n=8–12) whereas 1 mg kg−1 of dexamethasone had no significant effect on the neutrophil response to TNF-α (Figure 3, P>0.05 vs TNF-α alone, n=8–12). The effects of dexamethasone treatment on systemic leukocyte counts are shown in Table 2.
Table 1.
Total and relative numbers of leukocyte subtypes
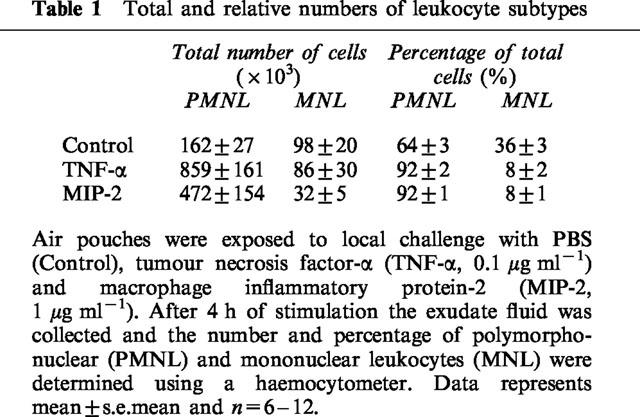
Figure 1.
Neutrophil accumulation in the air pouch exudate in response to different doses of TNF-α and PBS (0). One ml of tumour necrosis factor-α and PBS was injected into the pouches and after 4 h the exudate fluid was collected and the number of neutrophils was determined using a haemocytometer. Data are mean±s.e.mean and n=6–11. * P<0.05 vs PBS.
Figure 2.
Time-dependent recruitment of neutrophils into the air pouch in response to injection of 0.1 μg ml−1 of TNF-α. One ml of tumour necrosis factor-α was injected into the pouches and after different time-points the exudate fluid was collected and the number of neutrophils was determined using a haemocytometer. Data are mean±s.e.mean and n=4–12.
Figure 3.
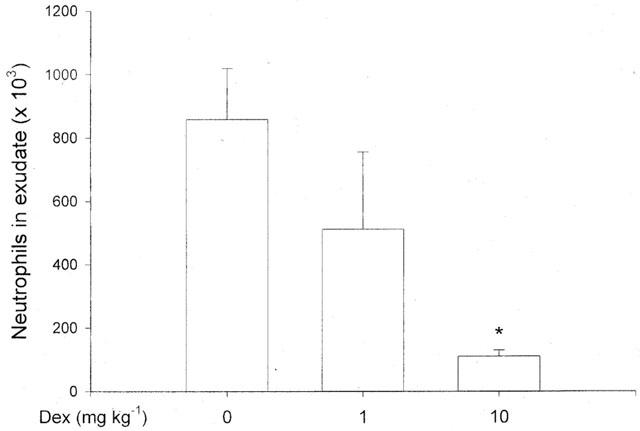
Effect of i.p. pretreatment of dexamethasone (Dex) for 2 h on tumour necrosis factor-α-induced neutrophil accumulation in the air pouch. One ml of TNF-α (0.1 μg ml−1) was injected into the pouches and after 4 h the exudate fluid was collected and the number of neutrophils was determined using a haemocytometer. Data are mean±s.e.mean and n=6–8. *P<0.05 vs O.
Table 2.
Systematic leukocyte counts
MIP-2 and IL-10 expression
The levels of MIP-2 and IL-10 in the pouch exudate fluid were determined by specific ELISA. We observed that administration of 0.1 μg ml−1 of TNF-α markedly increased production of MIP-2. At 1 h of TNF-α challenge the level of MIP-2 was 1711±323 ng pouch−1 exudate and after 4 h the expression returned to 95±26 ng pouch−1. Notably, i.p. pretreatment with 10 mg kg−1 of dexamethasone decreased the expression of MIP-2 by 65% at 1 h of TNF-α stimulation (Figure 4, P<0.05 vs TNF-α alone, n=6–9). After 4 h of TNF-α activation the levels of MIP-2 were not different between the groups (Figure 4, P>0.05 vs TNF-α alone, n=5–6). In addition, it was found that the expression of IL-10 was not significantly different in any of the groups (Table 3).
Figure 4.
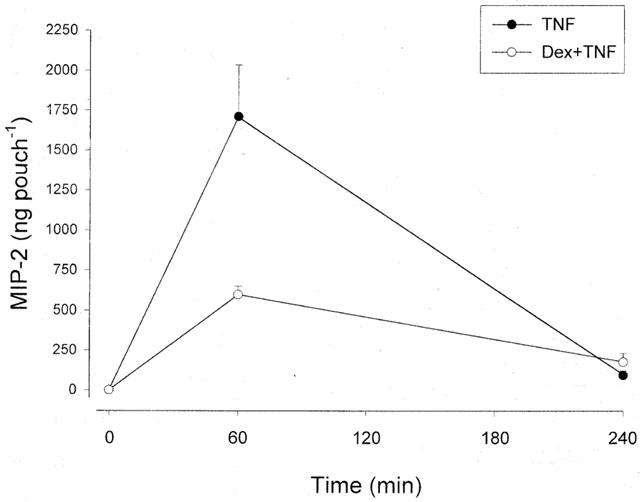
Production of MIP-2 in air pouch exudates in response TNF-α (0.1 μg ml−1). One group of mice were pretreated with i.p. dexamethasone (10 mg kg−1) 2 h before administration of TNF-α. One ml of TNF-α was injected into the pouches and after different time-points the exudate fluid was collected and the levels of MIP-2 protein were determined using a specific ELISA. Data are mean±s.e.mean and n=6–9.
Table 3.
IL-10 expression in pouch exudate
CD18 expression
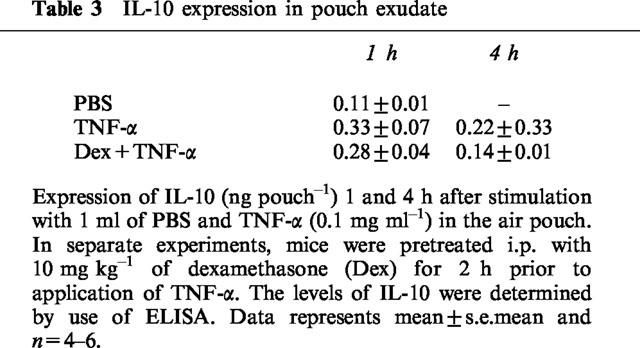
Firm adhesion of neutrophils to vascular endothelium is mediated by CD18 expressed on the neutrophil surface. We found that the surface density of CD18 on circulating neutrophils was uniformly high in PBS and TNF-α treated mice. Moreover, i.p. treatment with 10 mg kg−1 of dexamethasone 2 h prior to local TNF-α administration had no effect on the surface expression of CD18 on neutrophils (Table 4).
Table 4.
CD18 expression
MIP-2-induced neutrophil recruitment
As shown in Figure 5, local injection of MIP-2 into the air pouches caused a dose-dependent and transient increase in leukocyte accumulation (Figures 5 and 6). At 1 μg ml−1 of MIP-2, it was observed that the leukocyte response was greatest after 4 h of stimulation (Figure 5). Moreover, neutrophils were dominating in the cellular exudate, constituting more than 90% of the infiltrate (Table 1). Interestingly, we found that i.p. pretreatment for 2 h with 10 mg kg−1 of dexamethasone decreased MIP-2 (1 μg ml−1)-induced neutrophil recruitment by 62% (Figure 5, P<0.05 vs 1 μg ml−1 of MIP-2 alone, n=6–11).
Figure 5.
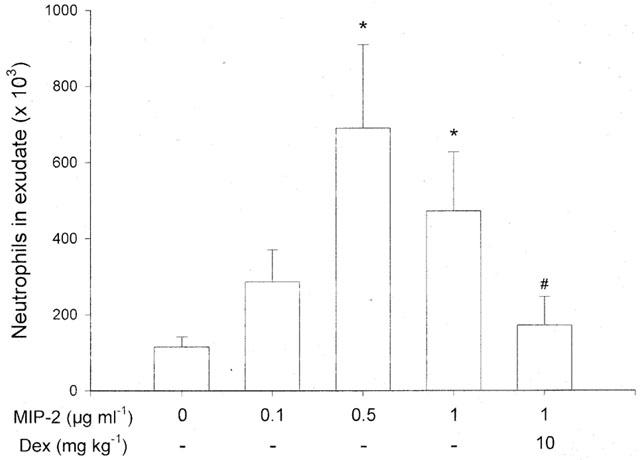
Neutrophil accumulation in the air pouch exudate in response to different doses of MIP-2 and PBS (0). One ml of MIP-2 and PBS was injected into the pouches and after 4 h the exudate fluid was collected and the number of neutrophils was determined using a haemocytometer. In separate experiments, mice were pretreated with i.p. dexamethasone (Dex) for 2 h before administration of MIP-2. Data are mean±s.e.mean and n=5–11. *P<0.05 vs PBS and #P<0.05 vs 1 μg ml−1 of MIP-2.
Figure 6.
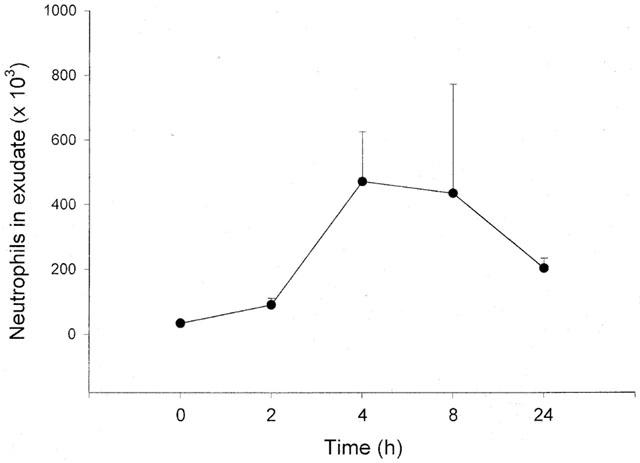
Time-dependent recruitment of neutrophils into the air pouch in response to injection of 1 μg ml−1 of MIP-2. One ml of MIP-2 was injected into the pouches and after different time-points the exudate fluid was collected and the number of neutrophils was determined using a haemocytometer. Data are mean±s.e.mean and n=4–11.
Discussion
TNF-α plays an important role in tissue injury by inducing activation and recruitment of leukocytes in inflammatory diseases. Dexamethasone is a potent anti-inflammatory agent known to attenuate cytokine-induced infiltration of inflammatory cells (Schleimer, 1993; Goulding et al., 1998). This study demonstrates that dexamethasone inhibits TNF-α-increased expression of MIP-2 in the air pouch exudate. On the other hand, the levels of IL-10 in the exudate and the surface density of CD18 on circulating neutrophils were insensitive to dexamethasone treatment. Moreover, dexamethasone administration inhibited MIP-2-induced accumulation of neutrophils in the extravascular space. Taken together, these findings expand on previous studies by showing that dexamethasone inhibits both the expression and function of MIP-2 in vivo, which, in turn, may help to further explain the powerful anti-inflammatory actions of glucocorticoids on leukocyte recruitment.
Several members of the chemokine superfamily, including KC, JE and MCP-1, have been demonstrated to be negatively regulated by glucocorticoids (Kawahara et al., 1991; Deng et al., 1994; Yi et al., 1996; Tailor et al., 1999). Although, previous studies have suggested an important role of MIP-2 in cytokine-induced neutrophil accumulation (Tessier et al., 1997; McColl & Clark-Lewis, 1999), the literature on the sensitivity of MIP-2 to dexamethasone treatment in vivo is complex and partly contradictory. For example, some authors have reported that LPS-induced expression of MIP-2 in the lung is insensitive to dexamethasone (O'Leary & Zuckerman, 1997; Rovai et al., 1998) whereas others cannot confirm such findings using ozone challenge in a similar model (Haddad et al., 1995), suggesting the existence of stimulus-specific differences in glucocorticoid sensitivity on MIP-2 expression in vivo. In the present study, we found that dexamethasone markedly reduced the expression of MIP-2 in the skin exudate in response to TNF-α, indicating that dermal expression of MIP-2 is negatively regulated by dexamethasone. Thus, our data suggest that the sensitivity of MIP-2 expression to glucocorticoids may not only be stimulus-dependent as discussed above, but also tissue-specific. This notion is in line with a recent study showing that the glucocorticoid-attenuation of LIX expression in septicaemia also appears to be tissue-dependent (Rovai et al., 1998). Interestingly, the basis for such a tissue-specific inhibition of MIP-2 expression exerted by dexamethasone is not presently known but it may, at least partly, be attributable to the fact that the levels of α and β isoforms of the glucocorticoid receptor is cell-specific (Bamberger et al., 1995; 1996).
The source of MIP-2 protein in TNF-α-induced inflammation is not clearly known. Herein, we found a transient expression of MIP-2 in the exudate supernatant, which was maximal at 1 h and returned to baseline 4 h after TNF-α injection into the air pouch. Notably, at 1 h following TNF-α exposure no increase in inflammatory cells was observed whereas at 4 h the neutrophil response reached its peak value. This inverse relationship between the presence of inflammatory cells and MIP-2 levels in the exudate fluid, suggest that recruited neutrophils do not likely contribute to a significant part of the MIP-2 expression in acute dermal inflammation provoked by TNF-α. Instead, using the same model as herein, a previous study has shown that (tissue) lining cells in the murine air pouch increase mRNA expression of MIP-2 within 30–60 min in response to TNF-α challenge and in vitro studies have implicated macrophages, fibroblasts, epithelial (Driscoll et al., 1993) and endothelial cells (Liu et al., 2000) as cellular sources of TNF-α-induced expression of MIP-2.
Chemokines activates and attracts leukocytes by directly ligating specific cell receptors, a process which is independent on de novo transcription of genes and synthesis of proteins. For example, the C-X-C chemokines bind to the C-X-C receptor 2 (IL-8 receptor) on the surface of neutrophils which results in increased intracellular Ca2+, elastase release, CD18 expression and migration (Huber et al., 1991; Cacalano et al., 1994; Jones et al., 1997). In fact, it has been documented that C-X-C receptor 2-deficient neutrophils do not respond to MIP-2 stimulation although the response to the complement fragment C5a is intact (Lee et al., 1995). In the present study, we demonstrated that local injection of MIP-2 triggered a dose- and time-dependent increase in neutrophil influx in the skin. Interestingly, administration of dexamethasone abolished this MIP-2-induced tissue recruitment of neutrophils, indicating that dexamethasone directly suppresses the function of MIP-2 in vivo. At present, the mechanism behind this inhibition of MIP-2 function exerted by dexamethasone is not known. Yet, it can be speculated that dexamethasone may reduce the C-X-C receptor 2 expression on neutrophils which would help to explain the abolished response to MIP-2 as observed in this study. Nonetheless, our novel data indicate that dexamethasone inhibits leukocyte recruitment provoked by TNF-α in vivo at two different levels. First, dexamethasone attenuates TNF-α-induced expression of MIP-2 and, second, the chemotactic effect on leukocytes of MIP-2 per se is inhibited by dexamethasone.
Inflammatory cell accumulation is not only regulated by proinflammatory cytokines, such as TNF-α and IL-1β, but is also under inhibitory influence exerted by counter-regulatory cytokines, such as IL-10. For example, it has been shown that IL-10 may attenuate the expression of TNF-α and chemokines, and reduce the tissue influx of leukocytes (Fiorentino et al., 1991; Kasama et al., 1994; Hickey et al., 1998). Although the anti-inflammatory mechanisms of action of IL-10 remains to be clearly described, it is interesting to note that some studies have reported increased levels of IL-10 following dexamethasone exposure (Tarabel et al., 1996; Dandona et al., 1999). Thus, it was reasonable to hypothesize that the dexamethasone-attenuated leukocyte response to TNF-α might be related to changes in IL-10 expression. However, in this study we found that dexamethasone did not alter the levels of IL-10 in the pouch exudate, indicating that the inhibitory impact of dexamethasone on neutrophil recruitment is not mediated by increased expression of IL-10.
Tissue infiltration of leukocytes is regulated by a coordinated expression of specific adhesion molecules. Firm adhesion of neutrophils to the vascular endothelium, which is a precondition for accumulation in the extravascular space, is dependent on the expression of CD18 and the functional significance of CD18 is illustrated in patients with LAD-1 syndrome characterized by an inability to recruit neutrophils and an increased susceptibility to recurrent bacterial infections (Bowen et al., 1982; Anderson et al., 1985; Harlan, 1993). Herein, we could demonstrate that the CD18 expression on the surface of neutrophils was insensitive to dexamethasone administration. Thus, our results suggest that the inhibitory effect of dexamethasone on tissue recruitment of neutrophils is not attributable to a down-regulation of CD18 on the surface of neutrophils. This notion is in line with previous investigations showing that CD18 expression is not sensitive to dexamethasone treatment (Roth et al., 1994; Trowald-Wigh et al., 1998) and, thus, not a likely anti-inflammatory mechanism of glucocorticoids. In this respect, it is important to note that these findings do not exclude other potential effects of glucocorticoids on leukocytes, which may be of relevance to the extravasation process of leukocytes.
Taken together, these data suggest that TNF-α-induced accumulation of neutrophils is attenuated at two separate steps by dexamethasone. First, dexamethasone inhibits the expression of MIP-2 provoked by TNF-α and, second, the chemotactic effect exerted by MIP-2 on leukocytes is dexamethasone-attenuated. However, we found that dexamethasone had no effect on the expression of IL-10 in the tissue and CD18 on neutrophils. Thus, our novel findings may improve the understanding of the anti-inflammatory mechanisms of action of dexamethasone with respect to inflammatory cell recruitment.
Acknowledgments
We thank Kerstin Norman for excellent technical assistance. This study was supported by Swedish Medical Research Council (K2000-04P-13411-01A and K98-27I-11610-03), Cancerfonden (4265-B99-01XAB), Allmäna sjukhusets i Malmö stiftelse för bekämpande av cancer, the Österlund Foundation, the Tore Nilsson Foundation, the Greta and Johan Kock Foundation. Malmö University Hospital and Lund University.
Abbreviations
- Dex
dexamethasone
- ELISA
enzyme-linked immunosorbent assay
- IL-10
interleukin-10
- i.p.
intraperitoneal
- LAD-1
leukocyte adhesion deficiency-1
- MIP-2
macrophage inflammatory protein-2
- TNF-α
tumour necrosis factor-α
References
- ANDERSON D.C., SCHMALSTEIG F.C., FINEGOLD M.J., HUGHES B.J., ROTHLEIN R., MILLER L.J., KOHL S., TOSI M.F., JACOBS R.L., WALDROP T.C., GOLDMAN A.S., SHEARER W.T., SPRINGER T.A. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J. Inf. Dis. 1985;152:668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- BACON K.B., OPPENHEIM J.J. Chemokines in disease models and pathogenesis. Cytokine Growth Factor Rev. 1998;9:167–173. doi: 10.1016/s1359-6101(98)00005-7. [DOI] [PubMed] [Google Scholar]
- BAMBERGER C.M., BAMBERGER A.M., DE CASTRO M., CHROUSOS G.P. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J. Clin. Invest. 1995;95:2435–2441. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAMBERGER C.M., SCHULTE H.M., CHROUSOS G.P. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocrin Rev. 1996;17:245–261. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- BOWEN T.J., OCHS H.D., ALTMAN L.C., PRICE T.H., VAN EPPS D.E., BRAUTIGAN D.L., ROSIN R.E., PERKINS W.D., BABIOR B.M., KLENBANOFF S.J., WEDGWOOD R.J. Severe recurrent bacterial infections associated with defective adherence and chemotaxis in two patients with neutrophils deficient in a cell-associated glycoprotein. J. Pediatrics. 1982;101:932–940. doi: 10.1016/s0022-3476(82)80013-9. [DOI] [PubMed] [Google Scholar]
- BURTON J.L., KEHRLI M.E., KAPIL S., HORST R.L. Regulation of L-selectin and CD18 on bovine neutrophils by glucocorticoids: effects of cortisol and dexamethasone. J. Leukoc. Biol. 1995;57:317–325. doi: 10.1002/jlb.57.2.317. [DOI] [PubMed] [Google Scholar]
- CACALANO G., LEE J., KIKLY K., RYAN A.M., PITTS-MEEK S., HULTGREN B., WOOD W.I., MOORE M.W. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- CARLOS T.M., HARLAN J.M. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- COCHRAN B.H., REFFEL A.C., STILES C.D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983;33:939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- DANDONA P., MOHANTY P., HAMOUDA W., ALJADA A., KUMBKARNI Y., GARG R. Effect of dexamethasone on reactive oxygen speices generation by leukocytes and plama interleukin-10 concentrations: a pharmacodynamic study. Clin. Pharmacol Therapeut. 1999;66:58–65. doi: 10.1016/S0009-9236(99)70054-8. [DOI] [PubMed] [Google Scholar]
- DAVENPECK K.L., ZAGORSKI J., SCHLEIMER R.P., BOCHER B.S. Lipopolysaccharide-induced leukocyte rolling and adhesion in the rat mesenteric microcirculation: regulation by glucocorticoids and role of cytokines. J. Immunol. 1998;161:6861–6870. [PubMed] [Google Scholar]
- DENG Z.W., DENKINGER D.J., PETERSON K.E., DEUEL T.F., KAWAHARA R. Glucocorticoids negatively regulate the transcription of KC, the mouse homolog of MGSA/GRO. Biochem. Biophys. Res. Commun. 1994;203:1809–1814. doi: 10.1006/bbrc.1994.2397. [DOI] [PubMed] [Google Scholar]
- DEVENTER S.J.H. Tumor necrosis factor and Crohn's disease. Gut. 1997;40:443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAB A., ABDALLA H., LI H.L., SHI F.D., ZHU J., HOJBERG B., LINDQUIST L., WRETLIND B., BAKHIET M., LINK H. Neutralization of macrophage inflammatory protein-2 (MIP-2) and MIP-1 alpha attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect. Immun. 1999;67:2590–2601. doi: 10.1128/iai.67.5.2590-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRISCOLL K.E., HASSENBEIN D.G., CARTER J., POYNTER J., ASQUITH T.N., GRANT R.A., WHITTEN J., PURDON M.P., TAKIGIKU R. Macrophage inflammatory proteins 1 and 2: expression by rat alveolar macrophages, fibroblasts, and epithelial cells and in rat lung after mineral dust exposure. Am. J. Resp. Cell Mol. Biol. 1993;8:311–318. doi: 10.1165/ajrcmb/8.3.311. [DOI] [PubMed] [Google Scholar]
- FENG L., XIA Y., YOSHIMURA T., WILSON C.B. Modulation of neutrophil influx in glomerulonephritis in the rat with anti-macrophage inflammatory protein-2 (MIP-2) antibody. J. Clin. Invest. 1995;95:1009–1017. doi: 10.1172/JCI117745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILEP J.G., DELALANDRE A., PAYETTE Y., FOLDES-FILEP E. Glucocorticoid receptor regulates expression of L-selectin and CD11/CD18 on human neutrophils. Circulation. 1997;96:295–301. doi: 10.1161/01.cir.96.1.295. [DOI] [PubMed] [Google Scholar]
- FIORENTINO D.F., ZLOTNIK A., MOSMANN T.R., HOWARD M., O'GARRA A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- GOULDING N.J., EUZGER H.S., BUTT S.K., PERRETTI M. Novel pathways for glucocorticoid effects on neutrophils in chronic inflammation. Inflamm. Res. 1998;47:S158–S165. doi: 10.1007/s000110050310. [DOI] [PubMed] [Google Scholar]
- HADDAD E.B., SALMON M., SUN J., LIU S., DAS A., ADCOCK I., BARNES P.J., CHUNG K.F. Dexamethasone inhibits ozone-induced gene expression of macrophage inflammatory protein-2 in rat lung. FEBS Lett. 1995;363:285–288. doi: 10.1016/0014-5793(95)00333-5. [DOI] [PubMed] [Google Scholar]
- HARLAN J.M. Leukocyte adhesion deficiency syndrome: insights into the molecular basis of leukocyte emigration. Clin. Immunol. Immunopathol. 1993;67:S16–S24. doi: 10.1006/clin.1993.1079. [DOI] [PubMed] [Google Scholar]
- HICKEY M.J., ISSEKUTZ A.C., REINHARDT P.H., FEDORAK R.N., KUBES P. Endogenous interleukin-10 regulates hemodynamic parameters, leukocyte-endothelial cell interactions, and microvascular permeability during endotoxemia. Circ. Res. 1998;83:1124–1131. doi: 10.1161/01.res.83.11.1124. [DOI] [PubMed] [Google Scholar]
- HUBER A.R., KUNKEL S.L., TODD R.F., WEISS S.J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- JONES S.A., DEWALD B., CLARK-LEWIS I., BAGGIOLINI M. Chemokine antagonists that discriminate between interleukin-8 receptors. J. Biol. Chem. 1997;272:16166–16169. doi: 10.1074/jbc.272.26.16166. [DOI] [PubMed] [Google Scholar]
- KASAMA T, , STREITER R.M., LUKACS N.W., BURDICK M.D., KUNKEL S.L. Regulation of neutrophil-derived chemokine expression by IL-10. J. Immunol. 1994;152:3559–3569. [PubMed] [Google Scholar]
- KAWAHARA R.S., DENG Z.W., DEUEL T.F. Glucocorticoids inhibit the transcriptional induction of JE, a platelet-derived growth factor-inducible gene. J. Biol. Chem. 1991;266:13261–13266. [PubMed] [Google Scholar]
- KSONTINI R., MACKAY S.L.D., MOLDAWER L.L. Revisiting the role of tumor necrosis factor α and the response to surgical injury and inflammation. Arch. Surg. 1998;133:558–567. doi: 10.1001/archsurg.133.5.558. [DOI] [PubMed] [Google Scholar]
- LEE J., CACALANO G., CAMERATO T., TOY K., MOORE M.W., WOOD W.I. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- LIU Q., WANG Y., THORLACIUS H. Dexamethasone inhibits tumor necrosis factor-alpha-induced expression of macrophage inflammatory protein-2 and adhesion of neutrophils to endothelial cells. Biochem. Biophys. Res. Commun. 2000;271:364–367. doi: 10.1006/bbrc.2000.2641. [DOI] [PubMed] [Google Scholar]
- MANUSCO F., FLOWER R.J., PERETTI M. Leukocyte transmigration, but not rolling or adhesion, is selectively inhibited by dexamethasone in the hamster post-capillary venule: involvement of endogenous lipocortin 1. J. Immunol. 1995;155:4377–4386. [PubMed] [Google Scholar]
- MCCOLL S.R., CLARK-LEWIS I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J. Immunol. 1999;163:2829–2835. [PubMed] [Google Scholar]
- O'LEARY E.C., ZUCKERMAN S.H. Glucocorticoid-mediated inhibition of neutrophil emigration in an endotoxin-induced rat pulmonary inflammation model occurs without an effect on airways MIP-2 levels. Am. J. Resp. Cell Mol. Biol. 1997;16:267–274. doi: 10.1165/ajrcmb.16.3.9070611. [DOI] [PubMed] [Google Scholar]
- OQUENDO P., ALBERTA J., WEN D.Z., GRAYCAR J.L., DERYNCK R., STILES C.D. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet alpha-granule proteins. J. Biol. Chem. 1989;264:4133–4137. [PubMed] [Google Scholar]
- ROLLINS B.J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- ROLLINS B.J., MORRISON E.D., STILES C.D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc. Natl. Acad. Sci. U.S.A. 1988;85:3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH J., GOEBELER M., ERPENSTEIN U., SORG C. Differential regulation of the macrophage-specific surface antigen RM3/1 by cyclosporine, azathioprine, and dexamethasone. Transplantation. 1994;57:127–133. doi: 10.1097/00007890-199401000-00020. [DOI] [PubMed] [Google Scholar]
- ROVAI L.E., HERSCHMAN H.R., SMITH J.B. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J. Leukocyt. Biol. 1998;64:494–502. doi: 10.1002/jlb.64.4.494. [DOI] [PubMed] [Google Scholar]
- SCHLEIMER R.P. An overview of glucocorticoid anti-inflammatory actions. Eur. J. Clin. Pharmacol. 1993;45:S3–S7. doi: 10.1007/BF01844196. [DOI] [PubMed] [Google Scholar]
- SCHMAL H., SHANLEY T.P., JONES M.L., FRIEDL H.P., WARD P.A. Role of macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J. Immunol. 1996;156:1963–1972. [PubMed] [Google Scholar]
- SCHNEIDER J., BRUCKMANN W., ZWINGENBERGER K. Extravasation of leukocytes assessed by intravital microscopy: Effect of thalidomide. Inflamm. Res. 1997;46:392–397. doi: 10.1007/s000110050209. [DOI] [PubMed] [Google Scholar]
- SHERRY B., TEKAMP-OLSON P., GALLEGOS C., BAUER D., DAVATELIS G., WOLPE S.D., MASIARZ F., COIT D., CERAMI A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 beta. J. Exp. Med. 1988;168:2251–2259. doi: 10.1084/jem.168.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART S.J., CASALE T.B. TNF-α-induced transendothelial neutrophil migration is IL-8 dependent. Am. J. Physiol. 1994;266:L238–L245. doi: 10.1152/ajplung.1994.266.3.L238. [DOI] [PubMed] [Google Scholar]
- TABARDEL Y., DUCHATEAU J., SCHMARTZ D., MARECAUX G., SHAHLA M., BARVAIS L., LECLERC J.L., VINCENT J.L. Corticosteroids increase blood interleukin-10 levels during cardiopulmonary bypass in men. Surgery. 1996;119:76–80. doi: 10.1016/s0039-6060(96)80217-0. [DOI] [PubMed] [Google Scholar]
- TAILOR A., FLOWER R.J., PERETTI M. Dexamethasone inhibits leukocyte emigration in rat mesenteric post-capillary venules: an intravital microscopy study. J. Leukoc. Biol. 1997;62:301–308. doi: 10.1002/jlb.62.3.301. [DOI] [PubMed] [Google Scholar]
- TAILOR A., TOMLINSON A., SALAS A., PANES J., GRANGER D.N., FLOWER R.J., PERRETTI M. Dexamethasone inhibition of leucocyte adhesion to rat mesenteric postcapillary venules: role of intercellular adhesion molecule 1 and KC. Gut. 1999;45:705–712. doi: 10.1136/gut.45.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEKAMP-OLSON P., GALLEGOS C., BAUER D., MCCLAIN J., SHERRY B., FABRE M., VAN DEVENTER S., CERAMI A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J. Exp. Med. 1990;172:911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESSIER P.A., NACCACHE P.H., CLARK-LEWIS I., GLADUE R.P., NEOTE K.S., MCCOLL S.R. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J. Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- TROWALD-WIGH G., HAKANSSON L., JOHANNISSON A., EDQVIST L.E. The effect of prednisolone on canine neutrophil function: in vivo and in vitro studies. Acta Vet. Scand. 1998;39:201–213. doi: 10.1186/BF03547793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YI E.S., REMICK D.G., LIM Y., TANG W., NADZIENKO C.E., BEDOYA A., YIN S., ULICH T. The intratracheal administration of endotoxin: X. Dexamethasone downregulates neutrophil emigration and cytokine expression in vivo. Inflammation. 1996;20:165–175. doi: 10.1007/BF01487403. [DOI] [PubMed] [Google Scholar]
- ZLOTNIK A., MORALES J., HEDRICK J.A. Recent advances in chemokines and chemokine receptors. Crit. Rev. Immunol. 1999;19:1–47. [PubMed] [Google Scholar]


