Abstract
This study made use of a nitric oxide-sensitive electrode to examine possible means of generating nitric oxide from nitroxyl anion (NO−) released upon the decomposition of Angeli's salt.
Our results show that copper ions (from CuSO4) catalyze the rapid and efficient oxidation of nitroxyl to nitric oxide. Indeed, the concentrations of copper required to do so (0.1–100 μM) are roughly 100-times lower than those required to generate equivalent amounts of nitric oxide from S-nitroso-N-acetyl-D,L-penicillamine (SNAP).
Experiments with ascorbate (1 mM), which reduces Cu2+ ions to Cu+, and with the Cu2+ chelators, EDTA and cuprizone, and the Cu+ chelator, neocuproine, each at 1 mM, suggest that the oxidation is catalyzed by copper ions in both valency states.
Some compounds containing other transition metals, i.e. methaemoglobin, ferricytochrome c and Mn(III)TMPyP, were much less efficient than CuSO4 in catalyzing the formation of nitric oxide from nitroxyl, while FeSO4, FeCl3, MnCl2, and ZnSO4 were inactive.
Of the copper containing enzymes examined, Cu-Zn superoxide dismutase and ceruloplasmin were weak generators of nitric oxide from nitroxyl, even at concentrations (2500 and 30 u ml−1, respectively) vastly greater than are present endogenously. Two others, ascorbate oxidase (10 u ml−1) and tyrosinase (250 u ml−1) were inactive.
Our findings suggest that a copper-containing enzyme may be responsible for the rapid oxidation of nitroxyl to nitric oxide by cells, but the identity of such an enzyme remains elusive.
Keywords: Angeli's salt, ceruloplasmin, copper, cuprizone, nitric oxide, neocuproine, nitroxyl anion, superoxide dismutase
Introduction
NO exists as three distinct redox forms, nitroxyl anion (NO−), nitric oxide free radical (NO.) and the nitrosonium cation (NO+) (Stamler et al., 1992; Hughes, 1999). Nevertheless, it is generally accepted that it is the free radical form that is produced endogenously through a 5-electron oxidation of L-arginine catalyzed by nitric oxide synthase (NOS) (Bredt & Snyder, 1990). Recently, however, it has been suggested, on the basis of a revised estimate of the quantity of NADPH consumed, that NOS carries out only a 4-electron oxidation, producing nitroxyl anion, with the final step to nitric oxide radical being carried out by superoxide dismutase (SOD) (Schmidt et al., 1996). Others too have reported that NOS can generate nitroxyl (Fukuto et al., 1992a). Furthermore, other biological reactions have been shown to produce nitroxyl. These include the decomposition of S-nitrosothiols in the presence of thiols (Arnelle & Stamler, 1995; Wolzt et al., 1999), and the reduction of nitric oxide radical by ferrocytochrome c (Sharpe & Cooper, 1998). Indeed, it has been proposed that this latter reduction of nitric oxide represent an important means by which mitochondrial respiration is regulated (Borutaite & Brown, 1996; Sharpe & Cooper, 1998).
Nitroxyl anion is a highly unstable species, making it difficult to study, but it can be generated readily following the decomposition of a number of N,O-diacylated-N-hydroxyarylsulphonamides and Angeli's salt (disodium trioxodinitrate) (Fukuto et al., 1992b; 1993). Interestingly, although nitroxyl cannot activate soluble guanylate cyclase (Dierks & Burstyn, 1996), these nitroxyl generators are powerful relaxants of vascular and non-vascular smooth muscle (Fukuto et al., 1992b,1992c; Pino & Feelisch, 1994; Li et al., 1999). Moreover, the relaxation they produce is associated with a rise in cyclic GMP content (Fukuto et al., 1992c) and is blocked by the inhibitor of soluble guanylate cyclase, ODQ (Li et al., 1999). It is therefore likely that nitroxyl is rapidly oxidized to nitric oxide radical by cells. Indeed, a number of possible routes for conversion have been proposed, including oxidation by SOD (Murphy & Sies, 1991; Schmidt et al., 1996), flavin adenine dinucleotide or methaemoglobin (Fukuto et al., 1993), but others are likely to exist. Accordingly, the aim of this study was to investigate other potential routes of oxidation of nitroxyl anion to biologically active nitric oxide radical.
Methods
Measurement of nitric oxide generation
Nitric oxide generation was measured using ISO-NOP200 amperometric electrodes fitted to an ISO-NO Mark II nitric oxide meter (World Precision Instruments Ltd, U.K.). The signals generated were captured and displayed on a MacLab (8e Series, AD Instruments, U.K.). The proper functioning of each electrode was tested routinely by generating nitric oxide according to the manufacturer's suggested protocol. This was performed at room temperature (22°C) by inserting the electrode tip into a stirring solution of CuSO4 (0.1 M; 10 ml) prepared in distilled water taken to pH 4 with sulphuric acid, and allowing the background current to stabilize (usually 3–5 min). S-nitroso-N-acetyl-D,L-penicillamine (SNAP) was then added in increasing concentrations (10 nM–10 μM) to generate nitric oxide. The rapid decline in the nitric oxide signals generated following the addition of each concentration of SNAP precluded the use of a cumulative protocol. As a consequence, increasing concentrations were added on the basis of ignoring the presence of the lower concentrations added previously. The maximum change in current (ΔpA) produced by each new addition was recorded. The threshold concentration of SNAP found to generate a nitric oxide signal was 3–10 nM, and the response remained linear up to the highest concentration tested (10 μM; see Figure 3a).
Figure 3.
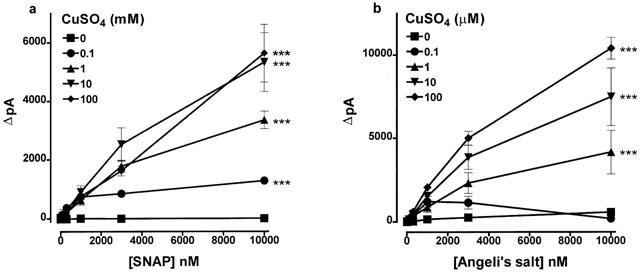
The generation of nitric oxide from (a) SNAP and (b) Angeli's salt stimulated by different concentrations of CuSO4. Control experiments in the absence of CuSO4 were conducted in the presence of 30 μM EDTA to chelate any contaminating copper ions. Each point is the mean and error bars indicate the s.e.mean of 5–8 experiments. ***P<0.001 indicates a significant difference from experiments conducted in the absence of CuSO4.
Experimental protocols
Experiments involving the generation of nitric oxide from Angeli's salt were conducted at 22°C (except where otherwise stated) with constant stirring in HEPES (N-[hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid])-buffered Krebs solution (pH 7.4) containing (mM): NaCl 118, KCl 4.8, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 2.4, D-glucose 11 and HEPES 5. Angeli's salt was added in increasing concentrations (10 nM–10 μM) and the generation of nitric oxide was assessed as a function of the maximum change in current (ΔpA) produced upon each new addition. As with the SNAP calibration curves described above, the transient nature of the signals generated dictated that each new addition was made on the basis of ignoring the presence of the lower concentrations added previously. When the effects of agents were to be examined on the generation of nitric oxide, these were added 3–5 min before the addition of Angeli's salt.
As will be seen from the Results, our initial experiments showed a highly variable generation of nitric oxide from Angeli's salt in HEPES-buffered Krebs solution, resulting from contamination of the ISO-NOP200 probes by copper ions following the routine standardization with the SNAP/CuSO4 reaction mixture. From that point on we ensured that probes were washed thoroughly with HEPES-buffered Krebs solution containing 30 μM EDTA in order to chelate this contaminating copper. Moreover, all subsequent experiments were conducted in the presence of 30 μM EDTA, with the exceptions of those in which the effects of a range of concentrations of CuSO4 were examined on the generation of nitric oxide from SNAP or Angeli's salt, and when the effects of the selective Cu+ chelator, neocuproine (1 mM), or the selective Cu2+ chelator, cuprizone (1 mM), were to be studied.
Drugs
Angeli's salt (sodium trioxodinitrate) was obtained from Alexis (Nottingham U.K.). Ascorbate (L-ascorbic acid), ascorbate oxidase (from Cucurbita), ceruloplasmin (bovine), cuprizone (bis-cyclohexanone oxaldihydrazone), ferricytochrome c (bovine), haemoglobin (bovine), neocuproine (2,9-dimethyl-1,10-phenanthroline) hydrochloride, S-nitroso-N-acetyl-D,L-penicillamine (SNAP), methaemoglobin (bovine), Mn (III) tetrakis [1-methyl-4-pyridyl] porphyrin (MnTMPyP), Cu-Zn superoxide dismutase (SOD; bovine erythrocytes), and tyrosinase (from mushroom) were obtained from Sigma (Poole, U.K.). All drugs were dissolved in 0.9 % saline except for Angeli's salt (0.01 M) which was dissolved in 0.01 M NaOH, cuprizone (0.01 M) which was dissolved in 50% ethanol, SNAP (0.01 M) which was dissolved at pH 9 in distilled water containing EDTA (0.54 mM) and haemoglobin (1 mM) which was dissolved in distilled water. Moreover, any contaminating methaemoglobin in this last solution was reduced to haemoglobin by treatment with the reducing agent, sodium dithionite (10 mM), and the dithionite was subsequently removed by dialysis against 100 volumes of distilled water for 2 h at 4°C.
Statistical analysis
Results are expressed as the mean±s.e.mean of n separate experiments. Comparisons were made by one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test, or by Student's unpaired t-test, as appropriate. A value of P<0.05 was considered significant.
Results
Variable generation of nitric oxide from Angeli's salt
Prior to experimentation, the responsiveness of each probe was checked in accordance with the manufacturer's instructions by generating nitric oxide by adding SNAP (10 nM–10 μM) to solutions of CuSO4 (100 mM) at pH 4 (see Figure 3a). When Angeli's salt (10 nM–10 μM) was added to HEPES (5 mM)-buffered Krebs (pH 7.4) immediately after preparing a SNAP calibration curve, a powerful nitric oxide signal was generated (17,000±2049 pA, n=5; Figure 1a). If, however, Angeli's salt (10 nM–10 μM) was added to HEPES-buffered Krebs following extensive washing of the probe, a much smaller nitric oxide signal was generated (2641±1125 pA, n=3, P<0.001; Figure 1b).
Figure 1.
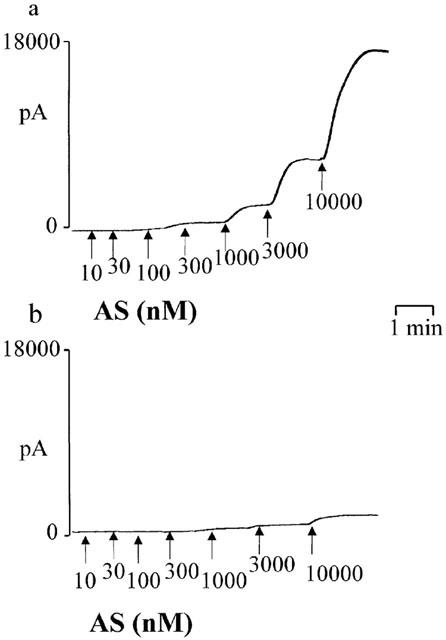
Individual experimental traces showing the variable generation of nitric oxide from Angeli's salt (AS), measured using a nitric oxide-sensitive electrode. (a) The signal detected by an electrode whose responsiveness had been assessed using the SNAP/CuSO4 reaction mixture (see Methods) immediately prior to the experiment with Angeli's salt. (b) The signal detected when the electrode had been subject to extensive washing.
Effects of copper on the generation of nitric oxide from Angeli's salt
The possibility that contamination of the probe by copper was responsible for the formation of nitric oxide from Angeli's salt was examined. When Angeli's salt (10 μM) was added to HEPES-buffered Krebs containing EDTA (30 μM) to chelate any contaminating copper, only a small nitric oxide signal was generated (571±65 pA, n=8; Figure 2). Subsequent addition of CuSO4 (100 μM) led, however, to a massive generation of nitric oxide (16,390±1118 pA, n=7; Figure 2). If the same experiment was conducted at 37°C rather than at room temperature, the signal generated was larger (22,760±1023 pA, n=7, P<0.005) but shorter in duration. Interestingly, CuSO4 (100 μM) failed to generate nitric oxide when added to Angeli's salt (10 μM) stabilized in 0.01 M NaOH (data not shown).
Figure 2.
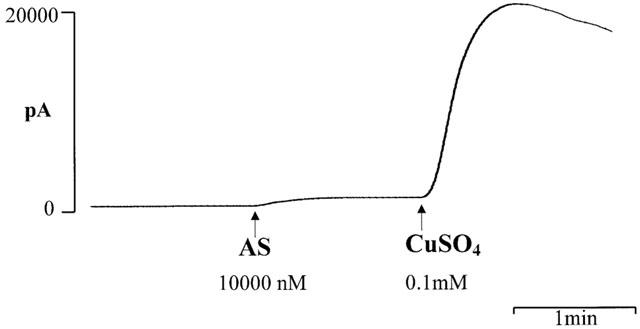
An individual experimental trace showing a barely detectable generation of nitric oxide when Angeli's salt (AS) was added to HEPES-buffered Krebs solution containing the copper chelator EDTA (30 μM). When CuSO4 (0.1 mM) was added subsequently, a massive generation of nitric oxide was seen.
The ability of CuSO4 to generate nitric oxide from SNAP and Angeli's salt was compared: 0.1–100 mM CuSO4 produced a concentration-dependent generation from SNAP (10 nM–10 μM), but lower concentrations (1–100 μM) were required to produce similar amounts from Angeli's salt (10 nM–10 μM; Figure 3). Figure 4 shows individual experimental traces demonstrating the poorer ability of CuSO4 (100 μM) to generate nitric oxide from SNAP than from Angeli's salt. The nitric oxide scavenger, haemoglobin (10 μM), inhibited by 85.6±3.2% (n=5, P<0.001) the nitric oxide signal generated from Angeli's salt (10 μM) by CuSO4 (10 μM). Haemoglobin was denatured by CuSO4 at 100 μM, and therefore its ability to quench the nitric oxide signal generated by this concentration of copper could not be determined.
Figure 4.
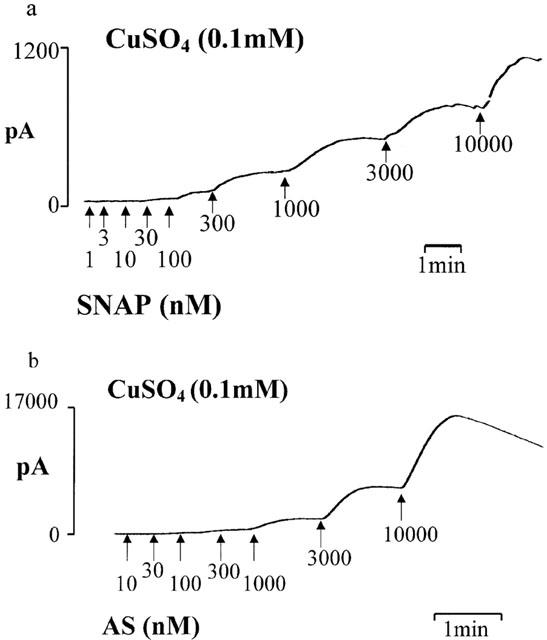
Individual experimental traces showing the poorer ability of CuSO4 (0.1 mM) to generate nitric oxide from (a) SNAP than from (b) Angeli's salt (AS).
Effects of ascorbate and of Cu+ and Cu2+ chelating agents
Adding ascorbate (1 mM) to CuSO4 (100 μM) to reduce the Cu2+ ions to Cu+ failed to affect its ability to generate nitric oxide from Angeli's salt (Figure 5a). The effects of reducing Cu2+ ions to Cu+ on the generation of nitric oxide from SNAP could not be assessed, however, because ascorbate itself liberated nitric oxide from this substance (Scorza et al., 1997).
Figure 5.
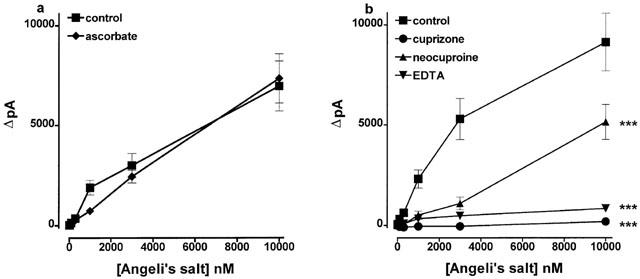
(a) The ability of CuSO4 (0.1 mM) to generate nitric oxide from Angeli's salt was unaffected following the reduction of Cu2+ ions to Cu+ by ascorbate (1 mM). (b) The effects of the Cu+ chelator, neocuproine, and the Cu2+ chelators, cuprizone and EDTA, each at 1 mM, on the ability of CuSO4 (0.1 mM) to stimulate the generation of nitric oxide from Angeli's salt. Each point is the mean and errors bars indicate the s.e.mean of 6–9 observations. ***P<0.001 indicates a significant difference from control.
The Cu2+ chelators, EDTA (1 mM) and cuprizone (1 mM), each almost abolished the ability of CuSO4 (100 μM) to generate nitric oxide from Angeli's salt (10 nM–10 μM; Figure 5b). Neocuproine (1 mM) was less effective but still produced substantial blockade.
Effects of copper containing enzymes
The ability of a number of copper containing enzymes to generate nitric oxide from Angeli's salt was investigated. Cu-Zn SOD at 250 u ml−1 (∼1.6 μM) failed to generate nitric oxide from Angeli's salt (10 nM–10 μM), but at 2500 u ml−1 (∼16 μM) it produced a weak but significant signal from Angeli's salt at concentrations of 10–300 nM; the magnitude of the generation did not increase when the concentration was elevated to 1 or 10 μM (Figure 6a,b). The results with ceruloplasmin were similar: at 3 u ml−1 (∼0.55 μM) no nitric oxide was generated from Angeli's salt, but at 30 u ml−1 (∼5.5 μM) a weak but significant signal was generated with Angeli's salt at concentrations of 10 nM–1 μM (Figure 6c). The magnitude of the generation did not increase at higher concentrations. Two other copper containing enzymes, ascorbate oxidase at 10 u ml−1 (∼36 nM) and tyrosinase at 250 u ml−1 (∼1 μM), produced almost no nitric oxide: maximum signals of 128±9 and 134±13 pA, respectively, n=6 for each.
Figure 6.
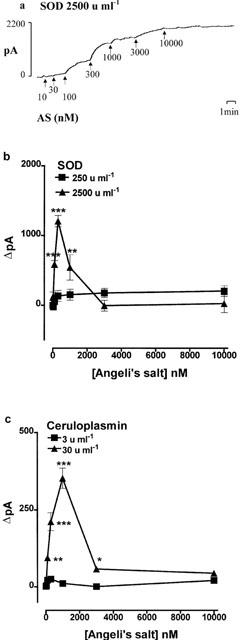
The generation of nitric oxide from Angeli's salt stimulated by superoxide dismutase (SOD) or ceruloplasmin. (a) An individual experimental trace showing the generation of nitric oxide by SOD (2500 u ml−1). (b) and (c) Combined data from a number of experiments showing the effects of SOD (250 and 2500 u ml−1) and ceruloplasmin (3 and 30 u ml−1), respectively. Each point is the mean and error bars indicate the s.e.mean of 6–10 experiments. *P<0.05, **P<0.01 and ***P<0.001 indicate a statistically significant generation of nitric oxide.
Effects of compounds containing iron, manganese and zinc
FeSO4, FeCl3 and MnCl2 each at 100 μM failed to generate nitric oxide from Angeli's salt (10 nM–10 μM), but methaemoglobin (10 μM), ferricytochrome c (10 μM) and the porphyrin compound MnTMPyP (100 μM) each generated a weak but significant signal (Figure 7). ZnSO4 (100 μM) failed to generate nitric oxide from Angeli's salt (data not shown).
Figure 7.
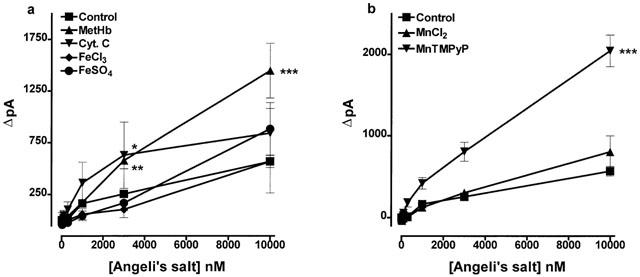
The generation of nitric oxide from Angeli's salt stimulated by (a) methaemoglobin (MetHb 10 μM), ferricytochrome c (Cyt. C 10 μM), ferric chloride (FeCl3 0.1 mM), or ferrous sulphate (FeSO4 0.1 mM) and (b) manganese chloride (MnCl2 0.1 mM) or MnTMPyP (0.1 mM). Each point is the mean and error bars indicate the s.e.mean of 5–9 observations. *P<0.05, **P<0.01 and ***P<0.001 indicate a statistically significant generation of nitric oxide.
Discussion
The most significant, yet unexpected finding from this study is that copper ions catalyze the one-electron oxidation of nitroxyl anion to nitric oxide radical. This was found quite by accident when we undertook a project to examine the means by which nitroxyl, generated from the spontaneous decomposition of Angeli's salt in HEPES-buffered Krebs solution at pH 7.4, could undergo conversion to nitric oxide radical. In some experiments we observed the expected result, i.e. that decomposition of Angeli's salt at pH 7.4 to produce nitroxyl did not lead to the spontaneous generation of nitric oxide radical, as assessed using a nitric oxide-sensitive electrode. Indeed, others have shown previously that nitroxyl decays to form nitrous oxide and nitrite (Fukuto et al., 1992b; Hughes, 1999). In other experiments, however, we detected a massive generation of nitric oxide from Angeli's salt. We observed that those occasions in which massive generation of nitric oxide took place occurred immediately following standardization of the electrode by nitric oxide generated following the decomposition of SNAP by 0.1 M CuSO4 (Dicks et al., 1996). Consequently, we suspected that residual copper contaminating the electrode might have catalyzed the oxidation of nitroxyl to nitric oxide. This indeed proved to be the case: when HEPES-buffered Krebs solution containing the copper chelator EDTA (30 μM) was used for further experiments, almost no nitric oxide signal was generated from Angeli's salt. However, when CuSO4 (100 μM) was subsequently added, an immediate massive generation of nitric oxide was seen. The signal generated was about 40% greater in magnitude but shorter in duration if the experiment was conducted at 37°C rather than at room temperature. Moreover, we could be certain that the signal recorded by the detector was indeed nitric oxide because it was quenched by haemoglobin (Martin et al., 1985). Interestingly, CuSO4 failed to generate nitric oxide when in 0.01 M NaOH in which Angeli's salt does not decompose to nitroxyl. We can therefore conclude that the action of copper is to oxidize nitroxyl to nitric oxide, with no effect of the metal ion on Angeli's salt itself.
We found only one passing reference to oxidation of nitroxyl to nitric oxide by copper in the literature (Fukuto et al., 1993), but this paper presented no experimental data. We therefore proceeded to characterize more fully the ability of copper to catalyze the oxidation of nitroxyl to nitric oxide. For comparison, parallel experiments were conducted with SNAP, since the ability of copper to release nitric oxide from S-nitrosothiols is well characterized (Dicks et al., 1996; Al-Sa'doni et al., 1997). We in fact found that CuSO4 was more that 100 times more potent in generating nitric oxide from nitroxyl than from SNAP. A likely explanation for this large difference is that the copper ions are in the wrong valency state to promote decomposition of SNAP: the Cu2+ ions in solution must first be reduced to Cu+ before the reaction can proceed, and this is achieved through a redox reaction with the parent thiol which almost always contaminates preparations of S-nitrosothiols (Al-Sa'doni et al., 1997). Interestingly, with CuSO4 at 0.1 μM, the nitric oxide signal generated by Angeli's salt at 10 μM was less than at 1 μM (Figure 3b). This might be explained by the ability of nitroxyl to react rapidly with nitric oxide (Hughes, 1999). Thus, when nitric oxide is being generated at low levels, there is the possibility that it will be scavenged by excess nitroxyl in solution.
Having established that Cu2+ ions promote the oxidation of nitroxyl to nitric oxide, the effects of Cu+ ions were examined by reducing solutions of CuSO4 with ascorbate. Our results suggest that Cu+ and Cu2+ ions are equally effective at catalysing the oxidation of nitroxyl to nitric oxide, perhaps according to reactions 1 and 2 below:
In support of this scheme, we found that copper metal was seen to precipitate from solution when a very high concentration of Angeli's salt (0.1 M) was added to an equal volume of CuSO4 (0.1 M) (data not shown). It could be argued, however, that the copper metal was produced by the disproportionation of Cu+ (i.e 2Cu+ → Cu+Cu2+), and that the generation of nitric oxide resulted solely from the actions of Cu2+. We think this unlikely, however, on the basis of the actions of chelators that discriminate between the two valency states of copper ions. Specifically, EDTA and cuprizone which selectively chelate Cu2+ (Peterson & Bollier, 1955) almost abolished the ability of CuSO4 to generate nitric oxide from nitroxyl, and neocuproine which selectively chelates Cu+ (Al-Sa'doni et al., 1997) produced powerful blockade. If reactions 1 and 2 above are correct, one would expect that reduction by ascorbate would half the oxidizing capacity of the copper ions in solution, thereby reducing the amount of nitric oxide formed. The relationship between copper concentration and oxidation of nitroxyl shown in Figure 3 indicates, however, that a 50% reduction in the copper concentration would have a negligible effect – around a 10 fold reduction would be required to attain a significant effect.
Compounds containing a number of other transition metals were examined for their ability to oxidise nitroxyl to nitric oxide. Fe(II)SO4 and Fe(III)Cl3 were inactive. We acknowledge that our experiments even with our freshly prepared solutions of Fe(III)Cl3 were not ideal because of visible formation of insoluble ferric oxides (rust). We overcame this particular problem and retained the ferric ion in solution by the use of citrate (15 mM) (Peterson & Bollier, 1955) but still no nitric oxide signal was generated (data not shown). In fact, even if the ferric ion did possess the ability to generate nitric oxide from Angeli's salt, its chelation by citrate would have abolished this effect since we found that citrate abolished the activity of CuSO4 (data not shown). In spite of these difficulties, we are confident that the ferric ion is not powerfully active, since metalloproteins containing this ion, i.e. methaemoglobin and ferricytochrome c produced weak, although statistically significant nitric oxide signals. The ability of methaemoglobin to generate nitric oxide from nitroxyl has previously been reported (Fukuto et al., 1993), and ferrocytochrome c is known to catalyze the reverse reaction efficiently, i.e. the reduction of nitric oxide to nitroxyl anion (Sharpe & Cooper, 1998). We also found Mn(II)Cl2 to be inactive but the metalloporphyrin superoxide dismutase mimetic, Mn(III)TMPyP (Faulkner et al., 1994; MacKenzie & Martin, 1998), was weakly effective. Zn(II)SO4, was, however, completely inactive. Thus, of the transition metal ions tested, copper was clearly the most active. Consequently, it is worth considering the possibility that endogenous copper is responsible for the oxidation of nitroxyl anion to nitric oxide which underlies the ability of Angeli's salt and other nitroxyl generators to relax smooth muscle (Fukuto et al., 1992b,1992c; Pino & Feelisch, 1994; Li et al., 1999). Moreover, such an oxidation may be physiologically important if nitric oxide synthase produces nitroxyl rather than nitric oxide per se (Schmidt et al., 1996). Indeed, the ability of the intracellular copper chelator, diethyldithiocarbamate, to inhibit the relaxation of rat aorta induced by the endothelium-dependent relaxant acetylcholine (MacKenzie & Martin, 1998) or by nitroxyl donors (Pino & Feelisch, 1994) supports this possibility. By analogy, experiments with the selective Cu+ chelator, neocuproine, have already provided evidence that endogenous copper contributes to the generation of nitric oxide from S-nitrosothiols in blood vessels and blood platelets (Al-Sa'doni et al., 1997; Gordge et al., 1996). Accordingly, on the basis that free copper is unlikely to exist in cells, we examined the ability of a number of copper containing enzymes to catalyze the oxidation of nitroxyl to nitric oxide. In keeping with previous reports (Schmidt et al., 1996; Murphy & Sies, 1991), we found that Cu-Zn SOD can indeed generate nitric oxide from nitroxyl, but the concentration required (2500 u ml−1) to produce even a weak signal is some 100-times greater than is present in cells (Halliwell & Gutteridge, 1989). Thus, Cu-Zn SOD is unlikely to be a physiologically relevant generator of nitric oxide from nitroxyl. Similar findings were obtained with ceruloplasmin: it too generated a weak nitric oxide signal from nitroxyl, but the concentration required to do so (30 u ml−1) is vastly greater than that found in the plasma (0.12 u ml−1) (Iskra & Majewski, 1999). Indeed, for Cu-Zn SOD (2500 u ml−1) and ceruloplasmin (30 u ml−1), the nitric oxide signals generated from Angeli's salt were somewhat smaller that those expected on the basis of their copper content (∼16.5 and 5.5 μM, respectively). Their enzymic nature therefore conferred no benefit in the generation of nitric oxide from Angeli's salt. Furthermore, two other copper containing enzymes, ascorbate oxidase (Gromov et al., 1999) and tyrosinase (Oetting et al., 1998), failed to generate nitric oxide from nitroxyl. Thus, although it remains possible that a copper-containing enzyme is responsible for the oxidation of nitroxyl to nitric oxide by cells, none of those tested so far appears to be a suitable candidate.
In conclusion, our findings show that copper ions catalyze the rapid and efficient oxidation of nitroxyl anion to nitric oxide free radical. It is therefore possible that a copper containing enzyme may be responsible for the endogenous conversion of nitroxyl to nitric oxide which underlies the smooth muscle relaxant properties of nitroxyl anion generators such as Angeli's salt (Fukuto et al., 1992b,1992c; Pino & Feelisch, 1994; Li et al., 1999), and which may even contribute to the formation of the nitric oxide from nitric oxide synthase (Schmidt et al., 1996). The identity of such a copper-containing enzyme remains elusive.
Acknowledgments
This work was supported by the British Heart Foundation and the Wellcome Trust. K. Buyukafsar held a grant under the NATO Science Fellowship Programme of the Scientific and Technical Research Council of Turkey (TUBITAK).
Abbreviations
- EDTA
ethylenediaminetetraacetic acid
- HEPES
N-[hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]
- MnTMPyP
Mn (III) tetrakis [1-methyl-4-pyridyl] porphyrin
- SNAP
S-nitroso-N-acetyl-D,L-penicillamine
- SOD
superoxide dismutase
References
- AL-SA'DONI H.H., MEGSON I.L., BISLAND S., BUTLER A.R., FLITNEY F.W. Neocuproine, a selective Cu (I) chelator, and the relaxation of rat vascular smooth muscle by S-nitrosothiols. Br. J. Pharmacol. 1997;121:1047–1050. doi: 10.1038/sj.bjp.0701218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARNELLE D.R., STAMLER J.S. NO+, NO, and NO- donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch. Biochem. Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- BORUTAITE V., BROWN G.C. Rapid reduction of nitric oxide by mitochondria, and reversible inhibition of mitochondrial respiration by nitric oxide. Biochem. J. 1996;315:295–299. doi: 10.1042/bj3150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREDT D.S., SNYDER S.H. Isolation of nitric oxide synthetase, a calcium-requiring enzyme. Proc. Natl. Acad. Sci. U.S.A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKS A.P., SWIFT H.R., WILLIAMS D.H.L., BUTLER A.R., AL-SA'DONI H.H., COX B.G. Identification of Cu+ as the effective agent in nitric oxide formation from S-nitrosothiols (RSNO) J. Chem. Soc. Perkin Trans. 1996;3:481–487. [Google Scholar]
- DIERKS E.A., BURSTYN J.N. Nitric oxide (NO.), the only nitrogen monoxide redox form capable of activating soluble guanylate cyclase. Biochem. Pharmacol. 1996;51:1593–1600. doi: 10.1016/0006-2952(96)00078-0. [DOI] [PubMed] [Google Scholar]
- FAULKNER K.M., LIOCHEV S.I., FRIDOVICH I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J. Biol. Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- FUKUTO J.M., CHIANG K., HSZIEH R., WONG P., CHAUDHURI G. The pharmacological activity of nitroxyl: a potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J. Pharmacol. Exp. Ther. 1992c;263:546–551. [PubMed] [Google Scholar]
- FUKUTO J.M., HOBBS A.J., IGNARRO L.J. Conversion of nitroxyl (HNO) to nitric oxide (NO) in biological systems: the role of physiological oxidants and relevance to the biological activity of HNO. Biochem. Biophys. Res. Commun. 1993;196:707–713. doi: 10.1006/bbrc.1993.2307. [DOI] [PubMed] [Google Scholar]
- FUKUTO J.M., HSZIEH R., GULATI P., CHIANG K.T., NAGASAWA H.T. N,O-Diacylated-N-hydroxyarylsulfonamides: nitroxyl precursors with potent smooth muscle relaxant properties. Biochem. Biophys. Res. Commun. 1992b;187:1367–1373. doi: 10.1016/0006-291x(92)90453-r. [DOI] [PubMed] [Google Scholar]
- FUKUTO J.M., WALLACE G.C., HSZIEH R., CHAUDHURI G. Chemical oxidation of N-hydroxyguanidine compounds. Release of nitric oxide, nitroxyl and possible relationship to the mechanism of biological nitric oxide generation. Biochem. Pharmacol. 1992a;43:607–613. doi: 10.1016/0006-2952(92)90584-6. [DOI] [PubMed] [Google Scholar]
- GORDGE M.P., HOTHERSHALL G.H., NEILD A.A., DUTRA N. Role of a copper (I)-dependent enzyme in the anti-platelet action of S-nitrosoglutathione. Br. J. Pharmacol. 1996;119:533–538. doi: 10.1111/j.1476-5381.1996.tb15704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMOV I., MARCHESINI A., FARVER O., PECHT I., GOLDFARB D. Azide binding to the trinuclear copper center in laccase and ascorbate oxidase. Eur. J. Biochem. 1999;266:820–830. doi: 10.1046/j.1432-1327.1999.00898.x. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B., GUTTERIDGE J.M.C. Free radicals in biology and medicine 1989Oxford: Clarendon Press; 2nd edn [Google Scholar]
- HUGHES M.N. Relationships between nitric oxide, nitroxyl ion, nitrosonium cation and peroxynitrite. Biochem. Biophys. Acta. 1999;1411:263–272. doi: 10.1016/s0005-2728(99)00019-5. [DOI] [PubMed] [Google Scholar]
- ISKRA M., MAJEWSKI W. Oxidase activity of ceruloplasmin and concentrations of copper and zinc in serum in chronic arterial occlusion of the lower limbs. J. Trace Elem. Med. Biol. 1999;13:76–81. doi: 10.1016/S0946-672X(99)80027-3. [DOI] [PubMed] [Google Scholar]
- LI C.G., KARAGIANNIS J., RAND M.J. Comparison of the redox forms of nitrogen monoxide with the nitrergic transmitter in the rat anococcygeus muscle. Br. J. Pharmacol. 1999;127:826–834. doi: 10.1038/sj.bjp.0702540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKENZIE A., MARTIN W. Loss of endothelium-derived nitric oxide in rabbit aorta by oxidant stress: restoration by superoxide dismutase mimetics. Br. J. Pharmacol. 1998;124:719–728. doi: 10.1038/sj.bjp.0701899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN W., VILLANI G.M., JOTHIANANDAN D., FURCHGOTT R.F. Blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation of rabbit aorta by certain ferrous hemoproteins. J. Pharmacol. Exp. Ther. 1985;233:679–685. [PubMed] [Google Scholar]
- MURPHY M.E., SIES H. Reversible conversion of nitroxyl anion to nitric oxide by superoxide dismutase. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10860–10864. doi: 10.1073/pnas.88.23.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OETTING W.S., FRYER J.P., KING R.A. Mutations of the human tyrosinase gene associated with tyrosinase related oculocutaneous albinism (OCA1) Hum. Mutat. 1998;12:433–434. doi: 10.1002/(SICI)1098-1004(1998)12:6<433::AID-HUMU14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- PETERSON R.E., BOLLIER M.E. Spectrophotometric determination of serum copper with biscyclohexanoneoxalyldihydrazone. Anal. Chem. 1955;27:1195–1197. [Google Scholar]
- PINO R.Z., FEELISCH M. Bioassay discrimination between nitric oxide (NO.) and nitroxyl (NO−) using L-cysteine. Biochem. Biophys. Res. Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., HOFMANN H., SCHINDLER U., SHUTENKO Z.S., CUNNINGHAM D.D., FEELISCH M. No NO from NO synthase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCORZA G., PIETRAFORTE D., MINETTI M. Role of ascorbate and protein thiols in the release of nitric oxide from S-nitroso-albumin and S-nitroso-glutathione in human plasma. Free Radical Biol. Med. 1997;22:633–642. doi: 10.1016/s0891-5849(96)00378-4. [DOI] [PubMed] [Google Scholar]
- SHARPE M.A., COOPER C.E. Reactions of nitric oxide with mitochondrial cytochrome c: A novel mechanism for the formation of nitroxyl anion and peroxynitrite. Biochem. J. 1998;332:9–19. doi: 10.1042/bj3320009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMLER J.S., SINGEL D.J., LOSCALZO J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- WOLZT M., MACALLISTER R.J., DAVIS D., FEELISCH M., MONCADA S., VALLANCE P., HOBBS A.J. Biochemical characterisation of S-nitrosohemoglobin. J. Biol. Chem. 1999;274:28983–28990. doi: 10.1074/jbc.274.41.28983. [DOI] [PubMed] [Google Scholar]


