Abstract
Experiments were designed to investigate the effects of the inducible nitric oxide synthase (iNOS) stimulator, lipopolysaccharide (LPS), on noradrenaline (NA) responses and on NOS activity and its expression in intact mesenteric resistance arteries (MRAs) from Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats.
In MRAs from WKY, LPS (10 μg ml−1; 1–5 h) reduced the vasoconstrictor responses to NA (0.1–30 μM) in the presence, but not in the absence of L-arginine (L-Arg, 10 μM). However, in SHR arteries, LPS induced an incubation-time dependent reduction of NA responses in the absence, as well as the presence, of L-Arg. The LPS inhibitory effect was reduced by the non-specific NOS inhibitor L-NG-nitroarginine methyl ester (L-NAME, 100 μM) and the selective iNOS inhibitor, aminoguanidine (100 μM).
L-NAME alone similarly shifted the concentration-response curve to NA leftward in arteries from both strains, while aminoguanidine had no effect. L-Arg shifted the curve to NA rightward only in SHR MRAs.
Basal activity of both iNOS and constitutive NOS (conversion of [3H]-L-Arg to [3H]-L-citrulline) was similar in arteries from both strains. After 5 h incubation with LPS, only iNOS activity in arteries from SHR was increased.
Basal iNOS protein expression was undetectable; basal endothelial (eNOS) protein expression was similar in arteries from both strains, while neuronal (nNOS) was greater in arteries from SHR. LPS induced iNOS protein expression, that was higher in arteries from SHR than in those from WKY.
These results indicate that NO production, via iNOS induction, is greater than in those from MRAs from SHR to WKY.
Keywords: Noradrenaline, lipopolysaccharide, hypertension, iNOS, rat mesenteric resistance arteries
Introduction
Nitric oxide (NO), generated from L-arginine (L-Arg) by NO synthase (NOS), is reported to play many physiological roles. NO can be synthesized in different cell types, including neurones, endothelial and smooth muscle cells (Moncada et al., 1991; Marín & Rodríguez-Martínez, 1997). Three NOS isoforms have been described, two that are constitutive (cNOS) and one that is inducible (iNOS) (Moncada et al., 1997; Marín & Rodríguez-Martínez, 1997). The cNOS isoform was initially described in the vascular endothelium and was designated eNOS; the other constitutive isoform is present in neurones and is designated nNOS (Moncada et al., 1997). These constitutive isoforms generate NO under basal conditions and in response to several physiological stimuli, whereas iNOS, expressed by exposure to bacterial endotoxin (lipopolysaccharide, LPS) and some proinflammatory cytokines, produces very large amounts of NO (Moncada et al., 1991; Marín & Rodríguez-Martínez, 1997).
It has been suggested that alterations in NO synthesis might be associated with elevations of vascular resistance and, thus, with hypertension (Moncada et al., 1991; Marín & Rodríguez-Martínez, 1997). In this sense, an impairment of endothelium-dependent relaxations has been observed in different vessels from spontaneously hypertensive rats (SHR) and from patients with essential hypertension (Marín & Rodríguez-Martínez, 1997). This suggests that endothelial function might be impaired in hypertension. However, there is growing evidence demonstrating that NO synthesis can be increased in SHR, probably as a counteregulatory mechanism activated to compensate for the increase of blood pressure. An increase in both basal iNOS activity and expression (Wu et al., 1996; Chou et al., 1998; Vaziri et al., 1998) as well as an increase of both eNOS and nNOS expressions in SHR have been reported (Nava et al., 1995; Hayakawa & Raij, 1997; Boulanger et al., 1998).
It has been described that the precursor of NO synthesis, L-Arg, produces inhibition of contractions and vasodilatation in precontracted vessels by the generation of NO (Jovanovic et al., 1994; Alonso et al., 1998; Briones et al., 1999). We and others have demonstrated that iNOS is the isoform involved in these effects (Jovanovic et al., 1994; Alonso et al., 1998; Briones et al., 1999). In addition, a greater fall in blood pressure or vasodilatation after L-Arg administration (Schleiffer et al., 1991; Briones et al., 1999), as well as an exaggerated increase in blood pressure after NOS inhibition (Lacolley et al., 1991) have been described in SHR. The isoform involved in these actions, iNOS, can be induced by certain substances, including bacterial endotoxin. Bernard et al. (1998) recently described a greater resistance to endotoxin shock in SHR than in Wistar Kyoto (WKY) rats, whereas Yen et al. (1997) reported a higher mortality in SHR after LPS administration. In view of these contradictory results, we considered it of interest to study the effects of LPS in SHR.
The aim of the present study was to assess the influence of hypertension on vascular NO synthesis, specially that generated by iNOS. For this, we have studied the effects of LPS on the vasoconstrictor responses induced by noradrenaline (NA) as well as on NOS expression and activity, in mesenteric resistance arteries (MRAs) from normotensive (WKY) and hypertensive (SHR) rats.
Methods
Six-month-old male WKY and SHR rats were obtained from colonies maintained at the Animal Quarters of the Facultad de Medicina of the Universidad Autónoma of Madrid. Systolic arterial pressure was measured by an automatic sphygmomanometer with a tail-cuff device placed on the tail of pretrained rats placed for 1 h in a warm chamber at 30°C, and restrained. The average systolic blood pressure (mean±s.e.mean in mmHg) was 226±4 and 144±5 for SHR and WKY rats, respectively (P<0.01). Rats were anaesthetized with diethyl ether (Panreac, Barcelona, Spain) and killed by exsanguination; thereafter, the mesenteric vascular bed was removed and placed in cold (4°C) Krebs-Henseleit solution (KHS) bubbled with a 95% O2–5% CO2 mixture.
For reactivity experiments the 4th branch of the mesenteric artery was dissected from the mesenteric bed and cleaned of connective tissue under a light microscope. For NOS activity and expression analysis, the 3rd and 4th branches of the mesenteric bed were carefully dissected out and cleaned. Arteries from one mesenteric arterial bed were used to perform two NOS determinations, either activity or expression. Some arteries were incubated for 5 h in KHS bubbled with a 95% O2–5% CO2 mixture, while the other group was incubated for 5 h in KHS containing 10 μg ml−1 LPS. Afterwards, the arteries were rapidly frozen in liquid nitrogen and kept at −70°C until the day of the experiment.
Reactivity experiments
Ring segments, approximately 2 mm in length, were mounted in a small vessel dual chamber myograph for measurement of isometric tension. Two myographs were used in parallel, permitting investigation of four vessels each day. Two tungsten wires (40 μm diameter) were introduced through the lumen of the segments and mounted according to the method described by Mulvany & Halpern (1977).
After a 30 min equilibration period in oxygenated KHS at 37°C and pH=7.4, segments were stretched to their optimal lumen diameter for active tension development. This was determined based on the internal circumference-wall tension ratio of the segments by setting their internal circumference, Lo, to 90% of that the vessels would have if they were exposed to a passive tension equivalent to that produced by a transmural pressure of 100 mmHg (Mulvany & Halpern 1977). The effective lumen diameter was calculated as Lo π−1.
Afterwards, the segments were washed three times and left to equilibrate for 30 min; then, contractility was tested by an initial exposure to a high K+ solution (120 mM K+-KHS). The presence of endothelium was determined by the ability of 10 μM acetylcholine (ACh) to produce relaxation in segments precontracted with 30 μM NA. At the end of the experiment, segments were washed and KHS was replaced by 120 mM K+-KHS; when the contraction was stable, 0.1 mM papaverine was added to determine the maximum response of segments.
Experimental protocol
In segments with functional endothelium, concentration-response curves to NA were constructed by the cumulative addition of NA (0.1–30 μM). Each NA concentration was applied once a steady-state with the previous one had been reached. Consecutive curves to NA were made at 1 h intervals over 6 h.
The effect of 10 μg ml−1 LPS on NA-induced contraction and the role of NO in this effect were investigated at different incubation times (1, 2, 3, 4 and 5 h). For this, parallel experiments were designed as follows: LPS was added, in the presence or absence of 10 μM L-Arg, 100 μM L-NG-nitroarginine methyl ester (L-NAME) or 100 μM aminoguanidine, after the first concentration-response curve to NA was performed. LPS was present in the bath through the successive NA curves; L-Arg was added 15 min before each concentration-response curve, while L-NAME or aminoguanidine were added 30 min before.
The involvement of NO in the concentration-response curves to NA was assessed in paired experiments. The effect of L-Arg was tested by administering increasing concentrations (1 μM–1 mM) of this aminoacid 15 min before the second to fifth NA response curves. In another group of arteries, the effect of L-NAME or aminoguanidine on the concentration-response curve to NA was also analysed; these substances were administered 30 min before the second NA response curve.
NOS activity assay
Mesenteric arteries were homogenized in a cold buffer (25 mM HEPES; 1 mM dithiothreitol, DTT; 50 mM sucrose, pH=7.4), containing protease inhibitors (10 μg ml−1 leupeptin, pepstatin, bestatin and chymostatin; 125 μg ml−1 soybean trypsin inhibitor and 100 μg ml−1 phenylmethylsulphonyl fluoride, PMSF). Aliquots of this homogenate were used for protein determination (Bradford, 1976) and NOS activity assay.
NOS activity was determined by measuring the conversion of [3H]-L-Arg to [3H]-L-citrulline after these aminoacids were separated by thin layer chromatography. Tissue homogenates (35–50 μg protein) were incubated with the same volume of reaction mixture (25 mM HEPES, 1 mM DTT, pH=7.4 containing 20 u ml−1 calmodulin, 4 mM EDTA, 20 μM CaCl2, 40 μM BH4, 20 μM FAD, 20 μM FMN, 2 mM NADPH, 4 μM L-Arg, 5 μCi ml−1 [3H]-L-Arg and 50 mM L-valine) for 30 min at 37°C. The reaction was stopped adding stop buffer (0.2 M sodium acetate buffer, pH=5.2, containing 2 mM EDTA and 0.5 mM citrulline) and 50 μl of aliquots were applied on thin layer chromatography plates (TLC aluminium sheets silica gel 60 F254, Merck KGaA, Darmstadt, Germany), including lanes for L-Arg and L-citrulline standards at 5 mg ml−1. After chromatography development was complete, the plates were removed from the chromatography tank and the lanes for standards were cut and visualized using ninhydrin reagent; the position of these bands was used as a reference and the corresponding bands of L-Arg and L-citrulline from the samples were cut and then incubated in 5 ml of 0.01 M HCl at 37°C overnight to eluate aminoacids from the plates. Afterwards, scintillation liquid (OptiPhase ‘HiSafe', Wallac, Loughborough, U.K.) was added to and thoroughly mixed with 1 ml of each eluate before measuring radioactivity in a beta scintillation counter (Beckman LS 2800, Beckman Instruments; Fullerton, CA, U.S.A.).
For the study of calcium-independent isoform activity, calcium and calmodulin were omitted from the reaction mixture. Similarly, when the activity of the calcium-dependent isoforms was determined, EDTA was removed from the reaction mixture.
Western blot analysis of NOS protein expression
Mesenteric arteries were homogenized in a boiling buffer composed of: 10 mM Tris (pH=7.4), 1% sodium lauryl sulphate (SDS) and the protease inhibitor sodium metavanadate (1 mM). Homogenates containing 15 μg protein were electrophoretically separated on a 7.5% SDS-polyacrylamide gel (SDS–PAGE) and then transferred to polyvinyl difluoride membranes overnight, using a Bio-Rad Trans-Blot Cell system (Bio-Rad Laboratories, Hercules, CA, U.S.A.) containing 25 mM Tris, 190 mM glycine, 20% methanol and 0.05% SDS. Prestained SDS–PAGE standards (BioRad Laboratories) were used as molecular mass standards. The membrane was blocked for 60 min at room temperature in Tris-buffered solution (10 mM Tris, 100 mM NaCl, 0.1% Tween 20) with 5% powdered non-fat milk and then incubated for 1 h at room temperature with mouse monoclonal antibody for iNOS (1 : 10,000 dilution), eNOS (1 : 2500 dilution) or nNOS (1 : 2500 dilution), all purchased from Transduction Laboratories, Lexington, U.K. After washing, the membrane was incubated with a 1 : 2000 dilution of antimouse IgG antibody conjugated to horseradish peroxidase (Transduction Laboratories). The membrane was thoroughly washed and the immunocomplexes were detected using an enhanced horseradish peroxidase/luminol chemiluminiscence system (ECL Plus, Amersham International plc, Little Chalfont, U.K.) and subjected to autoradiography (Hyperfilm ECL, Amersham International plc) for 30 min (iNOS), 3–5 min (eNOS) or 5–10 min (nNOS). Signals on the immunoblot were quantified with a NIH Image V1.56 computer program. The same membrane was used to determine α-actin expression, and the content of the latter was used to correct NOS expression in each sample, using a monoclonal antibody anti α-actin (1 : 30,000 dilution, Boehringer Mannheim, Mannheim, Germany).
Data analysis and statistics
Vasoconstrictor responses were expressed as active wall tension (calculated as the increase in vessel wall force above resting level divided by twice the vessel segment length) or as a percentage of the maximum response (determined by the difference between the tone generated by 120 mM K+ and that produced by 0.1 mM papaverine). Concentrations of NA producing 50% of maximum response (EC50 values) were calculated from individual concentration-response curves according to the method of Fleming et al. (1972). The concentration-ratio (ratio between EC50 values in the presence and in the absence of drugs) was also determined.
The results of NOS activity were expressed as pmol min−1 mg protein−1. For NOS expression, data are expressed as the ratio between optical density for NOS and for α-actin.
Results are expressed as mean±s.e.mean of the number of segments indicated in each case, and statistically significant differences were calculated by means of Student's t-test for paired or unpaired experiments, or by two way analysis of variance (ANOVA) to compare groups; a probability value of less than 5% was considered significant. At least four rats were used for each set of experiments. The EC50 values are expressed as means and 95% confidence intervals.
Drugs and solutions
KHS contained (mM): NaCl 115, NaHCO3 25, KCl 4.7, MgSO4.7H2O 1.2, CaCl2 2.5, KH2PO4 1.2, glucose 11.1 and Na2EDTA 0.01. The high K+ solution was identical to KHS except NaCl that was replaced by KCl in an equimolar basis. Drugs used were: Aminoguanidine hemisulphate, NA hydrochloride, L-Arg hydrochloride, D-Arg hydrochloride, ACh chloride, L-NAME dihydrochloride, papaverine hydrochloride, LPS (Escherichia coli, serotype 055:B5), HEPES, leupeptin, pepstatin A, bestatin, chymostatin, PMSF, soybean trypsin inhibitor, calmodulin, EDTA, BH4, FAD, FMN, NADPH, L-valine, L-citrulline, glycine, sodium metavanadate and DTT (Sigma Chemical, Co., St. Louis, MO, U.S.A.); Tween 20, Tris, SDS and acrylamide (BioRad, Laboratories, Hercules, CA, U.S.A.) and methanol and sucrose (Merck KGaA, Darmstadt, Germany). Drug solutions were made in bidistilled water except for NA, which was dissolved in saline (0.9% NaCl)-ascorbic acid (0.01% w v−1) solution. Stock solutions were kept at −20°C, and appropriate dilutions were made on the day of the experiment.
Results
Reactivity experiments
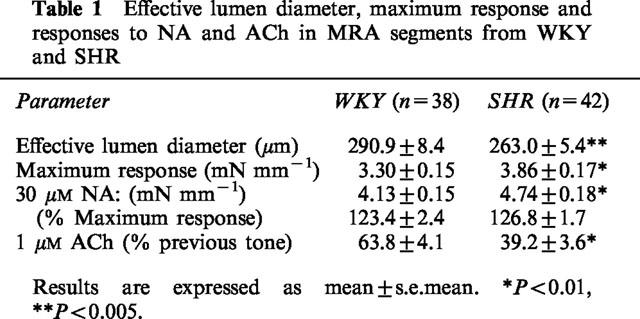
Data of effective lumen diameter, maximum response, contraction induced by the highest concentration of NA used (30 μM) and relaxation induced by 10 μM ACh in segments of MRAs from WKY and SHR, are shown in Table 1. The effective lumen diameter and the relaxation elicited by 10 μM ACh were higher in segments from normotensive than hypertensive rats, whereas the maximum response (difference between the response to 120 mM KCl and that of 0.1 mM papaverine added at the end of the experiment) was greater in the hypertensive strain. The percentage of contraction to 30 μM NA was similar in both strains; however, the response to NA was higher in the hypertensive strain when the comparison was made in active wall tension values (mN mm−1, Table 1).
Table 1.
Effective lumen diameter, maximum response and responses to NA and ACh in MRA segments from WKY and SHR
Concentration-response curves to NA (0.1–30 μM), expressed as a percentage of maximum response, were similar in mesenteric arteries from WKY and SHR rats (Figure 1). The EC50 values were also similar in arteries from either strain [WKY: 1.92 (1.66–2.23) μM; SHR: 2.13 (1.87–2.42) μM; in parentheses 95% confidence interval]. The results of the concentration-response curves to NA were the same at least six times each segment was tested, independently of the strain of origin for the MRAs (results not shown).
Figure 1.
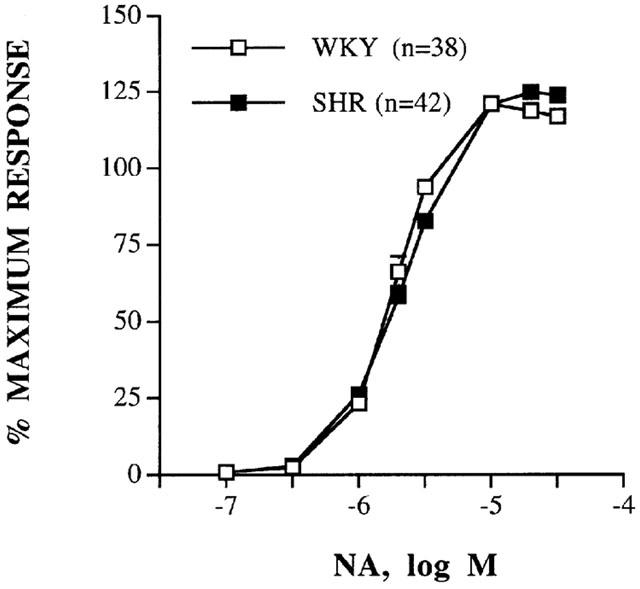
Concentration-response curves to noradrenaline (NA) in intact mesenteric arteries from WKY and SHR. Results (mean±s.e.mean) are expressed as a percentage of maximum response in each case (Table 1). n=number of arterial segments used.
Incubation with LPS (10 μg ml−1) for different time periods (1, 2, 3, 4 and 5 h) induced a slight decrease in the response to NA in MRAs from WKY rats that did not reach statistical significance. However, in SHR arteries, LPS induced a significant decrease in the response to NA that was incubation-time dependent (Figure 2 and Table 2). Only the effects of 1 and 5 h incubation with LPS on the concentration-response curve to NA in both types of arteries are shown. Five hours incubation with LPS did not affect the contraction to 120 mM KCl in MRAs from either WKY (control: 4.18±0.17 vs LPS: 4.35±0.28 mN mm−1, n=7) or SHR (control: 4.82±0.21 vs LPS: 5.15±0.22 mN mm−1, n=7).
Figure 2.
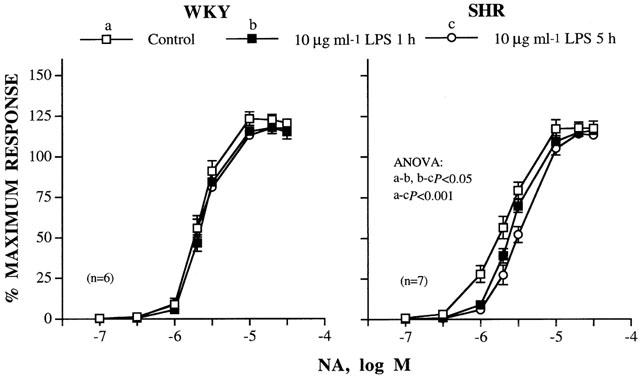
Concentration-response curves to noradrenaline (NA) in the absence and presence of 10 μg ml−1 LPS for 1 or 5 h in intact mesenteric arteries from WKY and SHR. Results (mean±s.e.mean) are expressed as a percentage of the maximum response in each case (WKY: 3.11±0.28 mN mm−1; SHR: 3.93±0.23 mN mm−1). n=number of arterial segments used.
Table 2.
Effect of LPS (1 and 5 h incubation) in the absence or presence of L-Arg, L-NAME or aminoguanidine on the contraction to NA in MRAs from WKY and SHR
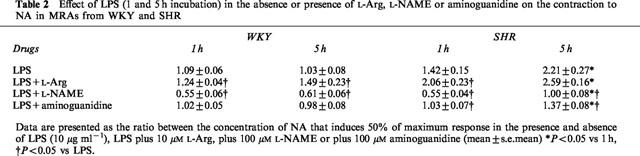
The effect of 1 and 5 h incubation with LPS on the concentration-response curve to NA in the presence of the NO synthesis precursor, L-Arg (10 μM), or the NOS inhibitors, L-NAME (100 μM) and aminoguanidine (100 μM), in segments from WKY and SHR is shown in Figure 3 and Table 2. In the presence of L-Arg, LPS reduced the response to NA in arteries from either strain. In WKY arteries, this reduction was independent of the incubation time with LPS, while in SHR arteries it increased with the length of LPS incubation time (Figure 3A and Table 2). In MRAs from SHR, the reduction of the response to NA induced by L-Arg plus LPS was greater than that observed with LPS after 1 h (Table 2). D-Arg (10 μM) did not modify the inhibitory effect of LPS (data not shown). The incubation with L-NAME (100 μM) shifted the concentration-response curves to NA leftward in arteries from either strain incubated with LPS for 1 h. This displacement was abolished, in arteries from SHR but not in those from WKY, when the incubation time with LPS was increased to 5 h (Figure 3B and Table 2). In the presence of aminoguanidine (100 μM), LPS (1–5 h) did not affect the concentration-response curve to NA in MRAs from WKY (Figure 3C and Table 2). In segments from SHR, aminoguanidine abolished the inhibitory effect induced by 1 h incubation with LPS, and reduced that observed after 5 h incubation (Figure 3C and Table 2).
Figure 3.

Concentration-response curves to noradrenaline (NA) in the absence and presence of 10 μg ml−1 LPS for 1 or 5 h plus (A) 10 μM L-Arg, (B) 100 μM L-NAME and (C) 100 μM aminoguanidine (AG) in intact mesenteric arteries from WKY and SHR. Results (mean±s.e.mean) are expressed as a percentage of the maximum response in each case (WKY: 3.34±0.20 mN mm−1, n=21; SHR: 3.81±0.24 mN mm−1, n=23). n=number of arterial segments used.
L-Arg (1 μM–1 mM) alone, but not D-Arg (results not shown), inhibited the concentration-response curve to NA only in segments from the hypertensive strain and this inhibition was independent of the L-Arg concentration. Figure 4A shows only the effect of 1 μM and 1 mM L-Arg on the concentration-response curve to NA; the effect of 10 and 100 μM was similar (results not shown). L-NAME (100 μM) did not modify arterial basal tone; however, this inhibitor similarly shifted the concentration-response curves to NA leftward in arteries from both strains (Figure 4B) (concentration ratio: WKY, 0.43±0.02; SHR, 0.55±0.06). Aminoguanidine (100 μM) alone did not modify the concentration-dependent response to NA either in MRAs from WKY or in those from SHR (Figure 4C).
Figure 4.
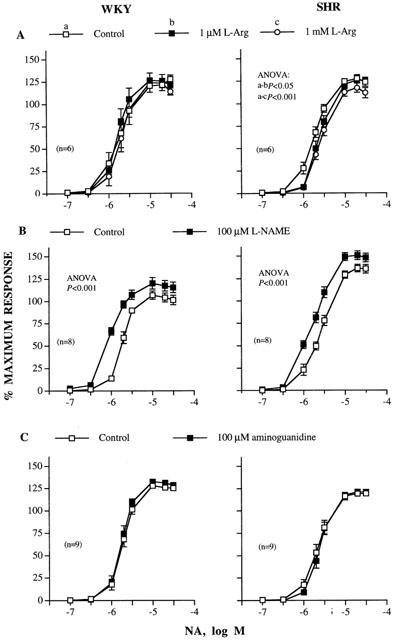
Concentration-response curves to noradrenaline (NA) in the absence and presence of (A) 1 μM and 1 mM L-Arg, (B) 100 μM L-NAME and (C) 100 μM aminoguanidine in intact mesenteric arteries from WKY and SHR. Results (mean±s.e.mean) are expressed as a percentage of the maximum response in each case (WKY: 3.50±0.17 mN mm−1, n=23, SHR: 4.01±0.13 mN mm−1, n=23). n=number of arterial segments used.
NOS activity
The activity of both constitutive and inducible NO isoforms was determined in basal conditions and after 5 h incubation with LPS (10 μg ml−1) in mesenteric arteries from WKY and SHR.
Basal activities of cNOS and iNOS were similar in arteries from both strains (Figure 5). Incubation for 5 h with LPS did not modify cNOS activity; however, LPS induced an increase of iNOS activity in arteries from SHR, but not in those from WKY (Figure 5).
Figure 5.

Activity of the NOS isoforms in intact mesenteric arteries from WKY and SHR rats incubated for 5 h in the absence (basal) or presence of 10 μg ml−1 LPS. NOS activity was determined by the conversion of [3H]-L-arginine into [3H]-L-citrulline in the absence (iNOS) or presence (cNOS) of Ca2+ and calmodulin. Results are expressed as mean±s.e.mean. Each determination was performed in duplicate. *P<0.05 vs WKY and +P<0.05 vs basal situation. n=number of arterial segments used.
Expression of NOS isoforms
Homogenates from mesenteric arteries showed a detectable basal expression of both eNOS and nNOS, as determined by Western blot analysis. The expression of eNOS was similar in arteries from WKY and SHR, while the expression of nNOS was approximately two times higher in mesenteric arteries from SHR than in those from WKY (Figure 6). Neither the expression of eNOS nor that of nNOS were modified by 5 h incubation with LPS (data not shown).
Figure 6.
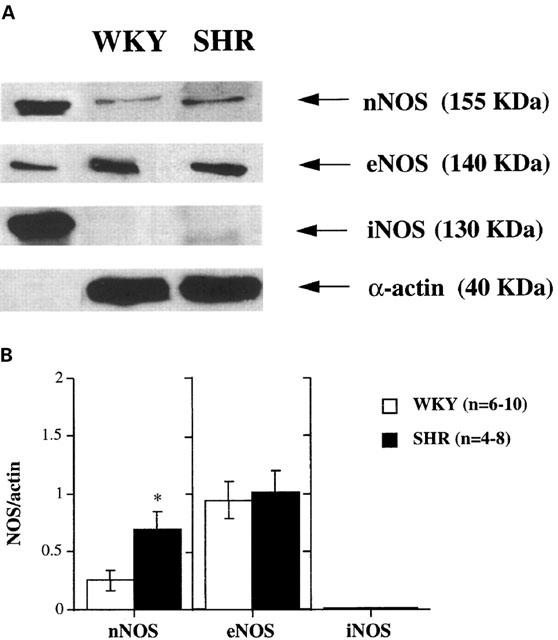
(A) Representative Western blot of nNOS, eNOS and iNOS protein expression in intact mesenteric arteries from WKY and SHR rats. The expression of α-actin is also shown as a loading control. The first lane shows the corresponding positive control for each protein (human endothelial cells, mouse macrophages and rat pituitary for eNOS, iNOS and nNOS, respectively). Arterial homogenates were subjected to SDS–PAGE followed by immunoblot analysis using anti-iNOS, anti-eNOS, anti-nNOS and anti-α-actin antibodies. (B) Densitometric analysis of the Western blot for nNOS, eNOS and iNOS protein expression. Results (mean±s.e.mean) are expressed as the ratio of the optical density of each protein versus that of α-actin. *P<0.05 vs WKY. n=number of arterial segments used.
No detectable basal expression of iNOS protein was observed in arteries from either WKY or SHR (Figures 6 and 7). After incubation of arteries with LPS for 5 h, there was a substantial induction of iNOS in mesenteric arteries from both strains, and this induction was significantly greater in arteries from SHR than in those from WKY (Figure 7).
Figure 7.
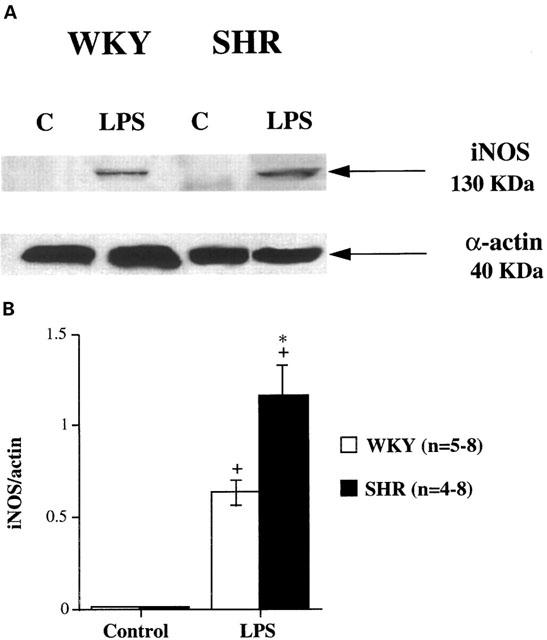
(A) Representative Western blot of iNOS protein expression in intact mesenteric arteries from WKY and SHR rats incubated for 5 h in the absence (Control, C) or presence of 10 μg ml−1 LPS (LPS). The expression of α-actin is also shown as a loading control. (B) Densitometric analysis of the Western blot of iNOS protein expression. Results (mean±s.e.mean) are expressed as the ratio of the signal of the iNOS protein versus that of α-actin. *P<0.05 vs WKY, +P<0.01 vs Control. n=number of arterial segments used.
Discussion
Bacterial LPS, which initiates septic shock, is one of the different stimuli that leads to induction of NOS through an over-production of NO (Radomski et al., 1990; Fleming et al., 1991); an over-production that can impair vasoconstrictor responses. In the present study, we investigated the effects of incubation with LPS on NA-induced vasoconstrictor responses and NOS activity and expression in mesenteric resistance arteries from SHR and WKY rats. The results show a greater effect of LPS on NA responses in SHR than in WKY mesenteric arteries, probably due to the higher iNOS expression and activity in the hypertensive strain. To our knowledge, this is the first report showing higher iNOS expression in resistance arteries from SHR after LPS stimulation. This suggests an alteration in the mechanisms of NO generation via iNOS with hypertension.
The contraction elicited by NA was greater in segments from SHR compared to those from WKY. These changes seem to be independent of receptor-signal transduction mechanisms since the maximum responses (difference between the contraction induced by K+ and the relaxation induced by papaverine) were also increased. Similar increases in the vasoconstrictor responses with hypertension have been described by some investigators in different peripheral arteries, including those of the mesenteric bed (Vila et al., 1993; Marquer-Domagala & Finet, 1997). However, a reduction in vasoconstrictor responses has also been described in rat cerebral arteries from SHR and stroke prone SHR (SHRSP) compared to WKY (Arribas et al., 1996; Briones et al., 1999). Hypertrophy of the media of large and small arteries from SHR and SHRSP has been established (Mulvany, 1990; Arribas et al., 1997). In agreement with these results, we have observed a reduction of the effective lumen diameter in segments from hypertensive rats. An impairment of the endothelium-dependent vasodilatation induced by ACh with hypertension has been described in different vascular preparations (Marín & Rodríguez-Martínez, 1997), while no change or enhancement have also been reported (Lüscher, 1992; Marín & Rodríguez-Martínez, 1997; Briones et al., 1999). In our preparation, a reduction of ACh-induced relaxation in segments from hypertensive rats is observed. It is unlikely that the higher vascular tone generated by NA is the cause of this impairment, since the percentage of maximum response was similar. The decrease in ACh-induced relaxation observed in our preparation with hypertension does not seem to be due to an alteration of eNOS isoform, because no change in basal cNOS activity or in eNOS expression was observed here in mesenteric arteries from SHR, as was also reported in aorta from SHR (Bauersachs et al., 1998). However, Chou et al. (1998) have found a reduction in both eNOS expression and activity in aorta from SHR, whereas Vaziri et al. (1998) and Kerr et al. (1999) found an increase in eNOS protein expression in aorta from hypertensive animals. It has been suggested that the endothelial dysfunction observed with hypertension is due to an excess of endothelium-derived contracting factors, i.e. superoxide anions (Tschudi et al., 1996; Kerr et al., 1999) or an impaired expression of soluble guanylate cyclase (Klöß et al., 2000), rather than to a decrease of NO generation. In addition, an endothelium-derived hyperpolarizing factor is involved in the relaxation to ACh in MRAs (Hwa et al., 1994). An impairment of the hyperpolarizing component has been suggested to possibly account for the reduction of the ACh-induced relaxation observed in MRAs from hypertensive rats (Fujii et al., 1992). This could explain the disagreement between the impairment of endothelium-dependent vasodilatation and the unaltered cNOS activity and eNOS expression observed in our preparation, although further experiments are necessary to clarify this point.
The precursor of NO synthesis, L-Arg, but not D-Arg, inhibited the response to NA but only in the hypertensive strain. These results agree with those found by Pucci et al. (1995) and Wu et al. (1996) who both observed that L-Arg caused relaxation in aortic rings from hypertensive but not from normotensive rats. In cerebral arteries, L-Arg inhibits vasoconstrictor responses and induces vasodilatation (Riedel et al., 1995; Alonso et al., 1998; Briones et al., 1999), which is increased in hypertension (Riedel et al., 1995; Briones et al., 1999). We and other authors have described the vasodepressor effects of L-Arg as being due to generation of NO, with inducible NOS being the isoform involved in these effects (Jovanovic et al., 1994; Alonso et al., 1998; Briones et al., 1999). Since iNOS, but not cNOS, depends on extracellular L-Arg (Schott et al., 1993; Durante et al., 1996) and D-Arg has no effect, it is possible that the inhibitory effect of L-Arg, which was only observed in SHR mesenteric arteries, was due to iNOS activation in unstimulated conditions in these arteries as has been described in SHR aorta (Wu et al., 1996; Chou et al., 1998). However basal iNOS expression was not found in any strain. Since iNOS isoform produces very large amounts of NO (Moncada et al., 1991; Marín & Rodríguez-Martínez, 1997), the expression of a quantity of protein too small to be detected by our method, could be responsible for the effects observed in the presence of L-Arg in SHR. Ex vivo induction of NOS by low levels of endotoxin present in the incubation medium was thought to be involved in the inhibitory effect of L-Arg (Rees et al., 1990). It is unlikely that this mechanism is involved in our study, since the inhibitory effect of L-Arg was only observed in mesenteric arteries from the hypertensive strain. In addition, the control responses to NA did not change with incubation time.
The NOS inhibitor, L-NAME, had no effect in basal conditions, although it did increase the NA-induced contraction. These results indicate that NO is not spontaneously released under basal conditions, at least to a significant extent, and that release of NO requires an active tone mediated by vasoconstrictors such as NA. These results agree with those of other authors, also in the rat mesenteric arterial bed (Mitchell et al., 1993; Marquer-Domagala & Finet, 1997). The similar increase of NA contraction elicited by L-NAME in both kinds of arteries and the lack of effect induced by the selective iNOS inhibitor, aminoguanidine, suggest the involvement of cNOS on the L-NAME-effect and confirm the non apparent dysfunction of this isoform with hypertension.
It has been established that LPS administration decreases vascular resistance, produces vascular hyporeactivity to different vasoconstrictors and promotes the overproduction of NO through the iNOS activation (Stoclet et al., 1993; Thiemermann, 1997). Our results show that, in arteries from normotensive rats, the contractile responses to NA were not modified by 1–5 h incubation with LPS, however, when L-Arg was present, LPS induced a reduction of contraction to NA. In contrast, in mesenteric arteries from hypertensive rats, LPS did not modify the contraction induced by high KCl but shifted the concentration-response curve to NA rightward, with no modification of the contraction induced by the highest concentration of NA used. This displacement was increased with L-Arg and with a longer incubation time. This reduction in the NA response was specific to LPS since no modification of control response to NA with the incubation time was observed. The results obtained in normotensive animals agree with those obtained by some investigators who also found, in mesenteric arteries from LPS-treated rats, no alteration in the contractile responses, unless L-Arg was added to the bath (Mitchell et al., 1993; Martínez et al., 1996). However, Mitolo-Chieppa et al. (1996), found that in vivo administration of LPS produced hyporesponsiveness to NA in the perfused rat mesenteric vascular bed. Other workers have also found that LPS inhibits vasoconstrictor responses in arteries from normotensive animals (Schott et al., 1993; Briones et al., 1999). We have recently demonstrated that the inhibitory effect of LPS on PGF2α-induced contraction, in rat cerebral arteries, is potentiated by hypertension (Briones et al., 1999). The increase in iNOS expression and activity after LPS stimulation supports the decrease in NA responses in the presence of LPS observed in arteries from SHR. It is possible that in mesenteric arteries from normotensive rats the degree of iNOS induction with LPS is not sufficient to inhibit vasoconstrictor responses. Thus, although an increase of iNOS expression was observed with LPS in normotensive arteries, this increase was lower than that observed in mesenteric arteries from SHR and no modification of iNOS activity in this strain was found.
L-NAME shifted the vasoconstrictor responses induced by NA leftward in arteries from the normotensive strain incubated with LPS during 1 or 5 h. However, in the hypertensive strain, this leftward displacement was only observed in arteries incubated with LPS for 1 h. These results support the previous assumption that NO production in the presence of LPS is greater in the hypertensive strain, and that NO production is increased with the time of exposure to LPS in SHR. The fact that aminoguanidine reduced the inhibitory effect of LPS in MRAs from SHR supports the participation of NO generated by iNOS in this inhibitory effect.
Taken together, the results with LPS indicate that mesenteric arteries from SHR reach a higher functional expression of iNOS in the presence of LPS. In agreement with our results, other investigators have found that intravenous infusion of LPS caused more severe hypotensive effects and a higher mortality in SHR than in WKY (Yen et al., 1997; Wu & Yen, 1999) and that these effects were associated with an elevated synthesis of NO in SHR. In addition, the increase in rat aorta of iNOS activity and expression and nitrate and cyclic GMP levels induced by LPS are greater in SHR than in WKY rats (Wu et al., 1996; Chou et al., 1998; Vaziri et al., 1998; Wu & Yen, 1999). We did not investigate why SHR mesenteric arteries are more sensitive to induction of iNOS by LPS than WKY arteries. However, some immune abnormalities have been reported in SHR. Thus, Wu et al. (1996) have proposed that the higher increase of TNFα levels found in SHR after challenge with LPS contributes to the more severe effect of LPS in this strain. On the other hand, Haller et al. (1995) found a higher macrophage infiltration in the perivascular space of hearts from SHR and these cells can be stimulated to produce NO from iNOS.
The expression of basal nNOS was greater in mesenteric arteries from SHR than from WKY, suggesting a possible increase in the production of NO through the nNOS isoform in hypertensive arteries. A similar increase in nNOS expression in vascular smooth muscle cells of carotid arteries from SHR was described by Boulanger et al. (1998). These authors also observed that angiotensin II stimulates the release of NO from this isoform only in arteries from SHR, leading to an impairment of the direct contractile effect of the peptide. So, we can not exclude the effect of NO derived from nNOS on the NA responses. However, this NO does not seem to be involved in the responses induced in the presence of LPS because no alteration of nNOS expression was observed in this situation.
In conclusion, the present work shows that LPS induces an inhibitory effect on vasoconstrictor responses to NA in mesenteric arteries from SHR whereas the effect is only observed in those from normotensive rats when the NOS substrate, L-Arg, is also present. These effects could be related to the increased iNOS expression and activity found in the hypertensive rat. The alterations observed in iNOS and nNOS expression of mesenteric arteries from the hypertensive animals suggest that the mechanisms that generate NO through these isoforms are increased with hypertension, although further experiments are necessary to determine the actual mechanism involved in this alteration.
Acknowledgments
Dedicated to Dr Jesús Marín López (1944–2000). This study has been supported by Grants from DGICYT (PM 97-0008 and PM 97/0011), FISS (98/0074-02) and Bayer España. We thank Dr M. Carmen Fernández-Criado for the care of animals. We also thank Prof A.F. Dominiczak and Dr S. Arribas for critical revision of the manuscript, Dr M.J. Brosnan for helpful advice in some experiments and Ms C.F. Warren for her linguistic assistance. All the experiments of this study comply with the current laws of Spain.
Abbreviations
- ACh
acetylcholine
- BH4
tetrahydrobiopterin
- cNOS
constitutive nitric oxide synthase
- DTT
dithiothreitol
- D-Arg
D-arginine
- EC50
Concentration producing 50% of maximum response
- EDTA
ethylenediamine-tetraacetic acid
- eNOS
endothelial nitric oxide synthase
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- iNOS
inducible nitric oxide synthase
- KHS
Krebs Henseleit solution
- L-Arg
L-arginine
- L-NAME
L-NG-nitroarginine methyl ester
- LPS
lipopolysaccharide
- MRAs
mesenteric resistance arteries
- NA
noradrenaline
- NADPH
reduced form of nicotinamide adenine dinucleotide phosphate
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- PAGE
polyacrylamide gel
- PMSF
phenylmethylsulphonyl fluoride
- SDS
sodium lauryl sulphate
- SHR
spontaneously hypertensive rats
- WKY
Wistar Kyoto rats
References
- ALONSO M.J., RODRÍGUEZ-MARTÍNEZ M.A., MARTÍNEZ-ORGADO J., MARÍN J., SALAICES M. The L-arginine inhibition of rat middle cerebral artery contractile responses is mediated by inducible nitric oxide synthase. J. Auton. Pharmacol. 1998;18:105–113. doi: 10.1046/j.1365-2680.1998.1820105.x. [DOI] [PubMed] [Google Scholar]
- ARRIBAS S., GORDON J., DALY C.J., DOMINICZAK A.F., McGRATH J.C. Confocal microscopic characterization of a lesion in cerebral vessel of the stroke-prone spontaneously hypertensive rat. Stroke. 1996;27:1118–1123. doi: 10.1161/01.str.27.6.1118. [DOI] [PubMed] [Google Scholar]
- ARRIBAS S.M., HILLIER C., GONZÁLEZ C., McGRORY S., DOMINICZAK A.F., McGRATH J.C. Cellular aspects of vascular remodeling in hypertension revealed by confocal microscopy. Hypertension. 1997;30:1455–1464. doi: 10.1161/01.hyp.30.6.1455. [DOI] [PubMed] [Google Scholar]
- BAUERSACHS J., BOULOUMIÉ A., MÜLSCH A., WIEMER G., FLEMING I., BUSSE R. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylate cyclase expression, and in superoxide anion production. Cardiovasc. Res. 1998;37:772–779. doi: 10.1016/s0008-6363(97)00250-2. [DOI] [PubMed] [Google Scholar]
- BERNARD C., MERVALL R., ESPINO B., TEDGUI A. Resistance to endotoxin shock in spontaneously hypertensive rats. Hypertension. 1998;31:1350–1356. doi: 10.1161/01.hyp.31.6.1350. [DOI] [PubMed] [Google Scholar]
- BOULANGER C.M., HEYME S.C., BENESSIANO J., GESKE R.S., LÉVY B.I., VANHOUTTE P.M. Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells. Activation by angiotensin II in hypertension. Circ. Res. 1998;83:1271–1278. doi: 10.1161/01.res.83.12.1271. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRIONES A.M., ALONSO M.J., MARÍN J., SALAICES M. Role of iNOS in the vasodilator responses induced by L-arginine in middle cerebral arteries from normotensive and hypertensive rats. Br. J. Pharmacol. 1999;126:111–120. doi: 10.1038/sj.bjp.0702281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOU T.C., YEN M.H., LI C.Y., DING Y.A. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–648. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- DURANTE W., LIAO L., IFTIKHAR I., O'BRIEN W.E., SCHAFER A.I. Differential regulation of L-arginine transport and nitric oxide production by vascular smooth muscle and endothelium. Circ. Res. 1996;78:1075–1082. doi: 10.1161/01.res.78.6.1075. [DOI] [PubMed] [Google Scholar]
- FLEMING I., JULOU-SCHAEFFER G., GRAY G.A., PARRAT J.R., STOCLET J.C. Evidence that an L-arginine/nitric oxide dependent elevation of tissue cyclic GMP content is involved in depression of vascular reactivity by endotoxin. Br. J. Pharmacol. 1991;103:1047–1052. doi: 10.1111/j.1476-5381.1991.tb12298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEMING W.W., WESTFALL D.P., DE LA LANDE I.S., JELLET L.B. Long-normal distribution of equieffective doses of norepinephrine and acetylcholine in several tissues. J. Pharmacol. Exp. Ther. 1972;181:330–345. [PubMed] [Google Scholar]
- FUJII K., TOMINAGA M., OHMORI S., KOBAYASHI K., KOGA T., TAKATA Y., FUJISHIMA M. Decreased endothelium-dependent hyperpolarization to acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circ. Res. 1992;70:660–669. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- HALLER H., BEHREND M., PARK J.K., SCHABERG T., LUFT F.C., DISTLER A. Monocyte infiltration and c-fms expression in hearts of spontaneously hypertensive rats. Br. J. Pharmacol. 1995;25:132–138. doi: 10.1161/01.hyp.25.1.132. [DOI] [PubMed] [Google Scholar]
- HAYAKAWA H., RAIJ L. The link among nitric oxide synthase activity, endothelial function and aortic and ventricular hypertrophy in hypertension. Hypertension. 1997;29:235–241. doi: 10.1161/01.hyp.29.1.235. [DOI] [PubMed] [Google Scholar]
- HWA J.J., GHIBAUDI L., WILLIAMS P., CHATTERJEE M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am. J. Physiol. 1994;35:H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- JOVANOVIC A., GRBOVIC L., TULIC I. L-Arginine induces relaxation of human uterine artery with both intact and denuded endothelium. Eur. J. Pharmacol. 1994;256:103–107. doi: 10.1016/0014-2999(94)90623-8. [DOI] [PubMed] [Google Scholar]
- KERR S., BROSNAN M.J., McINTYRE M., REID J.L., DOMINICZACK A.F., HAMILTON C.A. Superoxide anion production is increased in a model of genetic hypertension. Role of the endothelium. Hypertension. 1999;33:1353–1358. doi: 10.1161/01.hyp.33.6.1353. [DOI] [PubMed] [Google Scholar]
- KLÖß S., BOULOUMIÉ A., MÜLSCH A. Aging and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension. 2000;35:43–47. [PubMed] [Google Scholar]
- LACOLLEY P.J., LEWIS S.J., BRODY M.J. L-NG-nitro arginine produces an exaggerated hypertension in anesthetized SHR. Eur. J. Pharmacol. 1991;197:239–240. doi: 10.1016/0014-2999(91)90533-v. [DOI] [PubMed] [Google Scholar]
- LÜSCHER T.F. Heterogeneity of endothelial dysfunction in hypertension. Eur. Heart. J. 1992;13:50–55. doi: 10.1093/eurheartj/13.suppl_d.50. [DOI] [PubMed] [Google Scholar]
- MARÍN J., RODRÍGUEZ-MARTÍNEZ M.A. Role of nitric oxide in physiological and pathological conditions. Pharmacol. Ther. 1997;75:111–134. doi: 10.1016/s0163-7258(97)00051-x. [DOI] [PubMed] [Google Scholar]
- MARQUER-DOMAGALA F.L., FINET M. Comparison of the nitric oxide and cyclo-oxygenase pathway in mesenteric resistance vessels of normotensive and spontaneously hypertensive rats. Br. J. Pharmacol. 1997;121:588–594. doi: 10.1038/sj.bjp.0701173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTÍNEZ M.C., MULLER B., STOCLET J.C., ANDRIANTSITOHAINA R. Alteration by lipopolysaccharide of the relationship between intracellular calcium levels and contraction in rat mesenteric artery. Br. J. Pharmacol. 1996;118:1218–1222. doi: 10.1111/j.1476-5381.1996.tb15526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL J.A., KOHLHAAS K.L., SORRENTINO R., WARNER T.D., MURAD F., VANE J.R. Induction by endotoxin of nitric oxide synthase in the rat mesentery: lack of effect on action of vasoconstrictors. Br. J. Pharmacol. 1993;109:265–270. doi: 10.1111/j.1476-5381.1993.tb13563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITOLO-CHIEPPA D., SERIO M., POTENZA M.A., MONTAGNANI M., MANSI G., PECE S., JIRILLO E., STOCLET J.C. Hyporeactivity of mesenteric vascular bed in endotoxin-treated rats. Eur. J. Pharmacol. 1996;309:175–182. doi: 10.1016/0014-2999(96)00347-0. [DOI] [PubMed] [Google Scholar]
- MONCADA S., HIGGS A., FURCHGOTT R. International union of pharmacology nomenclature in nitric oxide research. Pharmacol. Rev. 1997;49:137–142. [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MULVANY M.J. Structure and function of small arteries in hypertension. J. Hypertens. 1990;8:S225–S232. [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NAVA E., NOLL G., LÜSCHER T.F. Increased activity of constitutive nitric oxide synthase in cardiac endothelium in spontaneous hypertension. Circulation. 1995;91:2310–2313. doi: 10.1161/01.cir.91.9.2310. [DOI] [PubMed] [Google Scholar]
- PUCCI M.L., DICK L.B., MILLER K.B., SMITH C.J., NASJLETTY A. Enhanced responses to L-arginine in aortic rings from rats with angiotensin-dependent hypertension. J. Pharmacol. Exp. Ther. 1995;274:1–7. [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1990;87:10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES D.D., CELLEK S., PALMER R.M.J., MONCADA S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem. Biophys. Res. Commun. 1990;173:541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- RIEDEL M.W., ANNESER F., HABERL R.L. Different mechanisms of L-arginine induced dilatation of brain arterioles in normotensive and hypertensive rats. Brain Res. 1995;671:21–26. doi: 10.1016/0006-8993(94)01292-p. [DOI] [PubMed] [Google Scholar]
- SCHLEIFFER R., PERNOT F., VAN-OVERLOOP B., GAIRARD A. In vivo involvement of endothelium-derived nitric oxide in spontaneously hypertensive rats: effects of NG-nitro-L-arginine methyl ester. J. Hypertens. 1991;9 Suppl. 6:S192–S193. [PubMed] [Google Scholar]
- SCHOTT C.A., GRAY G.A., STOCLET J.C. Dependence of endotoxin-induced vascular hyporeactivity on extracellular L-arginine. Br. J. Pharmacol. 1993;108:38–43. doi: 10.1111/j.1476-5381.1993.tb13436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOCLET J.C., FLEMING I., GRAY G.A., JULOU-SCHAEFFER G., SCHNEIDER F., SCHOTT C.A., SCHOTT C., PARRATT J.R. Nitric oxide and endotoxemia. Circulation. 1993;87 Suppl. V:77–88. [Google Scholar]
- THIEMERMANN C. Nitric oxide and septic shock. Gen. Pharmacol. 1997;29:159–166. doi: 10.1016/s0306-3623(96)00410-7. [DOI] [PubMed] [Google Scholar]
- TSCHUDI M.R., MESAROS S., LÜSCHER T.F., MALINSKI T. Direct in situ measurement of nitric oxide in mesenteric resistance arteries. Increased decomposition by superoxide in hypertension. Hypertension. 1996;27:32–35. doi: 10.1161/01.hyp.27.1.32. [DOI] [PubMed] [Google Scholar]
- VAZIRI N.D., NI Z., OVEISI F. Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension. 1998;31:1248–1254. doi: 10.1161/01.hyp.31.6.1248. [DOI] [PubMed] [Google Scholar]
- VILA E., TABERNERO A., IVORRA D. Inositol formation and contractile response linked to α1-adrenoceptors in tail artery and aorta from spontaneously hypertensive and Wistar-Kyoto rats. J. Cardiovasc. Pharmacol. 1993;22:191–197. doi: 10.1097/00005344-199308000-00003. [DOI] [PubMed] [Google Scholar]
- WU C.C., HONG H.J., CHOU T.C., DING Y.A., YEN M.H. Evidence for inducible nitric oxide synthase in spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 1996;228:459–466. doi: 10.1006/bbrc.1996.1682. [DOI] [PubMed] [Google Scholar]
- WU C.C., YEN M.H. Higher level of plasma nitric oxide in spontaneously hypertensive rats. Am. J. Hypertens. 1999;12:476–482. doi: 10.1016/s0895-7061(99)00008-4. [DOI] [PubMed] [Google Scholar]
- YEN M.H., LIU Y.C., HONG H.J., SHEU J.R., WU C.C. Role of nitric oxide in lipopolysaccharide-induced mortality from spontaneously hypertensive rats. Life Sci. 1997;60:1223–1230. doi: 10.1016/s0024-3205(97)00066-0. [DOI] [PubMed] [Google Scholar]


