Abstract
It is well known that extracellular ATP (ATPo) elevates the intracellular Ca2+ concentration ([Ca2+]i) by inducing Ca2+ influx or mobilizing Ca2+ from internal stores via activation of purinoceptors in the plasma membrane. This study shows that ATPo also activates the plasma membrane Ca2+ pumps (PMCPs) to bring the elevated [Ca2+]i back to the resting level in human embryonic kidney-293 (HEK-293) cells.
The duration of ATPo-induced intracellular Ca2+ transients was significantly increased by PMCP blockers, La3+ or orthovanadate. In contrast, replacement of extracellular Na+ with NMDG+, a membrane-impermeable cation, had no significant effect on duration, thus suggesting that Na+/Ca2+ exchangers do not participate in the ATPo-induced Ca2+ transient.
A rapid and significant decrease in [Ca2+]i, which was not dependent on extracellular Na+, was induced by ATPo in cells pretreated with thapsigargin (TG). This decrease was blocked by orthovanadate, indicating that it was caused by PMCPs rather than sarco/endoplasmic reticulum Ca2+ pumps (SERCPs).
UTP and ATPγS also caused a decrease in [Ca2+]i in cells pretreated with TG, although they were less effective than ATP. The effect of UTP implies the involvement of both P2Y1 and P2Y2 receptors, while the effect of ATPγS implies no significant role of ectophosphorylation and agonist hydrolysis in the agonist-induced [Ca2+]i decreases.
These results point to a role of PMCPs in shaping the Ca2+ signal and in restoring the resting [Ca2+]i level to maintain intracellular Ca2+ homeostasis after agonist stimulation.
Keywords: Purinoceptor, extracellular ATP, intracellular calcium, thapsigargin, plasma membrane calcium pump
Introduction
Extracellular ATP (ATPo) is a potent signal that modulates a variety of cellular functions through activation of plasma membrane P2-type purinoceptors (Ralevic & Burnstock, 1998). The P2-type purinoceptors have been subdivided into ATPo-gated ion channels and G protein-coupled receptors. Activation of the latter may trigger phosphoinositide-specific phospholipase C, which catalyzes production of inositol 1,4,5-trisphosphate (IP3) from the membrane lipid, phosphatidylinositol 4,5-bisphosphate. As a Ca2+ mobilizing messenger, IP3 diffuses into the cytoplasm and releases Ca2+ from intracellular stores to generate a Ca2+ signal (Ralevic & Burnstock, 1998). This Ca2+ signal is composed of two phases, one rising and one declining. The rising phase reflects activation of Ca2+ mobilizing systems, which in turn activate intracellular signalling mechanisms to induce a proper cellular response, while the declining phase reflects activation of Ca2+ sequestration systems, to avoid high and potentially toxic [Ca2+]i levels. For successful Ca2+ signalling, it is therefore essential that [Ca2+]i increase and reduction are closely integrated.
Increasing evidence has recently suggested that plasma membrane Ca2+ pumps (PMCPs) activated by Ca2+ mobilizing agonists play a significant role in controlling the Ca2+ signal. For example, an increase in Ca2+ extrusion via PMCP activation upon agonist stimulation has been demonstrated in human platelets (Rink & Sage, 1987), mouse pancreatic acinar cells (Tepikin et al., 1992), rat pancreatic acinar cells (Zhang et al., 1992), A7r5 smooth muscle cells (Broad et al., 1999), human U373 MG astrocytoma cells (Young et al., 1998), and GH3 pituitary cells (Nelson & Hinkle, 1994). These results imply that an agonist which causes an increase in [Ca2+]i may also accelerate Ca2+ extrusion by activating PMCPs (Broad et al., 1999).
In the present study, several lines of evidence are presented to show that ATPo, a potent purinoceptor agonist, can strongly and rapidly activate PMCPs in human embryonic kidney-293 (HEK-293) cells. The duration of ATPo-induced intracellular Ca2+ transients became longer in the presence of PMCP blockers. In the presence of a sarco/endoplasmic reticulum Ca2+ pump (SERCP) inhibitor, thapsigargin (TG), ATPo caused a rapid decrease in [Ca2+]i. A similar decrease in [Ca2+]i was also caused by UTP and ATPγS in TG-treated cells. These results suggest that agonist-activated PMCPs contribute significantly to terminating the initial [Ca2+]i increase and to rapidly bringing the increased [Ca2+]i back to the resting level to maintain intracellular Ca2+ homeostasis.
Methods
HEK-293 cells were grown on fibronectin-coated coverslips at 37°C in a humidified 5% CO2 atmosphere in DMEM-L (Dulbecco's modification of Eagle's medium with low glucose) culture medium supplemented with 10% foetal calf serum, 100 units ml−1 penicillin, and 100 μg ml−1 streptomycin (GIBCO BRL). After 2–4 days, the cells were transferred to a ‘standard' solution containing (mM): CaCl2 1.3, MgCl2 1.0, KC1 5.4, NaCl 140, HEPES 10.0 (pH adjusted to 7.4 with NaOH). For Ca2+-free solution, 0.1 mM EGTA was added to nominally Ca2+-free standard solution. For Na+-free solution, NMDG+ (N-methyl-D-glutamine) was used to replace Na+ in the standard solution. The osmolality of the solutions was adjusted to that of the culture medium (320 mOsm litre−1) using 40 mM D-glucose. Cells were incubated in the culture medium containing 3 μM fluo-3/AM and 0.02% Pluronic F-127 (Sigma Chemical Co.) for 30 min. Fluo-3 intensity (F, 480±15 nm excitation, 530±30 nm emission) was monitored every 2 s by a real time laser confocal microscope (Insight-IQ, Meridian Instruments, Inc.) and plotted as relative intensity with respect to that of a basal calcium level (F0). The results shown were representative of over 30 cells from at least three independent experiments. Each agonist was dissolved in the bath solution and drawn into a glass pipette (G-1, Narishige, Japan) with a tip diameter of ca. 2 μm. The pipette was placed at adjacence of approximately 70 μm from the closest cells for local application of agonist. The flow rate of the agonist through the pipette was controlled by a microinjection system (Eppendorf, Germany), and was adjusted to a value below the threshold for cell detachment from the coverslip. To prevent cells being influenced by agonist leakage, the pipette was raised 250 μm after each application. Experiments were carried out at 37°C.
Results and Discussion
It is generally accepted that PMCPs play a role in Ca2+ extrusion from the cytosol, particularly in non-excitable cells (Carafoli, 1994; Guerini, 1998). To evaluate the effect of PMCPs on ATPo-induced intracellular Ca2+ transients, we measured the duration of ATPo-induced intracellular Ca2+ transients in the presence of PMCP blockers. In preliminary experiments, we observed that the time courses of intracellular Ca2+ transients were quite variable among individual cells upon ATPo application at low concentrations (1–10 μM). To synchronize the time course of the intracellular Ca2+ transient, a relatively high concentration of ATPo (50 μM) was applied to the cells. Approximately 2 s after an ATPo application, a transient increase in [Ca2+]i was observed (Figure 1). This Ca2+ transient was not affected by the presence or absence of extracellular Ca2+ (data not shown), suggesting that the Ca2+ transient was caused by the mobilization of Ca2+ from internal stores, presumably via activation of purinoceptors in the plasma membrane. The duration of the ATPo-induced [Ca2+]i transient was measured in the absence and presence of orthovanadate and La3+, two widely used PMCP blockers (Carafoli, 1994; Guerini, 1998). Both blockers increased the duration of the ATPo-induced Ca2+ transient (Figure 1a,b). This increase can be seen clearly in the furthest right panel of each trace, where paired time courses of the ATPo induced Ca2+ transients respectively in the absence and presence of the blockers are overlapped. It is obvious that neither La3+ nor orthovanadate had any significant effect on the initial rising phase of the Ca2+ transient in response to ATPo, whereas both drugs significantly increased duration and slowed down the declining phase. It is well known that both La3+ and orthovanadate are blockers not only of PMCPs but also of other P-type pumps, including SERCPs (Carafoli, 1994). When more than 200 μM La3+ was contained in the Ca2+-free perfusing solution, the [Ca2+]i level increased without any stimulation. Furthermore, a much smaller increase in [Ca2+]i was evoked by ATPo after perfusion (data not shown). These observations imply that SERCPs were inhibited by 200 μM La3+. However, we observed no increase in [Ca2+]i in the above blocker-containing perfusing solutions and nearly the same increase in the ATPo-induced [Ca2+]i before and after perfusion. This suggests that the Ca2+ content of internal stores was not affected by the blockers, suggesting that these blockers had a negligible effect on SERCPs. Indeed, it has been previously shown that SERCPs are less sensitive to orthovanadate than PMCPs (Marin et al., 1999). Thus, the similar effect of these two blockers on the duration of the Ca2+ transient strongly suggests that the elongation of the Ca2+ transient is due to the blockade of PMCPs, because these two blockers inhibit PMCPs by completely different mechanisms (Carafoli, 1994; Guerini, 1998). In addition, the effect of orthovanadate can be partially recovered after a 15 min rinse (Figure 1a,d), providing further evidence that PMCPs participate in the ATPo induced Ca2+ transient. A similar conclusion, i.e. that La3+ increases the duration of the TRH (thyrotropin-releasing hormone)-evoked Ca2+ transient by inhibiting PMCP, was reached in GH3 pituitary cells (Nelson & Hinkle, 1994). Moreover, there was no significant change in the duration of the Ca2+ transient when the experiment was performed in Na+-free solution (Figure 1c,f). This result indicates that Ca2+ extrusion by Na+/Ca2+ exchangers has little effect on the ATPo-induced Ca2+ decrease in HEK-293 cells. It has been reported that overexpressing PMCPs in the Chinese hamster ovary cell line significantly decreases the activity of endogenous sarcoplasmic reticulum Ca2+-ATPase (Guerini et al., 1995). This result suggests that there is a compensation effect between PMCPs and SERCPs: i.e., inhibiting one will make the other more active. Therefore, the elongated Ca2+ transient caused by inhibiting PMCPs may have been underestimated in the above experiment, because SERCPs might have been activated more intensively to restore the basal [Ca2+]i level due to the blockade of PMCPs.
Figure 1.
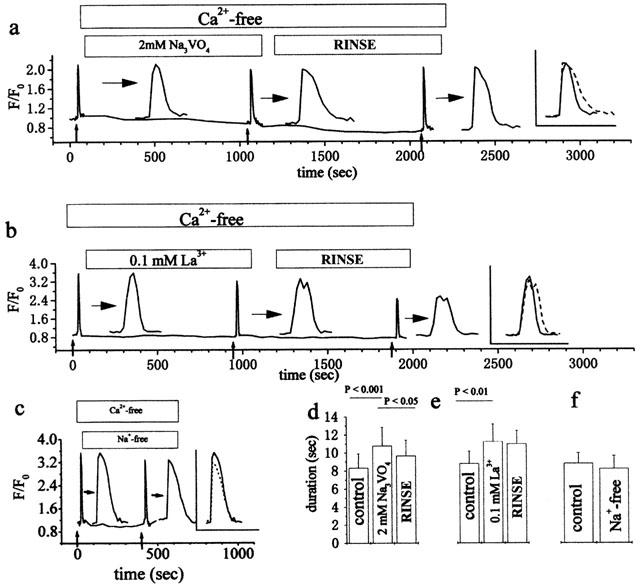
Effect of PMCP blockers and extracellular Na+ depletion on the duration of Ca2+ transients induced by ATPo. Comparison of intracellular Ca2+ transients induced by ATPo in the absence and presence of 2 mM orthovanadate (a), 100 μM La3+ (b) or Na+ depletion (c), respectively. For greater clarity, corresponding Ca2+ transients are expanded 10 times, as indicated by horizontal arrows (a, b and c). An overlap of paired Ca2+ transients induced by ATPo in the absence and presence of each corresponding blocker is shown in the furthest-right panel of a, b and c. Small vertical arrows indicate the time of a puff application of ATPo (50 μM, 5 s). Statistical results on the duration of the Ca2+ transient in a, b and c, which were obtained from at least three independent experiments over 40 cells, are summarized as means±s.d. in d, e and f, respectively. The duration of the Ca2+ transient was calculated as the mean time between the start of the [Ca2+]i increase (defined as the point when [Ca2+]i has increased 50% above the average basal [Ca2+]i) and the point where [Ca2+]i had returned halfway from the peak to the basal.
To evaluate the contribution of ATPo-activated PMCPs to the reduction of ATPo-stimulated cytoplasmic Ca2+ level, experiments were carried out using the following procedure (Figure 2a). First, a [Ca2+]i transient was induced by a puff application of ATPo (50 μM, 20 s) in a Ca2+-free solution to evaluate the level of ATPo-dependent [Ca2+]i increase. Then, a Ca2+-free solution containing 200 nM TG, a highly selective and irreversible inhibitor of SERCPs. (Thastrup et al, 1990), was continuously perfused for 15 min to completely inhibit SERCPs. [Ca2+]i was then elevated through capacitative Ca2+ entry triggered by restoration of extracellular [Ca2+] to 1.3 mM. Finally, after the elevated [Ca2+]i level had become stable, the same amount of ATPo as that used in the first step was applied to the cells. As shown in Figure 2a, the second application of ATPo rapidly decreased [Ca2+]i. It has already been shown that [Ca2+]i elevated by capacitative Ca2+ entry can activate PMCPs (Snitsarev & Taylor, 1999). This explains the stable level of high [Ca2+]i, which may represent a balance between Ca2+ entry and extrusion. The Ca2+ entry is through the capacitative pathway, while the Ca2+ extrusion is due to PMCP activation. However, this PMCP activation due to elevated [Ca2+]i only contributes to keeping the balance between the Ca2+ entry and extrusion, whereas it may not contribute to the rapid decrease in [Ca2+]i. The observation that the decrease may be inhibited by 2 mM orthovanadate (Figure 2b) suggests that the rapid decrease in [Ca2+]i is due to the ATPo-activated PMCPs. The trace in Figure 2a suggests that a large fraction of the increase in [Ca2+]i evoked by ATPo could be removed by ATPo activated PMCPs. This result is in good agreement with an early report that PMCPs can rapidly eject large amounts of Ca2+ during maximal receptor activation (Tepikin et al., 1992). We did not use La3+ to block PMCPs, because it also blocks the capacitative Ca2+ entry in HEK-293 cells as it does in other cells (Klishin et al., 1998). These results indicate that the ATPo activated PMCPs contribute significantly to the clearance of ATPo-induced [Ca2+]i increase.
Figure 2.
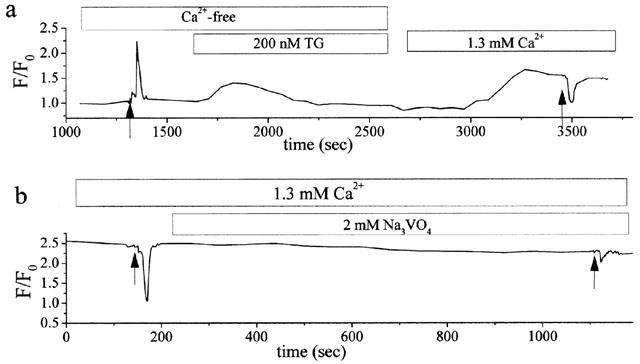
(a) Comparison of the amount of [Ca2+]i changes between ATPo (arrows: 50 μM, 20 s)-induced [Ca2+]i increase and decrease (see text for details). (b) Inhibition of ATPo-induced decrease in [Ca2+]i by 2 mM orthovanadate. Cells were incubated in culture medium containing 200 nM TG during 30-min Fluo-3 loading before rinsing with the Ca2+-free solution. [Ca2+]i was elevated with the standard solution containing 1.3 mM Ca2+ like that in (a). ATPo (arrows: 50 μM, 20 s) was applied to the cells once the [Ca2+]i had reached a steady-state level.
To evaluate whether the Na+-Ca2+ exchanger contributes to the rapid decrease in the ATPo induced [Ca2+]i increase, experiments were carried out in Na+-free solutions under the same experimental condition in Figure 2a. After the [Ca2+]i increase elevated by capacitative Ca2+ entry had reached a stable level, a rapid decrease in [Ca2+]i was induced by ATPo application to the TG pretreated cells (Figure 3a). This decrease was not affected by replacement of extracellular Na+ with NMDG+, a membrane-impermeant cation. Therefore, under a condition where the Na+-Ca2+ exchanger and SERCPs cannot be activated, ATPo can still stimulate rapid Ca2+ extrusion. Besides, Figure 3b shows that the decrease in [Ca2+]i by ATPo is dose-dependent, implying that PMCPs are activated by ATPo.
Figure 3.
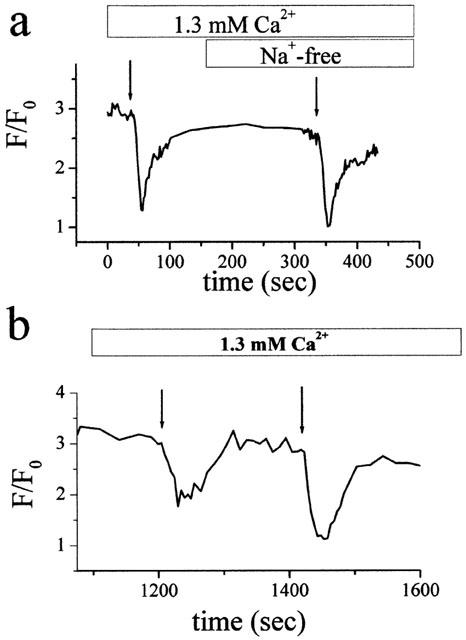
Reduction of steady-state [Ca2+]i by ATPo in the cells pretreated with TG. (a) Comparison of steady-state [Ca2+]i decreases by ATPo in the presence and absence of Na+. Each arrow indicates the time of a puff application of ATPo (50 μM, 10 s). (b) Dose dependence of ATPo-induced decrease in [Ca2+]i. First arrow: 6 μM ATPo, 20 s; second arrow: 50 μM ATPo, 20 s. Experimental procedures in both (a) and (b) as in Figure 2b.
At least two types of P2 receptors, i.e., P2Y1 and P2Y2, are endogenously expressed in HEK-293 cells (Gao et al., 1999; Schachter, 1997). It is well known that ATPo is a potent agonist at these two receptors (Ralevic & Burnstock, 1998). To investigate the involvement of P2Y1 and P2Y2 receptors in PMCP activation by ATPo, 50 μM UTP which is equipotent as ATP at P2Y2 (Janssens et al., 1999) but has almost no effect on P2Y1, was applied to the TG-treated cells. Figure 4a shows that UTP can also cause a decrease in [Ca2+]i, but its effect is smaller than that caused by the same amount of ATP. This result suggests that not only P2Y2 but also P2Y1 is involved in the activation of PMCPs by ATPo. To check if ectophosphorylation and ATP hydrolysis are involved in PMCP activation, ATPγS which is resistant to hydrolytic enzymes was applied to the TG-treated cells. Figure 4b shows that ATPγS also caused significant decrease in [Ca2+]i, although to a lesser extent. This result suggests that ectophosphorylation, the effect of which has been shown in T-lymphocyte (Redegeld et al., 1997) or ATP hydrolysis, plays an insignificant role in PMCP activation by ATPo. ATPγS is a less potent agonist at both P2Y1 (Schachter et al., 1996) and P2Y2 (Janssens et al., 1999) receptors. If the P2Y2 receptor alone is involved in PMCP activation by ATPo, then ATPγS will cause a much smaller decrease in [Ca2+]i than UTP. Consequently, the present result implies that both P2Y1 and P2Y2 receptors are involved in PMCP activation by ATPγS. These results strongly suggest that PMCPs are activated by ATPo through activation of P2Y-type receptors.
Figure 4.
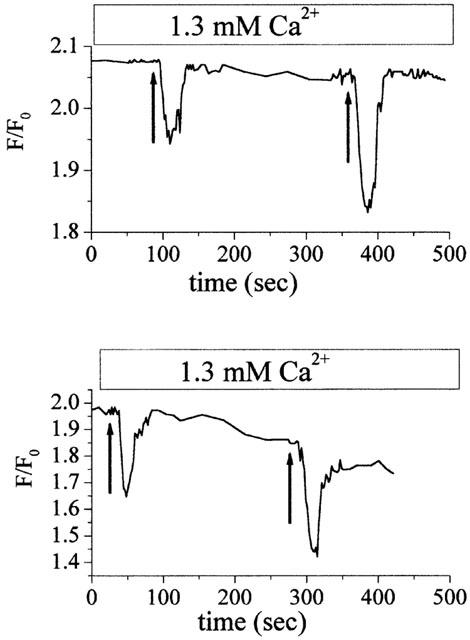
Reduction of steady-state [Ca2+]i by UTP and ATPγS in the cells pretreated with TG. (a) Comparison of steady-state [Ca2+]i decreases by UTP and ATPo. First arrow: 50 μM UTP, 20 s; second arrow: 50 μM ATP, 20 s. (b) Comparison of steady-state [Ca2+]i decreases by ATPγS and ATP. First arrow: 50 μM ATPγS, 20 s. Second arrow: 50 μM ATP, 20 s. Experimental procedures in both (a) and (b) as in Figure 2b.
It has been indicated that Ca2+-calmodulin, protein kinase A and C, acidic phospholipids, and proteases (for review see Carafoli, 1994 and Monteith & Roufogalis, 1995) could be involved in stimulating PMCPs. It has also been shown in human erythrocyte membranes that the activity of calmodulin-dependent PMCPs is Ca2+-dependent, i.e. the higher the [Ca2+]i, the higher the activity of the calmodulin-dependent PMCP (Scharff & Foder, 1982). Since calmodulin-binding sites are highly conserved among PMCPs (Carafoli, 1994), it is possible that calmodulin is involved in the activation of PMCPs by ATPo if the ATPo application can increase [Ca2+]i before ATPo-dependent PMCP activation. However, this possibility might be ruled out, because PMCPs were activated even without any trend toward an increase in [Ca2+]i (Figures 2 and 3). Other pathways for agonist activation of PMCPs remain contradictory. For example, in A7r5 cells, Arg8-vasopressin stimulates Ca2+ extrusion by a phospholipase C (PLC) and protein kinase C (PKC)-independent mechanism (Broad et al., 1999), while in U373 MG astrocytoma cells, PKC is involved in histamine stimulation of PMCPs (Young et al., 1998). Further investigations are required to gain insight into the mechanism of PMCP activation by ATPo.
In summary we have demonstrated that, in HEK-293 cells, ATPo has a dual effect on [Ca2+]i, evoking Ca2+ releases from intracellular stores through activation of purinoceptors and extrusion of Ca2+ by activating PMCPs. Whilst this extrusion process may be considered as a safety mechanism preventing excessive increases in [Ca2+]i, it might also be important in shaping the intracellular Ca2+ signal. These results, together with previous observations on agonist-dependent activation of PMCPs in both excitable and non-excitable cells, suggest that agonist-activated Ca2+ extrusion may be a universal component of the agonist-activated Ca2+ response. Therefore, agonist-activated PMCP plays an important role in generating a variety of Ca2+ signals, the most widely used means of controlling cellular activity (Berridge et al., 1998).
Acknowledgments
This work was supported by grants from CREST, ICORP, Future Program of JSPS and JSF (to M. Sokabe). Z. Qi is a research associate of the Future Program of JSPS.
Abbreviations
- ATPo
extracellular ATP
- ATPγS
adenosine 5′-O-(3-thiotriphosphate)
- [Ca2+]i
intracellular Ca2+ concentration
- HEK-293 cell
human embryonic kidney-293 cell
- PMCP
plasma membrane Ca2+ pump
- SERCP
sarco/endoplasmic reticulum Ca2+ pump
- TG
thapsigargin
- UTP
uridine 5′-triphosphate
References
- BERRIDGE M.J., BOOTMAN M.D., LIPP P. Calcium – a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- BROAD L.M., CANNON T.R., SHORT A.D., TAYLOR C.W. Receptors linked to polyphosphoinositide hydrolysis stimulate Ca2+ extrusion by a phospholipase C-independent mechanism. Biochem. J. 1999;342:199–206. [PMC free article] [PubMed] [Google Scholar]
- CARAFOLI E. Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J. 1994;8:993–1002. [PubMed] [Google Scholar]
- GAO Z., CHEN T., WEBER M.J., LINDEN J. A2B adenosine and P2Y2 receptors stimulate mitogen-activated protein kinase in human embryonic kidney-293 cells: cross-talk between cyclic AMP and protein kinase C pathways. J. Biol. Chem. 1999;274:5972–5980. doi: 10.1074/jbc.274.9.5972. [DOI] [PubMed] [Google Scholar]
- GUERINI D. The significance of the isoforms of plasma membrane calcium ATPase. Cell Tissue Res. 1998;292:191–197. doi: 10.1007/s004410051050. [DOI] [PubMed] [Google Scholar]
- GUERINI D., SCHRÖDER S., FOLETTI D., CARAFOLI E. Isolation and characterization of a stable Chinese hamster ovary cell line overexpressing the plasma membrane Ca2+-ATPase. J. Biol. Chem. 1995;270:14643–14650. doi: 10.1074/jbc.270.24.14643. [DOI] [PubMed] [Google Scholar]
- JANSSENS R., PAINDAVOINE P., PARMENTIER M., BOEYNAEMS J.-M. Human P2Y2 receptor polymorphism: identification and pharmacological characterization of two allelic variants. Br. J. Pharmacol. 1999;126:709–716. doi: 10.1038/sj.bjp.0702619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLISHIN A., MARINA S., BLATTER L.A. Time-dependent modulation of capacitative Ca2+ entry signals by plasma membrane Ca2+ pump in endothelium. Am. J. Physiol. 1998;274:C1117–C1128. doi: 10.1152/ajpcell.1998.274.4.C1117. [DOI] [PubMed] [Google Scholar]
- MARÍN J., ENCABO A., BRIONES A., GARCÍA-COHEN E.-C., ALONSO M.J. Mechanisms involved in the cellular calcium homeostasis in vascular smooth muscle: calcium pumps. Life Sci. 1999;64:279–303. doi: 10.1016/s0024-3205(98)00393-2. [DOI] [PubMed] [Google Scholar]
- MONTEITH G.R., ROUFOGALIS B.D. The plasma membrane calcium pump – a physiological perspective on its regulation. Cell Calcium. 1995;18:459–470. doi: 10.1016/0143-4160(95)90009-8. [DOI] [PubMed] [Google Scholar]
- NELSON E.J., HINKLE P.M. Thyrotropin-releasing hormone activates Ca2+ efflux: evidence suggesting that a plasma membrane Ca2+ pump is an effector for a G-protein-coupled Ca2+-mobilizing receptor. J. Biol. Chem. 1994;269:30854–30860. [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- REDEGELD F.A., SMITH P., APASOV S., SITKOVSKY M.V. Phosphorylation of T-lymphocyte plasma membrane-associated proteins by ectoprotein kinases: implications for a possible role for ectophosphorylation in T-cell effector functions. Biochim. Biophys. Acta. 1997;1328:151–165. doi: 10.1016/s0005-2736(97)00082-5. [DOI] [PubMed] [Google Scholar]
- RINK T.J., SAGE S.O. Stimulated calcium efflux from fura-2-loaded human platelets. J. Physiol. 1987;393:513–524. doi: 10.1113/jphysiol.1987.sp016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER J.B., LI Q., BOYER J.L., NICHOLAS R.A., HARDEN T.K. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinoceptor. Br. J. Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER J.B., SROMEK S.M., NICHOLAS R.A., HARDEN T.K. HEK293 human embryonic kidney cells endogenously express the P2Y1 and P2Y2 receptors. Neuropharmacology. 1997;36:1181–1187. doi: 10.1016/s0028-3908(97)00138-x. [DOI] [PubMed] [Google Scholar]
- SCHARFF O., FODER B. Rate constants for calmodulin binding to Ca2+-ATPase in erythrocyte membranes. Biochim. Biophys. Acta. 1982;691:133–143. doi: 10.1016/0005-2736(82)90222-x. [DOI] [PubMed] [Google Scholar]
- SNITSAREV V.A., TAYLOR C.W. Overshooting cytosolic Ca2+ signals evoked by capacitative Ca2+ entry result from delayed stimulation of a plasma membrane Ca2+ pump. Cell Calcium. 1999;25:409–417. doi: 10.1054/ceca.1999.0041. [DOI] [PubMed] [Google Scholar]
- TEPIKIN A.V., VORONINA S.G., GALLACHER D.V., PETERSEN O.H. Acetylcholine-evoked increase in the cytoplasmic Ca2+ concentration and Ca2+ extrusion measured simultaneously in single mouse pancreatic acinar cells. J. Biol. Chem. 1992;267:3569–3572. [PubMed] [Google Scholar]
- THASTRUP O., CULLEN P.J., DROBAK B.K., HANLEY M.R., DAWSON A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG K.W., PINNOCK R.D., GIBSON W.J., YOUNG J.M. Dual effects of histamine and substance P on intracellular calcium levels in human U373 MG astrocytoma cells: role of protein kinase C. Br. J. Pharmacol. 1998;123:545–557. doi: 10.1038/sj.bjp.0701620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG B.-X., ZHAO H., LOESSBERG P., MUALLEM S. Activation of the plasma membrane Ca2+ pump during agonist stimulation of pancreatic acini. J. Biol. Chem. 1992;267:15419–15425. [PubMed] [Google Scholar]


