Abstract
Haloperidol is a drug used in the management of several psychotic disorders and its use has been linked to Neuroleptic Malignant Syndrome. In the present study we have investigated the effect of a commercial preparation of haloperidol, Serenase, on skeletal muscle sarcoplasmic reticulum.
Addition of Serenase to isolated terminal cisternae caused a rapid release of calcium. We tested whether the active Ca2+-releasing substance was haloperidol or another compound present in the preparation.
Our results show that methyl p-hydroxybenzoate, one of the preservatives and a commonly used anti-microbial agent (E-218) is an activator of Ca2+ release (E.C.50=2.0 mM), mediated by a ruthenium red-sensitive Ca2+ release channel present in skeletal muscle terminal cisternae.
Keywords: Methyl p-hydroxybenzoate, ryanodine receptor, Ca2+release, Neuroleptic Malignant Syndrome
Introduction
Neuroleptic Malignant Syndrome (NMS) is a rare life-threatening complication of neuroleptic treatment characterized by fever, muscle rigidity, tachycardia and altered consciousness; its appearance has been linked to the use of high potency anti-psychotic drugs such as haloperidol and usually appears within 2 weeks of initiating neuroleptic treatment or increasing their dose (Kaufmann & Wyatt, 1987; Jahan et al., 1992; Levitt et al., 1990; Tanaka et al., 1998; Konikoff et al., 1984). Creatine phosphate kinase (skeletal muscle isoenzyme) is elevated in approximately 40% of the patients (Meltzer et al., 1996) and the use of dantrolene has had a favourable effect on NMS patients (Bismouth et al., 1984; Tanaka et al. 1998). This clinical picture closely resembles that of Malignant Hyperthermia (MH), a pharmacogenetic disease characterized by increase in body temperature and muscle contracture which is triggered by volatile inhalational anaesthetics and myorelaxants (Denborough & Lovell, 1960; MacLennan & Phillips, 1992; Michelson & Louis, 1996). In more than 50% of the cases, the latter inherited disorder has been linked to mutations in the ryanodine receptor gene (MacLennan et al., 1990; McCarthy et al., 1990) and to date more than 18 point mutations have been linked to human MH (for a recent review see Jurkat-Rott et al., 2000). The ryanodine receptor (RYR) is a homotetramer of four 565 kDa subunits that mediates Ca2+ release from intracellular stores (Smith et al., 1986). The primary sequence of this protein has been determined from a number of species and Northern blot analysis demonstrated that three isoforms exist, one specific for cardiac muscle, one present in the brain and one specific for skeletal muscle (McPherson & Campbell, 1993).
A large number of chemically diverse substances such as caffeine, thymol, halothane, 4-Cl-m-cresol, doxorubicin, ryanodine, bastadin and Ca2+ have been reported to activate the RYR leading to Ca2+ release (Palade, 1987; Palade et al., 1989; Mack et al., 1994; Zorzato et al., 1986, 1993; Zucchi & Ronchi-Testoni, 1997). One could thus postulate the presence of multiple activator binding sites present on the large hydrophilic amino terminal domain of the molecule. Because of the similarities between some of the clinical features of NMS and MH we hypothesized that the former syndrome may be due, at least in part, to the effect of the drug formulation on the RYR Ca2+ release channel. In the present report we show that a commercial preparation of haloperidol, a neuroleptic most frequently implicated in NMS, is capable of releasing Ca2+ from a ruthenium red-sensitive Ca2+ release channel selectively localized in terminal cisternae (TC). A closer investigation of the chemical substances present in this preparation, revealed that the compound inducing Ca2+-release was not haloperidol per se, but rather the preservative methyl p-hydroxybenzoate. These results support the view that in vivo the RYR could be the target of chemical substances present as preservatives and additives.
Methods
Materials
Serenase was from Lusofarmaco (Ist. Luso Farmaco Ita. Spa, Milano, Italy); 4-Cl-m-cresol, antipyrylazo-III, haloperidol, propyl p-hydroxybenzoate, methyl p-hydroxybenzoate were from Fluka (Buchs, Switzerland); doxorubicin and the Ca2+ ionophore A23187 were from Calbiochem (San Diego, CA, U.S.A.); fura-2/AM, ionomycin, creatine phosphate kinase, creatine phosphate, ATP, caffeine and ruthenium red were from Sigma Chemicals (St. Louis, MO, U.S.A.); phosphatidylethanolamine (PE), phosphatidylcholine (PC) and phosphatidylserine (PS) were from Avanti Polar Lipids (Alabaster, AL, U.S.A.). The EBV-transformed B-lymphoblastoid cell line GS-EBV was cultured in RPMI 1640, 10% FCS and was a generous gift of Prof Giulio Spagnoli, Universität Kantonsspital (Basel, Switzerland). All other chemicals were reagent or highest available grade.
Subcellular fractionation
Sarcoplasmic reticulum (SR) was isolated from the white muscles of New Zealand White rabbits and was fractionated into longitudinal tubules (LSR) and terminal cisternae (TC) in the presence of antiproteolytic agents as described by Saito et al. (1984). The SR fractions were resupended in 0.3 M sucrose, 5 mM imidazole pH 7.4, 100 μM PMSF, 1 μg ml−1 leupeptin and were stored in liquid nitrogen until used. Protein concentration was determined according to the method of Bradford, using bovine serum albumin as standard (Bradford, 1976).
Ca2+ release measurements
Ca2+ release from isolated SR fractions (TC and LSR) was measured in a Beckman DU7400 diode spectrophotometer by monitoring the A710–A790 value of the Ca2+ indicator antipyrylazo-III as described by Palade (1987). The assay was carried out at 37°C in a medium containing, in a final volume of 1.5 ml, 1 mM MgATP, 10 mM creatine phosphate, 10 μg ml−1 creatine phosphate kinase, 200 μg protein, 7.5 mM K-pyrophosphate pH 7.0, 18.5 mM MOPS pH 7.0, 100 mM KCl, 250 μM antipyrylazo-III and 0.1 mM CaCl2. Pulses of 25 nmol calcium were administered to load the SR fractions with approximately 2.3 μM Ca2+ mg protein−1. When steady state was reached Ca2+ release was triggered by the addition of different compounds. At the end of the experiment the signals were calibrated by the addition of 25 nmol Ca2+. Measurements of the initial rate of calcium release were performed with the ‘kinetics time program' software package of the Beckman DU7400 spectrophotometer, as previously described (Zorzato et al., 1993).
Intracellular Ca2+ measurements
Changes in the intracellular Ca2+ concentration of the B-lymphocyte cell line were monitored with the fluorescent calcium indicator fura-2/AM (final concentration 5 μM) as described (Grynkiewwicz et al., 1985; Zorzato et al., 1993). Fluorescent changes (ratio 340/380 nm) were measured in an LS50 Perkin Elmer spectrofluorimeter equipped with a magnetic stirrer and thermostated at 37°C. All measurements were made in Ca2+-free Krebs-Ringer containing 0.5 mM EGTA.
Planar lipid bilayer measurements
TC vesicles were incorporated into planar lipid bilayers of the Mueller/Rudin type (Mueller et al., 1963). Bilayers were formed across the aperture in a 12 μm Teflon septum, using a mixture at a 5 : 2 : 3 ratio of PE, PS and PC phospholipids (30 mg ml−1 in n-decane). For a full description of the conditions please refer to Buratti et al. (1995). Currents were recorded at 0 mV holding potential and are shown as upwards deflections. Single channel analysis was carried out according to Colquhoun & Sigworth (1983). Channel open probability (Po values) was determined from the areas of Gaussian distributions which best fit to peaks of amplitude histograms from recordings of 60–80 s duration.
Data analysis and curve fitting
Statistical analysis was performed using the student's t-test for paired samples. Dose-response curves were fit with the Sigmoidal, Boltzman and Gaussian fit functions using the Origin Microcal 4.1 software program.
Results
We tested the effect of the commercial preparation of haloperidol, Serenase on the initial rate of Ca2+ release from actively loaded TC vesicles, by following the Ca2+ fluxes spectrophotometrically with the indicator antipyrylazo-III. Addition of 1:10 dil of Serenase caused a rapid release of Ca2+ from the TC fraction (Figure 1). We subsequently wanted to establish: (i) what compound in the commercial drug preparation was responsible for this effect and (ii) the mechanism through which calcium was being released. Figure 2 shows the chemical structures of the compounds present in Serenase; each vial contains 2.66 mM haloperidol, 11.1 mM lactic acid, 3 mM propyl p-hydroxybenzoate and 3 mM methyl p-hydroxybenzoate. Each one of these substances was individually tested for its capacity to release Ca2+ from actively loaded TC vesicles. Figure 3 shows that neither haloperidol (trace a), nor lactic acid (trace b) nor propyl p-hydroxybenzoate (trace c) significantly affected Ca2+ release. The small upwards and downward deflections seen after the addition of haloperidol and lactic acid are due to the presence of small amounts of contaminating Ca2+ in these preparations. On the other hand methyl p-hydroxybenzoate (trace d) had a small Ca2+ releasing effect. We then tested in greater detail each of these substances for its Ca2+ releasing ability; higher concentrations of haloperidol (up to 3 mM), lactic acid (up to 10 mM) and propyl p-hydroxybenzoate (up to 5 mM) had no discernible effect on Ca2+ release from TC (not shown). At higher concentrations methyl p-hydroxybenzoate was capable of releasing Ca2+ from actively loaded TC vesicles (Figure 4a); the addition of successive increasing amounts of this compound did not result in a synergistic stimulation of Ca2+ release (Figure 4a). In fact addition of 2 mM or 0.4 plus 1.6 mM methyl p-hydroxybenzoate resulted in a net Ca2+-release rate of 0.59 (±0.12; n=6) and 0.63 (±0.12; n=6) μmoles of Ca2+ mg protein−1 min−1 (P=0.587). A similar additive effect was seen at higher concentrations of methyl p-hydroxybenzoate (not shown). Figure 4b shows the dose-dependent effect of methyl p-hydroxybenzoate on the kinetics of Ca2+ release from TC. The half-maximal stimulatory dose of Ca2+ release was 2 mM whereas at 4 mM this compound released approximately 2.4 μmoles of Ca2+ mg protein−1 min−1. Such a rate is in the same order of magnitude as that seen with 5 mM caffeine (Palade, 1987) and 1 mM 4-Cl-m-cresol (Zorzato et al. 1993). This Ca2+-releasing effect could be due to inhibition of the Ca2+ATPase, to a damage of the vesicles or to a direct effect of the compound on the RYR Ca2+ release channel. In order to address this problem LSR vesicles which are enriched in the Ca2+ATPase were tested. In this case, methyl p-hydroxybenzoate failed to elicit any Ca2+ release (Figure 5a) indicating that the above mentioned effect (i) could not be due to a damage inflicted on the vesicles and (ii) was not the consequence of an inhibitory action on the Ca2+ pump but most likely due to activation of the RYR Ca2+ channel. Figure 5b shows that pretreatment of TC vesicles with ruthenium red (final concentration 10 μM) a known inhibitor of the RYR, completely abolished the Ca2+-releasing action of methyl p-hydroxybenzoate confirming that its effect is modulated by the RYR intracellular calcium channel.
Figure 1.
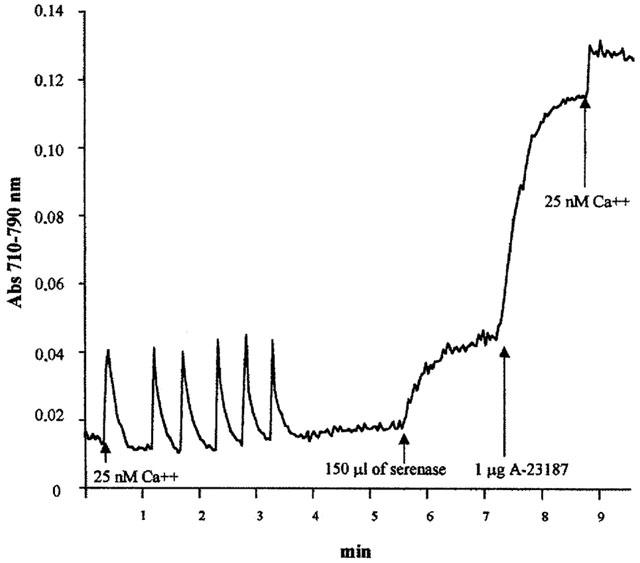
Serenase, a commercial preparation of haloperidol, stimulates Ca2+ release from isolated rabbit skeletal muscle terminal cisternae. The experiments were carried out as described in the Methods section, using the Ca2+ indicator antipyrylazo-III. Where indicated six consecutive additions of 25 nmol of CaCl2 were administered; after Ca2+ loading, 150 μl Serenase (1 : 10 dilution) were added. At the end of the experiment the Ca2+ ionophore A23187 was added to release remaining Ca2+, followed by the addition of 25 nmol CaCl2 used to calibrate the dye response. Upward deflections indicate Ca2+ release and downward deflections indicate Ca2+ accumulation.
Figure 2.
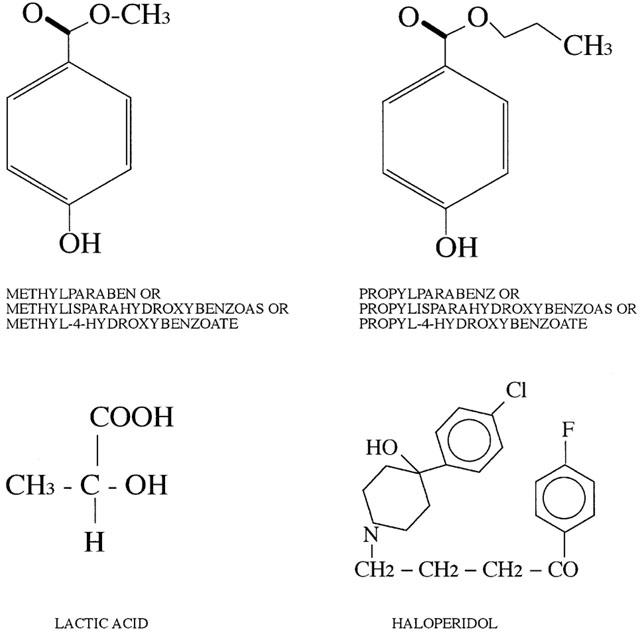
Chemical structure of the components present in a vial of Serenase.
Figure 3.
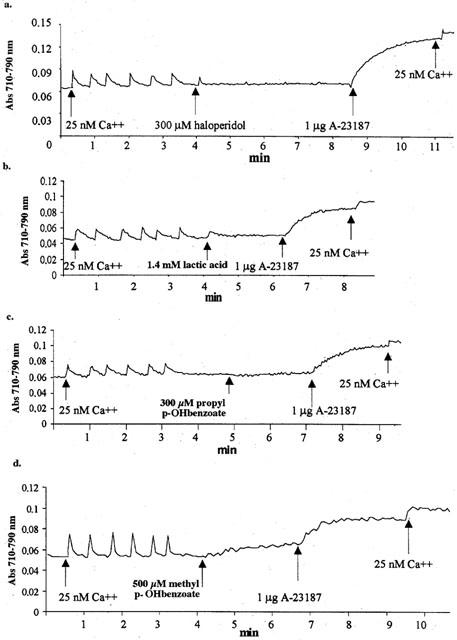
Ca2+-releasing properties of the single components present in Serenase. Conditions were as those described in Figure 1; where indicated the following compounds were added. Trace a, 300 μM haloperidol; trace b, 1.4 mM lactic acid; trace c, 300 μM propyl p-hydroxybenzoate and trace d, 500 μM methyl p-hydroxybenzoate. Traces are representative of experiments carried out at least four times.
Figure 4.
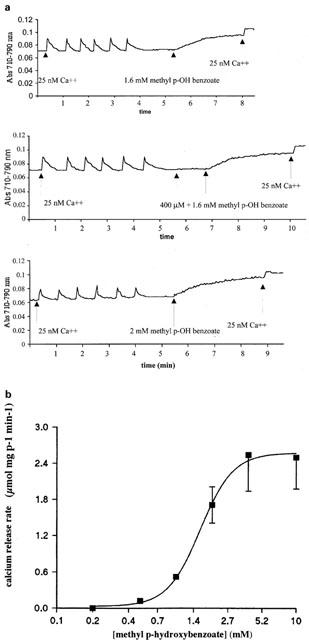
Dose dependence of methyl p-hydroxybenzoate-induced net Ca2+ release from rabbit skeletal muscle terminal cisternae. Experiments were carried out as described in Figure 1. (a): Ca2+ release was stimulated by the addition of the indicated concentrations of 4 mM methyl p-hydroxybenzoate. The traces are representative of experiments carried out six separate times. (b) Increasing concentrations of methyl p-hydroxybenzoate were added to terminal cisterane in order to obtain the initial rate of Ca2+ release. Results are the mean±standard deviation of four experiments.
Figure 5.
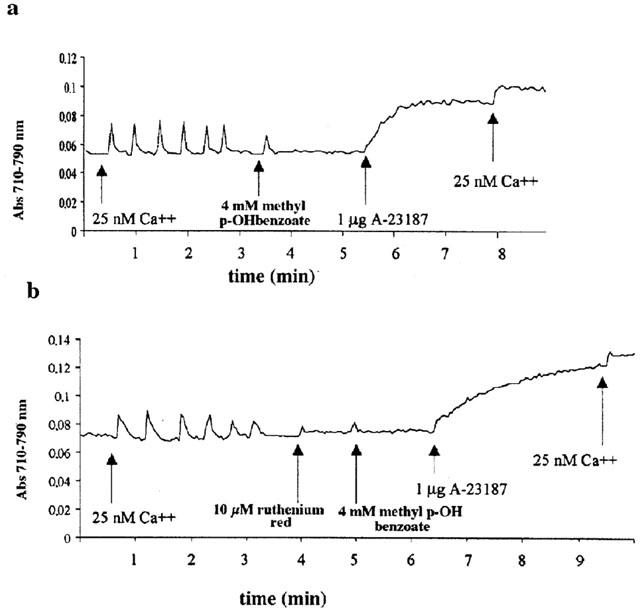
Characteristics of methyl p-hydroxybenzoate-induced Ca2+ release. No Ca2+ is released from isolated rabbit longitudinal sarcoplasmic reticulum (LSR, trace a) while its effect in terminal cisternae (TC) is blocked by pre-treatment with 10 μM ruthenium red (trace b). Conditions as described in Figure 1.
This effect of methyl p-hydroxybenzoate on the skeletal muscle RYR Ca2+ channel was also confirmed by testing the preservative on single channels incorporated into planar lipid bilayers. As shown in Figure 6 addition of 5 mM of the compound to the cis chamber increased the Po from 0.221 (controls) to 0.457 (5 mM methyl p-hydroxybenzoate), corresponding to an increment of 103±14% (n=4) of the RYR channel probability being in the open state. This effect was reversible and could be inhibited by the addition of 10 μM ruthenium red (bottom trace, Figure 6).
Figure 6.
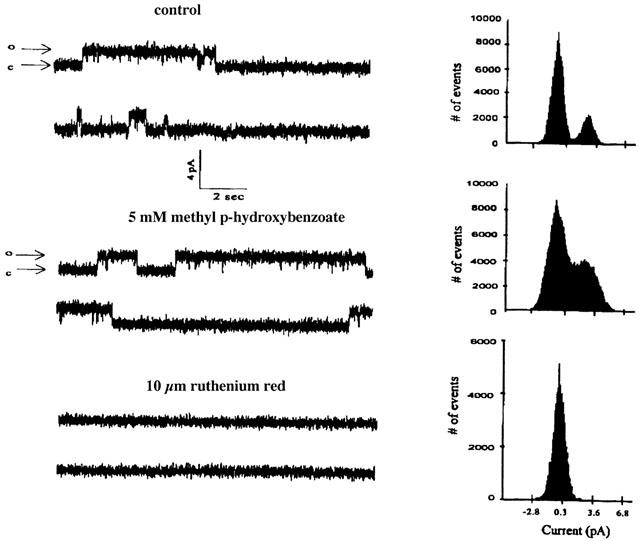
Effect of methyl p-hydroxybenzoate on the open probability of single channels incorporated into lipid bilayers. TC vesicles were fused with lipid bilayers and single channel currents and amplitude histograms were measured as described in the Methods section. Single channel activity, recorded at 0 mV holding potential, exhibited an open level having a conductance of 75 pS and an open probability (Po value) of 0.221 in the controls (top trace) and 0.457 in the presence of 5 mM methyl p-hydroxybenzoate (middle trace). The subsequent addition of 10 μM ruthenium red fully inhibited the current fluctuations (bottom trace). The charge carrier was Na+ in a gradient concentration in the trans/cis compartment of 50/250 mM NaCl; c=closed channel, o=open channel.
Because methyl p-hydroxybenzoate is present in a variety of formulations, we also tested whether its presence affected the calcium release rate of two other well known RYR activators, doxorubicin and caffeine. The curves depicted in Figure 7a show that the presence or absence of 2 mM methyl p-hydroxybenzoate did not significantly affect the calcium release dose response curve of isolated TC to caffeine. On the other hand, the presence of the preservatives had a small but significant effect on doxorubicin-induced calcium release from isolated TC fractions: in fact it shifted the EC50 of doxorubicin from 40 μM to about 25 μM, though the maximal effect was not altered (Figure 7b).
Figure 7.
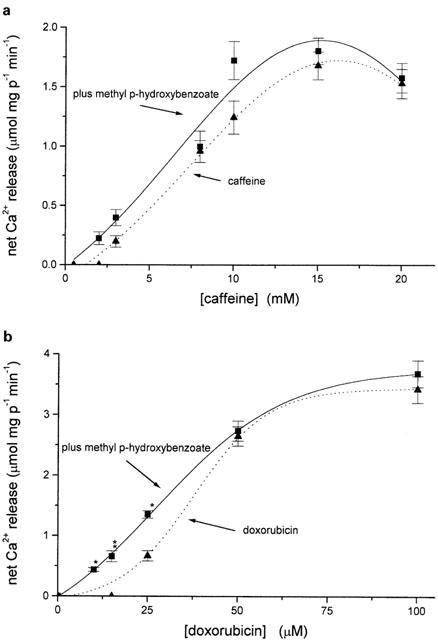
Methyl p-hydroxybenzoate decreases the EC50 of doxorubicin-induced Ca2+ release but does not affect caffeine-induced calcium release from isolated terminal cisternae. (a) Initial rates of calcium release from terminal cisternae induced by increasing concentrations of caffeine alone or caffeine plus 200 μM methyl p-hydroxybenzoate or (b) doxorubicin alone or doxorubicin plus 200 μM methyl p-hydroxybenzoate. Conditions as in Figure 4b. Results are the mean (±s.e.mean) of at least eight experiments. *P<0.0006; **P=0.026.
In a recent report Sei et al. (1999) show that human B-lymphocytes express the skeletal muscle type 1 ryanodine receptor. We thus tested whether methyl p-hydroxybenzoate affects the intracellular Ca2+ concentration, [Ca2+]i, of a human B-lymphocyte cell line. The addition of 4 mM methyl p-hydroxybenzoate caused a rapid increase in the [Ca2+]i of fura-2 loaded cells, which slowly (8–10 min) declined back to resting levels. The subsequent addition of 4-Cl-m-cresol, a specific activator of Ca2+ release via the skeletal muscle RYR Ca2+ channel, had no effect on the [Ca2+]i. On the other hand ionomycin was still capable of releasing Ca2+ (Figure 8a). Thus the lack of effect of 4-Cl-m-cresol was not due to depletion of all the intracellular Ca2+stores but rather these results suggest that methyl p-hydroxybenzoate and 4-Cl-m-cresol induce Ca2+ release by activating the same channel. Figure 8b shows that addition of 4-Cl-m-cresol to the B-cell line caused a large and sustained Ca2+ transient, completely abolished the effect of methyl p-hydroxybenzoate and greatly reduced the effect of ionomycin. These experiments demonstrate (i) that the intracellular Ca2+ pool that is sensitive to methyl p-hydroxybenzoate is the same as that which is sensitive to 4-Cl-m-cresol and (ii) that the latter compound is more potent at activating Ca2+ release. Pre-treatment of the B-cell line with ionomycin completely blocked the Ca2+-releasing effect of methyl p-hydroxybenzoate (Figure 8c); a result consistent with the observation that the methyl p-hydroxybenzoate-sensitive pool is contained within the ionomycin-sensitive one.
Figure 8.
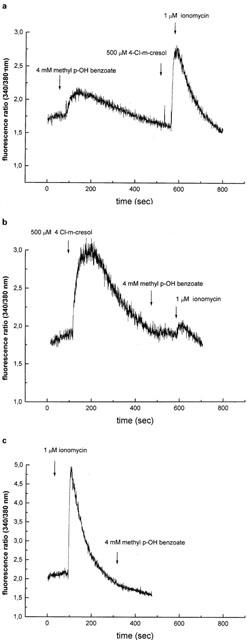
Effect of methyl p-hydroxybenzoate on the [Ca2+]i of the human B-lymphoblastoid cell line GC-BB. In each experiment 0.5×106 cells ml−1 loaded with 5 μM fura-2/AM were added to the thermostated, magnetically stirred cuvette and the fluorescence ratio recorded. Once a steady resting [Ca2+]i was reached, the effect of various agonists on calcium homeostasis was determined. (a) Where indicated, 4 mM methyl p-hydroxybenzoate were added. When [Ca2+]i returned back to the resting level, 4-chloro-m-cresol and ionomycin were added. (b) Same as (a) except that 4-Cl-m-cresol was added prior to methyl p-hydroxybenzoate. Ionomycin was added at the end to determine the status of the intracellular stores. (c) Same as (a), but ionomycin was added first to deplete all intracellular calcium stores. Experiments were performed in Ca2+-free medium containing 0.5 mM EGTA. The traces are representative of at least five separate experiments.
Discussion
The initial aim of our study was to investigate whether the pharmacological basis of Neuroleptic Malignant Syndrome, which has been associated with the use of substances such as haloperidol, could be explained by a direct effect of the drug on the RYR. This is the basis of the pharmacogenetic disease MH, which is triggered by volatile anaesthetics and succinylcholine in patients who have been shown to have functional alterations in their RYR Ca2+ release channel or other proteins involved in SR calcium homeostasis (MacLennan & Phillips, 1992; Michelson & Louis, 1996). Under our experimental conditions, we could not observe any effect of haloperidol on the RYR Ca2+ channel. We report however, that methyl p-hydroxybenzoate is capable of releasing Ca2+ from isolated terminal cisternae by directly activating the RYR Ca2+ release channel at a concentration similar to that of caffeine a well known RYR activator. The effect is specific since (i) pretreatment of TC vesicles with ruthenium red, an inhibitor of this Ca2+ release channel, completely blocks the effect of methyl p-hydroxybenzoate; (ii) the preservative has no effect on LSR vesicles and thus it does not interfere with the Ca2+ATPase and (iii) it does not damage the vesicles. Thus this compound can be added to the growing number of unrelated drugs and chemical substances which have been shown to activate the RYR and induce SR Ca2+ release.
Though methyl p-hydroxybenzoate can directly activate Ca2+ release, its recommended concentration in drug formulations is in the low millimolar range and thus at this concentration its use is unlikely to trigger activation of the RYR in individuals with a wild type Ca2+ channel. However, the association of drug formulations containing other activators of the RYR such as doxorubicin (Zorzato et al., 1986) and 4-Cl-m-cresol (Zorzato et al. 1993), could potentially cause activation of the Ca2+ release channel in certain individuals. This conclusion is supported by our observation of the potentiating effect of methyl p-hydroxybenzoate on doxorubicin-induced calcium release. As to its effect, our data suggest that even at such low subthreshold concentrations, the latter compound has a small but significant effect on the EC50 for doxorubicin-induced calcium release.
Methyl p-hydroxybenzoate is largely used as an antimicrobial agent in the food industry, where it is short handed E218. According to the Food and Drug Administration methyl p-hydroxybenzoate can be added to food up to a final concentration of 0.1%. Thus, ingestion of 200–300 g of food containing 0.1% E218 would result in the intake of 2–3 mmol of methyl p-hydroxybenzoate. If such an amount reaches the plasma volume, the concentration that would be achieved is quite close to that required for the half maximal-activation of the RYR in vitro.
Of interest, our results also show that addition of millimolar concentrations of methyl p-hydroxybenzoate to a human B-lymphocyte-derived cell line causes a rapid and transient increase in the [Ca2+]I. We think that this effect is most likely mediated by direct activation of their RYR1 type calcium channel since (i) in isolated skeletal muscle terminal cisternae and in reconstituted channels incorporated into lipid bilayers, the compound was shown to activate the RYR; (ii) its effect was blocked by ruthenium red, a well unknown inhibitor of the RYR and B-lymphocytes are endowed with type 1 RYR (Sei et al., 1999). The activation of B-lymphocytes could occur in the circulation or more importantly at the level of the mucosa and/or epithelium lining the gastrointestinal tract. Thus the adsorption of methyl p-hydroxybenzoate may have the undesired side effect of activating B-lymphocytes present in mucosa associated lymphoid tissues. In B-lymphocytes Ca2+ signalling has been implicated in various physiological responses including gene expression, cell proliferation and secretion of antigen-specific antibodies (Yamada et al., 1993; Dolmetsch et al., 1997). Sei et al. (1999) showed that the skeletal muscle ryanodine receptor present in human B-lymphocytes appears to be involved in calcium signalling via the B-cell receptor. Thus it may well be that in some patients who show intolerance or allergy to food, one triggering signal could be represented by the preservative added to the food. Since Ca2+ signalling determines the ultimate response of B-cells and particularly secretion of antibodies, the presence of a food additive which can directly cause Ca2+ release needs to be carefully reconsidered.
Acknowledgments
We would like to thank Dr Miodrag Filipovic and Prof Giulio Spagnoli for helpful suggestions. This work was supported by grants from Ministero dell'Universita' e della Ricerca Scientifica e Tecnologica 40 and 60% and the Anaesthesia Department of Basel University Hospital.
Abbreviations
- [Ca2+]i
intracellular calcium concentration
- LSR
longitudinal sarcoplasmic reticulum
- methyl p-OH benzoate
methyl p-hydroxybenzoate
- MH
malignant hyperthermia
- MNS
malignant neuroleptic syndrome
- RYR
ryanodine receptor
- SR
sarcoplasmic reticulum
- TC
terminal cisternae
References
- BISMOUTH C., DE ROHAN-CHABOT P., GOULON M., RAPHAEL J.C. Dantrolene-a new therapeutic approach to the neuroleptic malignant syndrome. Acta. Neurol. Scand. 1984;100 Suppl:193–198. [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BURATTI R., PRESTIPINO G., MENEGAZZI P., TREVES S., ZORZATO F. Calcium dependent activation of skeletal muscle Ca2+-release channel (ryanodine receptor) by calmodulin. Biochem. Biophys. Res. Commun. 1995;213:1082–1090. doi: 10.1006/bbrc.1995.2238. [DOI] [PubMed] [Google Scholar]
- COLQUHOUN D., SIGWORTH F.J. Single channel recording 1983New York, Plenum Press; 191–263.ed. Sakmann, B. and Neher, E. pp [Google Scholar]
- DENBOROUGH M.A., LOVELL R.R.H. Anesthetic deaths in a family. Lancet. 1960;2:45. [Google Scholar]
- DOLMETSCH R.E., LEWIS R.S., GOODNOW C.C., HEALY J.I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R. A new generation of Ca2+ indicators with greatly improved fluorescent properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- JAHAN M.S., FAROOQUE A.I., WAHID Z. Neuroleptic malignant syndrome. J. Natl. Med. Assoc. 1992;84:966–970. [PMC free article] [PubMed] [Google Scholar]
- JURKAT-ROTT K., MCCARTHY T., LEHMANN-HORN F. Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve. 2000;23:4–17. doi: 10.1002/(sici)1097-4598(200001)23:1<4::aid-mus3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- KAUFMANN C.A., WYATT R.J.Neuroleptic malignant syndrome Psychopharmacology: The third generation of progress 1987New York, Raven Press; 1421–1429.ed. Meltzer, H.Y. pp [Google Scholar]
- KONIKOFF F., KURITZKY A., JERUSHALMI Y., THEODOR E. Neroleptic malignant syndrome induced by a single injection of haloperidol. Br. Med. J. 1984;289:1228–1229. doi: 10.1136/bmj.289.6453.1228-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVITT A.J., MIDHA R., CRAVEN J.L. Neuroleptic malignant syndrome with intravenous haloperidol. Can. J. Psychiatry. 1990;35:789. doi: 10.1177/070674379003500914. [DOI] [PubMed] [Google Scholar]
- MACK M.M., MOLINSKI T.F., BUCK E.D., PESSAH I.N. Novel modulators of skeletal muscle FKBP12/calcium channel complex from Ianthella basta. Role of FKBP12 in channel gating. J. Biol. Chem. 1994;269:23236–23249. [PubMed] [Google Scholar]
- MACLENNAN D.H., DUFF C., ZORZATO F., FUJII J., PHILLIPS M., KORNELUK R.G., FRODIS W., BRITT B., WORTON R.G. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990;343:559–561. doi: 10.1038/343559a0. [DOI] [PubMed] [Google Scholar]
- MACLENNAN D.H., PHILLIPS M.S. Malignant hyperthermia. Science. 1992;256:789–794. doi: 10.1126/science.1589759. [DOI] [PubMed] [Google Scholar]
- MCCARTHY T.V., HEALY J.M.S., HEFFRON J.J.A., LEHANE M., DEUFEL T., LEHMANN-HORN F., FARALL M., JOHNSON K. Localization of the malignant hyperthermia susceptibility locus to human chromosome 19q12-13.2. Nature. 1990;343:562–564. doi: 10.1038/343562a0. [DOI] [PubMed] [Google Scholar]
- MCPHERSON P.S., CAMPBELL K.P. The ryanodine receptor/Ca2+ release channel. J. Biol. Chem. 1993;268:13765–13768. [PubMed] [Google Scholar]
- MELTZER H.Y., COLA P.A., PARSA M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15:395–405. doi: 10.1016/0893-133X(95)00276-J. [DOI] [PubMed] [Google Scholar]
- MICHELSON J.R., LOUIS CF. Malignant hyperthermia: excitation-contraction coupling, Ca2+ release channel and cell Ca2+-regulation defects. Physiol. Rev. 1996;76:537–591. doi: 10.1152/physrev.1996.76.2.537. [DOI] [PubMed] [Google Scholar]
- MUELLER P., RUDIN D.O., TIEN H.T., WESCOTT W.C. Methods for the formation of single biomolecular lipid membranes in aqueous solutions. J. Phys. Chem. 1963;67:534–535. [Google Scholar]
- PALADE P. Drug -induced Ca2+ release from isolated sarcoplasmic reticulum. II. Release involving a Ca2+-induced Ca2+ release channel. J. Biol. Chem. 1987;262:6142–6148. [PubMed] [Google Scholar]
- PALADE P., DETTBARN C., BRUNDER D., STEIN P., HALS G. Pharmacology of calcium release from sarcoplasmic reticulum. J. Biomembr. Bioenerg. 1989;21:295–320. doi: 10.1007/BF00812074. [DOI] [PubMed] [Google Scholar]
- SAITO A., SEILER S., CHU A., FLEISCHER S. Preparation and morphology of SR terminal cisternae from rabbit skeletal muscle. J. Cell. Biol. 1984;99:875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEI Y., GALLAGHER K.L., BASILE A.S. Skeletal muscle type ryanodine receptor is involved in calcium signaling in human B-lymphocytes. J. Biol. Chem. 1999;274:5995–6002. doi: 10.1074/jbc.274.9.5995. [DOI] [PubMed] [Google Scholar]
- SMITH J.S., CORONADO R., MEISSNER G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+ J. Gen. Physiol. 1986;88:573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA K., AKECHI T., YAMAZAKI M., HAYASHI R., NISHIWAKI Y., UCHITOMI Y. Neuroleptic malignant syndrome during haloperidol treatment in a cancer patient. A case report. Support. Care Cancer. 1998;6:536–538. doi: 10.1007/s005200050211. [DOI] [PubMed] [Google Scholar]
- YAMADA H., JUNE C.H., FINKELMAN F., BRUNSWICK M., RING M.S., LEES A., MOND J.J. Persistent calcium elevation correlates with the induction of surface immunoglobulin-mediated B cell DNA synthesis. J. Exp. Med. 1993;177:1613–1621. doi: 10.1084/jem.177.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZORZATO F., MARGRETH A., VOLPE P. Direct photoaffinity labelling of junctional sarcoplasmic reticulum with [14C]doxorubicin. J. Biol. Chem. 1986;261:13252–13257. [PubMed] [Google Scholar]
- ZORZATO F., SCUTARI E., TEGAZZIN V., CLEMENTI E., TREVES S. Chlorocresol: an activator of ryanodine receptor-mediated Ca2+ relase. Mol. Pharmacol. 1993;44:1192–1201. [PubMed] [Google Scholar]
- ZUCCHI S., RONCA-TESTONI S. The sarcoplasmic reticulum Calcium channel ryanodine receptor: modulation by endogenous effectros, drugs and diseases states. Pharmacol. Rev. 1997;49:1–51. [PubMed] [Google Scholar]


