Abstract
Hepatic stellate cells (HSC) and their transformed phenotype found in the chronically injured liver play important roles in hepatic physiology and pathology. HSC produce and react to a potent contractile peptide endothelin-1 (ET-1) and also synthesize a vasorelaxant nitric oxide (NO) upon stimulation with endotoxin. However, whether endotoxin affects ET-1 system of HSC and if this is a mechanism of endotoxin-induced hepatic injury is not known.
We characterized synthesis of ET-1 and NO and ET-1 receptors in cultured quiescent and transformed HSC subjected to endotoxin treatment. Endotoxin (1–1000 ng ml−1) stimulated synthesis of ET-1 and NO and up-regulated ET-1 receptors in both cell types.
Inhibition of NO synthesis by NG-monomethyl-L-homoarginine strongly inhibited endotoxin-induced increase in ET-1 receptors in transformed HSC but produced small additional increase in quiescent HSC. Inhibition of soluble guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one blocked the effect of endotoxin on ET-1 receptors in both cell types. Moreover, ET-1 receptors were increased in both cell types during earlier time points (1–4 h) of endotoxin treatment in the absence of the stimulation of NO synthesis.
These results demonstrate that endotoxin up-regulates ET-1 receptors in HSC by NO-dependent and -independent mechanisms. Such effects of endotoxin can be of importance in acute endotoxemia and during chronic injury of the liver.
Keywords: Endothelin, endotoxin, hepatic stellate cells, liver, nitric oxide, receptor
Introduction
Hepatic stellate cells (HSC) (also known as perisinusoidal cells, fat-storing cells, Ito cells) are found in the perisinusoidal areas in the space of Disse. HSC play an important role in regulating the sinusoidal blood flow by their contractile activity and in maintaining the architecture of the liver by producing components of the extracellular matrix (Geerts et al., 1994). During chronic liver injury, HSC shed their stored retinoids and transform into proliferating myofibroblast-like cells that express α-smooth muscle actin (Ramadori et al., 1990; Blomhoff & Wake, 1991). Cultured HSC isolated from the normal liver also lose retinoids gradually and transform into myofibroblast-like cells. The transformed HSC are extremely contractile and excessively fibrogenic and thus contribute to the pathologic developments in the diseased liver (Ramadori et al., 1990; Blomhoff & Wake, 1991; Geerts et al., 1994).
Endothelin-1 (ET-1), a potent constrictor of the hepatic vasculature (Gandhi et al., 1990; Tran-Thi et al., 1993), exerts contractile action on the quiescent (Sakamoto et al., 1993; Zhang et al., 1995) and transformed (Pinzani et al., 1992; Kawada et al., 1993; Housset et al., 1993a) HSC. Nitric oxide (NO), on the other hand, causes relaxation of HSC (Kawada et al., 1993) and vascular smooth muscle cells (Moncada et al., 1991). Amelioration of endotoxin-induced increase in portal resistance by ET-1 receptor antagonists and its augmentation upon inhibition of NO synthesis (Pannen et al., 1996a) suggest an imbalance in the synthesis and actions of these mediators in the liver. We and others have shown that the ET-1 system is up-regulated in human (Pinzani et al., 1996; Gandhi et al., 1996a; Leivas et al., 1998) and experimental (Gandhi et al., 1996b) liver cirrhosis, and that portal hypertension in the cirrhotic rats is reduced by ET-1 receptor antagonists (Rockey & Weisiger, 1996; Gandhi et al., 1998). Since endotoxin concentration is increased in cirrhosis (Fischer & Baldessarini, 1971; Bernardi et al., 1983; Liehr & Jacob, 1983), it is possible that a mechanism of the up-regulation of the ET-1 system may involve the action of endotoxin on the target cells such as HSC.
Although the actions of cytokines produced by macrophages is an important mechanism of endotoxin-mediated effects on various cell types, it has also been shown to exert direct effects on nonmacrophage cell types (Nolan, 1981). Endotoxin causes depression of contractile activity of cardiac myocytes (Yasuda & Lew, 1997), stimulates NO synthesis in endothelial cells (Moncada et al., 1991; Ros et al., 1997) and HSC (Kawada et al., 1998), and inhibits DNA synthesis in vascular smooth muscle cells (Paul et al., 1997) and HSC (Kawada et al., 1998). Considering the central role of HSC in liver pathophysiology and their responses to endotoxin (Kawada et al., 1998), NO (Kawada et al., 1993) and ET-1 (Pinzani et al., 1992; Housset et al., 1993a; Kawada et al., 1993; Sakamoto et al., 1993; Zhang et al., 1995; Mallat et al., 1996; Gabriel et al., 1998; 1999), interactions between these mediators and HSC can be important mechanisms underlying the pathophysiologic developments in the liver. Therefore, we investigated whether endotoxin can directly affect ET-1 receptors in normal and transformed HSC and whether NO plays a role in this phenomenon.
Methods
Materials
The following were purchased from the indicated sources: Protease type XIV (from Streptomyces griseus), Nycodenz, bovine serum albumin (fraction V) HEPES (N-[2-hydroxyethyl] piperazine-N′-[ethanesulphonic acid]), phenylmethylsulphonyl fluoride (PMSF), aprotinin, endotoxin (Escherichia coli lipopolysaccharide [LPS], serotype 0111:B4) (Sigma Chemical Co., St. Louis, MO, U.S.A.); collagenase type IV (from Clostridium histolyticum) (Worthington Biochemical Corporation, Freehold, NJ, U.S.A.); Dulbecco's modified Eagle's medium, penicillinG, streptomycin, foetal bovine serum and horse serum (Gibco-BRL, Grand Island, NY, U.S.A.); endothelin-1, BQ-123 and sarafotoxin S6c (American Peptide, Sunnyvale, CA, U.S.A.); [125I]-endothelin-1 (2200 Ci mmol−1) (Du Pont-NEN, Boston, MA, U.S.A.); L-NMMA (NG-monomethyl-L-homoarginine monoacetate) and ODQ ([1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one]) (Alexis Biochemicals, San Diego, CA, U.S.A.).
The experimental protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee in accordance with the guidelines of the National Institutes of Health.
Preparation of stellate cells
Stellate cells were prepared essentially as described previously (Gabriel et al., 1998; 1999). Briefly, the livers of male Sprague-Dawley rats (450–500 g) were digested with protease and collagenase. After removal of hepatocytes and cell debri by low speed centrifugation, HSC were purified from the other nonparenchymal cells by centrifugation on a Nycodenz gradient. The cells were suspended in DMEM containing 10% foetal bovine serum/10% horse serum and antibiotics, and plated in 24-well culture plates (1×106 cells well−1). The cells were plated in 100 mm culture dishes for the determination of protein and mRNA expression. The viability of the cells was greater than 95% as determined by Trypan Blue exclusion. The medium was renewed after overnight incubation and subsequently every 72 h. The purity of the cells was determined by light microscopy and vitamin A autofluorescence. Further characterization was done immunohistochemically using specific markers for endothelial cells (anti-factor VIII related antigen antibody; DAKO, Carpintoria, CA, U.S.A.), stellate cells (anti-desmin and -α-smooth muscle actin antibodies; DAKO), Kupffer cells (clone ED2; Serotec, Indianapolis, IN, U.S.A.) and epithelial cells (clone AE1/AE3; Boehringer Mannheim, Indianapolis, CA, U.S.A.) as described previously (Gandhi et al., 1999). The purity of the cells and the plating efficiency were greater than 95 and 75% respectively. Experiments were performed on day 2 (control cells) and on day 12 (transformed cells) of culture interval when more than 80% cells were transformed as indicated by their expression of α-smooth muscle actin.
Determination of ET-1
ET-1 was extracted from the culture medium, and its concentration determined by ELISA using a commercial kit (Peninsula laboratories, Belmont, CA, U.S.A.). Detailed procedure of the extraction and analysis of ET-1 has been described previously (Gabriel et al., 1998; 1999).
[125I]ET-1 binding assay
Cells were washed and placed in serum-free medium with or without LPS and other test agents (at concentrations indicated in the figure legends). After the incubation periods specified in the legends, cells were washed with HBSS containing 10 mM HEPES, pH 7.4, and 0.1% bovine serum albumin (HBSS/BSA) and placed in this medium containing 5–800 pM [125I]-ET-1±1 μM unlabelled ET-1 (saturation binding). In the competition binding assay, cells were incubated with 20 pM [125I]-ET- 1±20 pM–1 μM unlabelled ET-1, 100 μM–1 mM ETA antagonist BQ-123 (Ihara et al., 1992) or 20 pM–10 μM ETB agonist sarafotoxin S6c (Williams et al., 1991). After incubation at 22°C for 2 h, the cells were washed with HBSS/BSA and digested with 0.75 N NaOH for determination of radioactivity. Specific binding of [125I]-ET-1 was the difference between cell-associated radioactivity in the presence and absence of 1 μM unlabelled ET-1.
Determination of mRNA expression
Semiquantitative reverse transcriptase polymerase chain reaction (RT–PCR) assay was employed to assess the relative levels of preproET-1, ET-1 receptors and iNOS mRNA in LPS-stimulated and unstimulated HSC as described previously (Gabriel et al., 1998; 1999). Levels of β-actin mRNA were determined to ascertain the efficiency of cDNA synthesis and reverse transcription. The PCR primers specific for preproET-1 cDNA: 5′-CCAACTCTGGGCTCTCCATGCTGG-3′ (F) and 5′-GAATGGCACTGTGTCTCTGCTCTC-3′ (R) [241 bp (Sakurai et al., 1991)]; ETA cDNA: 5′-CCCTTCGAATACAAGGGCGA-3′ (F) and 5′-GAAGAGGGAACCAGCAC-3′ (R) [291 bp (Lin et al., 1991)]; ETB cDNA: 5′-GGCTGTTCAGTTTCTACTTCTGC-3′ (F) and 5′-AGAATCCTGCTGAGGTGAAGG-3′ (R) [210 bp (Sakurai et al., 1990)]; iNOS cDNA: 5′-AGAATGTTCCAGAATCCCTCCCTGGACA-3′ (F) and 5′-GAGTGAGCTGGTAGGTTCCTGTTG-3′ (R) [356 bp (Kosuga et al., 1994)]; and β-actin cDNA: 5′-TTCTACAATGAGCTGCGTGTG-3′ (F) and 5′-TTCATGGATGCCACAGGATTC-3′ (R) [561 bp (Nudel et al., 1983)] were used. Authenticity of the various PCR primers was confirmed by Southern analysis as described (Gabriel et al., 1998; 1999). PCR amplification reaction was performed essentially as described previously (Gabriel et al., 1998; 1999). PCR products were resolved in a 1.2% agarose gel and stained with 1×SYBR Green I (FMC Biproduct, Rockland, ME, U.S.A.). The gels were scanned under blue fluorescence light using a phosphorimager and the band intensity was quantified using ImageQuant software (Molecular Dynamics, Sunnyvale, CA, U.S.A.).
Determination of nitric oxide
The concentration of nitrite (an NO end product) was determined by Griess method as described (Green et al., 1982). Briefly, 100 μl of the Griess reagent was added to 100 μl of the culture supernatant in 96 well plates. The optical density was determined spectrophotometrically at 550 nm. A standard curve was developed using concentrations of sodium nitrite between 0 and 64 μM.
Western blot analysis for iNOS
Cells were scraped from the plate in ice-cold lysis buffer (Tris-HCl 10 mM, pH 7.6, containing 0.1 M NaCl, EDTA 1 mM, PMSF 0.5 mM and aprotinin 1 mM). Following homogenization in cold, the homogenate was kept in ice for 30 min and then centrifuged at 15,300×g for 10 min at 4°C. The supernatant was aspirated and its protein concentration was determined by Lowry's procedure. The lysate containing 10 μg protein was subjected to SDS–PAGE on a 8% acrylamide gel and the separated proteins were transferred on to a Immobilon-P membrane (Millipore, Bedford, MA, U.S.A.). The membrane was treated with a rabbit monoclonal anti-rat iNOS antibody (Transduction Laboratories Co., Lexington, KY, U.S.A.). After washing, the membrane was incubated with peroxidase-linked anti-rabbit IgG (Amersham-Pharmacia), and detection was achieved using an ECL chemiluminescence kit (Amersham-Pharmacia).
Data analysis
The values are presented as averages of duplicate or triplicate determinations with s.e.m. shown for triplicates. Each experiment was repeated at least three times using cells from different animals. Student's t-test was employed for statistical comparison of the paired samples. A P value of <0.05 was considered statistically significant.
Results
Effect of LPS on ET-1 and its receptors in HSC
LPS treatment caused an increase in ET-1 secretion by control and transformed HSC (Figure 1A), but the effect was statistically significant only in the former cell type. These results were consistent with the expression of preproET-1 mRNA transcript in the respective samples (Figure 1B,C).
Figure 1.
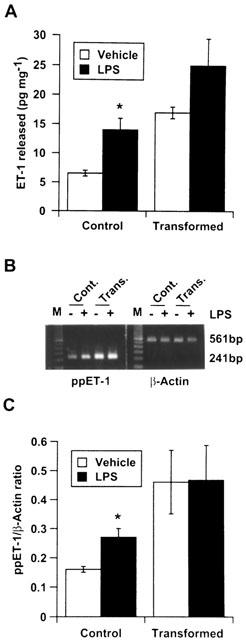
Effect of lipopolysaccharide (LPS) on endothelin-1 (ET-1) synthesis by stellate cells. Cells were placed in serum-free medium with or without 1 μg ml−1 LPS. After 24 h the medium was aspirated and analysed for ET-1 by enzyme-linked immunosorbant assay (A) while RNA was extracted from the cells for determination of preproET-1 mRNA expression by semiquantitative reverse transcriptase polymerase chain reaction assay (B). (C) Graphical presentation of the data shown in B. *P<0.05 vs vehicle. Cont., control cells; Trans., transformed cells.
Incubation of HSC with LPS also caused an increase in the ET-1 receptors. The effect was LPS concentration-dependent, and the extent of the increase was greater in transformed HSC (about 2 fold at 1 μg ml−1 LPS) than in control HSC (about 1.4 fold at 1 μg ml−1 LPS) (Figure 2).
Figure 2.
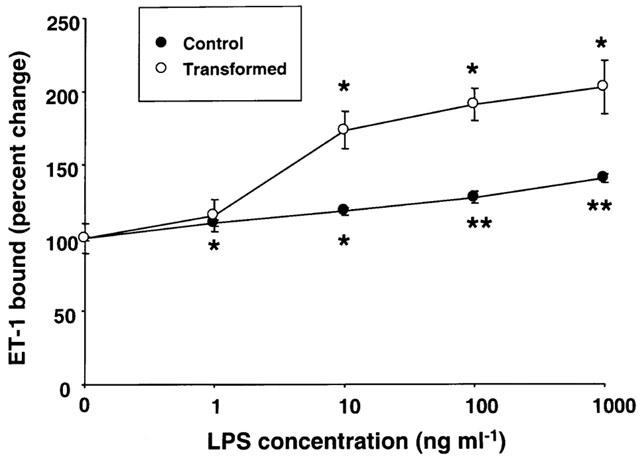
Effect of various concentrations of lipopolysaccharide (LPS) on endothelin-1 (ET-1) receptors. Stellate cells were placed in serum-free medium with indicated concentrations of LPS. After 24 h incubation, ET-1 binding assay was performed as described in the Methods section. *P<0.05 vs vehicle; **P<0.01 vs vehicle.
Figure 3 illustrates Scatchard plots of the results of [125I]-ET-1 saturation binding assays in HSC treated with 1 μg ml−1 LPS. In the control HSC, LPS caused an increase in the binding capacity (Bmax) from 1122±33 to 1660±90 fmol mg−1 while in transformed HSC, the Bmax increased from 673±43 to 1183±54 fmol mg−1. LPS treatment did not alter the affinity of the receptors which was in the range of 25–35 pM in both control and transformed HSC.
Figure 3.
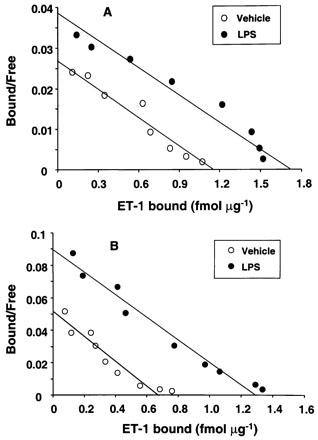
Scatchard plot of the endothelin-1 (ET-1) saturation binding data. Control and transformed stellate cells were treated with 1 μg ml−1 lipopolysaccharide (LPS) for 24 h in serum-free medium. Saturation binding assay for ET-1 was then performed as described in the Methods section.
Figure 4 shows the effect of LPS on ET-1 receptor subtypes in HSC. Both in control and transformed HSC, LPS caused an increase in ETB receptors and did not affect ETA receptors (Figure 4A). LPS-induced increase in ETB receptors was consistent with the increase in its mRNA transcript (Figure 4B,C).
Figure 4.
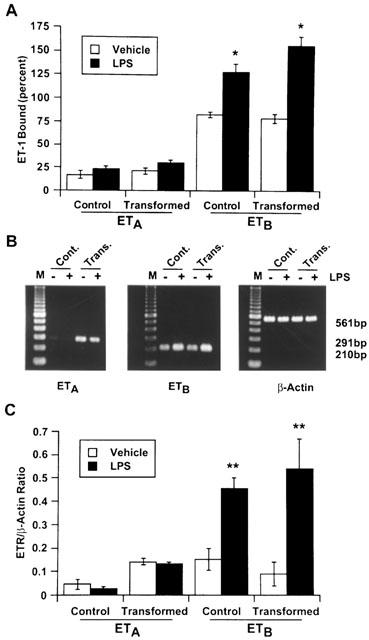
Effect of lipopolysaccharide (LPS) on endothelin-1 (ET-1) receptor subtypes of stellate cells. Control and transformed stellate cells were treated with 1 μg ml−1 LPS for 24 h in serum-free medium. Competition binding (A) or semiquantitative reverse transcriptase polymerase chain reaction (B) assays were performed as described in the Methods section. (C) Graphical presentation of the data shown in B. Both assays show significant increase in ETB receptors in LPS-stimulated cells. *P<0.01; **P<0.05 vs vehicle-treated cells. Cont., control cells; Trans., transformed cells.
Effect of LPS on NO synthesis by HSC
LPS stimulated NO synthesis (determined as nitrite) in control as well as transformed HSC concentration-dependently (Figure 5). The basal synthesis of NO2− at the end of 24 h incubation was much greater in control HSC (5.5–8.2 μM) as compared to the transformed HSC (1.8–2.5 μM). Unstimulated control HSC but not the transformed cells expressed iNOS protein (Figure 6A), and the mRNA expression also was quite abundant in control but barely detectable in the transformed HSC (Figure 6B). The expression of iNOS in unstimulated control cells could be due to cytokines produced as a result of the trauma of the isolation procedure. The extent of LPS-stimulated increase in NO2− was only 2 fold in control HSC and 7–8 fold in transformed HSC (Figure 5). Also, it is evident that the extent of LPS-induced expression of iNOS protein was much greater in transformed as compared to the control cells (Figure 6A). These observations are consistent with almost 10 fold increase in iNOS mRNA expression in transformed HSC and only 1.5 fold increase in control cells after LPS treatment (Figure 6B,C).
Figure 5.
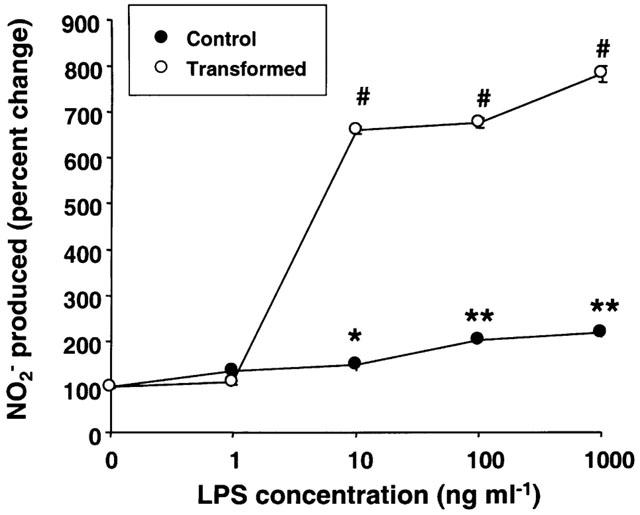
Effect of various concentrations of lipopolysaccharide (LPS) on nitrite (NO2−) synthesis. Control and transformed stellate cells were placed in serum-free medium with indicated concentrations of LPS. After 24 h incubation, NO2− was measured in the culture medium. *P<0.05; **P<0.01; #P<0.001 vs vehicle.
Figure 6.
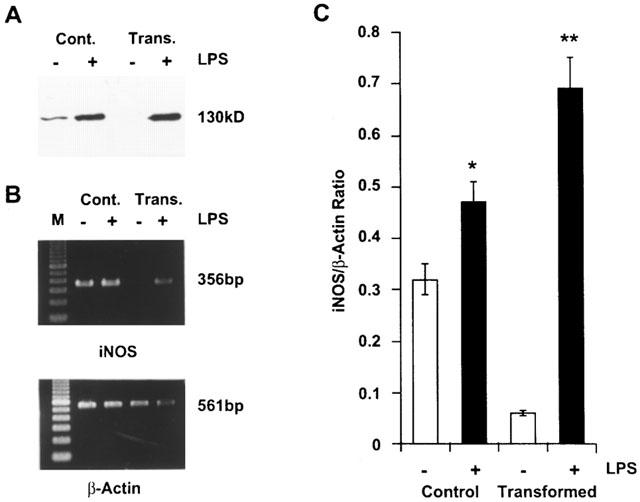
The inducible nitric oxide synthase (iNOS) protein and mRNA expression in lipopolysaccharide (LPS)-treated stellate cells. Control and transformed stellate cells were placed in serum-free medium with 1 μg ml−1 LPS. After 24 h incubation, Western analysis (A) or semiquantitative reverse transcriptase polymerase chain reaction assay (B) were performed as described in the Methods section. (C) Graphical presentation of the data shown in B. *P<0.05; **P<0.001 vs vehicle. Cont., control cells; Trans., transformed cells.
ET-1 stimulates NO synthesis via ETB receptors on endothelial cells (Hirata & Emori, 1993; Higuchi & Satoh, 1997). Since ETB subtype constitutes about 80% of the ET-1 receptors in rat HSC (Gabriel et al., 1998; 1999) and it is up-regulated upon LPS treatment, whether ET-1 stimulates NO synthesis in HSC was determined. No effect of ET-1 on basal or LPS-induced NO synthesis in either cell type was observed (results not shown).
Effect of L-NMMA on LPS-induced increase in NO2− synthesis and ET-1 receptors
In order to determine whether LPS-induced increase in ET-1 receptors in HSC is due to the autocrine action of NO, cells were stimulated with LPS in the presence of NOS inhibitor L-NMMA. L-NMMA (0.5 mM) caused inhibition of LPS-induced NO2− synthesis in both control and transformed HSC (Figure 7A). Interestingly, the concentration of NO2− formed in the presence of L-NMMA in the control cells treated with LPS was significantly lower than in the untreated cells (Figure 7A). However, L-NMMA treatment did not inhibit LPS-induced up-regulation of ET-1 receptors in control cells but caused a small additional increase (Figure 7B). In contrast, L-NMMA produced strong but incomplete inhibition of LPS-induced increase in ET-1 receptors in transformed cells (Figure 7B).
Figure 7.
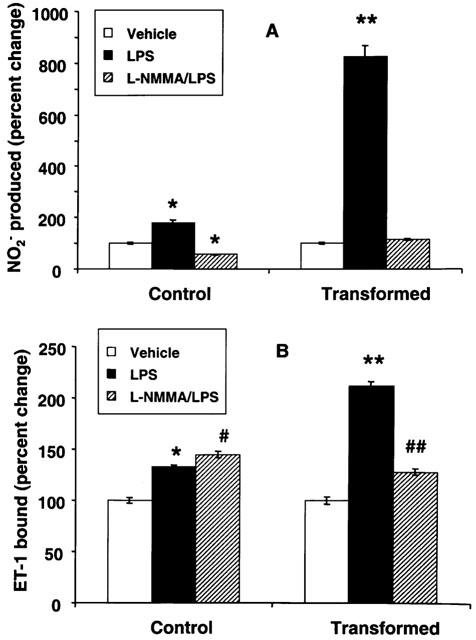
Effect of NG-monomethyl-L-homoarginine (L-NMMA) on lipopolysaccharide (LPS)-induced nitrite (NO2−) synthesis and endothelin-1 (ET-1) receptors in stellate cells. Control and transformed stellate cells were placed in serum-free medium with 1 μg ml−1 LPS±0.5 mM L-NMMA. After 24 h, NO2− concentration in the medium (A) and ET-1 receptors in the cell (B) were determined. *P<0.01; **P<0.001 vs vehicle; ##P<0.05 vs vehicle and <0.001 vs LPS. #P<0.05 vs LPS.
Effect of ODQ on LPS-induced increase in NO2− synthesis and ET-1 receptors
NO has been shown to exert many of its cellular effects via cyclic GMP (Schmidt et al., 1993). In order to determine whether LPS-induced increase in ET-1 receptors was mediated via cyclic GMP, the cells were incubated with LPS in the presence of an inhibitor of soluble guanylyl cyclase ODQ (Garthwaite et al., 1995). ODQ (5 μM) did not alter LPS-induced increase in NO2− synthesis in control or transformed HSC (Figure 8A) but inhibited up-regulation of ET-1 receptors in both cell types (Figure 8B). DBcGMP (1 mM) caused only modest increase in the ET-1 receptor density in control (12% increase compared to 40% by LPS) and in transformed (36% increase compared to 100% by LPS) HSC although it did not affect NO2− synthesis in both cell types.
Figure 8.
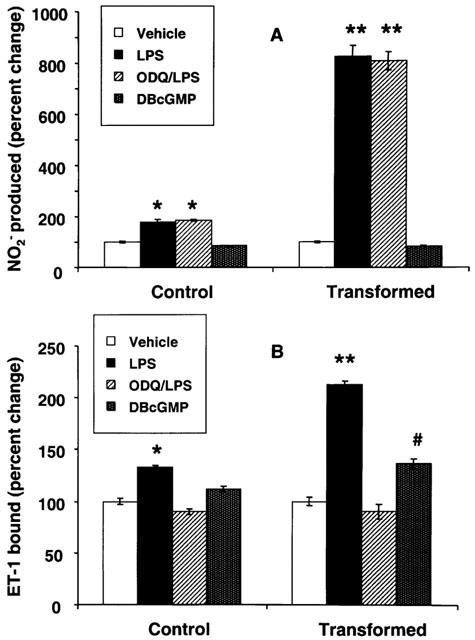
Effect of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) on lipopolysaccharide (LPS)-induced nitrite (NO2−) synthesis and endothelin-1 (ET-1) receptors in stellate cells. Control and transformed stellate cells were placed in serum-free medium with 1 μg ml−1 LPS±5 μM ODQ or with 1 mM dibutyryl cyclic GMP (DBcGMP) for 24 h. NO2− concentration in the medium (A) and ET-1 receptors in the cells (B) were then determined. #P<0.05; *P<0.01; **P<0.001 vs vehicle.
Time-course of the effect of LPS on NO2− synthesis and ET-1 receptors in HSC
The differential effect of L-NMMA on ET-1 receptors in control and transformed HSC suggested involvement of NO-dependent and -independent mechanisms. Since the synthesis of NO by HSC is increased upon induction of iNOS (Kawada et al., 1998), we determined the time course of the effect of LPS on NO2− synthesis and the increase in ET-1 receptors up to 24 h. As shown in Figure 9A, no change in NO2− synthesis over basal was observed up to 4 h in both control and transformed HSC. However, at 1 and 4 h of incubation with LPS, there was approximately 20–30% increase in ET-1 receptors in control as well as the transformed HSC (Figure 9B). The increase in the ET-1 receptors was near maximal at 8 h of stimulation in control cells while NO2− synthesis was still increasing. Conversely, the increase in ET-1 receptors of transformed cells did not plateau even at 24 h of treatment with LPS and was 40, 55 and 112% greater than the respective controls at 8, 12 and 24 h respectively (Figure 9B). The increase in ET-1 receptors in transformed HSC followed the increase in the NO2− synthesis which was respectively 15, 92 and 570% higher than the respective controls at 8, 12 and 24 h of stimulation.
Figure 9.
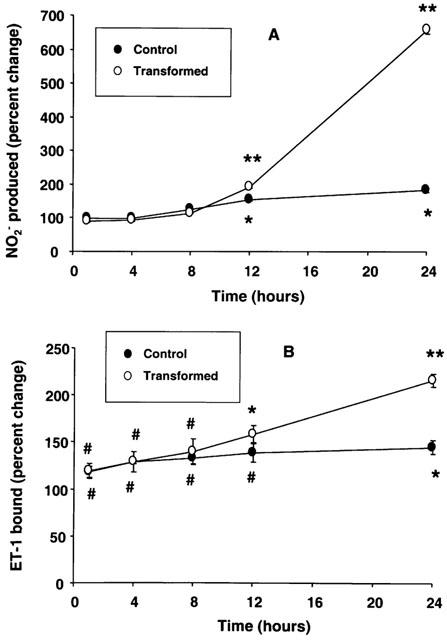
Time-course of lipopolysaccharide (LPS)-induced nitrite (NO2−) synthesis and endothelin-1 (ET-1) receptor up-regulation in stellate cells. Control and transformed stellate cells were placed in serum-free medium with 1 μg ml−1 LPS for indicated time periods. NO2− concentration in the medium (A) and ET-1 receptors in the cells (B) were then determined. #P<0.05; *P<0.01; **P<0.001 vs respective vehicle-treated cells.
Discussion
Elucidation of the interactions of HSC with ET-1, as a mechanism of hepatic microvascular pathophysiology, has been a topic of intense investigation in the recent years. Considering the possibility that the actions of endotoxin on HSC may be a major factor in causing increased portal resistance in endotoxemia and liver cirrhosis, we investigated the effects of LPS on ET-1 system in control and transformed HSC. The phenotypical and biochemical characteristics of the transformed HSC are very distinct from their precursor cells. The transformed HSC are devoid of retinoids, express α-smooth muscle actin, and are excessively fibrogenic and contractile as compared to the control HSC. Furthermore, upon their transformation, HSC lose their capacity to synthesize hepatocyte growth factor but express TGF-α and TGF-β at much greater levels (Bachem et al., 1991). In the present investigation, we demonstrated that LPS-induced expression of ET-1 receptors and iNOS, and the synthesis of NO were much greater in transformed HSC than in control HSC. These observations indicate that the sensitivity of HSC to LPS is augmented upon their transformation into the cirrhotic phenotype.
Amelioration of LPS-induced increase in portal resistance by ET-1 receptor antagonist (Pannen et al., 1996b) suggested a role of ET-1 in this pathologic condition. Although LPS-stimulated synthesis of ET-1 in HSC may be an important mechanism of increased ET-1 in endotoxin-treated rat liver, possibility of the contribution by other hepatic cell types such as endothelial cells (Rieder et al., 1991) and bile duct epithelial cells (Caligiuri et al., 1998; Housset et al., 1993b) cannot be ruled out. In fact, medium conditioned by Kupffer cells in the presence of endotoxin was shown to enhance synthesis of ET-1 by cultured hepatic endothelial cells (Rieder et al., 1991; Eakes & Olson, 1998). However, endotoxin alone did not stimulate ET-1 synthesis in hepatic endothelial cells (Eakes & Olson, 1998). Since TGF-β 1 stimulates ET-1 synthesis in cultured HSC (Pinzani et al., 1996; Gandhi et al., 1996b) and endothelial cells (Rieder et al., 1991; Eakes & Olson, 1998), the effect of this and other cytokines, produced by Kupffer cells in response to endotoxin, can be an additional mechanism of increased hepatic ET-1 in vivo.
Increased ET-1 receptor density on HSC is another mechanism of increased resistance to the blood flow through the liver in endotoxin-treated rats. Sinusoidal contractility due to HSC contributes significantly to the regulation of hepatic blood flow (Bhathal & Grossman, 1985; Geerts et al., 1994). Several investigators have demonstrated potent contractile action of ET-1 on HSC (Pinzani et al., 1992; Housset et al., 1993a; Kawada et al., 1993; Sakamoto et al., 1993; Zhang et al., 1995). Interestingly, only ETB receptors increased in cultured HSC upon endotoxin exposure, while ET-1-induced decrease in the sinusoidal diameter was reported to be mediated by activation of ETA and not ETB receptors (Zhang et al., 1995). This observation suggests that ETB receptor up-regulation in HSC may not be responsible for sinusoidal contraction in endotoxin-treated rats. However, ET-1 or ET-3-induced sinusoidal contraction was assessed in vitamin-A positive stellate cells (Zhang et al., 1995; Pannen et al., 1996a,1996b) that are present in abundance in the periportal area (Wake, 1988; Geerts et al., 1991). HSC present in the midzonal and pericentral areas contain little or no vitamin A (Wake, 1988; Geerts et al., 1991). Considering the contractile effect of ET-1 on transitional (5–8 days in culture) HSC via both ETA and ETB receptors (Rockey, 1995), it is quite likely that HSC in the midzonal and pericentral areas may contract upon ETB activation. Therefore, it is reasonable to conclude that up-regulated ETB receptors on the vitamin A-deficient or -negative HSC may contribute to the increased resistance to the blood flow in endotoxin-treated rats. Conversely, transformed (passaged) HSC derived from human liver were shown to contract only by ETA stimulation (Pinzani et al., 1996). In yet another study, ETA receptor blockade was found to worsen endotoxin-induced liver injury (Nishida et al., 1998). If ETA is primarily responsible for sinusoidal constriction in rats, its antagonism (Nishida et al., 1998) should have improved the pathological changes associated with endotoxin exposure. Taken together, these observations imply that further investigation is necessary to determine precise identification of ET-1 receptors on HSC coupled to their contraction.
Previously, NO was shown to cause up-regulation of ETA receptors in vascular smooth muscle cells (Redmond et al., 1996). Therefore, we hypothesized that LPS-induced up-regulation of ET-1 receptors in HSC could be due to a similar mechanism involving the action of NO. However, L-NMMA did not inhibit but actually produced an additional small increase in ET-1 receptor density in control HSC indicating existence of mechanism(s) other than the action of NO. Conversely, partial inhibition of LPS-induced ET-1 receptor up-regulation in transformed HSC by L-NMMA suggests that the mechanism of this effect, at least in part, involves NO similar to that in smooth muscle cells (Redmond et al., 1996). The NO-independence of LPS-induced ET-1 receptor up-regulation in both cell types became further evident in the time-course experiment in which about 20–30% increase in ET-1 receptors occurred even before an increase in NO synthesis (Figure 9). NO-independent actions of endotoxin were previously reported in which some of its hypotensive/vasodilator actions were unmasked by ET-1 receptor antagonism but not by inhibition of NOS (Gardiner et al., 1996). Moreover, blockade of NO synthesis caused only partial inhibition of endotoxin-induced inhibition of DNA synthesis in HSC and did not affect the activation of AP-1 and NF-κB (Kawada et al., 1998).
NO elicits several of its biological effects through cyclic GMP as a signalling molecule (Schmidt et al., 1993). Although inhibition of soluble guanylyl cyclase by ODQ completely blocked LPS-induced ET-1 receptor up-regulation, failure of L-NMMA to do so in normal HSC and to cause only partial inhibition in transformed HSC indicate that cyclic GMP may be generated in response to LPS via NO-independent pathway. However, only a modest increase in the ET-1 receptors by DBcGMP suggests that factors other than cyclic GMP produced due to LPS actions are required for the maximal effect.
Among the various biological mediators produced by HSC (e.g., HGF, TGF-α, TGF-β, PGI2 and PGE2) (Bachem et al., 1991; Schirmacher et al., 1992; Mallat et al., 1996), only TGF-β has been shown to affect the expression of ET-1 receptors in certain cell types including HSC (Cristiani et al., 1994; Gabriel et al., 1999). We demonstrated TGF-β1-induced down-regulation of ET-1 receptors in cultured HSC, the effect being much more pronounced for ETB subtype (Gabriel et al., 1999). Interestingly, LPS was found to cause decrease in the expression of TGF-β1 in both control and transformed HSC (T. Uemura and C. R. Gandhi, unpublished observation). Thus increased expression of ETB receptors in the HSC can be postulated to be due to decreased synthesis of TGF-β1 by these cells. However, a major portion (nearly 90%) of TGF-β1 released by HSC was found to be in the latent form (Gabriel et al., 1999), and its proportion did not change in LPS-treated cells. Therefore, inhibition of TGF-β1 synthesis may not be a factor responsible for LPS-induced up-regulation of ET-1 receptors in cultured HSC.
In liver cirrhosis, the concentration of endotoxin is elevated due to its reduced clearance by the macrophages of the liver (Fischer & Baldessarini, 1971; Bernardi et al., 1983; Liehr & Jacob, 1983). In this situation, endotoxin can act directly on nonmacrophage cell types (Nolan, 1981). Therefore, endotoxin-induced ET-1 receptor up-regulation in transformed HSC suggests that a mechanism of their increase in the cirrhotic liver (Gandhi et al., 1996a,1996b; 1998) may involve a direct action of LPS on HSC, and a major portion of this effect may be dependent on NO. Although NO production by ecNOS activity in the hepatic endothelial cells is reduced in experimental liver cirrhosis (Rockey & Chung, 1998), large amounts of NO can be produced by induction of iNOS in HSC and endothelial cells by the actions of endotoxin as well as cytokines and inflammatory mediators released in the injured liver. This suggestion is supported by the observation that endotoxin induces iNOS activity in endothelial cells (Radmoski et al., 1990; Rees et al., 1990) and HSC (Kawada et al., 1998).
The mechanisms of increased portal resistance in endotoxemia and of portal hypertension in liver cirrhosis are not completely understood. However, antagonists of ET-1 receptors ameliorate the increased portal resistance in both conditions (Pannen et al., 1996b; Rockey & Weisiger, 1996; Gandhi et al., 1998). The results of this study demonstrating a direct effect of endotoxin on control and transformed HSC indicate that the up-regulation of ET-1 receptor density in them can be an important mechanism of the resistance to the blood flow through the liver. Although these effects occur via both NO-dependent and -independent mechanisms, whether endotoxin stimulates synthesis of cytokines such as interleukin-1 and TNF-α, similar to its effects on Kupffer cells (Decker, 1990), and if these cytokines affect the ET-1 system in HSC remains to be determined.
Acknowledgments
This work was supported by a grant from NIH (DK 54411) and a VA Merit award to C.R. Gandhi. We thank Ms Jennifer Costantine and Ms Kristin Anselmi for excellent technical assistance.
Abbreviations
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbant assay
- ET
endothelin
- GBSS
Gay's balanced salt solution
- HBSS
Hank's balanced salt solution
- HSC
hepatic stellate cells
- LPS
lipopolysaccharide
- NO
nitric oxide
- PMSF
phenylmethylsulphonyl fluoride
- RT–PCR
reverse transcriptase polymerase chain reaction
- SDS–PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
References
- BACHEM M., MEYER D., RIESS U., MELCHIO R., SELL K., BITTINGER A.Paracrine stimulation of fat-storing cells via soluble mediators produced by myofibroblast-like cells Cells of the hepatic sinusoid 1991Rijswijk: Kupffer Cell Foundation; 164–170.ed. Wisse, E. & Knook, D. pp [Google Scholar]
- BERNARDI M., TREVISANI F., LANDINI C. Plasma norepinephrine, weak neurotransmitters and renin activity in liver cirrhosis. Relationship with cardiovascular homeostasis and renal function. Hepatology. 1983;3:55–64. doi: 10.1002/hep.1840030109. [DOI] [PubMed] [Google Scholar]
- BHATHAL P.S., GROSSMAN H.J. Reduction of the increased portal vascular resistance of the isolated perfused cirrhotic rat liver by vasodilators. J. Hepatol. 1985;1:325–337. doi: 10.1016/s0168-8278(85)80770-4. [DOI] [PubMed] [Google Scholar]
- BLOMHOFF R., WAKE K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991;5:271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- CALIGIURI A., GLASER S., RODGERS R.E., PHINIZY J.L., ROBERTSON W., PAPA E., PINZANI M., ALPINI G. Endothelin-1 inhibits secretin-stimulated ductal secretion by interacting with ETA receptors on large colangiocytes. Am. J. Physiol. 1998;275:G835–G846. doi: 10.1152/ajpgi.1998.275.4.G835. [DOI] [PubMed] [Google Scholar]
- CRISTIANI C., VOLPI D., LANDIONIO A., BERTOLERO F. Endothelin-1-selective binding sites are downregulated by transforming growth factor-β and upregulated by basic fibroblast growth factor in a vascular smooth muscle-derived cell line. J. Cardiovasc. Pharmacol. 1994;23:988–994. doi: 10.1097/00005344-199406000-00018. [DOI] [PubMed] [Google Scholar]
- DECKER K. Biologically active products of stimulated liver macrophages (Kupffer cells) Eur. J. Biochem. 1990;192:245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- EAKES A.T., OLSON M.S. Regulation of endothelin synthesis in hepatic endothelial cells. Am. J. Physiol. 1998;274:G1068–G1076. doi: 10.1152/ajpgi.1998.274.6.G1068. [DOI] [PubMed] [Google Scholar]
- FISCHER J.E., BALDESSARINI R.J. False neurotransmitters and hepatic failure. Lancet. 1971;2:75–80. doi: 10.1016/s0140-6736(71)92048-4. [DOI] [PubMed] [Google Scholar]
- GABRIEL A., KUDDUS R.H., RAO A.S., GANDHI C.R. Down-regulation of endothelin receptors by transforming growth factor β1 in hepatic stellate cells. J. Hepatol. 1999;30:440–450. doi: 10.1016/s0168-8278(99)80103-2. [DOI] [PubMed] [Google Scholar]
- GABRIEL A., KUDDUS R.H., RAO A.S., WATKINS W.D., GANDHI C.R. Superoxide-induced changes in endothelin (ET) receptors in hepatic stellate cells. J. Hepatol. 1998;29:614–627. doi: 10.1016/s0168-8278(98)80157-8. [DOI] [PubMed] [Google Scholar]
- GANDHI C.R., KANG Y., DE WOLF A., MADARIAGA J., AGGARWAL S., SCOTT V., FUNG J. Altered endothelin homeostasis in patients undergoing liver transplantation. Liver Transplant. Surg. 1996a;2:362–369. doi: 10.1002/lt.500020506. [DOI] [PubMed] [Google Scholar]
- GANDHI C.R., KUDDUS R., SUBBOTIN V.M., PRELICH J., MURASE N., RAO A.S., NALESNIK M.A., WATKINS S.C., DELEO A., TRUCCO M., STARZL T.E. A fresh look at augmenter of liver regeneration in rats. Hepatology. 1999;29:1435–1445. doi: 10.1002/hep.510290522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANDHI C.R., NEMOTO E.M., WATKINS S.C., SUBBOTIN V.M. An endothelin receptor antagonist TAK-044 ameliorates carbon tetrachloride-induced acute liver injury and portal hypertension in rats. Liver. 1998;18:39–48. doi: 10.1111/j.1600-0676.1998.tb00125.x. [DOI] [PubMed] [Google Scholar]
- GANDHI C.R., SPROAT L.A., SUBBOTIN V.M. Increased hepatic endothelin-1 levels and endothelin receptor density in cirrhotic rats. Life Sci. 1996b;58:55–62. doi: 10.1016/0024-3205(95)02255-4. [DOI] [PubMed] [Google Scholar]
- GANDHI C.R., STEPHENSON K., OLSON M.S. Endothelin, a potent peptide agonist in the liver. J. Biol. Chem. 1990;265:17432–17435. [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Influence of aminoguanidine and endothelin antagonist, SB 209670, on the regional haemodynamic effects of endotoxemia in conscious rats. Br. J. Pharmacol. 1996;118:1822–1828. doi: 10.1111/j.1476-5381.1996.tb15609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARTHWAITE L.J., SOUTHAM E., BOULTON C.L., NIELSEN E.B., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- GEERTS A., DE BLESER P., HAUTEKEETE M.L., NIKI T., WISSE E.Fat-storing (Ito) cell biology The Liver: Biology and Pathobiology 1994New York: Raven Press, Ltd; 819–838.ed. Arias, I.M., Boyer, J.L., Fasuto, N., Jakoby, W.B., Schachter, D.L. & Shafritz, D.A. pp [Google Scholar]
- GEERTS A., LAZOU J., DE BLESER P., WISSE E. Tissue distribution, quantitation and proliferation kinetics of fat-storing cells in CCl4 injured rat liver. Hepatology. 1991;13:1193–1202. [PubMed] [Google Scholar]
- GREEN L.C., WAGNER D.A., GLOGOWSKI J., SKIPPER P.L., WISHNOK J.S., TANNENBAUM S. Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- HIGUCHI H., SATOH T. Endothelin-1 induces vasoconstriction and nitric oxide release via endothelin ETB receptors in isolated perfused rat liver. Eur. J. Pharmacol. 1997;328:175–182. doi: 10.1016/s0014-2999(97)83043-9. [DOI] [PubMed] [Google Scholar]
- HIRATA Y., EMORI T. Cellular mechanism of endothelin-induced nitric oxide synthesis by cultured bovine endothelial cells. J. Cardiovasc. Pharmacol. 1993;22:S225–S228. doi: 10.1097/00005344-199322008-00060. [DOI] [PubMed] [Google Scholar]
- HOUSSET C., CARAYON A., HOUSSET B., LEGENDRE C., HANNOUN L., POUPON R. Endothelin-1 secretion by human gallbladder epithelial cells in primary culture. Lab. Invest. 1993b;69:750–755. [PubMed] [Google Scholar]
- HOUSSET C., ROCKEY D.C., BISSELL D.M. Endothelin receptors in rat liver: Lipocytes as a contractile target for endothelin 1. Proc. Natl. Acad. Sci. U.S.A. 1993a;90:9266–9270. doi: 10.1073/pnas.90.20.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHARA M., NOGUCHI K., SAEKI T., FUKURODA T., TSUCHIDA S., KIMURA S., FUKAMI T., ISHIKAWA K., NISHIKIBE M., YANO M. Biological profiles of highly potent novel endothelin antagonists selective for the ETA receptor. Life Sci. 1992;50:247–255. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- KAWADA N., SEKI S., KUROKI T., INOUE M. Regulation of stellate cell proliferation by lipopolysaccharide: Role of endogenous nitric oxide. J. Gastroenterol. Hepatol. 1998;13 Suppl:S6–S13. doi: 10.1111/jgh.1998.13.s1.6. [DOI] [PubMed] [Google Scholar]
- KAWADA N., TRAN-THI T.-A., KLEIN H., DECKER K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur. J. Biochem. 1993;213:815–823. doi: 10.1111/j.1432-1033.1993.tb17824.x. [DOI] [PubMed] [Google Scholar]
- KOSUGA K., YUI Y., HATTORI R., SASE K., EIZAWA H., AOYAMA T., INOUE R., SASAYAMA S. Cloning of an inducible nitric oxide synthase from rat polymorphonuclear neutrophils. Endothelium. 1994;2:217–221. [Google Scholar]
- LEIVAS A., JIMENEZ W., BRUIX J., BOIX L., BOSCH J., ARROYO V., RIVERA F., RODES J. Gene expression of endothelin1 and ETA and ETB receptors in human cirrhosis: relationship with hepatic hemodynamics. J. Vasc. Res. 1998;35:186–193. doi: 10.1159/000025583. [DOI] [PubMed] [Google Scholar]
- LIEHR H., JACOB A.L.Endotoxin and renal failure in liver disease The kidney in liver disease 1983New York: Elsevier Biomedical; 535–551.edn 2. ed. Epstein, M. pp [Google Scholar]
- LIN H.Y., KAJI E.H., WINKEL G.K., IVES H.E., LODISH H.F. Cloning and functional expression of a vascular smooth muscle endothelin 1 receptor. Proc. Natl. Acad. Sci. U.S.A. 1991;88:3185–3189. doi: 10.1073/pnas.88.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALLAT A., PREAUX A.-M., SERRADEIL-LE GAL C., RAUFASTE D., GALLOIS C., BRENNER D.A., BRADHAM C., MACLOUF J., IOURGENKO V., FOUASSIER L., DHUMEAUX D., MAVIER P., LOTERSZTAJN S. Growth inhibitory properties of endothelin-1 in activated human hepatic stellate cells: A cyclic adenosine monophosphate-mediated pathway. J. Clin. Invest. 1996;98:2771–2778. doi: 10.1172/JCI119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- NISHIDA T., HUANG T.P., SEIYAMA A., HAMADA E., KAMIIKE W., UESHIMA S., KAZUO H., MATSUDA H. Endothelin A-receptor blockade worsens endotoxin-induced hepatic microcirculatory changes and necrosis. Gastroenterology. 1998;115:412–420. doi: 10.1016/s0016-5085(98)70208-2. [DOI] [PubMed] [Google Scholar]
- NOLAN J.P. Endotoxin, reticuloendothelial function, and liver injury. Hepatology. 1981;1:458–465. doi: 10.1002/hep.1840010516. [DOI] [PubMed] [Google Scholar]
- NUDEL U., ZAKUT R., SHANI M., NEUMAN S., LEVY Z., YAFFER D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983;11:1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANNEN B.H.J., BAUER M., ZHANG J.X., ROBOTHAM J.L., CLEMENS M.G. Endotoxin pretreatment enhances portal venous contractile response to endothelin-1. Am. J. Physiol. 1996a;271:H7–H15. doi: 10.1152/ajpheart.1996.270.1.H7. [DOI] [PubMed] [Google Scholar]
- PANNEN B.H.J., BAUER M., ZHANG J.X., ROBOTHAM J.L., CLEMENS M.G. A time-dependent balance between endothelins and nitric oxide regulating portal resistance after endotoxin. Am. J. Physiol. 1996b;271:H1953–H1961. doi: 10.1152/ajpheart.1996.271.5.H1953. [DOI] [PubMed] [Google Scholar]
- PAUL A., BRYANT C., LAWSON M.F., CHILVERS E.R., PLEVIN R. Dissociation of lipopolysaccharide-mediated nitric oxide synthase and inhibition of DNA synthesis in RAW264.7 macrophages and rat aortic smooth muscle cells. Br. J. Pharmacol. 1997;120:1439–1444. doi: 10.1038/sj.bjp.0701070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINZANI M., FAILLI P., RUOCCO C., CASINI A., MILANI S., BALDI E., GIOTTI A., GENTILINI P. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J. Clin. Invest. 1992;90:642–646. doi: 10.1172/JCI115905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINZANI M., MILANI S., DE FRANCO R., GRAPPONE C., CALIGURI A., GENTILINI P., TOSTI-GUERRA C., MAGGI M., FAILLI P., RUOCCO C., GENTILINI P. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534–548. doi: 10.1053/gast.1996.v110.pm8566602. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. Glucocorticoids inhibit the expression of an inducible, but not constitutive, nitric oxide synthase in vascular endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1990;87:10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMADORI G., VEIT T., SCHWOGLER D., DIENES H.P., KNITTEL T., RIEDER H., MEYER ZUM BUSCHENFELDE K.H. Expression of the gene of the α-smooth muscle actin isoform in rat liver and in rat fat-storing cells. Wirchows Arch. 1990;59:349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- REDMOND E.M., CAHILL P.A., HODGES R., ZHANG S., SITZMANN J.V. Regulation of endothelin receptors by nitric oxide in cultured rat vascular smooth muscle cells. J. Cell. Physiol. 1996;166:469–479. doi: 10.1002/(SICI)1097-4652(199603)166:3<469::AID-JCP1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- REES D.D., CELLEK S., PALMER R.M.J., MONCADA S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem. Biophys. Res. Commun. 1990;173:541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- RIEDER H., RAMADORI G., MEYER ZUM BUSCHENFELDE K.H. Sinusoidal endothelial liver cells in vitro release endothelin- Augmentation by transforming growth factor β and Kupffer cell-conditioned media. Klin. Wochenschr. 1991;69:387–391. doi: 10.1007/BF01647411. [DOI] [PubMed] [Google Scholar]
- ROCKEY D.C. Characterization of endothelin receptors mediating rat hepatic cell contraction. Biochem. Biophys. Res. Commun. 1995;207:725–731. doi: 10.1006/bbrc.1995.1247. [DOI] [PubMed] [Google Scholar]
- ROCKEY D.C., CHUNG J.J. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–351. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- ROCKEY D.C., WEISIGER R.A. Endothelin induces contractility of stellate cells from normal and cirrhotic liver: Implications for regulation of portal pressure and resistance. Hepatology. 1996;24:233–240. doi: 10.1002/hep.510240137. [DOI] [PubMed] [Google Scholar]
- ROS J., LEIVAS A., JIMENEZ W., MORALES M., BOSCH-MARCE M., ARROYO V., RIVERA F., RODES J. Effect of bacterial lipopolysaccharide on endothelin-1 production in human vascular endothelial cells. J. Hepatol. 1997;26:81–87. doi: 10.1016/s0168-8278(97)80013-x. [DOI] [PubMed] [Google Scholar]
- SAKAMOTO M., UENO T., KIN M., OHIRA H., TORIMURA T., INUZUKA S., SATA M., TANIKAWA K. Ito cell contraction in response to endothelin-1 and substance P. Hepatology. 1993;18:978–983. doi: 10.1002/hep.1840180432. [DOI] [PubMed] [Google Scholar]
- SAKURAI T., YANAGISAWA M., INOUE A., RYAN U.S., KIMURA S., MITSUI Y., GOTO K., MASAKI T. cDNA cloning, sequence analysis and tissue distribution of rat preproendothelin-1 mRNA. Biochem. Biophys. Res. Commun. 1991;175:44–47. doi: 10.1016/s0006-291x(05)81197-0. [DOI] [PubMed] [Google Scholar]
- SAKURAI T., YANAGISAWA M., TAKUWA Y., MIYAZAKI H., KIMURA S., GOTO K., MASAKI T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- SCHIRMACHER P., GEERTS A., PIETRANGELO A., ROGLER C.E. Analysis of hepatocyte growth factor/hepatopoietin A expression in rat liver. Hepatology. 1992;15:5–11. doi: 10.1002/hep.1840150103. [DOI] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., LOHMANN S.M., WALTER U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim. Biophys. Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- TRAN-THI T.A., KAWADA N., DECKER K. Regulation of endothelin-1 action on the perfused rat liver. FEBS Lett. 1993;318:353–357. doi: 10.1016/0014-5793(93)80544-5. [DOI] [PubMed] [Google Scholar]
- WAKE K.Liver perivascular cells revealed by gold- and silver-impregnation methods and electron microscopy Biopathology of the liver: an ultrastructural approach 1988Lancaster, UK: Kluwer Academics; 23–36.ed. Motta, P. pp [Google Scholar]
- WILLIAMS D.L., JR, JONES K.L., PETTIBONE D.J., LIS E.V., CLINESCHMIDT B.V. Sarafotoxin S6c: An agonist which distinguishes between endothelin receptor subtypes. Biochem. Biophys. Res. Commun. 1991;175:556–561. doi: 10.1016/0006-291x(91)91601-8. [DOI] [PubMed] [Google Scholar]
- YASUDA S., LEW W.Y. Endothelin-1 ameliorates contractile depression by lipopolysaccharide in cardiac myocytes. Am. J. Physiol. 1997;273:H1403–H1407. doi: 10.1152/ajpheart.1997.273.3.H1403. [DOI] [PubMed] [Google Scholar]
- ZHANG J.X., BAUER M., CLEMENS M.G. Vessel- and target cell-specific actions of endothelin-1 and endothelin-3 in rat liver. Am. J. Physiol. 1995;269:G269–G277. doi: 10.1152/ajpgi.1995.269.2.G269. [DOI] [PubMed] [Google Scholar]


