Abstract
Curcumin, an anti-inflammatory, antioxidant, was evaluated for its ability to suppress bleomycin (BLM)-induced pulmonary fibrosis in rats. A single intratracheal instillation of BLM (0.75 U 100−1 g, sacrificed 3, 5, 7, 14 and 28 days post-BLM) resulted in significant increases in total cell numbers, total protein, and angiotensin-converting enzyme (ACE), and alkaline phosphatase (AKP) activities in bronchoalveolar lavage fluid. Animals with fibrosis had a significant increase in lung hydroxyproline content. Alveolar macrophages from BLM-administered rats elaborated significant increases in tumour necrosis factor (TNF)-α release, and superoxide and nitric oxide production in culture medium. Interestingly, oral administration of curcumin (300 mg kg−1 10 days before and daily thereafter throughout the experimental time period) inhibited BLM-induced increases in total cell counts and biomarkers of inflammatory responses in BALF. In addition, curcumin significantly reduced the total lung hydroxyproline in BLM rats. Furthermore, curcumin remarkably suppressed the BLM-induced alveolar macrophage production of TNF-α, superoxide and nitric oxide. These findings suggest curcumin as a potent anti-inflammatory and anti-fibrotic agent against BLM-induced pulmonary fibrosis in rats.
Keywords: Bleomycin, curcumin, hydroxyproline, nitric oxide, superoxide anion, tumor necrosis factor-α
Introduction
Bleomycin (BLM), an anti-tumour antibiotic, causes a dose-dependent pulmonary fibrosis characterized by the presence of alveolar macrophages (AM), neutrophils and eosinophils within the alveolar structures (Crouch, 1990). Tumour necrosis factor-α (TNF-α), secreted by activated AM, has been strongly implicated in the pathogenesis of pulmonary fibrosis (Zhang et al., 1993) since anti-TNF antibody was demonstrated to inhibit BLM-induced pulmonary fibrosis (Piguet et al., 1989; Piguet, 1990). Therefore, TNF-α appears to be a potential target for therapeutic intervention of pulmonary fibrosis. Fibrosis associated with BLM treatment has also been linked to toxic reactive oxygen and nitrogen species produced by infiltrating inflammatory cells (Yamazaki et al., 1998). Thus, agents that depress oxidative stress are also of potential clinical value, and could have additional protective effects against BLM-induced pulmonary fibrosis.
Curcumin, a yellow curry pigment from turmeric (Curcuma longa), has been demonstrated to be a powerful anti-inflammatory, anti-cancer and anti-oxidant agent, and is under preclinical trial for cancer prevention and anti-inflammation (Gescher et al., 1998). Interestingly, curcumin was reported to inhibit lipopolysaccharide (LPS)-induced production of TNF-α by a human monocytic-macrophage cell line (Chan, 1995). Recently, curcumin was shown to inhibit the production of various chemokines and cytokines, including TNF-α, by monocytes and alveolar macrophages (Abe et al., 1999). Curcumin is also well known for its free radical scavenging activity (Sharma, 1976). These observations prompted us to investigate whether curcumin might play a protective role against the development of BLM-induced pulmonary fibrosis by blocking the release of TNF-α and cytotoxic free radicals.
Methods
Healthy male Wistar rats weighing 325–350 g were divided into four groups: (i) a control group which received a single intratracheal dose of 0.4 ml of sterile physiological saline; (ii) a curcumin (suspended in 1% gum acacia and administered by gastric intubation) group which received 300 mg kg−1 of curcumin; (iii) a single dose of bleomycin (0.75 U 100−1 g body weight, in 0.4 ml of saline) was intratracheally instilled to the third group and (iv) a curcumin+bleomycin group which received 300 mg kg−1 of curcumin 10 days before BLM and daily thereafter throughout the experimental period. The decision to select the dose of curcumin (300 mg kg−1) was based upon results from dose response study.
Alveolar macrophage culture
At 3, 5, 7, 14 and 28 days after BLM administration, groups of six rats per treatment group were killed by an overdose of sodium pentobarbital (75 mg kg−1) and severing of the inferior vena cava according to standard ethical procedures. The lungs were then lavaged five times with calcium and magnesium-free phosphate-buffered saline, pH 7.4. at a volume of 5 ml wash−1. The pooled BALF was filtered through sterile gauze to remove mucus and particulates and then centrifuged at 300×g for 10 min, the erythrocytes were lysed, and after washing three times in RPMI-1640 media, the pelleted cells were resuspended in RPMI-1640 (GIBCO, Grand Island, NY, U.S.A.) media containing 25 mM HEPES buffer. Aliquots of the cell suspension were counted by a haemocytometer and cell viability (>95%) measured by trypan blue exclusion. Differential cell counts were performed on cytocentrifuge preparations that were fixed in methanol and stained with Diff-Quik (Sigma, St. Louis, MO, U.S.A.).
The BAL cells were resuspended in RPMI-1640 media supplemented with 2 mM glutamine, 100 u ml−1 penicillin, 100 μg ml−1 streptomycin and 10% heat-inactivated foetal bovine serum. The cells were seeded (1×106 AM ml−1) to a 24-well tissue culture plate and allowed to adhere for 2 h at 37°C in a humidified atmosphere in 95% O2 : 5% CO2, after which the non-adherent cells were removed by washing thrice with RPMI media. The AM-enriched monolayers were incubated in fresh RPMI media for an additional 24 h. The macrophage-conditioned media was collected, centrifuged, and the supernatants were stored in aliquots at −70°C for biochemical analysis.
Biochemical assays
Cell-free BALF was analysed at 3, 5 and 7 days post-BLM treatment, which reflect the time course of acute inflammation before the development of fibrotic changes, for total protein content, and activities of angiotensin-converting enzyme (ACE), alkaline phosphatase (AKP) using standard laboratory methods. Total lung collagen was determined by analysis of hydroxyproline content in lung tissues (Woessner, 1961). TNF-α protein level in AM-culture media was assayed using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, MN, U.S.A.) according to the manufacturer's protocol. Superoxide anion release by AM was measured spectrophotometrically using the ferricytochrome C reduction technique (Babior et al., 1973). To assess nitric oxide production, AM were cultured (in DMEM because RPMI-1640 contains calcium nitrate) in 96-well plates (1×106 cells ml−1) for 24 h and the accumulation of nitrite, its oxidation product, in the culture medium was assayed using Greiss reaction with sodium nitrite as the standard (Carreras et al., 1996).
Statistical analysis
All data are expressed as means±standard deviation of six independent observations. Statistical analysis was done using an unpaired t-test or by one way ANOVA for multiple comparisons followed by post-hoc Bonferroni test.
Results
Curcumin-treated BLM rats showed no signs of respiratory distress, enhanced survival, and a striking reduction in body weight loss (data not shown). These data suggest that curcumin was protective against BLM-induced general toxicity and lethality. Animals with fibrosis showed severely damaged lung with infiltration of inflammatory cells followed by infiltration of the interstitium with fibroblasts with excessive collagen deposition, whereas these inflammatory and fibrotic changes were significantly reduced by curcumin treatment. Total cell counts in BALF were significantly increased at days 3, 5, and peaked at day 7 after BLM administration (Table 1). Furthermore, the increase of total cell counts was associated with significant elevation of BALF protein (increased vascular permeability and transudation of proteins from the blood stream), ACE (vascular endothelial cell injury) and AKP (type II alveolar epithelial cell destruction) levels during the same time period (Table 1). Results in Figure 1 demonstrate that curcumin administration inhibited BLM-induced increases in lung hydroxyproline content (a classical biochemical marker of pulmonary fibrosis) in a dose-dependent fashion. The highest dose (300 mg kg−1) of curcumin significantly restored lung hydroxyproline content to near normal levels, compared to 200 and 100 mg kg−1 doses, in BLM rats. These data indicate that curcumin inhibited BLM-induced pulmonary fibrosis. BLM treatment resulted in a significant increase in the AM release of TNF-α at days 3 and 5, peaking at day 7, but this increase decreased on days 14 and 28, although the production of TNF-α remained elevated over controls. Similarly AM production of superoxide anion and nitric oxide was higher in BLM rats, peaking at day 7 and day 14 for superoxide anion and nitric oxide, respectively. AM release of superoxide anion and nitric oxide was lower in curcumin-treated controls compared to saline-treated controls. Interestingly, curcumin suppressed BLM-induced AM release of TNF-α, superoxide anion and nitric oxide.
Table 1.
Protective effect of curcumin on BLM-induced changes in BALF biomarkers
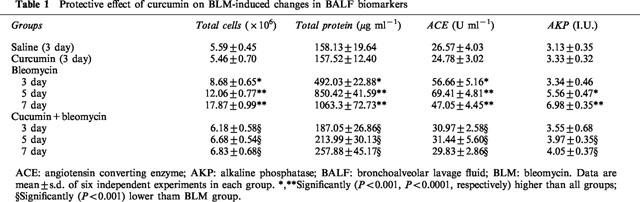
Figure 1.
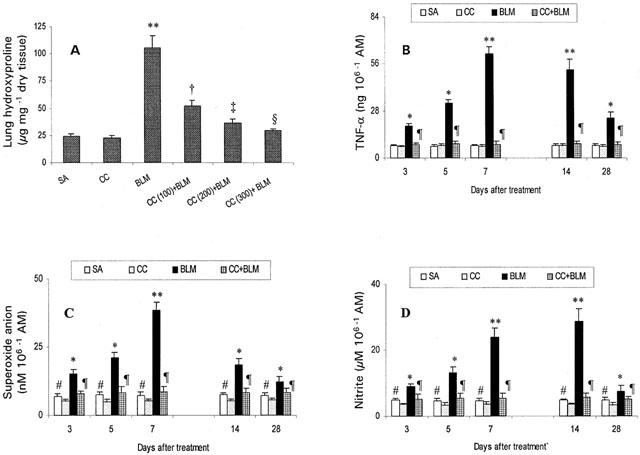
Protective effect of curcumin against BLM-induced changes in lung collagen (A), and alveolar macrophage release of TNF-α (B), superoxide anion (C) and nitrite (D). Results shown are mean±s.d. of six independent observations in each group. AM: alveolar macrophage; SA: saline; CC: curcumin; BLM: bleomycin. *,**Significantly (P<0.001, P<0.0001, respectively) higher than all groups; †,‡,§significantly (P<0.01, P<0.005, P<0.001, respectively) lower than BLM; ¶significantly (P<0.001) lower than BLM; #significantly (P<0.01) higher than curcumin-treated controls.
Discussion
This is the first study in rats to demonstrate the novel observation that curcumin prevented the development of BLM-induced pulmonary fibrosis. Curcumin-mediated reduction of total cell numbers in BALF may be due to the inhibition of migration of inflammatory cells through endothelial and epithelial basement membrane to the site of inflammation. This could be attributed to the membrane-stabilizing property of curcumin (Nirmala & Puvanakrishnan, 1996). In addition, we observed a striking reduction in protein content, ACE and AKP levels in BALF of curcumin-treated BLM rats suggesting that curcumin prevents not only the inflammatory reaction but also the primary toxic effect of BLM on the capillary endothelium and alveolar epithelium and thus prevent transudation of proteins from the blood stream.
Curcumin treatment led to a marked suppression in the release of TNF-α by BLM-activated macrophages. These findings are consistent with earlier reports of curcumin attenuation of inflammatory cytokine and chemokine production, including TNF-α by blood monocytes and alveolar macrophages (Abe et al., 1999). It is possible that curcumin could block the initiation and progression of the BLM-induced inflammatory response by suppressing TNF-α release by activated macrophages, which in turn could inhibit inflammatory cell recruitment and modulate the expression of adhesion molecules on both leukocytes and endothelial cells. Supporting this contention, curcumin has been reported to inhibit TNF-α-induced, expression of adhesion molecules on human umbilical vein endothelial cells (Kumar et al., 1998). The present study does not analyse the mechanism by which curcumin suppresses TNF-α production, however, recent findings demonstrate that curcumin inhibits the activation of NF-κB (Kumar et al., 1998), a transcription factor implicated in the regulation of pro-inflammatory gene products.
BLM is known to activate macrophages, which in turn can release large amounts of cytotoxic mediators, including superoxide anion and nitric oxide (Yamazaki et al., 1998). Administration of curcumin completely prevented the effects of BLM on superoxide and nitric oxide release by alveolar macrophages. Our results are consistent with previous data indicating that curcumin is capable of lowering the generation of both superoxide and nitric oxide from rat peritoneal macrophages (Joe & Lokesh, 1994). BLM treatment resulted in a significant increase in lung hydroxyproline content, while curcumin treatment markedly attenuated BLM-induced increases in lung collagen in a dose-dependent manner. Bleomycin has been shown to generate toxic free radicals, and the involvement of free radical in the formation of hydroxylated proline was demonstrated earlier (Bhatnagar & Liu, 1972). Thus, treatment of rats with curcumin could block production of free radicals and contribute to the decreased lung collagen in BLM-treated animals. These findings argue for the role of curcumin as an antifibrotic compound in BLM-induced pulmonary fibrosis in rats. Recently, curcumin has also been shown to modulate collagen metabolism in isoproterenol-induced myocardial toxicity in rats (Nirmala et al., 1999).
In summary, our findings clearly indicate that the administration of curcumin prevents BLM-induced pulmonary fibrosis: (1) by interfering with the influx of inflammatory cells, (2) by suppressing the activation of alveolar macrophages and subsequent release of toxic mediators, and (3) by preventing excess collagen accumulation in lungs. In the absence of any reported serious side-effects, combined with its excellent anticancer activity, curcumin could be useful as a potent antifibrotic agent against pulmonary fibrosis. Further studies are in progress in our laboratory.
Acknowledgments
The authors would like to thank the Director, CLRI for his interest and permission to publish this work, Mr V. Elango for his help in animal experiments and Dr V. Arumugam for statistical analysis.
Abbreviations
- ACE
angiotensin converting enzyme
- AKP
alkaline phosphatase
- AM
alveolar macrophage
- ANOVA
analysis of variance
- BALF
bronchoalveolar lavage fluid
- BLM
bleomycin
- CC
curcumin
- DMEM
Dulbecco's modified eagle's medium
- ELISA
enzyme-linked immunosorbent assay
- LPS
lipopolysaccharide
- RPMI
Rosewell park memorial institute
- SA
saline
- TNF-α
tumour necrosis factor-α
References
- ABE Y., HASHIMOTO S., HORIE T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol. Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- BABIOR B.M., KIPNES R.S., CURNUTTE J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 1973;2:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATNAGAR R.S., LIU T.Z. Evidence for free radical involvement in the hydroxylation of proline: Inhibition by nitro blue tetrazolium. FEBS Lett. 1972;26:32–34. doi: 10.1016/0014-5793(72)80535-0. [DOI] [PubMed] [Google Scholar]
- CARRERAS M.C., PODEROSO J.J., CADENAS E., BOVERIS A. Measurement of nitric oxide and hydrogen peroxide production from human neutrophils. Methods Enzymol. 1996;269:65–75. doi: 10.1016/s0076-6879(96)69010-7. [DOI] [PubMed] [Google Scholar]
- CHAN M.M.Y. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem. Pharmacol. 1995;49:1551–1556. doi: 10.1016/0006-2952(95)00171-u. [DOI] [PubMed] [Google Scholar]
- CROUCH E. Pathobiology of pulmonary fibrosis. Am. J. Physiol. 1990;259:L159–L184. doi: 10.1152/ajplung.1990.259.4.L159. [DOI] [PubMed] [Google Scholar]
- GESCHER A., PASTORINO U., PLUMMER S.M., MANSON M.M. Suppression of tumour development by substances derived from the diet-mechanisms and clinical implications. Br. J. Clin. Pharmacol. 1998;45:1–12. doi: 10.1046/j.1365-2125.1998.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOE B., LOKESH B.R. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim. Biophys. Acta. 1994;1224:255–263. doi: 10.1016/0167-4889(94)90198-8. [DOI] [PubMed] [Google Scholar]
- KUMAR A., DHAWAN S., HARDEGEN N.J., AGGARWAL B.B. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem. Pharmacol. 1998;55:775–783. doi: 10.1016/s0006-2952(97)00557-1. [DOI] [PubMed] [Google Scholar]
- NIRMALA C., ANAND S., PUVANAKRISHNAN R. Curcumin treatment modulates collagen metabolism in isoproterenol induced myocardial necrosis in rats. Mol. Cell. Biochem. 1999;197:31–37. doi: 10.1023/a:1006960106929. [DOI] [PubMed] [Google Scholar]
- NIRMALA C., PUVANAKRISHNAN R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol. Cell. Biochem. 1996;159:85–93. doi: 10.1007/BF00420910. [DOI] [PubMed] [Google Scholar]
- PIGUET P.F. Is “tumor necrosis factor” the major effector of pulmonary fibrosis. Eur. Cytokine Netw. 1990;1:257–258. [PubMed] [Google Scholar]
- PIGUET P.F., COLLART M.A., GRAU G.E., KAPANCI Y., VASSALLI P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J. Exp. Med. 1989;170:655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARMA O.P. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976;25:1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- WOESSNER J.F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI Y., HOSHINO J., SEKIGUCHI T., HORI Y., MIYAUCHI S., MIZUNO S., HORIE K. Production of superoxide and nitric oxide by alveolar macrophages in the bleomycin-induced interstitial penumonia mice model. Jpn. J. Pharmacol. 1998;78:69–73. doi: 10.1254/jjp.78.69. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., LEE T.C., GUILLEMIN B., YU M., ROM W.N. Enhanced IL-1β and tumor necrosis factor-α release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J. Immunol. 1993;150:4188–4196. [PubMed] [Google Scholar]


