Abstract
The cardiac electrophysiological effects of S-oxybutynin, a single-enantiomer drug under evaluation for the management of urinary incontinence, have been investigated and compared with those of terodiline, an incontinence agent withdrawn following reports of QT lengthening and ventricular tachyarrhythmia. Membrane currents were recorded from whole-cell configured guinea-pig and rabbit ventricular myocytes, and action potentials were recorded from guinea-pig and rabbit papillary muscles.
L-type Ca2+ current (ICa,L), rapidly-activating K+ current (IKr) and slowly-activating K+ current (IKs) were unaffected by submicromolar S-oxybutynin and inhibited by higher concentrations; IC50 values were 17.8 μM for ICa,L, 12 μM for IKr, and 41 μM for IKs. Terodiline IC50 values were somewhat lower for ICa,L (15.2 μM) and IKs (30 μM), but 24 fold lower in the case of IKr (0.5 μM).
The durations of action potentials in guinea-pig and rabbit papillary muscles driven at 1 Hz were unaffected or moderately shortened by 0.1–100 μM S-oxybutynin, but lengthened by terodiline. Terodiline (⩽10 μM) also depressed maximal upstroke velocity.
The action potential plateau shortened by an average of 23% when control rabbit papillary muscles were driven at 0.4 Hz instead of 1 Hz. Plateau shortening was significantly smaller in the presence of drugs (30 μM S-oxybutynin, 3 and 30 μM terodiline), suggesting that they suppress the transient outward current (Ito) involved in rate-dependent shortening. In experiments on rabbit ventricular myocytes, 3 and 30 μM S-oxybutynin inhibited Ito by 9±2% and 35±3%, respectively, whereas 3 and 30 μM terodiline inhibited the current by 31±3% and 87±3%, respectively.
The results indicate that S-oxybutynin has relatively weak non-specific effects on cardiac ion channels, and that clinically relevant submicromolar concentrations are unlikely to have terodiline-like proarrhythmic actions on the myocardium.
Keywords: S-Oxybutynin, terodiline, papillary muscles, ventricular myocytes, cardiac action potentials, cardiac membrane currents, delayed-rectifier K+ currents, calcium current, action potential duration, rate dependence
Introduction
Micturation dysfunction is a major medical and social problem that has a particularly high incidence in young children and older adults (Thomas et al., 1980; Fonda, 1989; Caione et al., 1997; Hjalmas, 1997; Theodorou et al., 1998). Management with anticholinergic drugs is a long-standing practice (Yarker et al., 1995; Wein, 1998), and it is believed that anticholinergics with Ca2+ antagonistic activity are particularly effective (Andersson, 1984; Smith et al., 1998). One such dual-action drug, terodiline, secured a prominent share of the market after its introduction in 1986 (Langtry & McTavish, 1990). However, it was withdrawn five years later amid reports of cardiotoxicity (conduction disturbances, QT prolongation, ventricular tachyarrhythmia (torsades de pointes)) (Connolly et al., 1991; McLeod et al., 1991; Stewart et al., 1992). In the interim, oxybutynin has emerged as a drug of choice for relief of symptoms such as urinary urge with or without incontinence, nocturia, enuresis, postsurgical bladder dysfunction, neurogenic spastic bladder, and detrusor muscle instability (Yarker et al., 1995; Åmark et al., 1998; Theodorou et al., 1998). Like terodiline, it is a tertiary amine that has anticholinergic activity and direct relaxant effects on smooth muscle (Lish et al., 1965; Fredericks et al., 1975; Tonini et al., 1987; Noronha-Blob & Kachur, 1991; Smith et al., 1998).
A major problem in the management of voiding dysfunction with anticholinergic agents is the high incidence of side-effects (e.g. dry mouth) that may lead patients to discontinue treatment (Langtry & McTavish, 1990; Yarker et al., 1995; Buyse et al., 1998). Compared to the incidence of such side-effects with oxybutynin, a lower incidence is the major advantage claimed for the recently-introduced terodiline derivative, tolterodine (Abrams et al., 1998), as well as for S-oxybutynin, a stereoisomer that is currently undergoing evaluation as a urinary incontinence agent (Koch et al., 1998; Smith et al., 1998). As part of that process, we have investigated the effects of the drug on cardiac membrane currents and action potentials, and compared them with those of terodiline.
Methods
All procedures were carried out in accordance with national and university regulations on the care and treatment of laboratory animals. Male guinea-pigs (250–300 g) were killed by cervical dislocation, whereas male New Zealand white rabbits (ca. 1.5 kg) were anaesthesized by injection of sodium pentobarbitone (40 mg kg−1) into a marginal ear vein, and killed by removal of the heart. Papillary muscles and ventricular myocytes were prepared as described below.
Papillary muscles
Hearts were placed in oxygenated (95% O2-5% CO2) Krebs' solution that contained (mM): NaCl 113.1, KCl 4.6, CaCl2 2.45, MgCl2 1.15, NaHCO3 21.9, NaH2PO4 3.48 and glucose 10.0 (pH 7.4), and papillary muscles were excised from right ventricles. The muscles were mounted in a Perspex bath (0.25 ml volume) perfused with solution (36°C±0.2°C) at 4–6 ml/min, stimulated at 1 Hz with 3-ms long pulses of 1.2 times threshold strength via a bipolar Ag-AgCl electrode, and equilibrated for 60–90 min prior to data collection. Action potentials were recorded with a high-input impedance amplifier (model 750, WP Instruments, New Haven, CT, U.S.A.) using conventional microelectrodes filled with 3 M KCl (resistance 8–15 Mω), and the upstroke was electronically differentiated to record upstroke velocity ( ). The action potentials were displayed on a storage oscilloscope (model 5103N, Tektronix, Beaverton, OR, U.S.A.) and recorded on film, by a chart recorder (model RS 3400, Gould, Cleveland, OH, U.S.A.), and/or by computer via Axoscope (Axon Instruments, Foster City, CA, U.S.A.).
). The action potentials were displayed on a storage oscilloscope (model 5103N, Tektronix, Beaverton, OR, U.S.A.) and recorded on film, by a chart recorder (model RS 3400, Gould, Cleveland, OH, U.S.A.), and/or by computer via Axoscope (Axon Instruments, Foster City, CA, U.S.A.).
Ventricular myocytes
Whole-cell membrane currents were recorded from guinea-pig and rabbit ventricular myocytes. The myocytes were enzymatically isolated as described previously (Ogura et al., 1995). Excised hearts were mounted on a Langendorff column, and retrogradely perfused through the aorta with Ca2+-free Tyrode's solution (37°C) containing collagenase (0.08–0.12 mg/ml: Yakult Pharmaceutical Co., Tokyo, Japan) for 10–15 min. The cells were dispersed and stored at 22°C in a high-K+, low-Na+ solution supplemented with 50 mM glutamic acid and 20 mM taurine. A few drops of the cell suspension were placed in a 0.3-ml perfusion chamber mounted on an inverted microscope stage. After the cells had settled to the bottom, the chamber was perfused (2 ml/min) with Tyrode's solution at 36°C. The Tyrode's solution contained (in mM): NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 1, glucose 10, and N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) 5 (pH 7.4 with NaOH).
All of the experiments on guinea-pig ventricular myocytes were conducted with normal or modified Tyrode's solution warmed to 36°C. The modified solutions included K+-free Tyrode's solution (KCl omitted) and K+-, Ca2+-free Tyrode's solution (KCl and CaCl2 omitted) that also contained 0.2 mM Cd2+ to suppress Ca2+ channel current. The experiments on rabbit ventricular myocytes were designed for measurement of transient outward current. To isolate the current, the bathing solution (normal Tyrode's at 24 or 36°C) contained 0.2 mM Cd2+, 0.1 mM Ba2+, 3 μM E4031, and 20 μM tetrodotoxin.
Membrane currents were recorded using an EPC-7 amplifier (List Electronic, Darmstadt, Germany) in the whole-cell patch configuration. Recording pipettes were fab-ricated from thick-walled borosilicate glass capillaries (H15/10/137, Jencons Scientific Ltd., Bedfordshire, U.K.) and filled with (i) K+ solution that contained (in mM): KCl 40, po-tassium aspartate 106, MgCl2 1, K2-ATP 4, ethylene glycol-bis(b-aminoethyl ether)-N,N,N,N-tetraacetic acid (EGTA) 5, and HEPES 5 (pH 7.2 with KOH), or (ii) Cs+ solution (K+ replaced by Cs+). The pipettes had resistances of 1.5–2.5 Mω when filled with pipette solution, and liquid junction potentials between external and pipette-filling solution were nulled prior to patch formation. Series resistance ranged between 3 and 7 Mω and was compensated by 60–80%. Cell capacitance ranged from 80 to 130 pF. Membrane current signals were filtered at 3 kHz, and digitized with an A/D converter (Digidata 1200A, Axon Instruments) and pCLAMP software (Axon Instruments) at a sampling rate of 8 kHz prior to analysis.
Drugs
S-oxybutynin and terodiline were supplied as hydrochloride salts by Sepracor Inc. (Marlborough, MA, U.S.A.), and E4031 was supplied by Eisai (Tokyo, Japan). These agents were dissolved in dimethyl sulphoxide (DMSO) (Sigma Chemical Co., St. Louis MO, U.S.A.) immediately prior to addition to the superfusate. The highest final concentration of DMSO in the superfusate was 0.03% (100 μM drug), a concentration of DMSO that has no significant effect on electrical activity or membrane currents in guinea-pig ventricular cells (Ogura et al., 1995). Tetrodotoxin (Calbiochem, La Jolla, CA, U.S.A.) and 4-aminopyridine (Sigma) were dissolved in distilled water.
Statistics
Results are expressed as means±s.e.mean. Single comparisons were made using Student's t-test, and multiple comparisons were made using one-way ANOVA followed by the Bonferroni test. Differences were considered significant when P<0.05.
Results
Effects of S-oxybutynin and terodiline on membrane current in guinea-pig ventricular myocytes
Figure 1a,b shows examples of whole-cell membrane currents recorded when myocytes were depolarized from prepulse −40 mV to more positive potentials for 500 ms before and after addition of S-oxybutynin and terodiline. Identifiable components in the records include inward L-type Ca2+ current (ICa,L) that reached maximal amplitude on depolarizations to ca. 0 mV, and time-dependent outward current that increased with positive potential and deactivated when the membrane was repolarized to −40 mV. Applications of 10 and 50 μM S-oxybutynin (Figure 1a) or of 10 and 50 μM terodiline (Figure 1b) inhibited these current components in a concentration-dependent manner. These drugs also reduced the outward current at prepulse −40 mV (Figure 1a,b), and this was most likely due to concentration-dependent inhibitions of inwardly-rectifying K+ current (IK1) (Figure 1c,d).
Figure 1.

Inhibition of whole-cell membrane currents by S-oxybutynin and terodiline. Guinea-pig ventricular myocytes bathed and dialyzed with K+-containing solutions were held at −80 mV, and pulsed at 0.1 Hz following 200-ms prepulses to −40 or −50 mV. (a) Records obtained on 500-ms depolarizations from prepulse −40 mV to more positive potentials before (control) and 8–10 min after additions of 10 and 50 μM S-oxybutynin (S-OXB). The dashed line indicates zero-current level. (b) Records from a similar experiment with 10 and 50 μM terodiline (TER). (c) Amplitudes of peak inward current on 500-ms depolarizations, and late (500 ms) current on depolarizations and hyperpolarizations. The representative myocyte was exposed to 3 μM terodiline for 8 min (data in left hand plot), washed for 14 min to obtain new pre-drug control values, and then exposed to 3 μM S-oxybutynin for 8 min (right hand plot). (d) Inhibition of IK1 in myocytes pretreated with 0.2 mM Cd2+. Currents were elicited by 1-s pulses from prepulse −50 mV to potentials V, and the end-of-pulse current amplitudes were measured with respect to zero current. Number of myocytes: S-oxybutynin (8), terodiline (7). †P<0.01 (one-way ANOVA, Bonferroni test).
Effects on peak ICa,L
We have previously established that terodiline inhibits peak ICa,L with an IC50 of 15.2 μM (Ogura et al., 1999). To determine the effects of S-oxybutynin on peak ICa,L, myocytes were bathed and dialyzed with either K+ or K+-free solutions, depolarized for 200 ms from prepulse −40 to 0 mV at 0.1 Hz except for periodic determinations of I-V relationships, and exposed to one or two concentrations of the drug. S-oxybutynin had rapid inhibitory effects that were relatively independent of voltage and reversible on washout of the drug (Figure 2a–c). The Hill equation describing the data has an IC50 of 17.8 μM and a coefficient of 0.99 (Figure 2d).
Figure 2.
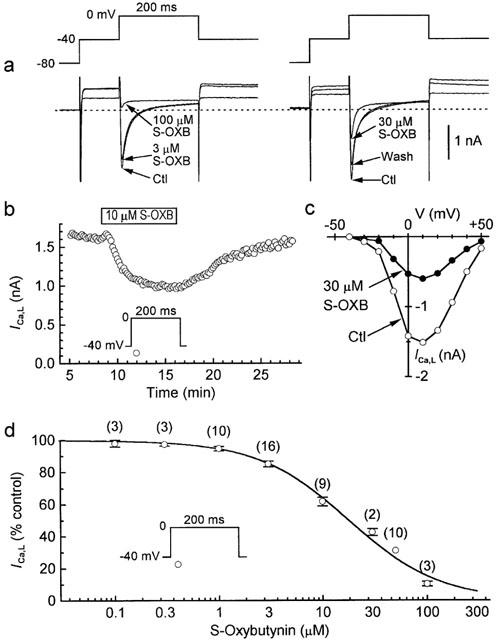
Inhibition of ICa,L by S-oxybutynin. Myocytes were held at −80 mV, depolarized to prepulse −40 mV for 100 ms, and then pulsed at 0.1 Hz. (a) Records from experiments. Left: effects of sequential applications of 3 μM (10 min) and 100 μM (5 min) drug; the lowest traces at −40 mV were obtained with 100 μM drug. Right: reversible effects of 30 μM drug (drug treatment 12 min; washout 8 min); records at −40 mV were obtained during (top to bottom) control, 30 μM, and washout treatments. The experiments were performed using K+-containing external and internal solutions. The dashed lines indicate zero-current levels. (b) Time course of reversible inhibition of peak ICa,L by 10 μM S-oxybutynin in a myocyte bathed and dialyzed with K+-free solutions. (c) I-V relationships determined before and 6 min after addition of 30 μM S-oxybutynin. The myocyte was bathed and dialyzed with K+-free solutions. (d) Effect of drug concentration on peak ICa,L elicited by pulses to 0 mV. Drug exposure times ranged from 6–10 min (high concentrations) up to 15 min (low concentrations). Myocytes were exposed to a single concentration of the drug, and 27 of the 56 experiments were performed with K+-free solutions. The Hill equation describing the data has an IC50 of 17.8 μM and a coefficient 0.99. The numbers of myocytes are given in parentheses.
Effects on delayed-rectifier K+ currents
An overview of the effects of S-oxybutynin and terodiline on total delayed-rectifier K+ current (IK) was obtained by measuring the amplitudes of the IK tail (IK,tail) that followed 500-ms depolarizations to test potentials (V) between −30 and +70 mV. Based on selective suppression of the rapidly-activating component of IK (IKr) by E4031 (3 μM) (Follmer & Colatsky, 1990; Sanguinetti & Jurkiewicz, 1990; Heath & Terrar, 1996), the I-V relationships of IK,tail were comprised of an IKr-dominated phase at low test voltages and IKr plus slowly-activating IK (IKs) at high test voltages (Figure 3a). S-oxybutynin up to 3 μM had negligible effects, whereas higher concentrations inhibited both components (Figure 3b–d). Although the effects of terodiline on the I-V relationship of IK,tail were also dependent on drug concentration, they were evident at considerably lower concentrations than observed with S-oxybutynin (Figure 3e,f).
Figure 3.
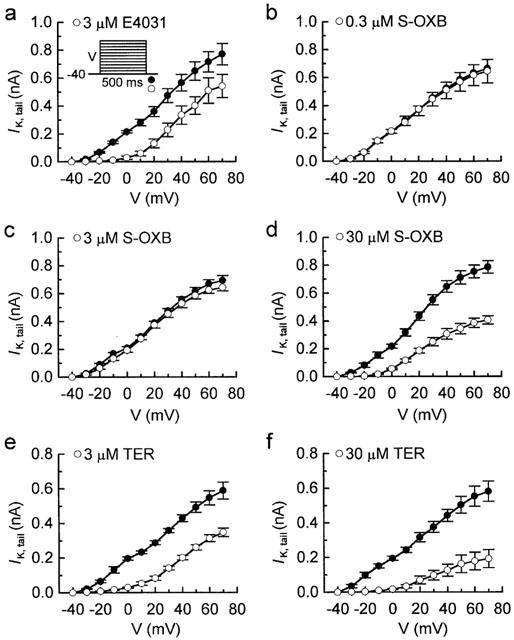
I-V relationships of IK,tail from experiments on myocytes treated with E4031, S-oxybutynin, and terodiline. The amplitudes of IK,tail following 500-ms depolarizations to test potentials (V) up to +70 mV were measured before and after drug treatments that lasted for 5–8 min. (a) Results with 3 μM E4031 (n=6). (b–d) Results with 0.3 μM (n=5), 3 μM (n=4) and 30 μM (n=5) S-oxybutynin. (e,f) Results with 3 μM (n=5) and 30 μM (n=6) terodiline. Note the relative inactivity of 3 μM S-oxybutynin compared to 3 μM E4031 and 3 μM terodiline.
The amplitude of IK,tail after short (200 ms) depolarizations from −40 to 0 mV was reduced to 11±2% control (n=18) by 3 μM E4031 (e.g. Figure 4a), and was therefore selected as a useful indicator of drug action on IKr. IKr,tail was inhibited by both terodiline and S-oxybutynin, but there was a marked difference in the potency of the two compounds; the estimated IC50 values were 0.5 μM for terodiline and 12 μM for S-oxybutynin (Figure 4b–d).
Figure 4.
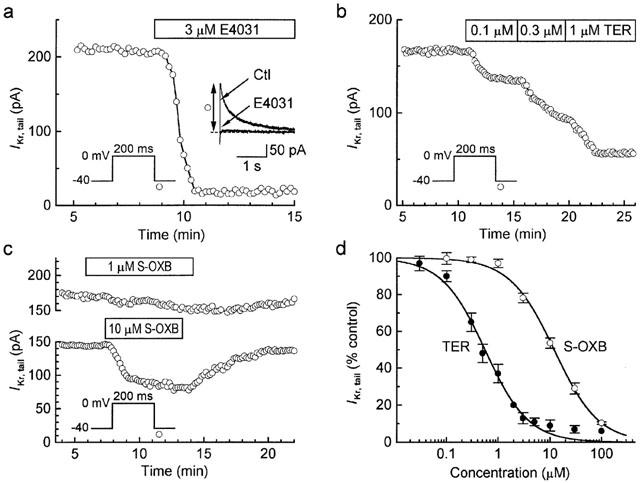
Inhibition of IKr by terodiline and S-oxybutynin. (a) Identification of IK,tail measured after 200-ms depolarizations to 0 mV as predominantly IKr,tail based on its sensitivity to 3 μM E4031. (b) Marked inhibition of IKr,tail by 0.1, 0.3 and 1 μM terodiline. (c) Data from representative experiments with 1 μM (upper panel) and 10 μM (lower panel) S-oxybutynin. (d) Concentration-response relationships. The Hill equation fitting the S-oxybutynin data (n=3–16) has an IC50 of 12 μM and a Hill-coefficient of 1.01, and that fitting the terodiline data (n=1 at 2 μM and 100 μM; otherwise n=3–18) has an IC50 of 0.5 μM and a Hill-coefficient of 1.04.
The magnitude of IKs was estimated in two ways: (i) as the time-dependent current during 500-ms depolarizations to ca. +70 mV (where time-dependent ICa,L and inwardly-rectifying IKr are expected to be relatively small), and (ii) as the time-dependent current during 500-ms depolarizations to +30 mV in myocytes superfused with K+-, Ca2+-free Cd2+ solution to minimise IKr and enhance IKs (see Sanguinetti & Jurkiewicz, 1992; Jones et al., 1998). The results obtained with the two methods were similar, and since I-V relations obtained under the K+-free conditions revealed little dependence of block on voltage (tests with 30 μM drug; data not shown), the data were pooled. The representative results and summary of pooled-data in Figure 5 indicate that S-oxybutynin was a somewhat weaker inhibitor of IKs than was terodiline. The Hill fits to the data have an IC50 of 43 μM for S-oxybutynin, and a significantly lower (P<0.01) 30 μM for terodiline. These results are consistent with the effects of the drugs on the high-voltage component of tail IK-V relationships (Figure 3).
Figure 5.
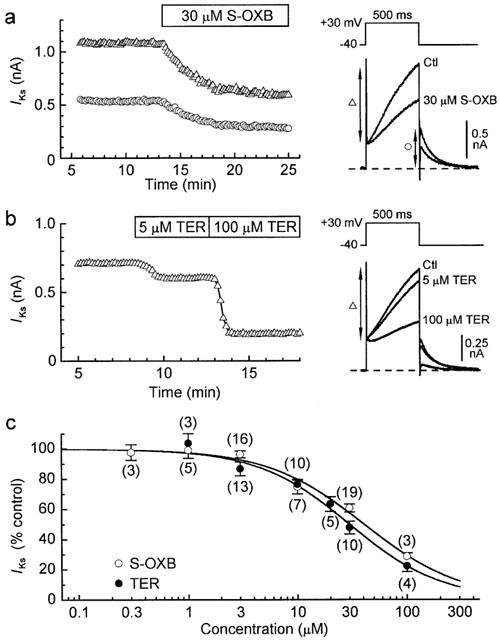
Inhibition of IKs by S-oxybutynin and terodiline. (a,b) Effects of S-oxybutynin and terodiline on IKs in myocytes superfused with K+-, Ca2+-free Cd2+ solution. The measurements taken are indicated by the vertical arrows on the records. (c) Concentration-response relationships. IKs was measured as the time-dependent outward current during 500-ms pulses to +30 mV (as in a,b) (37 of 98 myocytes), or as the time-dependent current during 500-ms pulses to +70 mV under standard conditions (see records in Figure 1a,b). Fits to the pooled data indicate an IC50 of 43 μM and a Hill-coefficient of 0.98 for S-oxybutynin (n=3–16), and an IC50 of 30 μM and Hill-coefficient of 1.11 for terodiline (n=3–10).
Effects of S-oxybutynin and terodiline on action potentials in guinea-pig papillary muscles
Action potentials were recorded in control and test groups of guinea-pig papillary muscles for measurement of action potential duration at 20 and 90% repolarization levels (APD20, APD90), action potential amplitude (APA), and maximal upstroke velocity ( max). The observation periods lasted for 30 min, and data were normalized to the values measured at 0 min. APD20 and APD90 were stable in control muscles (30-min values of 100±1% and 99±1% (n=8), respectively), and not significantly affected by ⩽3 μM S-oxybutynin (Figure 6a,c). Higher concentrations shortened the action potential in a concentration-dependent manner, with the largest changes occurring at the plateau level (e.g., APD20 81±3% (P<0.001) and APD90 94±2% (P<0.05) (n=10, t-tests) with 30 μM drug) (Figure 6a,c).
max). The observation periods lasted for 30 min, and data were normalized to the values measured at 0 min. APD20 and APD90 were stable in control muscles (30-min values of 100±1% and 99±1% (n=8), respectively), and not significantly affected by ⩽3 μM S-oxybutynin (Figure 6a,c). Higher concentrations shortened the action potential in a concentration-dependent manner, with the largest changes occurring at the plateau level (e.g., APD20 81±3% (P<0.001) and APD90 94±2% (P<0.05) (n=10, t-tests) with 30 μM drug) (Figure 6a,c).  max was stable in the control muscles, and unaffected by S-oxybutynin (Figure 6c).
max was stable in the control muscles, and unaffected by S-oxybutynin (Figure 6c).
Figure 6.
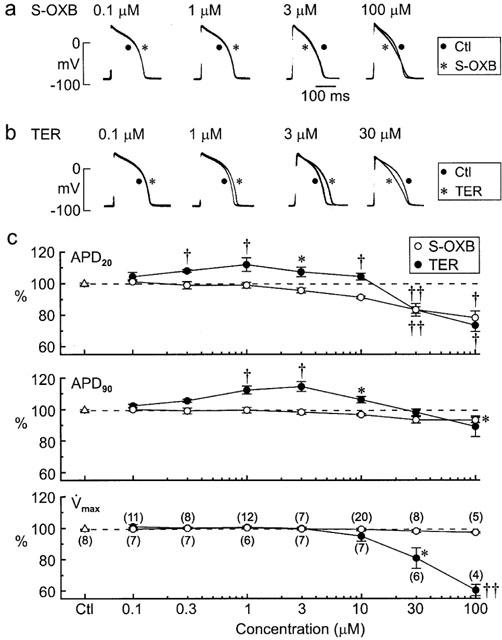
Effects of S-oxybutynin and terodiline on action potentials in guinea-pig papillary muscles. The muscles were stimulated at 1 Hz and treated with a single concentration of the drug. (a,b) Superimposed sets of action potentials; the traces were recorded from muscles before and 30 min after addition of drug. (c) APD20, APD90 and  max of action potentials from control muscles (no drug treatment) and muscles treated with single concentrations of S-oxybutynin and terodiline. Values measured after 30-min observation periods are expressed as percentages of pre-drug values. Numbers of muscles are shown in parentheses on the bottom plot. Significance was assessed using t-tests: *P<0.05, †P<0.01, ††P<0.001.
max of action potentials from control muscles (no drug treatment) and muscles treated with single concentrations of S-oxybutynin and terodiline. Values measured after 30-min observation periods are expressed as percentages of pre-drug values. Numbers of muscles are shown in parentheses on the bottom plot. Significance was assessed using t-tests: *P<0.05, †P<0.01, ††P<0.001.
The effects of terodiline on the action potential in guinea-pig papillary muscles differed from those of S-oxybutynin in two major respects: (i) concentrations ⩽10 μM lengthened the action potential (e.g., peak APD90 of 115±3% (n=7) with 3 μM drug, P<0.01), and (ii) concentrations>10 μM markedly depressed  max (Figure 6b,c). These terodiline data are in agreement with findings in a previous study (Shuba et al., 1999).
max (Figure 6b,c). These terodiline data are in agreement with findings in a previous study (Shuba et al., 1999).
Effects of S-oxybutynin and terodiline on action potentials in rabbit papillary muscles
Further information on the cardiac actions of S-oxybutynin and terodiline were sought by examining the effects of the drugs on the action potential in rabbit papillary muscles. This tissue was chosen because it has a different blend of membrane currents governing repolarization than guinea-pig papillary muscle; in particular, a transient outward current (Ito) that promotes early repolarization is prominent in rabbit but not guinea-pig ventricular cells (Hiraoka & Kawano, 1987; Carmeliet, 1993).
Concentration-dependent effects
After 60–90 min equilibration, muscles were driven at 1 Hz and monitored for 30 min. Action potential parameters were unaffected by ⩽3 μM S-oxybutynin, and the only significant effect of the highest concentration tested (30 μM) was a reduction in APD20 to 95±2% of pre-drug control (n=9) (P<0.05, t-test) (Figure 7a,c). In marked contrast, both low and high concentrations of terodiline lengthened the APD20 and APD90, with peak lengthening (APD90 128±5%, n=6; P<0.01, t-test) observed at 10 μM (Figure 7b,c). High concentrations of terodiline also depressed  max (e.g., to 69±6% (n=8; P<0.01) at 30 μM), whereas high concentrations of S-oxybutynin had little effect (Figure 7c).
max (e.g., to 69±6% (n=8; P<0.01) at 30 μM), whereas high concentrations of S-oxybutynin had little effect (Figure 7c).
Figure 7.
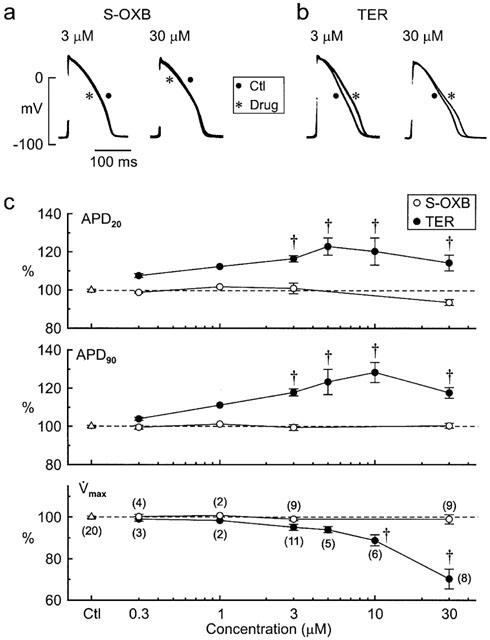
Concentration-dependent effects of S-oxybutynin and terodiline on action potentials in rabbit papillary muscles stimulated at 1 Hz. (a,b) Superimposed sets of action potentials recorded before and 30 min after addition of drug. (c) APD20, APD90 and  max of action potentials from control muscles and muscles treated with single concentrations of S-oxybutynin and terodiline. Values measured after 30-min observation periods are expressed as percentages of pre-drug values. The numbers of muscles are indicated in parentheses on the bottom plot. Significance was assessed with the t-test: *P<0.05, †P<0.01.
max of action potentials from control muscles and muscles treated with single concentrations of S-oxybutynin and terodiline. Values measured after 30-min observation periods are expressed as percentages of pre-drug values. The numbers of muscles are indicated in parentheses on the bottom plot. Significance was assessed with the t-test: *P<0.05, †P<0.01.
Rate-dependent effects
After the 30-min monitoring period (1-Hz stimulation), control and drug-treated muscles were driven at 0.4 Hz for 5 min and then 3 Hz for 3 min to evaluate whether drug treatment affected the response of the action potential plateau to changes in driving rate. Records from a control muscle (Figure 8a, top row) indicate that slowing the stimulation rate from 1 to 0.4 Hz lowered plateau amplitude by a few millivolts and shortened plateau duration by about 25%. This rate-dependent abbreviation (Gibbs & Johnson, 1961) is generally attributed to an increase in the availability of transient outward current (Ito) following a lengthening of the diastolic interval (Kukushkin et al., 1983; Hiraoka & Kawano, 1987; Giles & Imaizumi, 1988). Consistent with this interpretation, the action potential plateau recovered when the stimulation rate was subsequently increased to 3 Hz. A similar pattern of plateau abbreviation and recovery was evident in a muscle treated with 3 μM S-oxybutynin, but less so in a muscle treated with 3 μM terodiline (Figure 8a).
Figure 8.

Effects of stimulation rate on the configuration of action potentials in control and drug-treated rabbit papillary muscles. (a) Top to bottom rows: Records from control, S-oxybutynin and terodiline treated muscles. Left to right: the records were obtained just before (0 min) and at the end of successive stimulation periods (30 min at 1 Hz, 5 min at 0.4 Hz, and 3 min at 3 Hz). Time (top) is continuous from 0 min. The calibration bars indicate 50 mV and 100 ms. (b) Superimposed sets of action potentials; the traces were recorded after 30 min drug treatment at 1 Hz stimulation rate and 5 min after reducing the rate from 1 to 0.4 Hz. The calibration bars indicate 50 mV and 100 ms. (c) Summary of the percentage changes in plateau duration at 0 mV (APD0mV) induced by changing the driving rate from 1 to 0.4 Hz for 5 min and then to 3 Hz for 3 min. All data are referenced to APD0mV values measured before the 30-min (control or drug) 1-Hz trial periods. Numbers of muscles: control (16), S-oxybutynin 3 and 30 μM (15 each), terodiline 3 and 30 μM (9,10). Significance was assessed using one-way ANOVA, Bonferroni test: ††P<0.001.
Since there were definite (though small) changes in action potential amplitude at different stimulation rates, the effects of rate on plateau duration were assessed by measuring duration at a fixed (0 mV) repolarization level (APD0mV) (Figure 8b,c). When referenced to the duration at the start of the 30-min period at 1 Hz, the APD0mV at the end of each frequency trial in control muscles (n=16) was 99±2%, 77±2% and 97±2% at 1, 0.4 and 3 Hz, respectively. The durations in muscles treated with S-oxybutynin followed the same pattern: 100±3%, 75±4% and 98±3%, respectively, at 0.3 μM (n=4) (not shown), 99±2%, 75±2% and 98±2%, respectively, at 3 μM (n=15), and 95±1%, 77±2% and 92±2%, respectively, at 30 μM (n=15). However, APD0mV in muscles treated with 3 and 30 μM terodiline was less likely to shorten when the rate was lowered from 1 to 0.4 Hz (e.g., 110±3% (1 Hz) and 102±2% (0.4 Hz) (n=9) at 30 μM), and more likely to shorten (e.g. to 83±2% at 30 μM) rather than lengthen when the stimulation rate was increased from 0.4 to 3 Hz. Analysis of the data (one-way ANOVA followed by the Bonferroni test) indicates that (APD0mV) (0.4 Hz) was significantly longer (P<0.001) in muscles treated with 3 and 30 μM terodiline than in control muscles, whereas APD0mV (3 Hz) was significantly shorter (P<0.001) in muscles treated with 30 μM terodiline than in control muscles.
Effects of S-oxybutynin and terodiline on transient outward current in rabbit ventricular myocytes
Differences in the responses of the action potential in rabbit and guinea-pig papillary muscles to drug treatments (see Discussion) suggested that the drugs might have concentration-dependent inhibitory effects on Ito in rabbit papillary muscles. This possibility was examined by measuring the whole-cell Ito in rabbit ventricular myocytes. The myocytes were bathed in a modified Tyrode's solution that contained 20 μM tetrodotoxin to block Na+ current, 0.2 mM Cd2+ to block ICa,L, 0.1 mM Ba2+ to block IK1, and 3 μM E4031 to block IKr. In a series of experiments at 36°C, myocytes were held at −80 mV, depolarized to potentials up to +50 mV, and exposed to 3 and/or 30 μM drug for times (5–7 min) long enough to reach new steady-state Ito. Figure 9a indicates that 3 μM S-oxybutynin reduced Ito by 9±2% (n=6) (P<0.05, t-test), and 30 μM reduced it by 35±3% (n=4) (P<0.001). Terodiline had a more potent inhibitory action, with 3 μM reducing the current by 31±3% (n=11) and 30 μM reducing it by 87±3% (n=8) (both P<0.001) (Figure 9b).
Figure 9.
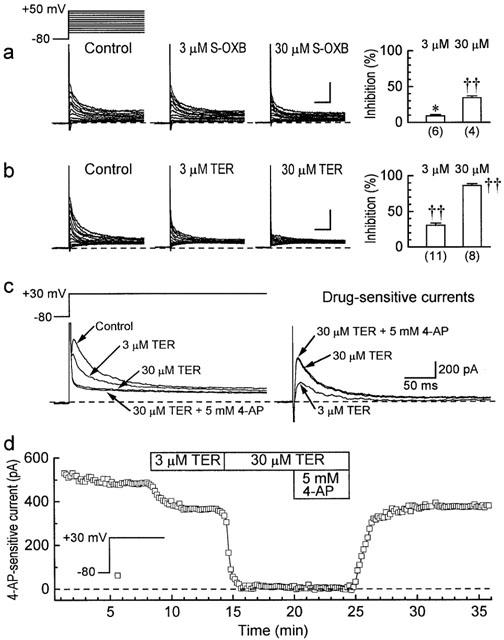
Effects of S-oxybutynin and terodiline on Ito in rabbit ventricular myocytes. The myocytes were superinfused with Tyrode's solution that contained 0.2 mM Cd2+, 0.1 mM Ba2+, 3 μM E4031 and 20 μM tetrodotoxin. (a,b) Records from experiments conducted at 36°C. The myocytes were held at −80 mV, and depolarized to more positive potentials for 400 ms at 0.1 Hz before and approximately 6 min after exposures to 3 and/or 30 μM drug. Left: representative results. Right: percentage inhibition of Ito amplitude. The amplitude was measured as the time-dependent current (10 ms to 400 ms) elicited on pulses to +50 mV. The calibration bars indicate 0.5 nA and 100 ms. Numbers of myocytes in parentheses. Significance was assessed with the t-test: *P<0.05, †P<0.01, ††P<0.001. (c,d) Results from representative experiments conducted at 24°C. The myocytes were held at −80 mV and depolarized to +30 mV for 400 ms at 0.1 Hz. Ito at +30 mV was measured as the current sensitive to 5 mM 4-aminopyridine.
In the foregoing series, Ito was measured as the time-dependent current (10 ms to end-of-pulse) at +50 mV. For improved resolution, experiments were conducted at 24°C (to slow Ito kinetics) and 5 mM 4-aminopyridine was added at the end of the experiment to allow measurement of Ito on pulses to +30 mV (0.1 Hz) as 4-aminopyridine-sensitive current. Using this protocol, the results with 3 and 30 μM terodiline (Figure 9c) were virtually identical to those obtained at 36°C (35±3% inhibition with 3 μM (n=8), and 88±3% inhibition with 30 μM (n=8) (both P<0.001)).
Discussion
We have compared the effects of S-oxybutynin and terodiline on membrane currents in guinea-pig and rabbit ventricular myocytes and on action potential configuration in guinea-pig and rabbit papillary muscles. Although both S-oxybutynin and terodiline inhibited Ca2+ and K+ currents, terodiline was the more potent of the two, especially in regard to IKr and Ito. Action potentials in guinea-pig and rabbit papillary muscles were unaffected by ⩽10 μM S-oxybutynin and moderately shortened by higher concentrations, whereas action potentials were lengthened by ⩽10 μM terodiline and either shortened (guinea-pig) or lengthened (rabbit) by higher concentrations. An additional finding was that rate-dependent changes in rabbit ventricular action potentials were strongly modified by terodiline. We discuss these findings, and consider their implications in relation to clinical safety of the drugs.
Effects on membrane currents and action potentials in guinea-pig ventricular preparations
The results of the voltage-clamp experiments on guinea-pig ventricular myocytes indicate that S-oxybutynin is a relatively weak, non-specific inhibitor of cardiac membrane currents (IC50 values of 17.8 μM for ICa,L, and 12, 41 and ca. 50 μM for IKr, IKs, and IK1, respectively). The relatively high IC50 values explain why concentrations of the drug ⩽3 μM had little effect on the configuration of action potentials in guinea-pig papillary muscles. Higher concentrations reduced the APD20 by up to 22%, suggesting that a plateau shortening influence related to inhibition of ICa,L outweighed plateau lengthening influences related to inhibitions of IKr and IKs. The APD90 was less affected, suggesting that reductions in IK1 at high concentrations of the drug contributed to a slowing of phase 3 repolarization.
The IC50 values for terodiline were moderately lower than those for S-oxybutynin in regard to ICa,L and IKs, but 24 fold lower (0.5 μM) in regard to IKr. The consequence of this selective inhibition was a lengthening of the action potential at terodiline concentrations ⩽10 μM, with the largest lengthening (10–15%) occurring with 1–3 μM drug. It seems likely that the lengthening was restricted by the concomitant inhibition of ICa,L since specific inhibition of IKr by 3 μM E4031 lengthens the duration by 27±2% (n=5) (Shuba et al., 1999).
Effects on action potentials in rabbit papillary muscles
The shape of the action potential in rabbit papillary muscles was almost insensitive to 0.3–30 μM S-oxybutynin. This finding suggests that the action potential shortening and lengthening influence caused by inhibitions of Ca2+ and K+ currents, respectively, were almost in balance over this range of drug concentration. By contrast, the action potential was prolonged by 0.3–30 μM terodiline, suggesting relatively larger inhibition of K+ currents by this drug.
The terodiline-induced lengthening of the action potential was larger and peaked at a higher drug concentration in rabbit (10 μM) than in guinea-pig (1–3 μM) papillary muscles. The most likely reason for this species difference is that rabbit ventricular cells have a prominent Ito, and the shortening influence related to concentration-dependent inhibition of ICa,L in rabbit muscles is counterbalanced by the lengthening influence related to concentration-dependent inhibition of Ito.
Inhibition of Ito by terodiline also provides a basis for understanding the behaviour of the action potential plateau when rabbit papillary muscles were driven at different rates. In control muscles, slowing of the driving rate from 1 to 0.4 Hz shortened APD0mV by an average of 23%, i.e., net outward plateau current was enhanced at the slower rate. This enhancement of net outward plateau current was almost certainly due to an increase in Ito that outweighed other possible changes induced by the slowing of the driving rate (e.g., increase in ICa,L, reduction in IK: Hiraoka & Kawano, 1987; Boyett et al., 1994; Ogura et al., 1999). In drug-treated muscles, the rate-related abbreviation of the plateau was unaffected by 3 μM S-oxybutynin, moderately depressed by 30 μM S-oxybutynin and 3 μM terodiline, and strongly depressed by 30 μM terodiline. This set of results is consistent with the concentration-dependent effects of the drugs on Ito (see Figure 9a,b, right hand panels).
When control and S-oxybutynin-treated muscles were driven at 3 Hz, the duration of the plateau was only slightly shorter than at 1 Hz, indicating that likely rate-induced shortening influences (smaller ICa,L, larger IK) were almost fully offset by the lengthening influence of smaller Ito (weaker recovery during the shorter diastolic intervals). In marked contrast, the plateau duration in terodiline-treated muscles was 15% (3 μM) to 26% (30 μM) shorter at 3 Hz than at 1 Hz, suggesting a concentration-dependent removal of the lengthening influence of rate-dependent attenuation of Ito. A rough indication of the normal magnitude of this lengthening influence is that the APD20 in Ito-deficient guinea-pig papillary muscles shortens by 27% when the stimulation rate is increased from 1 to 3 Hz (Shuba et al., 1999). In summary (and independent of whether inhibition of Ito is the sole factor at play), terodiline induces a ‘reverse' rate dependence in rabbit papillary muscles, i.e. the degree of action potential lengthening is accentuated at lower stimulation rates.
Relation to clinical safety
A recent pharmacokinetic study on human volunteers (Koch et al., 1998) indicates that the peak plasma concentration Cmax) of orally-administered S-oxybutynin was similar to that found in earlier studies on oxybutynin. In the case of patients receiving therapeutic doses of oxybutynin, there is considerable inter-patient variability but average Cmax is approximately 0.01 μM (Hughes et al., 1992; Lukkari et al., 1997a, 1997b). On that basis, the negligible effects of 1 μM S-oxybutynin on muscle action potential  max and myocyte ICa,L suggest that cardiac function dependent on Na+ and Ca2+ channel activity is highly unlikely to be compromised by the drug. The situation with terodiline is less clearcut because Cmax is near 1 μM (Connolly et al., 1991),
max and myocyte ICa,L suggest that cardiac function dependent on Na+ and Ca2+ channel activity is highly unlikely to be compromised by the drug. The situation with terodiline is less clearcut because Cmax is near 1 μM (Connolly et al., 1991),  max is much more sensitive to terodiline than to S-oxybutynin, and this sensitivity is enhanced by lower resting potential and higher stimulation rates (Shuba et al., 1999).
max is much more sensitive to terodiline than to S-oxybutynin, and this sensitivity is enhanced by lower resting potential and higher stimulation rates (Shuba et al., 1999).
A large number of chemically and therapeutically unrelated drugs are known to cause QT lengthening that can lead to ventricular tachyarrhythmia (torsades de pointes) (Zipes, 1987; Zehender et al., 1991; Roden et al., 1996). Although electrolyte disturbances, dietary deficiencies and heart disease can be contributing factors to torsades, and the precise events triggering the arrhythmia are still uncertain, it is highly likely that drug-induced inhibition of repolarizing K+ currents, especially IKr, play a pivotal role (Roden et al., 1996). In that regard, the results of the present study identify a major difference in the cardiac effects of terodiline and S-oxybutynin: terodiline inhibited IKr with IC50 0.5 μM, whereas S-oxybutynin inhibited it with IC50 12 μM. When this difference is considered in relation to the respective Cmax values of 1 and 0.01 μM (see above) by taking the ratio of Cmax to the IC50 for IKr, the outcome is 2.0 for terodiline and ca. 0.001 for S-oxybutynin. For comparison, the ratio for chlorpheniramine, a first-generation antihistamine with a good safety record, appears to be about 0.02 (Cmax of 0.03 μM (Yasuda et al., 1995); IC50 for IKr of 1.6 μM (Salata et al., 1995)), whereas the approximate ratio for the second-generation, torsades-inducing terfenadine is a much larger 0.4 (Cmax near 0.02 μM (Woosley et al., 1993) and IC50 for IKr of 0.05 μM (Salata et al., 1995)). A final point is that the frequency response of the rabbit ventricular action potential was unaffected by 0.3–3 μM S-oxybutynin, and only moderately affected by a 30 μM concentration, suggesting that the Ito-dominated frequency response of human myocardial cells (Shibata et al., 1989; Beuckelmann et al., 1993; Wettwer et al., 1994) is also unlikely to be affected by S-oxybutynin. This is important in view of the link between drug-induced ‘reverse' rate-dependence, bradycardia and torsades de pointes (Jackman et al., 1988; Stewart et al., 1992; Thomas et al., 1995; Roden et al., 1996), and the likelihood that many elderly patients receiving incontinence drugs have aging-related cardiac hypertrophy (Svanborg, 1997) and lowered density of Ito (Beuckelmann et al., 1993; McIntosh et al., 1998).
Acknowledgments
We thank Ms Gina Dickie and Dr An-Chi Guo for excellent technical assistance. This study was supported by Sepracor Inc., the Medical Research Council of Canada, and the Heart and Stroke Foundation of New Brunswick.
Abbreviations
- APA
action potential amplitude
- APD20
action potential duration at 20% repolarization level
- APD90
action potential duration at 90% repolarization level
- APD0mV
action potential duration at 0 mV
- DMSO
dimethyl sulphoxide
- EGTA
ethylene glycol-bis(b-aminoethyl)-N,N,N,N-tetraacetic acid
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- IC50
concentration that produces 50% of maximal inhibition
- ICa,L
L-type Ca2+ current
- IK
delayed-rectifier K+ current
- IK1
inward-rectifying K+ current
- IKr
rapidly-activating component of IK
- IKs
slowly-activating component of IK
- Ito
transient outward current
- I-V
current-voltage relationship
- P
probability (significance level)
- s.e.m.
standard error of the mean
- V̇
upstroke velocity
- V̇max
maximal upstroke velocity
References
- ABRAMS P., FREEMAN R., ANDERSTROM C., MATTIASON A. Tolterodine, a new antimuscarinic agent: as effective but better tolerated than oxybutynin in patients with an overactive bladder. Br. J. Urol. 1998;81:801–810. doi: 10.1046/j.1464-410x.1998.00717.x. [DOI] [PubMed] [Google Scholar]
- ÅMARK P., BUSSMAN G., EKSBORG S. Follow-up of long-time treatment with intravesical oxybutynin for neurogenic bladder in children. Eur. Urol. 1998;34:148–153. doi: 10.1159/000019701. [DOI] [PubMed] [Google Scholar]
- ANDERSON K.E. Clinical pharmacology of terodiline. Scan. J. Urol. Nephrol. Suppl. 1984;87:13–20. [PubMed] [Google Scholar]
- BEUCKELMANN D.J., NABAUER M., ERDMANN E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ. Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- BOYETT M.R., HONJO H., HARRISON S.M., ZANG W.J., KIRBY M.S. Ultra-slow voltage-dependent inactivation of the calcium current in guinea-pig and ferret ventricular myocytes. Pflügers Arch. 1994;428:39–50. doi: 10.1007/BF00374750. [DOI] [PubMed] [Google Scholar]
- BUYSE G., WALDECK K., VERPOORTEN C., BJORK H., CASAER P., ANDERSSON K.E. Intravesical oxybutynin for neurogenic bladder dysfunction: less systemic side effects due to reduced first pass metabolism. J. Urol. 1998;160:892–896. doi: 10.1016/S0022-5347(01)62828-3. [DOI] [PubMed] [Google Scholar]
- CAIONE P., ARENA F., BIRAGHI M., CIGNA R.M., CHENDI D., CHIOZZA M.L., DE LISA A., DE GRAZIA E., FANO M., FORMICA P., GAROFALO S., GRAMENZI R., VON HELAND M., LANZA P., LANZA T., MAFFEI S., MANIERI C., MERLINI E., MIANO L., NAPPO S., PAGLIARULO A., PAOLINI PAOLETTI F., PAU A.C., PORRU D., RICCIPETITONI G., SCARPA R.M., SEIMANDI P., ARTIBANI W. Nocturnal enuresis and daytime wetting: a multicentric trial with oxybutynin and desmopressin. Eur. Urol. 1997;31:459–463. doi: 10.1159/000474507. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. Mechanisms and control of repolarization. Eur. Heart J. 1993;14:3–13. doi: 10.1093/eurheartj/14.suppl_h.3. [DOI] [PubMed] [Google Scholar]
- CONNOLLY M.J., ASTRIDGE P.S., WHITE E.G., MORLEY C.A., COWAN J.C. Torsades de pointes ventricular tachycardia and terodiline. Lancet. 1991;338:344–345. doi: 10.1016/0140-6736(91)90481-4. [DOI] [PubMed] [Google Scholar]
- FOLLMER C.H., COLATSKY T.J. Block of delayed rectifier potassium current, IK, by flecainide and E-4031 in cat ventricular myocytes. Circ. 1990;82:289–293. doi: 10.1161/01.cir.82.1.289. [DOI] [PubMed] [Google Scholar]
- FONDA D. The prevalence and significance of dependent urinary incontinence. Austr. New Zeal. J. Med. 1989;17:600. [Google Scholar]
- FREDERICKS C.M., ANDERSON G.F., KREULEN D.L. A study of the anticholinergic and antispasmodic activity of oxybutynin (Ditropan) on rabbit detrusor. Invest. Urol. 1975;12:317–319. [PubMed] [Google Scholar]
- GIBBS C.L., JOHNSON E.A. Effect of changes in frequency of stimulation upon rabbit ventricular action potential. Circ. Res. 1961;9:165–170. doi: 10.1161/01.res.9.1.165. [DOI] [PubMed] [Google Scholar]
- GILES W.R., IMAIZUMI Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J. Physiol. 1988;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEATH B.M., TERRAR D.A. Separation of the components of the delayed rectifier potassium current using selective blockers of IKr and IKs in guinea-pig isolated ventricular myocytes. Exp. Physiol. 1996;81:587–603. doi: 10.1113/expphysiol.1996.sp003961. [DOI] [PubMed] [Google Scholar]
- HIRAOKA M., KAWANO S. Mechanism of increased amplitude and duration of the plateau with sudden shortening of diastolic intervals in rabbit ventricular cells. Circ. Res. 1987;60:14–26. doi: 10.1161/01.res.60.1.14. [DOI] [PubMed] [Google Scholar]
- HJALMAS K. Pathophysiology and impact of nocturnal enuresis. Acta Paediatr. 1997;86:919–922. doi: 10.1111/j.1651-2227.1997.tb15170.x. [DOI] [PubMed] [Google Scholar]
- HUGHES K.M., LANG J.C.T., LAZARE R., GORDON D., STANTON S.L., MALONE-LEE J., GERAINT M. Measurement of oxybutynin and its N-desethyl metabolite in plasma, and its application to pharmacokinetic studies in young, elderly and frail elderly volunteers. Xenobiot. 1992;22:859–869. doi: 10.3109/00498259209053145. [DOI] [PubMed] [Google Scholar]
- JACKMAN W.M., FRIDAY K.J., ANDERSON J.L., ALIOT E.M., CLARK M., LAZZARA R. The long QT syndromes: A critical review, new clinical observations and a unifying hypothesis. Prog. Cardiovasc. Diseas. 1988;XXXI:115–172. doi: 10.1016/0033-0620(88)90014-x. [DOI] [PubMed] [Google Scholar]
- JONES S.E., OGURA T., SHUBA L.M., MCDONALD T.F. Inhibition of the rapid component of the delayed-rectifier K+ current by therapeutic concentrations of the antispasmodic agent terodiline. Br. J. Pharmacol. 1998;125:1138–1143. doi: 10.1038/sj.bjp.0702173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH P., MCCULLOUGH J.R., BLUM P.S., DEGRAW S., KRAUSE A.C., RUBIN P.D. Pharmacokinetics and safety of (S)-oxybutynin in normal healthy volunteers. F.A.S.E.B. J. 1998;12:A142. [Google Scholar]
- KUKUSHKIN N.I., GAINULLIN R.Z., SOSUNOV E.A. Transient outward current and rate dependence of action potential duration in rabbit cardiac ventricular muscle. Pflügers Arch. 1983;399:87–92. doi: 10.1007/BF00663902. [DOI] [PubMed] [Google Scholar]
- LANGTRY H.D., MCTAVISH D. Terodiline: a review of its pharmacological properties, and therapeutic use in the treatment of urinary incontinence. Drugs. 1990;40:748–761. doi: 10.2165/00003495-199040050-00008. [DOI] [PubMed] [Google Scholar]
- LISH P.M., LABUDDE J.A., PETERS E.L., ROBBINS S.I. Oxybutynin–a musculotropic antispasmodic drug with moderate anticholinergic action. Arch. Int. Pharmacodyn. Ther. 1965;156:467–488. [PubMed] [Google Scholar]
- LUKKARI E., ARANKO K., JUHAKOSKI A., HAKONEN T., NEUVONEN P.J. Effect of time interval between food and drug ingestion on the absorption of oxybutynin from a controlled-release tablet. Pharmacol. Toxicol. 1997b;81:31–34. doi: 10.1111/j.1600-0773.1997.tb00027.x. [DOI] [PubMed] [Google Scholar]
- LUKKARI E., JUHAKOSKI A., ARANKO K., NEUVONEN P.J. Itraconazole moderately increases serum concentrations of oxybutynin but does not affect those of the active metabolite. Eur. J. Clin. Pharmacol. 1997a;52:403–406. doi: 10.1007/s002280050309. [DOI] [PubMed] [Google Scholar]
- MCINTOSH M.A., COBBE S.M., KANE K.A., RANKIN A.C. Action potential prolongation and potassium currents in left- ventricular myocytes isolated from hypertrophied rabbit hearts. J. Mol. Cell. Cardiol. 1998;30:43–53. doi: 10.1006/jmcc.1997.0570. [DOI] [PubMed] [Google Scholar]
- MCLEOD A.A., THOROGOOD S., BARNETT S. Torsades de pointes complicating treatment with terodiline. Br. Med. J. 1991;302:1469. doi: 10.1136/bmj.302.6790.1469-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORONHA-BLOB L., KACHUR J.F. Enantiomers of oxybutynin: in vitro pharmacological characterization at M1, M2 and M3 muscarinic receptors and in vivo effects on urinary bladder contraction, mydriasis and salivary secretion in guinea pigs. J. Pharmacol. Exp. Ther. 1991;256:562–567. [PubMed] [Google Scholar]
- OGURA T., JONES S., SHUBA L.M., MCCULLOUGH J.R., MCDONALD T.F. Block and modified gating of cardiac calcium channel currents by terodiline. Br. J. Pharmacol. 1999;127:1837–1845. doi: 10.1038/sj.bjp.0702713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGURA T., SHUBA L.M., MCDONALD T.F. Action potentials, ionic currents and cell water in guinea pig ventricular preparations exposed to dimethyl sulfoxide. J. Pharmacol. Exp. Ther. 1995;273:1273–1286. [PubMed] [Google Scholar]
- RODEN D.M., LAZZARA R., ROSEN M., SCHWARTZ P.J., TOWBIN J., VINCENT G.M. Multiple mechanisms in the long-QT syndrome: current knowledge, gaps, and future directions. Circ. 1996;94:1996–2012. doi: 10.1161/01.cir.94.8.1996. [DOI] [PubMed] [Google Scholar]
- SALATA J.J., JURKIEWICZ N.K., WALLACE A.A., STUPIENSKI R.F., GUINOSSO P.J., LYNCH J.J. Cardiac electrophysiological actions of the histamine H1-receptor antagonists astemizole and terfenadine compared with chlorpheniramine. Circ. Res. 1995;76:110–119. doi: 10.1161/01.res.76.1.110. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Role of external Ca2+ and K+ in gating of cardiac delayed rectifier K+ currents. Pflügers Arch. 1992;420:180–186. doi: 10.1007/BF00374988. [DOI] [PubMed] [Google Scholar]
- SHIBATA E.F., DRURY T., REFSUM H., ALDRETE V, GILES W. Contributions of a transient outward current to repolarization in human atrium. Am. J. Physiol. 1989;257:H1773–H1781. doi: 10.1152/ajpheart.1989.257.6.H1773. [DOI] [PubMed] [Google Scholar]
- SHUBA L.M., KASAMAKI Y., JONES S.E., OGURA T., MCCULLOUGH J.R., MCDONALD T.F. Action potentials, contraction, and membrane currents in guinea pig ventricular preparations treated with the antispasmodic agent terodiline. J. Pharmacol. Exp. Ther. 1999;290:1417–1426. [PubMed] [Google Scholar]
- SMITH E.R., WRIGHT S.E., ABERG G., FANG Y., MCCULLOUGH J.R. Comparison of the antimuscarinic and antispasmodic actions of racemic oxybutynin and desethyloxybutynin and their enantiomers with those of racemic terodiline. Arzneim. 1998;48:1012–1018. [PubMed] [Google Scholar]
- STEWART D.A., TAYLOR J., GHOSH S., MACPHEE G., ABDULLAH I., MCLENACHAN J.M., STOTT D.J. Terodiline causes polymorphic ventricular tachycardia due to reduced heart rate and prolongation of QT interval. Eur. J. Clin. Pharmacol. 1992;42:577–580. doi: 10.1007/BF00265918. [DOI] [PubMed] [Google Scholar]
- SVANBORG A. Age-related changes in cardiac physiology. Can they be postponed or treated by drugs? [see comments] Drugs Aging. 1997;10:463–472. doi: 10.2165/00002512-199710060-00006. [DOI] [PubMed] [Google Scholar]
- THEODOROU C., MOUTZOURIS G., FLORATOS D., PLASTIRAS D., KATSIFOTIS C., MERTZIOTIS N. Incontinence after surgery for benign prostatic hypertrophy: the case for complex approach and treatment. Eur. Urol. 1998;33:370–375. doi: 10.1159/000019618. [DOI] [PubMed] [Google Scholar]
- THOMAS S.H., HIGHAM P.D., HARTIGAN GO K., KAMALI F., WOOD P., CAMPBELL R.W., FORD G.A. Concentration dependent cardiotoxicity of terodiline in patients treated for urinary incontinence. Br. Heart J. 1995;74:53–56. doi: 10.1136/hrt.74.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS T.M., PLYMAT K.R., BLANNIN J., MEADE T.W. Prevalence of urinary incontinence. Br. Med. J. 1980;281:1243–1245. doi: 10.1136/bmj.281.6250.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONINI M., RIZZI C.A., PERUCCA E., DE PONTI F., D'ANGELO L., DEL VECCHIO A., CREMA A. Depressant action of oxybutynin on the contractility of intestinal and urinary tract smooth muscle. J. Pharm. Pharmacol. 1987;39:103–107. doi: 10.1111/j.2042-7158.1987.tb06953.x. [DOI] [PubMed] [Google Scholar]
- WEIN A.J. Pharmacologic options for the overactive bladder. Urol. 1998;51:43–47. doi: 10.1016/s0090-4295(98)90009-7. [DOI] [PubMed] [Google Scholar]
- WETTWER E., AMOS G.J., POSIVAL H., RAVENS U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ. Res. 1994;75:473–482. doi: 10.1161/01.res.75.3.473. [DOI] [PubMed] [Google Scholar]
- WOOSLEY R.L., CHEN Y., FREIMAN J.P., GILLIS R.A. Mechanism of the cardiotoxic actions of terfenadine. J.A.M.A. 1993;269:1532–1536. [PubMed] [Google Scholar]
- YARKER Y.E., GOA K.L., FITTON A. Oxybutynin. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in detrusor instability. Drugs Aging. 1995;6:243–262. doi: 10.2165/00002512-199506030-00007. [DOI] [PubMed] [Google Scholar]
- YASUDA S.U., WELLSTEIN A., LIKHARI P., BARBEY J.T., WOOSLEY R.L. Chlorpheniramine plasma concentration and histamine H1-receptor occupancy. Clin. Pharmacol. Ther. 1995;58:210–220. doi: 10.1016/0009-9236(95)90199-X. [DOI] [PubMed] [Google Scholar]
- ZEHENDER M., HOHNLOSER S., JUST H. QT-interval prolonging drugs: mechanisms and clinical relevance of their arrhythmogenic hazards. Cardiovasc. Drugs Ther. 1991;5:515–530. doi: 10.1007/BF03029779. [DOI] [PubMed] [Google Scholar]
- ZIPES D.P. Proarrhythmic effects of antiarrhythmic drugs. Amer. J. Cardiol. 1987;59:26E–31E. doi: 10.1016/0002-9149(87)90198-6. [DOI] [PubMed] [Google Scholar]


