Abstract
Current analgesic therapy is dominated by NSAIDs and opiates, however these agents have limited efficacy in the treatment of neuropathic pain. The novel anticonvulsant agent gabapentin (Neurontin) has been shown to be an effective treatment for neuropathic pain in the clinic. Recent studies have demonstrated that gabapentin selectively interacts with the α2δ subunit of voltage dependent calcium channels (VDCCs) which may be important in its mechanism of action.
Previous studies have identified a gabapentin analogue, 3-methyl gabapentin, that stereoselectively interacts with the α2δ subunit of VDCCs. Thus, whilst (1S,3R) 3-methyl gabapentin binds to the α2δ protein with high affinity (IC50=42 nM), the corresponding (1R,3R) isomer is 300 times weaker (Bryans et al., 1998: J. Med. Chem., 41, 1838–1845). The present study examines the activity of diastereoisomers of 3-methyl gabapentin in two rat models of neuropathic pain to assess the importance of an interaction with the α2δ subunit of VDCCs.
(1S,3R) 3-methyl-gabapentin dose-dependently (10–100 mg kg−1, p.o.) blocked the maintenance of static allodynia in the rat streptozocin and Chung models of neuropathic pain with MEDs of 30 mg kg−1. This isomer also dose-dependently blocked the maintenance of dynamic allodynia in both models with respective MEDs of 30 and 100 mg kg−1. In contrast, (1R,3R) 3-methyl gabapentin (100 mg kg−1, p.o.) failed to block either static or dynamic allodynia in the streptozocin model.
It is concluded that these data further support the hypothesis that the α2δ subunit of VDCCs plays an important role in the maintenance of mechanical hypersensitivity in models of neuropathic pain.
Keywords: Streptozocin, Chung model, von Frey hair, cotton bud, α2δ subunit of VDCCs, neuropathic pain, 3-methyl gabapentin, allodynia
Introduction
Current analgesic therapy is dominated by two classes of drugs, NSAIDs and opiates. These agents dominate this field despite possessing limited efficacy in some pain states (e.g. neuropathic pain) and having unacceptable side effects. Thus, for some time considerable resources have been spent understanding the pathophysiology of pain in order to discover novel treatments with increased efficacy and improved side effect profiles. Gabapentin (Neurontin) is fast establishing itself as one such treatment. Recent preclinical studies indicate that unlike opiates neither gabapentin nor the related compound pregabalin (formally S-isobutylgaba and Cl-1008) affect transient physiological pain responses (Field et al., 1997b; Hunter et al., 1997; Shimoyama et al., 1997). However, it has been reported that these compounds possess antihyperalgesic and antiallodynic properties in animal models of inflammatory, surgical and neuropathic pain (Xiao & Bennett, 1995; Singh et al., 1996; Field et al., 1997a,1997b; 1999a,1999b; Hunter et al., 1997; Shimoyama et al., 1997; Hwang & Yaksh, 1997). More recently, controlled clinical trials have revealed that gabapentin can reduce pain associated with diabetic neuropathy and PHN (Backonja et al., 1998; Rowbotham et al., 1998).
Several hypotheses have been put forward regarding the mechanism of action of gabapentin (for review see Taylor et al., 1998). Thus, despite its structural similarities with GABA, it does not interact with GABA receptors. However, some evidence does suggest that gabapentin can increase GABA release, thereby enhancing inhibitory neurotransmission (Gotz et al., 1993). More recent studies have shown that the antiallodynic action of gabapentin is not sensitive to either GABAA or GABAB receptor antagonists (Hwang & Yaksh 1997). This suggests that the increase in CNS GABA levels may not be responsible for the action of gabapentin in models of pain. Other studies have suggested that gabapentin is able to block excitatory neurotransmission. Thus, it has been shown that the antihyperalgesic actions of gabapentin can be blocked by D-serine, an agonist at the glycine/NMDA receptor (Singh et al., 1996; Partridge et al., 1998). This effect of D-serine is likely to be indirect as biochemical studies have shown that gabapentin does not interact with the glycine/NMDA receptor. More recently, it has been shown that gabapentin binds with high affinity and specificity to the α2δ subunit of voltage dependent calcium channels (VDCC) (Gee et al., 1996). However, the role of this protein in the maintenance of hypersensitivity induced by nerve damage requires further clarification. We have synthesized a series of novel ligands that show high affinity and specificity for this site. This work led to the identification of 3-methyl gabapentin (Bryans et al., 1998). We have previously reported that this compound interacts with the α2δ subunit of VDCC in a stereoselective manner. Thus, whilst the (1S,3R) diastereoisomer has high (42 nM) affinity, the (1R,3R) is approximately 300 times weaker (Bryans et al., 1998). We have previously demonstrated that both static and dynamic allodynia can be detected in models of neuropathic pain in the rat (Field et al., 1999a,1999b). Here, we evaluate the activity of each diastereoisomer on both types of allodynia in two models of neuropathic pain. Part of this work has previously been published in abstract form (Field et al., 1999c).
Methods
Animals
Male Sprague Dawley rats were used in all the studies and obtained from Charles River (Margate, Kent, U.K.). Animals were housed in groups of six under a 12 h light/dark cycle (lights on at 0700 h) with food and water ad libitum. All experiments were carried out by an observer blind to drug treatments. All experiments were carried out in accordance with the Home Office Animals (Scientific Procedures) Act 1986.
Animal models of neuropathic pain
Chung model
Animals (125–150 g) were anaesthetized with a 2% isofluorane O2 mixture maintained during surgery via a nose cone. Animals were placed on a homeothermic blanket for the duration of the procedure. The L5 and L6 spinal nerves were ligated as previously described by Kim & Chung 1992. Briefly, following surgical preparation the left paraspinal muscles were separated by an incision from spinal processes at L4 to S2 levels. Using a dissecting microscope the L6 transverse process was exposed and partially removed with the use of microrongeurs. The L5 and L6 spinal nerves were isolated and tightly ligated with 4-0 silk thread. The muscle and skin were then sutured and the wound treated with topical antibiotics. In the present study we have used the animal's contralateral paw for reference following Chung surgery rather than separate sham animals, as this reduces the total number of animals required for each experiment. We have previously used the contralateral paw of Chung animals for reference and have shown that it remains unaffected by the surgery for the duration of our experiments (Field et al., 1999a)
Streptozocin model
Diabetes was induced in animals (225–250 g) by a single i.p. injection of streptozocin (50 mg kg−1). Control animals received a similar administration of isotonic saline. No animals were culled due to ill health following streptozocin treatment. Animals displayed increased urination and some animals tended to put less body weight on than vehicle controls, but otherwise the animals appeared normal.
Measurement of static and dynamic allodynia
Animals were habituated to test cages prior to the assessment of allodynia. Static allodynia was evaluated by application of von Frey hairs (Stoelting, Wood Dale, IL, U.S.A.) in ascending order of force (0.7, 1.2, 1.5, 2, 3.6, 5.5, 8.5, 11.8, 15.1 and 29 g) to the plantar surface of hind paws. Each von Frey hair was applied to the paw for 6 s, or until a withdrawal response occurred. Once a withdrawal response was established, the paw was re-tested, starting with the next descending von Frey hair until no response occurred. The highest force of 29 g lifted the paw as well as eliciting a response, thus represented the cut off point. Each animal had both hind paws tested in this manner. The lowest amount of force required to elicit a response was recorded as paw withdrawal threshold (PWT) in grams. Static allodynia was defined as present if animals responded to or below the previously innocuous 3.63 g von Frey hair. The von Frey hairs used in the present study give applied force in a logarithmic scale. Thus the data obtained in the current study was represented as medians and quartiles using a log scale. The quartiles indicate the range of values and all calculations were performed using Prism Software (GraphPad, San Diego, CA, U.S.A.).
Dynamic allodynia was assessed by lightly stroking the plantar surface of the hind paw with a cotton bud. Care was taken to perform this procedure in fully habituated rats that were not active to avoid recording general motor activity. At least three measurements were taken at each time point the mean of which represented the paw withdrawal latency (PWL). If no reaction was exhibited within 15 s the procedure was terminated and animals were assigned this withdrawal time. Thus, 15 s effectively represents no withdrawal. A withdrawal response was often accompanied with repeated flinching or licking of the paw. Dynamic allodynia was considered to be present if animals responded to the cotton stimulus within 8 s of stroking.
Effect of compounds on static and dynamic allodynia
The ability of (1S,3R)-3-methyl gabapentin or (1R,3R)-3-methyl gabapentin to block static and dynamic allodynia were examined in the Chung and/or streptozocin models. Animals were used 3 weeks post-streptozocin treatment or Chung surgery. At this time it has previously been shown both static and dynamic allodynia are fully developed (Field et al., 1999a, 1999b). Baseline PWT to von Frey hairs or PWL to cotton bud stimulus were determined on each test day before drug administration. Animals were administered either vehicle, (1S,3R)-3-methyl gabapentin (10–100 mg kg−1, p.o.) or (1R,3R)-3-methyl gabapentin (100 mg kg−1, p.o.) and allodynia was assessed for up to 4 h.
Effect of (1S,3R)-3-methyl gabapentin on the rota-rod
Rats were trained to stay on an accelerating rota-rod (Ugo Basile, Italy) for 120 s. On the test day animals were baselined and re-evaluated on the rota-rod at 0.5, 1 and 2 h post (1S,3R)-3-methyl gabapentin (30–300 mg kg−1, p.o.) or CDP (7.5 mg kg−1, i.p.).
Drugs used
(1S,3R)-3-methyl gabapentin and (1R,3R)-3-methyl gabapentin were synthesized at Pfizer GRD, Cambridge, U.K. Gabapentin was synthesized at Pfizer GRD, MI, U.S.A. All compounds were dissolved in water. Chlordiazepoxide was obtained from Sigma (Poole, U.K.) and was suspended in 1% w v−1, carboxymethylcellulose containing 0.1% v v−1 Tween 80. Drug administrations were made in a volume of 1 ml kg−1.
Data analysis
Data obtained from dynamic allodynia and rota-rod studies were analysed using a one-way ANOVA followed by a Dunnett's t-test. Data from static allodynia studies were subjected to Kruskal–Wallis test followed by an individual Mann–Whitney U-test. In each case the drug treated groups were compared with the appropriate vehicle treated group.
Results
Effect of (1S,3R)-3-methyl gabapentin on static allodynia
The oral administration of (1S,3R)-3-methyl gabapentin dose-dependently (10–100 mg kg−1) blocked the maintenance of static allodynia in both streptozocin and Chung animals with MEDs of 30 mg kg−1 (Figure 1a,b). The dose of 100 mg kg−1 (1S,3R)-3-methyl gabapentin completely blocked the static allodynia in both the Chung and streptozocin models and the antiallodynic effect lasted for over 3 h (Figure 1a,b). (1S, 1R)-3-methyl-gabapentin had no effect on the PWTs for the contralateral paw in the Chung model (data not shown).
Figure 1.
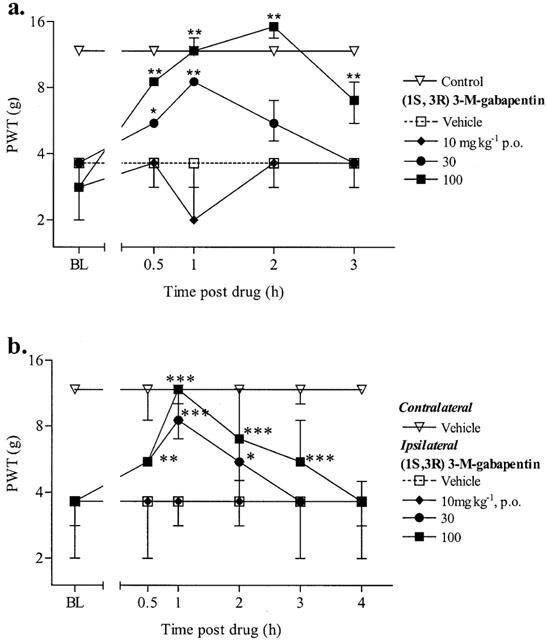
Effect of (1S,3R) 3-methyl-gabapentin on the maintenance of (a) streptozocin and (b) Chung-induced static allodynia. Baseline (BL) paw withdrawal thresholds (PWT) to von Frey hairs were determined in both paws of the Chung animals and in the right paw of control and streptozocin treated animals before drug administration. (1S,3R) 3-M-gabapentin was administered p.o. and PWT were re-examined for up to 4 h. For clarity only the vehicle contralateral paw is shown for the Chung data. The data are expressed as median force (g) required to induce a withdrawal in 8–10 animals per group (vertical bars represent 1st and 3rd quartiles). *P<0.05, **P<0.01, ***P<0.001 significantly different (Kruskal–Wallis test followed by a Mann–Whitney U-test). All comparisons were carried out comparing drug treated groups with the vehicle group at each time point.
Effect of (1S,3R)-3-methyl gabapentin on dynamic allodynia
The oral administration of (1S,3R)-3-methyl gabapentin dose-dependently (10–100 mg kg−1) blocked the maintenance of dynamic allodynia in streptozocin and Chung animals with respective MEDs of 30 and 100 mg kg−1 (Figure 2a,b). These antiallodynic effects lasted over 2 h (Figure 2a,b).
Figure 2.
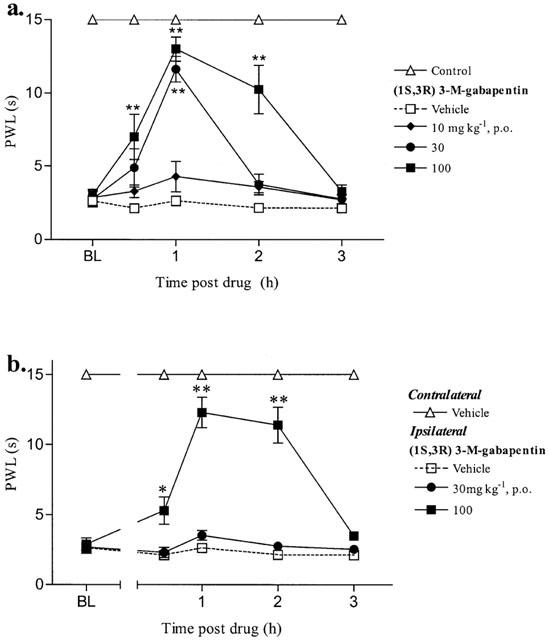
Effect of (1S,3R)-methyl-gabapentin on the maintenance of (a) streptozocin and (b) Chung-induced dynamic allodynia. Baseline (BL) paw withdrawal latencies (PWL) to cotton bud stimulus were determined in both paws of the Chung animals and in the right paw of control and streptozocin treated animals before drug administration. (1S,3R) 3-M-gabapentin was administered p.o. and PWL were re-examined for up to 3 h. For clarity only the vehicle contralateral paw is shown for the Chung data. The dynamic data are expressed as the mean PWL (s) of 6–10 animals per group (vertical bars represent±s.e.mean). *P<0.05, **P<0.01 significantly different (ANOVA followed by Dunnett's t-test). All comparisons were carried out comparing drug treated groups with the vehicle group at each time point.
Effect of (1R,3R)-3-methyl gabapentin on streptozocin-induced static and dynamic allodynia
(1R,3R)-3-methyl-gabapentin failed to have any effect on the maintenance of static or dynamic allodynia in the streptozocin model at the dose of 100 mg kg−1 (Figure 3). Gabapentin (100 mg kg−1, p.o.) was used as a positive control on the day of each experiment and it blocked the maintenance of both static and dynamic allodynia with similar effects to those previously reported (Field et al., 1999b).
Figure 3.
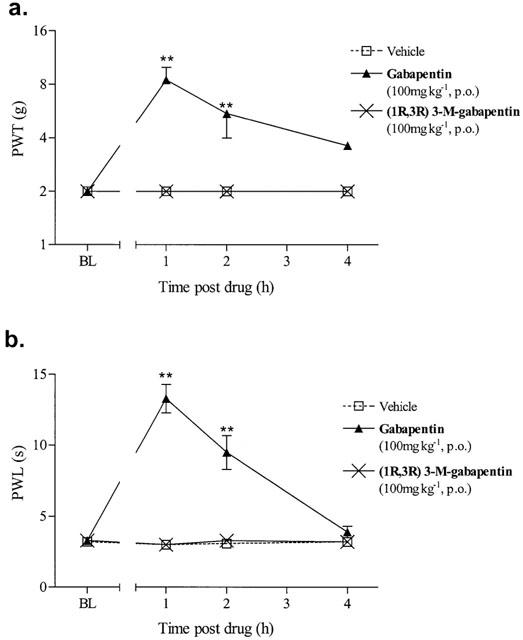
Effect of (1R,3R)-3-methyl-gabapentin on the maintenance of streptozocin-induced (a) static and (b) dynamic allodynia. Baseline (BL) paw withdrawal thresholds (PWT) to von Frey hairs or paw withdrawal latencies (PWL) to cotton bud stimulus were determined in the right paw of streptozocin treated animals before drug administration. (1R,3R) 3-M-gabapentin was administered p.o. and PWT and PWL were re-examined for up to 4 h. Static allodynia data are expressed as median force (g) required to induce a paw withdrawal (n=6/group vertical bars represent 1st and 3rd quartiles). **P<0.01, significantly different (Kruskal-Wallis test followed by a Mann–Whitney U-test). The dynamic data are expressed as the mean PWL (s) of 6–10 animals per group (vertical bars represent±s.e.mean). **P<0.01 significantly different (ANOVA followed by Dunnett's t-test). All comparisons were carried out comparing drug treated groups with the vehicle group at each time point.
Effect of (1S,3R)-3-methyl gabapentin on the rota-rod
The oral administration of (1S,3R)-3-methyl gabapentin failed to have any effect on the rota-rod at the doses used in the pain studies (Figure 4). Only the highest dose of 300 mg kg−1 had any effect and this was only seen at the 2 h time point (Figure 4). The positive control of 7.5 mg kg−1 chlordiazepoxide (CDP) significantly reduced the time spent on the rota-rod at 0.5 and 1 h post administration indicating sedation/ataxia (Figure 4).
Figure 4.
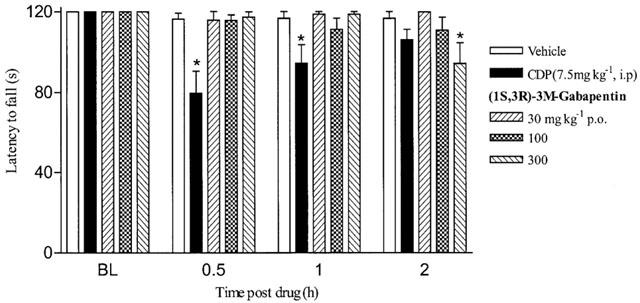
Effect of (1S,3R)-methyl-gabapentin on rota-rod performance. Animals were trained to stay on an accelerating rota-rod for 120 s. On the test day animals were baselined (BL) and re-evaluated on the rota-rod at 0.5, 1 and 2 h post (1S,3R)-3-M-gabapentin or chlordiazepoxide (CDP) administration. Data is expressed as mean latency to fall off the rota-rod (vertical bars represent±s.e.mean). *P<0.05 **P<0.01, one way ANOVA followed by a Dunnett's t-test compared to vehicle group.
Discussion
The results of the present study indicate that antiallodynic action of 3-methyl gabapentin resides in the (1S,3R) diastereoisomer with high binding affinity for the α2δ subunit of VDCCs. This finding supports and extends our previous observations. Thus, it has been shown that pregabalin has high affinity for the α2δ protein and possesses antihyperalgesic and antiallodynic properties. In contrast, the R-isomer of isobutylgaba has weaker binding affinity and was either inactive or weakly active in animal models of pain (Field et al., 1997b; 1999b; Partridge et al., 1998). The antiallodynic effects of (1S,3R)-3-methyl gabapentin were seen at doses that did not induce sedation or ataxia as measured in the rota-rod test. This selective antiallodynic action is similar to that seen with other compounds which selectively interact with the α2δ subunit of VDCCs (Field et al., 1997b).
Recent electrophysiological data also support an involvement of the α2δ subunit in mediating effects of gabapentin. It has been shown that gabapentin can block Ca2+ currents in cortical neurones (Stefani et al., 1998). It is also interesting to note that there is an upregulation of the α2δ subunit mRNA following chronic constriction injury (CCl), another preclinical model of neuropathic pain (Philp et al., 1999). Furthermore, studies carried out in our laboratories have shown that there is an increase in [3H]-gabapentin binding sites in dorsal horn of spinal cord on the ipsilateral side of CCl (R. Williams personal communication). Taken together, these studies suggest that the α2δ protein may play an important role in chronic pain syndromes. However, further studies are required to corroborate this hypothesis. For example, it would be interesting to examine whether the upregulation of α2δ subunits is reduced by chronic administration of gabapentin in models of neuropathic pain. It is known that the α2δ subunit is common to all VDCCs (Hofmann et al., 1994; Isom et al., 1994). Therefore, it is possible that actions of gabapentin involve more than one VDCC. It is clear that these channels play an important role in neurotransmission (e.g. control of neurotransmitter release) and are widely distributed throughout both the periphery and the CNS. Both preclinical and clinical studies indicate that gabapentin has a clean side effect profile (Field et al., 1997b). This would suggest that it is unlikely gabapentin interacts with all α2δ subtypes. Recently, it has been reported that there are at least three subtypes of α2δ subunits (Klugbauer et al., 1999). Gabapentin and related compounds may interact specifically with one α2δ subtype associated with the pain pathway accounting for its clean side effect profile.
Neuropathic pain patients suffer many types of pain but allodynia (pain to previously innocuous stimuli) is a classic symptom and is one of the most debilitating. It has previously been shown that in animals and humans, two types of mechanical allodynia can be detected following nerve damage. They have been termed static and dynamic after the type of stimuli required for their detection (Koltzenburg et al., 1992; Ochoa & Yarnitsky, 1993; Field et al., 1999a). It has been shown that they are signalled by different sensory fibres. Thus, whilst static allodynia involves capsaicin sensitive Aδ-fibres, the dynamic type appears to be signalled by the large diameter myelinated Aβ-sensory neurones (Koltzenburg et al., 1992; Ochoa & Yarnitsky, 1993; Field et al., 1999b). The results of the present study show that (1S,3R)-3-methyl gabapentin can block both components of mechanical allodynia. This is consistent with previous studies showing that this is also the case with gabapentin and pregabalin (Field et al., 1999a,1999b). This wide spectrum of antiallodynic activity is a distinct advantage over morphine and amitriptyline. These compounds appear to be only effective against static allodynia (Field et al., 1999a,1999b).
Recently the streptozocin model has been criticised as it has been suggested that the profound ill-health of the animals compromize its use as a model of neuropathic pain (Fox et al., 1999). We feel the dose of streptozocin used and the weight of the animal before dosing are major factors in obtaining animals which display allodynia but are not profoundly ill. In the present study we used animals weighing between 225–250 g and a lower dose of streptozocin (50 mg kg−1) to induce diabetes compared to the dose used by Fox et al., (65 mg kg−1). We have used this lower dose in our laboratory successfully for a number of years now. We have shown that this protocol induces hyperglycaemia followed by the development of static and dynamic allodynia (Field et al., 1999b). The streptozocin treated animals do require extra husbandry due to excessive urination and some animals have limited weight gain but are otherwise difficult to distinguish from the saline treated controls. Thus we feel that using the correct dose of streptozocin reduces the severity of illness and side effects to an acceptable level and under these conditions the streptozocin model is a very useful and valid model of neuropathic pain.
In conclusion, the results of the present study are consistent with the involvement of α2δ subunit of VDCCs in mediating the action of gabapentin and related compounds. However, further studies are necessary to determine which subtype(s) is responsible for their effects in models of pain. It is known that gabapentin and pregabalin at high doses produce sedation/ataxia. It is unclear at present whether the same α2δ subtype is responsible for the efficacy in models of pain and these side effects. It remains to be seen whether it will be possible to design compounds with superior efficacy with improved side effect profile for the treatment of chronic pain.
Acknowledgments
We would like to acknowledge Steve Bramwell and Scott McCleary for their excellent technical assistance throughout these experiments.
Abbreviations
- CCl
chronic constriction injury
- CDP
chlordiazepoxide
- CNS
central nervous system
- MED
minimum effective doses
- NSAID
nonsteroidal antiinflammatory drugs: PHN, post herpetic neuralgia
- PWL
paw withdrawal latencies
- PWT
paw withdrawal thresholds
- VDCC
voltage dependent calcium channels
References
- BACKONJA M., BEYDOUN A., EDWARDS K.R., SCHWARTZ S.L., FONSECA V., HES M., LAMOREAUX L., GAROFALO E. Gabapentin for the treatment of painful neuropathy in patients with diabetes mellitus. JAMA. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- BRYANS J.S., DAVIS N., GEE N., DISSANAYAKE V.U.K., RATCLIFFE G.S., HORWELL D.C., KNEEN C.O., MORRELL A.I., OLES R.J., O'TOOLE J.C., PERKINS G.M., SINGH L., SUMAN-CHAUHAN N., O'NEILL J.A. Identification of novel ligands for the gabapentin binding site on the α2δ subunit of a calcium channel and their evaluation as anticonvulsant agents. J. Med. Chem. 1998;41:1838–1845. doi: 10.1021/jm970649n. [DOI] [PubMed] [Google Scholar]
- FIELD M.J., BRAMWELL S., HUGHES J., SINGH L. Detection of static and dynamic components of mechanical allodynia in rat models of neuropathic pain: Are they signalled by distinct primary sensory neurones. Pain. 1999a;83:303–311. doi: 10.1016/s0304-3959(99)00111-6. [DOI] [PubMed] [Google Scholar]
- FIELD M.J., HOLLOMAN E.F., MCCLEARY S., HUGHES J., SINGH L. Evaluation of gabapentin and S-(+)-3-isobutylgaba in a rat model of postoperative pain. J. Pharmacol. Exp. Ther. 1997a;282:1242–1246. [PubMed] [Google Scholar]
- FIELD M.J., MCCLEARY S., HUGHES J., SINGH L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999b;80:391–398. doi: 10.1016/s0304-3959(98)00239-5. [DOI] [PubMed] [Google Scholar]
- FIELD M.J., MCCLEARY S., SINGH L. The gabapentin analogue 3-methyl-gabapentin blocks both static and dynamic components of mechanical allodynia in a rat model of neuropathic pain. Br. J. Pharmacol. 1999c;128 Suppl:235P. [Google Scholar]
- FIELD M.J., OLES R.J., LEWIS A.S., MCCLEARY S., HUGHES J., SINGH L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Brit. J. Pharmacol. 1997b;121:1513–1522. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX A., EASTWOOD C., GENTRY C., MANNING D., URBAN L. Critical evaluation of the streptozocin model of painful diabetic neuropathy in the rat. Pain. 1999;81:307–316. doi: 10.1016/S0304-3959(99)00024-X. [DOI] [PubMed] [Google Scholar]
- GEE N.S., BROWN J.P., DISSANAYAKE V.U.K., OFFORD J., THURLOW R., WOODRUFF G.N. The novel anticonvulsant drug, Gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J. Biol. Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- GOTZ E., FEUERSTEIN T.J., LAIS A., MEYER D.K. Effects of gabapentin on release of gamma-aminobutyric acid from slices of rat neostriatum. Ameimittelforschung. 1993;43:636–638. [PubMed] [Google Scholar]
- HOFMANN F., BIEL M., FLOCKERZI V. Molecular basis for Ca2+ channel diversity. Annu. Rev. Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- HUNTER J.C., GORGAS K.R., HEDLEY L.R., JACOBSON L.O., KASSOTAKIS L., THOMPSON J., FONTANA D.J. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. Eur. J. Pharmacol. 1997;324:153–160. doi: 10.1016/s0014-2999(97)00070-8. [DOI] [PubMed] [Google Scholar]
- HWANG J.H., YAKSH T.L. Effect of subarachnoid gabapentin on tactile-evoked allodynia in a surgically induced neuropathic model in the rat. Regional Anaesthesia. 1997;22:249–256. doi: 10.1016/s1098-7339(06)80010-6. [DOI] [PubMed] [Google Scholar]
- ISOM L.L., DE-JONGH K.S., CATTERALL W.A. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12:1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- KIM S.H., CHUNG J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- KLUGBAUER N., LACINOVA L., MARAIS E., HOBOM M., HOFMANN F. Molecular diversity of the calcium channel alpha2delta subunit. J. Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLTZEBURG M., LUNDBERG L.E.R., TOREBIORK H.E. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain. 1992;51:207–219. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- OCHOA J.L., YARNITSKY D. Mechanical hyperalgesias in neuropathic pain patients; Dynamic and static subtypes. Ann. Neurol. 1993;33:465–472. doi: 10.1002/ana.410330509. [DOI] [PubMed] [Google Scholar]
- PARTRIDGE B.J., CHAPLAN S.R., SAKAMOTO E., YAKSH T.L. Characterization of the effects of gabapentin and 3-isobutyl-γ-aminobutyric acid on substance P-induced thermal hyperalgesia. Anesthesiology. 1998;88:196–205. doi: 10.1097/00000542-199801000-00028. [DOI] [PubMed] [Google Scholar]
- PHILP L., HOLLOMAN E., MEECHAM K., BLYTH K., PINNOCK R., HUGHES J., WILLIAMS R. [3H]-Gabapentin binding and α2δ immunoreactivity in the spinal cord of the rat following chronic constriction injury of the sciatic nerve. Brit. Neurosci. Assoc. Abstr. 1999;15:46.08. [Google Scholar]
- ROWBOTHAM M., HARDEN N., STACEY B., BERNSTEIN P., MAGNUS-MILLER L. Gabapentin for the treatment of postherpetic neuralgia. JAMA. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- SHIMOYAMA N., SHIMOYAMA M., DAVIS A.M., INTURRISI C.E., ELLIOTT K.J. Spinal gabapentin is antinociceptive in the rat formalin test. Neurosci. Lett. 1997;222:65–67. doi: 10.1016/s0304-3940(97)13331-6. [DOI] [PubMed] [Google Scholar]
- SINGH L., FIELD M.J., FERRIS P., HUNTER J.C., OLES R.J., WILLIAMS R.G., WOODRUFF G.N. The antiepileptic agent gabapentin (Neurontin) possesses anxiolytic-like and antinociceptive actions that are reversed by D-Serine. Psychopharmacology. 1996;127:1–9. doi: 10.1007/BF02805968. [DOI] [PubMed] [Google Scholar]
- STEFANI A., SPADONI F., BERARDI G. Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology. 1998;37:83–91. doi: 10.1016/s0028-3908(97)00189-5. [DOI] [PubMed] [Google Scholar]
- TAYLOR C.P., GEE N.S., SU T.Z., KOCSIS J.D., WELTY D.F., BROWN J.P., DOOLEY D.J., BODEN P., SINGH L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29:233–249. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- XIAO W., BENNETT G.I. Gabapentin relieves abnormal pains in a rat model of painful peripheral neuropathy. Soc. Neurosci. Abstr. 1995;21:356. [Google Scholar]


