Abstract
The pharmacological properties of fatty acid amidohydrolase (FAAH) were investigated in brains of 35-day-old chickens, since nothing is known about the enzyme in avian species.
FAAH activity towards both [3H]-palmitoylethanolamide (PEA) [KM=1.5 μM] and [3H]-anandamide (AEA) [KM=5.4 μM] was demonstrated in the chicken brains. The chicken FAAH was inhibited by the substrate analogues oleyl trifluoromethylketone (OTMK) and diazomethylarachidonyl ketone (DAK) with similar potencies to the rat FAAH. However, in contrast to the rat brain, phenylmethylsulphonyl fluoride (PMSF) and the enantiomers of ibuprofen had very weak effects on chicken brain FAAH.
Indomethacin and niflumic acid were found to inhibit rat brain AEA hydrolysis. The inhibition by indomethacin was reversible and competitive, with a Ki value of 120 μM. Chicken FAAH was less sensitive to indomethacin than its rodent counterpart, but the inhibition was also competitive (Ki).
It is concluded that chicken FAAH activity has different pharmacological properties to its rodent counterpart.
Keywords: Anandamide, palmitoylethanolamide, fatty acid amidohydrolase, ibuprofen, indomethacin
Introduction
Anandamide (AEA, arachidonyl ethanolamide) and palmitoylethanolamide (PEA) belong to a class of biologically active endogenous fatty acid amides. AEA is an agonist at cannabinoid CB1 and CB2 receptors (for review, see Di Marzo et al., 1998) and has recently been shown to activate vanilloid receptors (Zygmunt et al., 1999). PEA has been shown to prevent mast cell activation in vitro (Facci et al., 1995) and to reduce inflammatory pain in vivo (Mazzari et al., 1996; Calignano et al., 1998; Jaggar et al., 1998). Both AEA and PEA are metabolized by fatty acid amidohydrolase (FAAH, EC 3.5.1.4, also known as anandamide amidase and anandamide amidohydrolase), which in the rat brain has a cellular localization complementary, albeit with little or no actual co-localization, to the CB1 receptors (Hillard et al., 1995; Egertová et al., 1998), and is located in large neurons (Tsou et al., 1998). FAAH expression by non-neuronal epithelial cells has also been reported for the choroid plexus (Egertová et al., 2000). The enzyme is inhibited by substrate analogues, such as oleyl trifluoromethyl ketone (OTMK) and diazomethylarachidonyl ketone (DAK) (Cravatt et al., 1996; Edgemond et al., 1998; see also Koutek et al., 1994; Boger et al., 1999), as well as by phenylmethylsulphonyl fluoride (PMSF) (Deutsch & Chin, 1993) and by various non-steroidal anti-inflammatory agents such as ibuprofen, ketoprofen and flurbiprofen (Fowler et al., 1997a,1997b; 1999).
Most of our present knowledge concerning the pharmacological properties of FAAH has been derived from studies in mammalian species, and nothing is known as to their equivalents in avian species. In consequence, in the present study, we have investigated the pharmacological properties of chicken brain FAAH.
Methods
Materials
DAK (diazomethylarachidonyl ketone) was a kind gift from Dr Cecilia Hillard, Medical College of Wisconsin, U.S.A. Palmitoylethanolamide-[1-3H] ([3H]PEA) and arachidonyl-ethanolamide-[1-3H] ([3H]AEA) (both specific activity 30 Ci mmol−1), were obtained from American Radiolabelled Chemicals Inc., St. Louis, MO, U.S.A. The enantiomers of ibuprofen (α-methyl-4-(2-methylpropyl) benzeneacetic acid) were obtained from Research Biochemicals International, Natick, MA, U.S.A. Niflumic acid (2-[[3-(trifluoromethyl)phenyl]-amino]-3-pyridinecarboxylic acid) was obtained from the Alexis Corp., San Diego, CA, U.S.A. PMSF (phenylmethylsulphonyl fluoride), non-radioactive PEA, indomethacin and fatty acid free bovine serum albumin were obtained from the Sigma Chemical Co. (St Louis, MO, U.S.A.). Non radioactive AEA was obtained from Tocris-Cookson, Bristol, U.K. Oleyl trifluoromethylketone (OTMK) was obtained from the Cayman Chemical Company, Ann Arbor, MI, U.S.A. The compounds were dissolved in ethanol.
Preparation of chicken and rat brain homogenates and membranes
Frozen skulls from 35-day-old Ross chickens were obtained from Kronfågel AB, Kristianstad, Sweden. Upon thawing, the brains were removed and weighed. Cerebral cortices obtained from adult Sprague-Dawley rats (Umeå University) stored at −20°C, were thawed on ice and weighed. For the experiments shown in Figures 1–3, brains were homogenized in 10 mM Tris-HCl, pH 7.6 containing 1 mM EDTA, and aliquots stored frozen at −70°C. For the experiments shown in Figures 4 and 5, the brain tissues were homogenized at 4°C in homogenization buffer (20 mM HEPES pH 7.0+1 mM MgCl2) using an Ultra-Turrax or glass homogenizer. These tissue homogenates were centrifuged at 36,000×g for 20 min at 4°C. The pellets were resuspended in 20 ml homogenization buffer and recentrifuged again at 36,000×g for 20 min at 4°C. Pellets were resuspended in 10 ml homogenization buffer and incubated at 37°C for 15 min. After recentrifugation at 36,000×g for 20 min at 4°C, pellets were resuspended in homogenization buffer to a final concentration of 200 mg wet weight ml−1. Membrane preparations were stored in microcentrifuge tubes at −20°C until used for assay. This protocol was used partly to allow concomitant measurement of cannabinoid receptor density in the samples (results to be presented elsewhere), but also to allow the use of a membrane preparation which was necessary for the reversibility studies shown in Figure 4.
Figure 1.
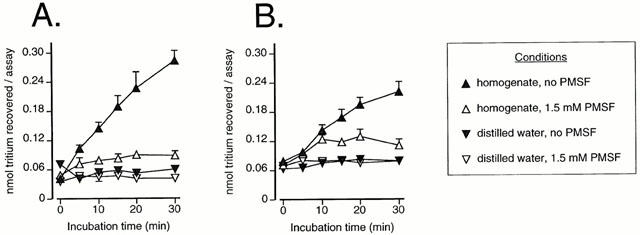
Time-dependent hydrolysis of 2 μM PEA (A) and 2 μM AEA (B) by chicken brain FAAH. Samples (homogenates or distilled water) were incubated in the absence or presence of 1.5 mM PMSF. Data are means±s.e.mean (when not enclosed by the symbols), n=3.
Figure 4.
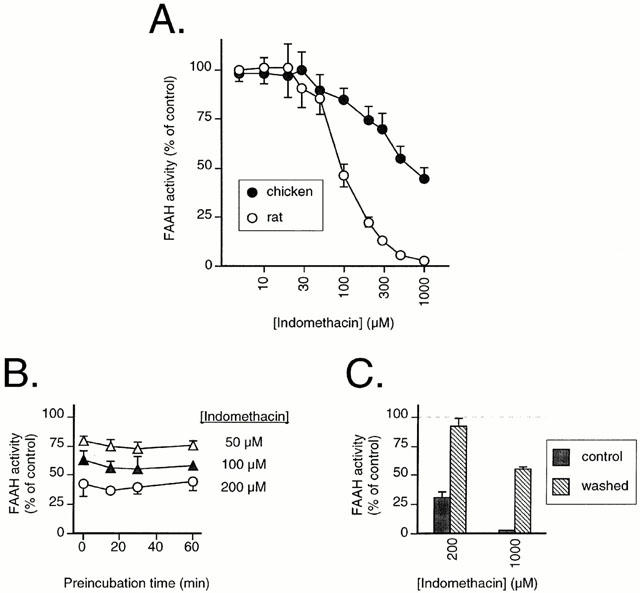
(A) Inhibition of FAAH in rat and chicken brain membranes by indomethacin. (B) effect of preincubation upon the inhibition FAAH in rat brains by indomethacin. (C) reversibility of the inhibition of FAAH in rat brain membranes by indomethacin. The membranes were preincubated for 60 min with either 0, 200 or 1000 μM indomethacin and then either left on ice (‘control') or washed (by centrifugation followed by resuspension in the same volume) prior to addition of substrate and assay for FAAH activity. The values are given as per cent of the corresponding values for samples treated in the same way (i.e. ±washing as appropriate) in the absence of indomethacin. All panels show results as means±s.e.mean (when not enclosed by the symbols), n=3, using 2 μM AEA as substrate. In all cases, blank values were those for the rat brain samples in the presence of 1.5 mM PMSF.
Figure 5.
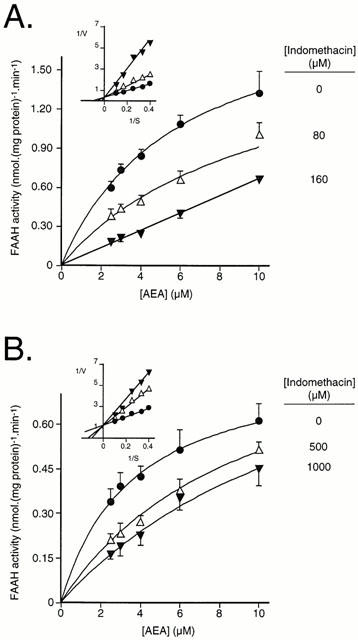
Mode of inhibition of FAAH by indomethacin. (A) rat brain membranes; (B) chicken brain membranes. Blank values were obtained for distilled water in place of the homogenates. Data are means±s.e.mean, n=3. Secondary Lineweaver-Burk replots of the mean data are shown as insets.
Assay of FAAH activity in chicken and rat brain homogenates
FAAH was assayed essentially as described by Omeir et al. (1995) who used carbon-14 labelled AEA as substrate (for full descriptions using the present tritiated substrates, see Fowler et al., 1997b; Tiger et al., 2000). Briefly, assay mixtures contained homogenate (10–60 μg protein asssay−1), test compound (when appropriate) (25 μl), and radiolabelled AEA or PEA (25 μl, containing 10 mg ml−1 fatty acid-free bovine serum albumin). Test compounds were diluted with ethanol and compared with controls containing the same concentration of ethanol (25 μl in a 200 μl assay volume). Blanks contained either PMSF (final concentration 1.5 mM) or distilled water instead of homogenate, as indicated. The mixtures were incubated at 37°C for 10 min (unless otherwise stated) after which reactions were stopped by placing the tubes in ice and adding 400 μl of chloroform : methanol (1:1 v v−1). The tubes were vortex mixed, after which the phases were separated by centrifugation in a bench centrifuge. Aliquots (200 μl) of the methanol/buffer phase were removed for analyses of radioactivity by liquid scintillation spectroscopy with quench correction.
Protein determination
Protein contents were measured using the method of Harrington (1990) using bovine serum albumin as standard.
Determination of Km, Vmax and pI50 values
Km and Vmax values for FAAH activities were calculated using the Direct Linear Plot analysis of Eisenthal & Cornish-Bowden (1974) using the Enzyme Kinetics v 1.4 software package, Trinity Software, Campton, NH, U.S.A. Given that this analysis is non-parametric in nature, KM and Vmax values have been given as medians and ranges. pI50 values were calculated by plotting data (in the 9–91% contiguous per cent activity remaining range, to avoid bias at high and low levels of inhibition) as log10 (inhibitor concentration) vs log10 (100% activity remaining) per cent activity remaining−1). From the regression lines, pI50 values were determined.
Results
Assay of FAAH activity in the chicken and rat brain homogenates
Initial experiments were undertaken to determine the optimal conditions for assay of initial velocities. In the presence of chicken brain homogenate (10 μg assay−1), a linear increase in the metabolism of both PEA (Figure 1A) and AEA (Figure 1B) over 20 min was found. No such increase was seen when distilled water was used in place of the homogenates. In general, PMSF (1.5 mM) is used to define blank values, since this concentration is much higher than its reported IC50 value (in the range of 3–25 μM; Hillard et al., 1995; Desarnaud et al., 1995; Fowler et al., 1997b) for rat brain samples. However, a residual activity was seen when chicken brain homogenates were incubated with this concentration of PMSF. This can be seen most clearly in Figure 2A,B, where a residual activity over blank (defined using distilled water instead of homogenate) was found for the chicken at all substrate concentrations tested. In contrast, there was no residual activity found for the rat brain homogenates (Figure 2A,B). In consequence, in the remaining experiments, blanks were either defined as the radioactivity recovered in the absence of homogenate, or (in Figures 3 and 4), by running chicken and rat experiments concomitantly, and using the rat PMSF blank, since this is equivalent to the distilled water blank. In the remaining experiments, an incubation time of 10 min was used. Using these conditions, the rates of substrate hydrolysis were saturable, with median KM values of 1.5 (chicken) and 5.0 (rat) μM being found for PEA as substrate (Figure 2A). For AEA, the median KM values were 5.4 (chicken) and 2.6 (rat) μM (Figure 2B).
Figure 2.
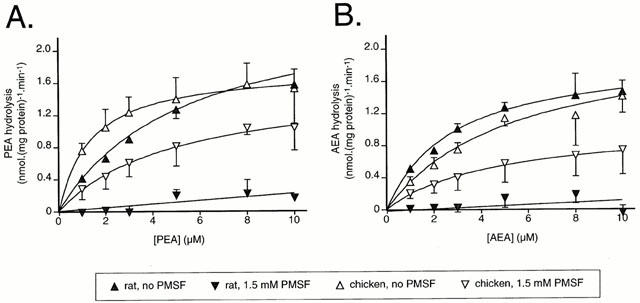
Concentration-dependence of the metabolism of PEA (A) and AEA (B) by rat and chicken brain FAAH in the absence or presence of 1.5 mM PMSF. Blank values were obtained for distilled water in place of the homogenates. Data are means±s.e.mean (when not enclosed by the symbols), n=3.
Figure 3.
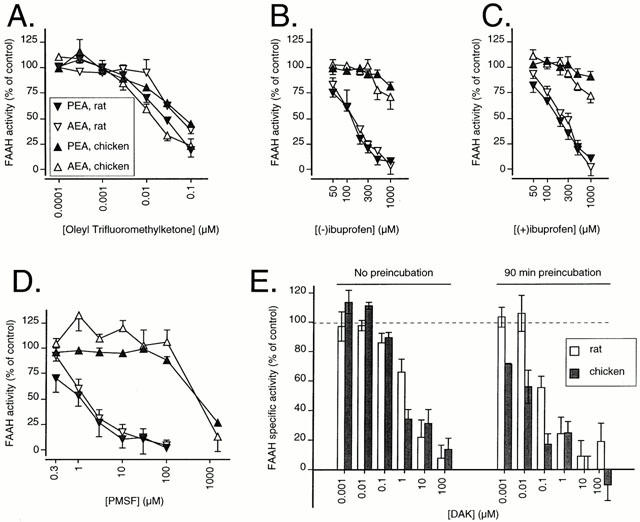
Inhibition of FAAH in the rat and chicken brains by OTMK (A); (−)ibuprofen (B); (+)ibuprofen (C) and PMSF (D). The substrate concentration was 2 μM. The explanation of the symbols is enclosed within A. In E, the inhibition of 2 μM PEA by DAK is shown. Blank values were those for the rat brain samples in the presence of 1.5 mM PMSF. Data are means±s.e.mean (when not enclosed by the symbols), n=3, or means and ranges, n=2, towards 2 μM AEA and PEA.
Inhibition of chicken and rat brain FAAH activity by OTMK, PMSF, ibuprofen and DAK
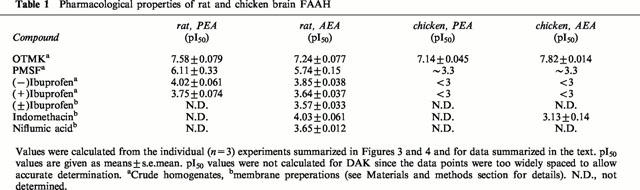
The inhibition of rat and chicken brain FAAH activity by OTMK, (−)ibuprofen, (+)ibuprofen, PMSF and DAK is shown in Figure 3A–E and the pI50 values (with the exception of DAK) are given in Table 1. The substrate analogues OTMK and DAK inhibited chicken FAAH with roughly the same potency as for rat FAAH (Figure 3A,E). DAK, which is an irreversible inhibitor of FAAH (Edgemond et al., 1998) showed the expected time-dependency (Figure 3E). In contrast to the substrate analogues, PMSF and the enantiomers of ibuprofen were very much less potent in the chicken than in the rat (Figure 3B–D). These results were seen regardless of whether PEA or AEA was used as substrate (data for DAK and AEA not shown).
Table 1.
Pharmacological properties of rat and chicken brain FAAH
Inhibition of chicken and rat brain FAAH activity by indomethacin
In order to determine whether the species difference in sensitivity to inhibition by the ibuprofen enantiomers was a property common to all non-steroidal anti-inflammatory drugs (NSAIDs), we investigated whether indomethacin could inhibit FAAH. In these experiments, membrane preparations at a pH of 7.0 were used. However, initial experiments in the rat indicated that the sensitivity of FAAH to ibuprofen was retained, concentrations of 50, 100, 150, 200, 300, 500 and 1000 μM (±)ibuprofen giving rates of FAAH activity (towards 2 μM [3H]-AEA) of 82±3, 76±3, 61±2, 57±3, 44±0.3, 36±1 and 23±4% of control, respectively (means±s.e.mean, n=3). The rat membranes were also sensitive to inhibition by niflumic acid, with concentrations of 50, 100, 200, 300, 500 and 1000 μM giving values of 75±8, 64±12, 61±6, 37±4, 34±5 and 20±6% of control, respectively.
Using 2 μM AEA as substrate, indomethacin was found to be a more potent inhibitor of rat FAAH activity than (±)ibuprofen, with a mean pI50 value of 4.03 being found (Figure 4A, Table 1). The degree of inhibition produced by indomethacin was not increased by preincubation for up to 60 min (Figure 4B) and could be reduced by washing of the membranes following a 60 min preincubation (Figure 4C) indicative of a reversible mode of action. Chicken FAAH was less sensitive than rat FAAH to indomethacin, a mean pI50 value of 3.13 being found (Figure 4A, Table 1). The mode of inhibition of AEA hydrolysis was competitive for both rat (Figure 5A) and chicken (Figure 5B), with Ki values of 120 and 330 μM, respectively, being found for secondary replots of KMapp (calculated by direct linear plot analysis of the mean data for 0 and 80 μM indomethacin (rat) and for 0, 500 and 1000 μM indomethacin (chicken)) vs the indomethacin concentration. The 160 μM indomethacin concentration was not used in these calculations, since at that concentration the KMapp>> the highest AEA concentration used, making its accurate determination impossible. For the control membranes, the KM values (medians, n=3, with ranges in parentheses) towards AEA were 5.2 (4.3–8.6) μM and 3.4 (3.2–3.4) for rat and chicken, respectively.
Discussion
In a relatively short time, much has been learnt about FAAH in mammals. Rat, human and mouse FAAH have a high degree of homology and a similar (but not identical) substrate selectivity (Giang & Cravatt, 1997). Rat and human FAAH have been the subject of detailed investigations into their substrate (e.g. Giang & Cravatt, 1997; Lang et al., 1999) and inhibitor (e.g. Koutek et al., 1994; Maccarrone et al., 1998) specificities. Recent mutagenesis studies have in addition identified a number of amino acids that are essential for mammalian FAAH activity (Goparaju et al., 1999; Omeir et al., 1999; Patricelli et al., 1999). In contrast, little is known about FAAH in non-mammalian species, although an activity sensitive to PMSF (∼50% inhibition at 250 μM) capable of metabolizing both PEA and AEA has been reported in sea urchin ovaries (Bisogno et al., 1997).
In the present study, the properties of chicken brain FAAH have been compared with those of rat brain. In the rat brain, the KM and Vmax values, and the potencies of the inhibitors (OTMK≈DAK [90 min preincubation]>PMSF≈DAK [0 min preincubation]>(−)ibuprofen>(+)ibuprofen are consistent with the literature (Hillard et al., 1995; Desarnaud et al., 1995; Fowler et al., 1997b; 1999; Edgemond et al., 1998; Tiger et al., 2000). The finding that indomethacin and niflumic acid are also capable of inhibiting FAAH extends the list of NSAIDs that share this property. In theory, inhibition of FAAH might contribute to the anti-inflammatory properties of these drugs, given that both PEA and AEA have positive effects in inflammatory pain (Mazzari et al., 1996; Calignano et al., 1998; Jaggar et al., 1998), and that blockade of FAAH potentiates the behavioural effects of AEA in vivo (Compton & Martin, 1997). However, significant inhibition of FAAH is unlikely to be found in vivo after administration of a therapeutic dose of indomethacin, given that the peak plasma concentration (e.g. ∼12 μM after administration of a 75 mg controlled release preparation to elderly volunteers, Bruguerolle et al., 1986) is much lower than the Ki value found here.
In the case of the chicken FAAH, the enzyme shows similar KM values towards AEA and PEA as is seen in the rat, whereas PMSF, (−)ibuprofen and (+)ibuprofen are very much less potent as inhibitors of PEA and AEA metabolism. One possible explanation for this difference is simply that when using brain homogenates, the activity of more than one FAAH isoform may be measured, and that the difference between species observed may reflect differences in the relative abundances of these enzyme species. Detailed pharmacological investigations in different subcellular fractions and in different regions of the rat brain, however, have failed to show obvious differences in the sensitivity of the FAAH to inhibition by ibuprofen (Fowler et al., 1999; Tiger et al., 2000), which would argue against the notion of expression to any great extent of a ‘chicken-like' FAAH activity in the rat brain. Recently, Ueda et al. (1999) described an enzymic activity in human CMK megakaryoblastic cells that could hydrolyze both AEA and PEA, and that was rather insensitive to PMSF. However, this enzyme was not inhibited by the substrate analogue methyl arachidonyl fluorophosphonate (Ueda et al., 1999), which would suggest that it is a different enzyme to that described here in the chicken, which is highly sensitive to the related substrate analogue OTMK. Ibuprofen inhibits rat brain FAAH in a mixed manner (Fowler et al., 1997b; 1999), and a suggestion could be made that whilst the active site of chicken brain FAAH is rather similar to its mammalian equivalent, there may be species differences in parts of the molecule mediating the inhibitory effects of PMSF and ibuprofen. Indomethacin, however, was a competitive inhibitor of both rat and chicken FAAH, but was more potent towards the rodent enzyme. It is to be hoped that the molecular characterization of avian FAAH will shed light upon the mechanism behind this species difference.
Acknowledgments
We thank Dr Cecilia Hillard for her kind gift of DAK, and Kronfågel AB, Kristianstad for their help in providing the adult chicken brains. This work was supported by grants from the Swedish Medical Research Foundation (Grants nos. 12158 and 12548), Strokeförbundet, Loo and Hans Ostermans Fund, Stiftelsen Sigurd and Elsa Goljes Minne, Magnus Bergvalls Stiftelse, Stiftelse Åke Wiberg and the Research Fund of the Medical Faculty, Umeå University. The excellent technical assistence of Britt Jacobsson is much appreciated.
Abbreviations
- AEA
anandamide
- DAK
diazomethylarachidonyl ketone
- FAAH
fatty acid amidohydrolase, NSAID, non-steroidal anti-inflammatory drug
- OTMK
oleyl trifluoromethylketone
- PEA
palmitoylethanolamide
- PMSF
phenylmethylsulphonyl fluoride.
References
- BISOGNO T., VENTRIGLIA M., MILONE A., MOSCA M., CIMINO G., DI MARZO V. Occurrence and metabolism of anandamide and related acyl-ethanolamides in ovaries of the sea urchi. Paracentrotus lividus. Biochim. Biophys. Acta. 1997;1345:338–348. doi: 10.1016/s0005-2760(97)00009-x. [DOI] [PubMed] [Google Scholar]
- BOGER D.L., SATO H., LERNER A.E., AUSTIN B.J., PATTERSON J.E., PATRICELLI M.P., CRAVATT B.F. Trifluoromethyl ketone inhibitors of fatty acid amide hydrolase: a probe of structural and conformational features contributing to inhibition. Bioorg. Med. Chem. Letts. 1999;9:265–270. doi: 10.1016/s0960-894x(98)00734-3. [DOI] [PubMed] [Google Scholar]
- BRUGUEROLLE B., BARBEAU G., BELANGER P.M., LABRECQUE G. Pharmacokinetics of a sustained-release product of indomethacin in the elderly. Gerontology. 1986;32:277–285. doi: 10.1159/000212802. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., LA RANA G., GIUFFRIDA A., PIOMELLI D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- COMPTON D.R., MARTIN B.R. The effect of the enzyme inhibitor phenylmethylsulfonyl fluoride on the pharmacological effect of anandamide in the mouse model of cannabimimetic activity. J. Pharmacol. Exp. Ther. 1997;283:1138–1143. [PubMed] [Google Scholar]
- CRAVATT B.F., GIANG D.K., MAYFIELD S.P., BOGER D.L., LERNER R.A., GILULA N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- DESARNAUD F., CADAS H., PIOMELLI D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J. Biol. Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- DEUTSCH D.G., CHIN S.A. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem. Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., MELCK D., BISOGNO T., DE PETROCELLIS L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–526. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- EDGEMOND W.S., GREENBERG M.J., MCGINLEY P.J., MUTHIAN S., CAMPBELL W.B., HILLARD C.J. Synthesis and characterization of diazomethylarachidonyl ketone: an irreversible inhibitor of N-arachidonylethanolamine amidohydrolase. J. Pharmacol. Exp. Ther. 1998;286:184–190. [PubMed] [Google Scholar]
- EGERTOVÁ M., CRAVATT B.F., ELPHICK M.R. Fatty acid amide hydrolase expression in rat choroid plexus: possible role in regulation of the sleep-inducing action of oleamide. Neurosci. Letts. 2000;282:13–16. doi: 10.1016/s0304-3940(00)00841-7. [DOI] [PubMed] [Google Scholar]
- EGERTOVÁ M., GIANG D.K., CRAVATT B.F., ELPHICK M.R. A new perspective on cannabinoid signalling: complimentary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc. R. Soc. Lond. B. 1998;265:2081–2085. doi: 10.1098/rspb.1998.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENTHAL R., CORNISH-BOWDEN A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem. J. 1974;139:715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FACCI L., DAL TOSO R., ROMANELLO S., BURIANI A., SKAPER S.D., LEON A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. U.S.A. 1985;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOWLER C.J., JANSSON U., JOHNSON R.M., WAHLSTRÖM G., STENSTRÖM A., NORSTRÖM Å., TIGER G. Inhibition of anandamide hydrolysis by the enantiomers of ibuprofen, ketorolac and flurbiprofen. Arch. Biochem. Biophys. 1999;362:191–196. doi: 10.1006/abbi.1998.1025. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J., STENSTRÖM A., TIGER G. Ibuprofen inhibits the metabolism of the endogenous cannabimimetic agent anandamide. Pharmacol. Toxicol. 1997a;80:103–107. doi: 10.1111/j.1600-0773.1997.tb00291.x. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J., TIGER G., STENSTRÖM A. Ibuprofen inhibits rat brain deamidation of anandamide at pharmacologically relevant concentrations. Mode of inhibition and structure-activity relationship. J.Pharmacol. Exp. Ther. 1997b;283:729–734. [PubMed] [Google Scholar]
- GIANG D.K., CRAVATT B.F. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOPARAJU S.K., KURAHASHI Y., SUZUKI H., UEDA N., YAMAMOTO S. Anandamide amidohydrolase of porcine brain: cDNA cloning, functional expression and site-directed mutagenesis. Biochim. Biophys. Acta. 1999;1441:77–84. doi: 10.1016/s1388-1981(99)00143-2. [DOI] [PubMed] [Google Scholar]
- HARRINGTON C.R. Lowry protein assay containing sodium dodecyl sulfate in microtiter plates for protein determination on fractions from brain tissue. Analyt. Biochem. 1990;186:285–287. doi: 10.1016/0003-2697(90)90081-j. [DOI] [PubMed] [Google Scholar]
- HILLARD C., WILKISON D., EDGEMOND W., CAMPBELL W. Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochim. Biophys. Acta. 1995;1257:249–256. doi: 10.1016/0005-2760(95)00087-s. [DOI] [PubMed] [Google Scholar]
- JAGGAR S.I., HASNIE F.S., SELLATURAY S., RICE A.S.C. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- KOUTEK B., PRESTWICH G.D., HOWLETT A.C., CHIN S.A., SALEHANI D., AKHAVAN N., DEUTSCH D.G. Inhibitors of arachidonoyl ethanolamide hydrolysis. J. Biol. Chem. 1994;269:22937–22940. [PubMed] [Google Scholar]
- LANG W., QIN C., LIN S., KHANOLKAR A.D., GOUTOPOULOS A., FAN P., ABOUZID K., MENG Z., BIEGEL D., MAKRIYANNIS A. Substrate specificity and stereo-selectivity of rat brain microsomal anandamide amidohydrolase. J.Med. Chem. 1999;42:896–902. doi: 10.1021/jm980461j. [DOI] [PubMed] [Google Scholar]
- MACCARRONE M., VAN DER STELT M., ROSSI A., VELDINK G.A., VLIEGENTHART J.F.G., FINAZZI AGRÒ A. Anandamide hydrolysis by human cells in culture and brain. J.Biol. Chem. 1998;273:32332–32339. doi: 10.1074/jbc.273.48.32332. [DOI] [PubMed] [Google Scholar]
- MAZZARI S., CANELLA R., PETRELLI L., MARCOLONGO G., LEON A. N-(2-hydroxyethyl)hexadecamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur. J. Pharmacol. 1996;300:227–236. doi: 10.1016/0014-2999(96)00015-5. [DOI] [PubMed] [Google Scholar]
- OMEIR R.L., ARREAZA G., DEUTSCH D.G. Identification of two serine residues involved in catalysis by fatty acid amide hydrolase. Biochem. Biophys. Res. Commun. 1999;264:316–320. doi: 10.1006/bbrc.1999.1524. [DOI] [PubMed] [Google Scholar]
- OMEIR R.L., CHIN S., HONG Y., AHERN D.G. , DEUTSCH D.G. Arachidonoyl ethanolamide-[1,2-14C] as a substrate for anandamide amidase. Life Sci. 1995;56:1999–2005. doi: 10.1016/0024-3205(95)00181-5. [DOI] [PubMed] [Google Scholar]
- PATRICELLI M.P., LOVATO M.A., CRAVATT B.F. Chemical and mutagenic investigations of fatty acid amide hydrolase: evidence for a family of serine hydrolases with distinct catalytic properties. Biochemistry. 1999;38:9804–9812. doi: 10.1021/bi990637z. [DOI] [PubMed] [Google Scholar]
- TIGER G., STENSTRÖM A., FOWLER C.J. Pharmacological properties of rat brain fatty acid amidohydrolase in different subcellular fractions using palmitoylethanolamide as substrate. Biochem. Pharmacol. 2000;59:647–653. doi: 10.1016/s0006-2952(99)00373-1. [DOI] [PubMed] [Google Scholar]
- TSOU K., NOGUERON M., MUTHIAN S., SAÑUDO-PEÑA M., HILLARD C., DEUTSCH D., WALKER J. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunochemistry. Neurosci. Letts. 1998;254:137–140. doi: 10.1016/s0304-3940(98)00700-9. [DOI] [PubMed] [Google Scholar]
- UEDA N., YAMANAKA K., TERASAWA Y., YAMAMOTO S. An acid amidase hydrolyzing anandamide as an endogenous ligand for cannabinoid receptors. FEBS Letts. 1999;454:267–270. doi: 10.1016/s0014-5793(99)00820-0. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H-H., SØRGÅRD M., DI MARZO V., JULIUS D., HÖGESTÄTT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


