Abstract
Studies in vitro suggest that cephalosporin antibiotics release the gut hormone cholecystokinin. Cholecystokinin is known to inhibit gastric emptying. Here we examine the effects of cefaclor on gastric emptying and intestinal motility.
Male Sprague-Dawley rats were fitted with gastric cannulas. Following a 3-week recovery, the rate of gastric emptying of saline, peptone (4.5%) or cefaclor was determined after instillation into the gastric cannula, while intestinal transit was measured by using the propagation of arabic gum + charcoal mixture given intraduodenally.
Gastric emptying of saline was significantly delayed by the addition of cefaclor (3, 10, 30 or 100 mM). The CCK-A antagonist SR-27897B (1 mg kg−1, i.p.) reversed the delay induced by 10 mM cefaclor, whereas the CCK-B antagonist CI-988 (1 mg kg−1, i.p.) had no significant effect. In capsaicin-treated rats, 10 mM cefaclor emptied more rapidly than in vehicle-treated animals.
Thirty-minute intestinal transit was increased at 30 and 100 mM of cefaclor, while the gastric acid secretion following cefaclor instillation was no different than the group which received saline.
The cephalosporin antibiotic cefaclor appears to be a potent stimulant of CCK release from gut endocrine cells, resembling the effects of peptone. Cefaclor delays gastric emptying via capsaicin-sensitive afferent pathways, which involve CCK-A receptor interaction.
Keywords: Cephalosporins, gastric emptying, cholecystokinin (CCK), CCK-A receptors, capsaicin
Introduction
Cholecystokinin (CCK) is a gastrointestinal hormone that plays a key role in the digestion and assimilation of nutrients. CCK is secreted from specific endocrine cells (I cells) in the proximal small intestine in response to food intake (Walsh, 1994; Yoshida et al., 1999). There is species variation in the action of different luminal nutrients in releasing CCK (Douglas et al., 1988). In the rat, dietary proteins are the major stimulus of CCK release (Liddle et al., 1986). However, the mechanism by which proteins stimulate CCK release is not clear. Several CCK-releasing peptides that are secreted luminally have been isolated, which may be intraluminal regulators of hormone release in the intestine (Liddle, 1995).
Several studies indicate that among the individual food components, peptones (i.e. acid or enzyme hydrolysate of proteins) were the most potent in stimulating CCK release in man, rat, and pig (Miazza et al., 1985; Cuber et al., 1990a; Li & Owyang, 1996). Moreover, it has been shown that CCK release is potently stimulated by peptones in the isolated vascularly perfused rat duodeno-jejunum preparation (Cuber et al., 1990b). Peptones also stimulate CCK secretion and gene transcription in the intestinal CCK-producing enteroendocrine cell line, STC-1 (Cordier-Bussat et al., 1997), which demonstrates many features of native intestinal CCK-producing cells (Rindi et al., 1990; Chang et al., 1994). Recently, a variety of peptidomimetic cephalosporin antibiotics were shown to be strong stimulants of CCK release from STC-1 cells (Nemoz-Gaillard et al., 1998; Murai et al., 2000). The cellular mechanisms involve pertussis toxin-sensitive G protein(s) and require Ca2+ availability. It was suggested that peptones might stimulate CCK release via a direct or an indirect mechanism, which may involve the participation of a CCK-releasing factor(s) (Nemoz-Gaillard et al., 1998).
Erythromycin, a macrolide antibiotic, was noted to be a promotility agent in the gastric antrum and upper gastrointestinal tract and has recently been used in the treatment for diabetic gastroparesis (Richards et al., 1993; Janssens et al., 1990; Otterson & Sarna, 1990). Because of the prevalence of significant gastrointestinal side effects with erythromycin and related macrolides, cephalosporins were studied as candidates for the treatment of diabetic and idiopathic gastroparesis (Kuo et al., 1998). Several cephalosporins appear to have a significant acceleration on the gastric emptying rate, while gastric emptying rate is delayed at very high or maximum antibiotic doses (Kuo et al., 1998).
Endogenous CCK is widely thought to play a physiological role in the control of gastric emptying in the rat, and because cephalosporins release CCK from STC-1 cells, the present study was designed to determine whether cephalosporins inhibit gastric emptying, and if so to determine the mechanisms of action. The results suggest cefaclor inhibits gastric emptying by releasing CCK which acts via vagal afferent neurons.
Methods
Animals
Adult male Sprague-Dawley rats (180–200 g) were housed individually in a light- and temperature-controlled room on a 12 : 12 h light-dark cycle, where the temperature (22±2°C) and relative humidity (65–70%) were kept constant. The animals were fed a standard pellet lab chow, and food was withdrawn overnight before preparative surgery and emptying experiments, but free access to water was allowed. Experiments were approved by The Marmara University, School of Medicine, Animal Care and Use Committee.
Surgery
Rats were anaesthetized by intraperitoneal (i.p.) injection of a mixture of ketamine (100 mg kg−1) and chlorpromazine (12.5 mg kg−1) and aseptically prepared for abdominal surgery. For gastric emptying experiments, a small stainless steel Gregory cannula was installed in the corpus of the stomach as previously described (Debas et al., 1975). Animals were allowed at least 3 weeks to recover from the operation before experiments were commenced. In another group of rats, a pediatric feeding tube (Mallinckrodt Laboratories, Athlone, Ireland) was placed intraluminally 1 cm distal to pyloric sphincter using a purse-string suture of 5/0 silk around the catheter. The catheter was tunnelled subcutaneously to the midscapular region where it was exteriorized via a cutaneous puncture wound. Exposed end of the tube was closed with a blunt ended pin. Before the intestinal transit measurement, each rat was allowed a recovery period of 5–6 days, during which a constant amount of saline was perfused daily to ensure the tubing to remain patent. During this period rats were also adapted to experimental conditions (e.g. manipulation of their catheters).
Administration of drugs
Cefaclor (kindly provided by Lilly & Co., İstanbul) was prepared in saline at varying concentrations. CCK-A receptor antagonist SR-27897B (1 mg kg−1; a generous gift from Sanofi Recherche, Montpellier, France) and CCK-B/gastrin receptor antagonist CI-988 (1 mg kg−1; a generous gift from Parke-Davis Neuroscience Research Centre, Cambridge, U.K.) were dissolved in 3.3% dimethyl sulphoxide (DMSO; Sigma Chemical Co., St. Louis, MO, U.S.A.) and given i.p. 10 min before performing emptying studies of peptone or cefaclor solutions. The rationale for selecting the doses of cefaclor depend upon the in vitro studies (Nemoz-Gaillard et al., 1998), while the doses of the antagonists are those that were found to be effective in reversing the physiologic effects of CCK (Bozkurt et al., 1999).
Measurement of gastric emptying
Trained rats were fasted overnight and lightly restrained in Bollman-type cages. The stomach was flushed with warm (37°C) physiological saline until clean and was allowed to drain freely for 45 min. Test solutions were instilled into the gastric cannula in a volume of 3 ml containing phenol red (PR; 60 mg l−1) as a nonabsorbable dilution marker. The rate of gastric emptying of saline (0.9% NaCl, 300 mOsm kg−1 H2O), or peptone (4.5% (w v−1) meat peptone; Sigma), or cefaclor (3, 10, 30 and 100 mM) was examined using the method described previously (Green et al., 1988). Gastric emptying was determined from the volume and PR concentrations recovered from the cannula 5 min after instillation of the test solutions, as reported previously (Green et al., 1988). Systemic effects were determined by intraperitoneal administration of 10 mM cefaclor.
Capsaicin treatment
In a group of rats capsaicin or vehicle pretreatment was performed 3 days after the gastric cannula placement. Fresh solutions of capsaicin (Sigma) in 10% Tween 80 (Sigma), 10% absolute ethanol and 80% saline, at a concentration of 12.5 mg ml−1 were injected subcutaneously over a 36 h period (125 mg kg−1) in rats lightly anaesthetized with ether (Barquist et al., 1992). The first injection consisted of 25 mg kg−1, followed by two injections of 50 mg kg−1, 12 h apart. Rats also received atropine (1 mg kg−1, i.p.) before the first capsaicin or vehicle injection to decrease the acute effects of capsaicin on the respiratory and cardiovascular systems. Before the emptying experiments, capsaicin- and vehicle-pretreated rats were tested for impaired chemosensitivity by the eye-wiping test. In capsaicin-treated animals, the corneal afferents were no longer sensitive to a solution of 1% NH4OH (Holzer, 1991).
Measurement of gastric acid secretion and intestinal transit
Gastric acid secretion was monitored in fasted rats fitted with gastric cannulas at 10 min following gastric instillation of saline or cefaclor solution (10 mM). Gastric perfusates were collected by the flushing technique and titrated to pH 7.0 with 0.01 N NaOH. Gastric acid output was calculated as μmol in 10 min (Çorak et al., 1997).
Intestinal transit studies were performed by giving 1 ml of a mixture of Arabic gum (gum Arabic from Acacia tree, Sigma Chemical) and activated charcoal mixture through the intraduodenal catheter at 10 min following the orogastric administration of saline or cefaclor solution (10, 30 or 100 mM) by gavage (Udassin et al., 1994). Twenty minutes later, rats were killed by decapitation, the abdomen was opened, and ligatures were made around the pylorus and ileocecal valve. The small intestine was dissected and freed from its mesentery, with its continuity retained. The intestine was then measured by laying it longitudinally. To avoid movement of intraluminal contents, the intestine was not stretched. The total length of the small bowel and the length of small bowel filled with the black meal were recorded. Intestinal transit index (%) was expressed by the fraction of the total length of the small bowel filled with the black material.
Statistical analysis
The results are expressed as means±s.e.mean with seven to nine rats per group. One-way analysis of variance (ANOVA) with the Tukey-Kramer (post hoc) test was used for multiple comparisons. The concentration causing 50% of the maximal response of cefaclor was calculated using a computer-assisted probit transformation (Pharmacological Calculations) and is represented as ED50. Differences were considered statistically significant if P<0.05.
Results
Effect of cefaclor on gastric emptying
In control gastric fistula rats, the emptying of saline was rapid (2.87±0.05 ml 5 min−1; 96%) and similar to that described previously (Forster et al., 1990). The emptying of cefaclor was dose-dependently delayed at 3 mM (P<0.05) and at 10, 30 and 100 mM (P<0.001; Figure 1), however, 1 mM concentration was found to be ineffective on gastric emptying rate. The ED50 of cefaclor was calculated as 2.55 mM.
Figure 1.

The gastric emptying of cefaclor was dose-dependently delayed at 3, 10, 30 and 100 mM, with an ED50 of 2.55 mM. Results are expressed as means±s.e.mean. *P<0.05, **P<0.01 and ***P<0.001; compared to gastric emptying of saline.
As previously described, peptone instilled into the stomach of control rats delayed gastric emptying compared with physiological saline (Figure 2a). In accordance with the previous findings, when the rats were pretreated with the CCK-A antagonist, SR-27897B, the inhibitory effect of peptone on gastric emptying was abolished (2.77±0.10 ml 5 min−1), while the CCK-B antagonist CI-988, had no effect on peptone-induced delay in gastric emptying. The delay in gastric emptying of cefaclor was also abolished by treatment with the CCK-A antagonist (P<0.01), whereas the CCK-B antagonist had no significant effect (Figure 2b). Systemic administration of cefaclor at a dose of 40 mg kg−1 (i.p.) did not alter the gastric emptying of saline (2.82±0.14 ml 5 min−1) when compared with that of vehicle-treated rats (2.72±0.08 ml 5 min−1). The cefaclor-induced delay in gastric emptying was reversed by capsaicin pretreatment, which was used to ablate C fibres (P<0.01; Figure 3).
Figure 2.

Effect of CCK-A (SR-27897B; 1 mg kg−1) and CCK-B receptor antagonist (CI-988; 1 mg kg−1) on gastric emptying rate of peptone (a) and cefaclor (b). Results are expressed as means± s.e.mean. **P<0.01 and ***P<0.001; compared to gastric emptying of saline. ++P<0.01 and +++P<0.001; compared to the vehicle- (DMSO) treated group.
Figure 3.
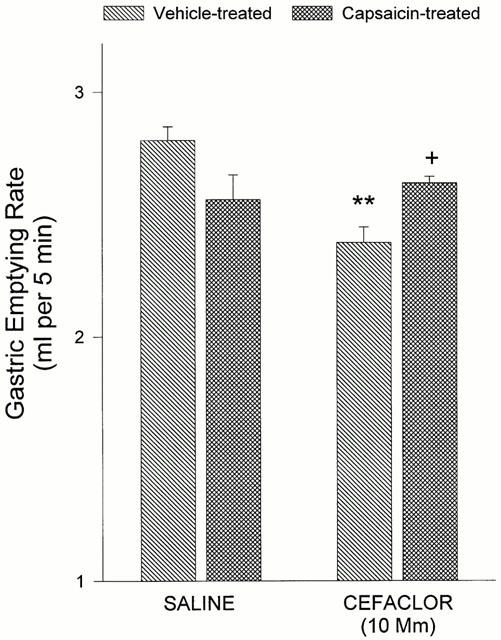
Reversal of cefaclor-induced delay in gastric emptying by systemic capsaicin pretreatment (125 mg kg−1 injected subcutaneously over a 36 h period). Results are expressed as means± s.e.mean. **P<0.01; compared to gastric emptying of saline in vehicle-treated group. +P<0.05; compared to the vehicle- (10% Tween 80, 10% ethanol and 80% saline) treated group.
Effect of cefaclor on acid secretion and intestinal transit
Cefaclor (intragastric, 10 mM) which significantly delayed gastric emptying had no significant effect on the gastric acid secretion at 10 min following cefaclor administration (16.0±1.65 μmol), when compared with saline (19.5±2.4 μmol). Intragastric administration of cefaclor facilitated the intestinal transit at 30 and 100 mM doses (P<0.05–0.01), while the systemic administration of an equivalent dose (40 mg kg−1, i.p.) had no significant effect on intestinal motility (Figure 4).
Figure 4.
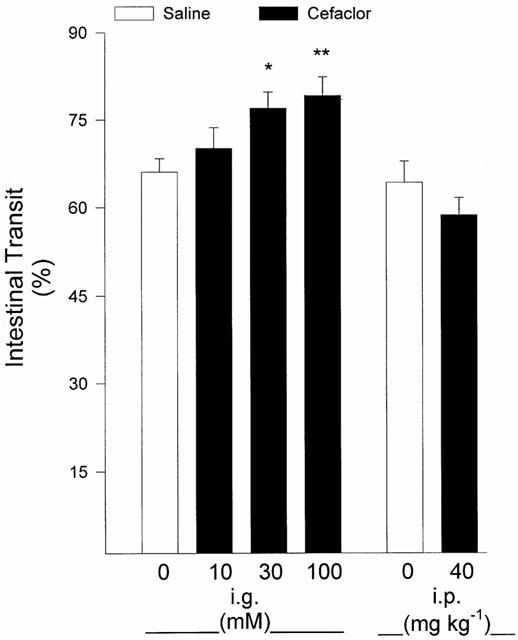
Intragastric administration (i.g.) of cefaclor at 30 and 100 mM doses facilitated the intestinal transit, but intraperitoneal (i.p.; 40 mg kg−1) administration was not different from saline-treated group. Results are expressed as means±s.e.mean. *P<0.05 and **P<0.01; compared to intestinal transit following intragastric saline.
Discussion
The results of the present study indicate that cefaclor delays gastric emptying in rats via capsaicin-sensitive afferent pathways, which involve CCK-A receptor interaction. As it was demonstrated in the intestinal CCK-producing STC-1 cells, cefaclor is likely to release CCK from gut endocrine cells. The results are compatible with the idea that cefaclor acts in vivo to release CCK which in turn inhibits gastric emptying by well recognized pathways. The facilitatory effect of cefaclor on intestinal transit further supports the idea that cefaclor releases CCK, which has been shown to stimulate intestinal motor activity when administered exogenously (Parker & Beneventano, 1976). The results suggest that the inhibition of gastric emptying by intragastric cefaclor is not due to an alteration in gastric acid secretion, since the acid secretory capacity does not change during the corresponding period of cefaclor exposure.
Peptones are potent stimulants of CCK release in rats, both in vivo and ex vivo in a model of isolated vascularly perfused duodeno-jejunum preparation and in vitro in the intestinal CCK-producing cell line STC-1 (Cuber et al., 1989; 1990b; Cordier-Bussat et al., 1997). Protein hydrolysates from various origins (meat, casein, soybean, and ovalbumin) dose-dependently increased CCK release (Nemoz-Gaillard et al., 1998). Intraluminal proteins exert their stimulatory effect on CCK secretion via trypsin inhibition, and this has been postulated to be the major link in the feedback loop regulating the intestinal phase of pancreatic exocrine secretion (Green & Lyman, 1972; Green et al., 1973). It was suggested that molecules sharing a peptidomimetic structure are capable of triggering peptide release from CCK-producing cells, either directly or indirectly by the participation in a CCK-releasing factor pathway. A variety of peptidomimetic antibiotics, namely cephalosporins, also stimulated the release of CCK over the concentration range 1–20 mM in vitro (Nemoz-Gaillard et al., 1998). Cephalosporin-induced CCK release in the STC-1 cell line was reduced after pertussis toxin treatment and was abolished by using intra- and extracellular Ca2+ chelators (Nemoz-Gaillard et al., 1998). In the present study, the cefaclor-induced delay in gastric emptying was abolished by the CCK-A antagonist, but not the CCK-B antagonist, which resembles peptone-induced inhibition of gastric emptying. These data suggest that cefaclor delays gastric emptying via CCK-A receptors, as does intraluminal peptone. The functional effect of cefaclor on gastric emptying and intestinal transit is in accordance with previous in vitro and ex vivo results, where it was found to be potent as peptones in stimulating CCK release.
Endogenous CCK clearly plays a physiological role in the control of gastric emptying in the rat as the administration of CCK antagonists results in accelerated emptying (Green et al., 1988; Forster et al., 1990; Forster & Dockray, 1992). The involvement of capsaicin-sensitive fibres has been shown in the regulation of gastric emptying (Forster et al., 1990; Bozkurt et al., 1999) and inhibition of gastric motility in response to injections of CCK (Raybould & Tache, 1988). Autoradiographic studies have provided clear evidence that vagal afferent fibres in the rat express CCK binding sites, mainly of the A-type, as found in the pancreas, pyloric sphincter and in the brainstem regions of the area postrema and nucleus tractus solitarius (Moran et al., 1985; 1987; 1990). It is likely to suggest that cefaclor inhibits gastric emptying in rats by stimulating CCK secretion and gene transcription in the intestinal I cells, which then interacts with low affinity CCK receptor sites. Moreover, cefaclor may be acting on the intraluminal regulators of the hormone release, which include the luminal CCK-releasing factor (Miyasaka et al., 1989; Liddle 1995; Spanngel et al., 1996), CCK monitor peptide (Iwai et al., 1987; Bouras et al., 1992; Tsuzuki et al., 1992; Liddle 1995), and diazepam-binding inhibitor (Herzig et al., 1996; Yoshida et al., 1999). The cellular mechanisms activated by cephalosporins and peptones were shown to be similar for stimulation of CCK release (Nemoz-Gaillard et al., 1998; Yoshida et al., 1999), and these molecules share a peptidomimetic structure capable of triggering peptide release from CCK-producing cells. Their mode of action on gastric motility is also alike in acting via capsaicin-sensitive small diameter afferents and CCK-A-type receptors (Forster et al., 1990).
In an attempt to develop new agents for gastroparesis, erythromycin has been used as a treatment for diabetic gastroparesis (Janssens et al., 1990; Urbain et al., 1990; Otterson & Sarna, 1990; Richards et al., 1993). It accelerates gastric emptying in patients with gastroparesis by binding to the receptor for the gastrointestinal peptide motilin (Feighner et al., 1999). Although cephalosporins are not interacting with the motilin receptors, several cephalosporins were shown to have a significant acceleration on the gastric emptying rate over the range 2–200 mg kg−1 (i.p.) in mice, while at higher doses (200–1000 mg kg−1) a significant delay in gastric emptying could be detected (Kuo et al., 1998). The present results indicate that intragastric cefaclor inhibits gastric emptying similar to those detected in the higher dose range of its systemic administration. It seems likely that in order to reach this intraluminal dose range that would induce endocrine CCK release, higher systemic doses are required. Moreover, this finding does not rule out the possibility that cephalosporins may be candidates for the treatment of gastroparesis at lower doses, as it was previously suggested that the cephalosporins might have a larger therapeutic window with delay in gastric emptying not seen until the higher doses (Kuo et al., 1998). Nevertheless, the inhibitory effect of cephalosporins on gastric emptying, which involves a physiologic feedback control of gastric motility through the activation of specific CCK-receptors, suggests that further experimental and clinical studies may be indicated to evaluate the potential efficacy of cephalosporins in the treatment of gastrointestinal disorders.
As it was demonstrated in the intestinal cell lines, the cephalosporin antibiotic cefaclor appears to be a potent stimulant of CCK release from gut endocrine cells, resembling the effects of the food component, peptone. Cefaclor delays gastric emptying via capsaicin-sensitive afferent pathways, which involve CCK-A receptor interaction. Therefore, cefaclor may be a useful experimental tool to study the CCK-dependent physiological events in vivo, since cellular mechanisms involved in CCK release are also common for both peptones and cephalosporins.
Acknowledgments
The authors are grateful to Professor Graham J. Dockray, Liverpool, U.K., for his invaluable guidance throughout the project. The authors thank Dr Tamer Coskun for his technical assistance. We also acknowledge Lilly & Co., İstanbul (Cefaclor), Sanofi Recherche, Montpellier, France (SR-27897B) and Parke-Davis Neuroscience Research Centre, Cambridge, U.K. (CI-988) for supplying the chemicals. These data were presented, in part, at the Physiological Society Meeting, Newcastle, U.K., 1999.
Abbreviations
- CCK
cholecystokinin
- CI-988
CCK-B antagonist
- DMSO
dimethyl sulphoxide
- i.g.
intragastric
- i.p.
intraperitoneal
- PR
phenol red
- SR-27897B
CCK-A antagonist
- STC-1 cells
intestinal CCK-producing cell line
References
- BARQUIST E., ZINNER M., RIVIER J., TACHE Y. Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am. J. Physiol. 1992;262:G616–G620. doi: 10.1152/ajpgi.1992.262.4.G616. [DOI] [PubMed] [Google Scholar]
- BOURAS E.P., MISUKONIS M.A., LIDDLE R.A. Role of calcium in monitor peptide-stimulated cholecystokinin release from perfused intestinal cells. Am. J. Physiol. 1992;262:G791–G796. doi: 10.1152/ajpgi.1992.262.5.G791. [DOI] [PubMed] [Google Scholar]
- BOZKURT A., OKTAR B.K., KURTEL H., ALICAN I., COSKUN T., YEĞEN B.Ç. Capsaicin-sensitive vagal fibres and 5-HT3-, gastrin releasing peptide- and cholecystokinin A-receptors are involved in distension-induced inhibition of gastric emptying in the rat. Regul. Peptides. 1999;83:81–86. doi: 10.1016/s0167-0115(99)00050-6. [DOI] [PubMed] [Google Scholar]
- CHANG C.H., CHEY W.Y., SUN Q., LEITER A., CHANG T.M. Characterization of the release of cholecystokinin from a murine neuroendocrine cell line, STC-1. Biochim. Biophys. Acta. 1994;1221:339–347. doi: 10.1016/0167-4889(94)90259-3. [DOI] [PubMed] [Google Scholar]
- CORAK A., COSKUN T., ALICAN I., KURTEL H., YEĞEN B.Ç. The effect of nitric oxide synthase blockade and indomethacin on gastric emptying and gastric contractility. Pharmacology. 1997;54:298–304. doi: 10.1159/000139499. [DOI] [PubMed] [Google Scholar]
- CORDIER-BUSSAT M., BERNARD C., HAOUCHE S., ROCHE C., ALBELLO J., CHAYVIALLE J.A., CUBER J.C. Peptones stimulate cholecystokinin secretion and gene transcription in the intestinal cell line STC-1. Endocrinology. 1997;138:1137–1144. doi: 10.1210/endo.138.3.5023. [DOI] [PubMed] [Google Scholar]
- CUBER J.C., BERNARD G., FUSHIKI T., BERNARD C., YAMANISHI R., SUGIMOTO E., CHAYVIALLE J.A. Luminal CCK-releasing factors in the isolated vascularly perfused rat duodenojejunum. Am. J. Physiol. 1990b;259:G191–G197. doi: 10.1152/ajpgi.1990.259.2.G191. [DOI] [PubMed] [Google Scholar]
- CUBER J.C., BERNARD C., LEVENEZ F., CHAYVIALLE J.A. Intraduodenal infusion of fats, proteins and carbohydrates stimulates the release of intestinal cholecystokinin in the pig. Reprod. Nutr. Dev. 1990a;30:267–275. [PubMed] [Google Scholar]
- CUBER J.C., VILAS F., CHARLES N., BERNARD C., CHAYVIALLE J.A. Bombesin and nutrients stimulate release of CCK through distinct pathways in the rat. Am. J. Physiol. 1989;256:G989–G996. doi: 10.1152/ajpgi.1989.256.6.G989. [DOI] [PubMed] [Google Scholar]
- DEBAS H.T., FAROOQ O., GROSSMAN M.I. Inhibition of gastric emptying is a physiological action of cholecystokinin. Gastroenterology. 1975;68:1211–1217. [PubMed] [Google Scholar]
- DOUGLAS B.R., WOUTERSEN R.A., JANSEN J.B.M.J., DE JONG A.J.L., LAMERS C.B.H.W. The influence of different nutrients on plasma cholecystokinin secretion in the rat. Experientia. 1988;44:21–23. doi: 10.1007/BF01960229. [DOI] [PubMed] [Google Scholar]
- FEIGHNER S.D., TAN C.P., MCKEE K.K., PALYHA O.C., HRENIUK D.L., PONG S.S., AUSTIN C.P., FIGUEROA D., MACNEIL D., CASCIERI M.A., NARGUND R., BAKSHI R., ABRAMOVITZ M., STOCCO R., KARGMAN S., O'NEILL G., VAN DER PLOEG L.H., EVANS J., PATCHETT A.A., SMITH R.G., HOWARD A.D. Receptor for motilin identified in the human gastrointestinal system. Science. 1999;284:2184–2188. doi: 10.1126/science.284.5423.2184. [DOI] [PubMed] [Google Scholar]
- FORSTER E.R., DOCKRAY G.J. The role of cholecystokinin in inhibition of gastric emptying by peptone in the rat. Exp. Physiol. 1992;77:693–699. doi: 10.1113/expphysiol.1992.sp003635. [DOI] [PubMed] [Google Scholar]
- FORSTER E.R., GREEN T., ELLIOT M., BREMNER A., DOCKRAY G.J. Gastric emptying in rats: role of afferent neurons and cholecystokinin. Am. J. Physiol. 1990;258:G552–G556. doi: 10.1152/ajpgi.1990.258.4.G552. [DOI] [PubMed] [Google Scholar]
- GREEN G.M., LYMAN R.L. Feedback regulation of pancreatic enzyme secretions as a mechanism for trypsin inhibitor-induced hypersecretion in rats. Proc. Soc. Exp. Biol. Med. 1972;140:6–12. doi: 10.3181/00379727-140-36384. [DOI] [PubMed] [Google Scholar]
- GREEN G.M., OLDS B.A., MATTHEWS G., LYMAN R.L. Protein, as a regulator of pancreatic enzyme secretion in the rat. Proc. Soc. Exp. Biol. Med. 1973;142:1162–1167. doi: 10.3181/00379727-142-37199. [DOI] [PubMed] [Google Scholar]
- GREEN T., DIMALINE R., PEIKIN S., DOCKRAY G.J. Action of cholecystokinin antagonist L364, 718 on gastric emptying in the rat. Am. J. Physiol. 1988;255:G685–G689. doi: 10.1152/ajpgi.1988.255.5.G685. [DOI] [PubMed] [Google Scholar]
- HERZIG K.H., SCHON I., TATEMOTO K., OHE Y., LI Y., FOLSCH U.R., OWYANG C. Diazepam binding inhibitor is a potent cholecystokinin-releasing peptide in the intestine. Proc. Natl. Acad. Sci. U.S.A. 1996;93:7927–7932. doi: 10.1073/pnas.93.15.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- IWAI K., FUKUOKA S., FUSHIKI T., TSUJIKAVA M., HIROSE M., TSUNASAWA S., SEKIYAMA F. Purification and sequencing of a trypsin-sensitive cholecystokinin-releasing peptide from rat pancreatic juice. J. Biol. Chem. 1987;262:8956–8959. [PubMed] [Google Scholar]
- JANSSENS J., PEETERS T.L., VANTRAPPEN G., TACK J., URBAIN J.L., ROO M.D., MULS E., BOUILLON R. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. N. Engl. J. Med. 1990;322:1028–1031. doi: 10.1056/NEJM199004123221502. [DOI] [PubMed] [Google Scholar]
- KUO W.H., WADWA K.S., FERRIS C.D. Cephalosporin antibiotics accelerate gastric emptying in mice. Dig. Dis. Sci. 1998;43:1690–1694. doi: 10.1023/a:1018811114815. [DOI] [PubMed] [Google Scholar]
- LI Y., OWYANG C. Peptones stimulate CCK-releasing peptide secretion by activating intestinal submucosal cholinergic neurons. J. Clin. Invest. 1996;97:1463–1470. doi: 10.1172/JCI118568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIDDLE R.A. Regulation of cholecystokinin secretion by intraluminal releasing factors. Am. J. Physiol. 1995;269:G319–G327. doi: 10.1152/ajpgi.1995.269.3.G319. [DOI] [PubMed] [Google Scholar]
- LIDDLE R.A., GREEN G.M., CONRAD C.K., WILLIAMS J.A. Proteins but not amino acids, carbohydrates, or fats stimulate cholecystokinin secretion in the rat. Am. J. Physiol. 1986;251:G243–G248. doi: 10.1152/ajpgi.1986.251.2.G243. [DOI] [PubMed] [Google Scholar]
- MIAZZA B., PALMA R., LACHANCE J.R., CHAYVIALLE J.A., JONNARD P.P., MODIGLIANI R. Jejunal secretory effect of intraduodenal food in humans. A comparison of mixed nutrients, proteins, lipids and carbohydrates. Gastroenterology. 1985;88:1215–1222. doi: 10.1016/s0016-5085(85)80082-2. [DOI] [PubMed] [Google Scholar]
- MIYASAKA K., GUAN D., LIDDLE R.A., GREEN G.M. Feedback regulation by trypsin: evidence of intraluminal CCK-releasing peptide. Am. J. Physiol. 1989;257:G175–G181. doi: 10.1152/ajpgi.1989.257.2.G175. [DOI] [PubMed] [Google Scholar]
- MORAN T.H., NORGREN R., CROSBY R.J., MCHUGH P.R. Central and peripheral transport of cholecystokinin binding sites occurs in afferent fibres. Brain Res. 1990;526:95–102. doi: 10.1016/0006-8993(90)90253-8. [DOI] [PubMed] [Google Scholar]
- MORAN T.H., ROBINSON P.H., GOLDRICH M.S., MCHUGH P.R. Two brain CCK receptors: implications for behavioral actions. Brain Res. 1985;362:175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- MORAN T.H., SMITH G.P., HOSTETLER A.M., MCHUGH P.R. Transport of cholecystokinin (CCK) binding sites in subdiaphragmatic vagal branches. Brain Res. 1987;415:149–152. doi: 10.1016/0006-8993(87)90278-2. [DOI] [PubMed] [Google Scholar]
- MURAI A., NOBLE P.-J.M., DEAVALL D.G., DOCKRAY G.J. Control of c-fos expression in STC-1 cells by peptiolomimetic stimuli. Eur. J. Pharmacol. 2000;394:27–34. doi: 10.1016/s0014-2999(00)00076-5. [DOI] [PubMed] [Google Scholar]
- NEMOZ-GAILLARD E., BERNARD C., ALBELLO J., CORDIER-BUSSAT M., CHAYVIALLE J.A., CUBER J.C. Regulation of cholecystokinin secretion by peptones and peptidomimetic antibiotics in STC-1 cells. Endocrinology. 1998;139:932–938. doi: 10.1210/endo.139.3.5802. [DOI] [PubMed] [Google Scholar]
- OTTERSON M.F., SARNA S.K. Gastrointestinal motor effects of erythromycin. Am. J. Physiol. 1990;259:G355–G363. doi: 10.1152/ajpgi.1990.259.3.G355. [DOI] [PubMed] [Google Scholar]
- PARKER J.C., BENEVENTANO T.C. Acceleration of the small bowel intestinal motility by CCK. Gastroenterology. 1976;58:679–684. [PubMed] [Google Scholar]
- RAYBOULD H.E., TACHE Y. CCK inhibits gastric motility and emptying via a capsaicin-sensitive pathway in rats. Am. J. Physiol. 1988;255:G242–G246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- RICHARDS R.D., DAVENPORT K.G., MCCALLUM R.W. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am. J. Gastroenterol. 1993;88:203–207. [PubMed] [Google Scholar]
- RINDI G., GRANT S.G.N., YANGOU Y., GHATEI M.A., BLOOM S.R., BAUTCH V.L., SOLCIA E., POLAK J.M. Development of neuroendocrine tumors in the gastrointestinal tract of transgenic mice. Am. J. Pathol. 1990;136:1349–1363. [PMC free article] [PubMed] [Google Scholar]
- SPANNGEL A.W., GREEN G.M., GUAN D., LIDDLE R.A., FAULL K., REEVE J.R. Purification and characterization of a luminal cholecystokinin-releasing factor from rat intestinal secretion. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4415–4420. doi: 10.1073/pnas.93.9.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUZUKI S., MIURA Y., FUSHIKI T., OOMORI T., SATOH T., NATORI Y., SUGIMOTO E. Molecular cloning and characterization of genes encoding rat pancreatic cholecystokinin (CCK)-releasing peptide (monitor peptide) and pancreatic secretory trypsin inhibitor (PSTI) Biochim. Biophys. Acta. 1992;1132:199–202. doi: 10.1016/0167-4781(92)90012-o. [DOI] [PubMed] [Google Scholar]
- UDASSIN R., EIMERI D., SCHIFFMAN J., HAKEL Y. Epidural anesthesia accelerates the recovery of postischemic bowel motility in the rat. Anesthesiology. 1994;80:832–836. doi: 10.1097/00000542-199404000-00016. [DOI] [PubMed] [Google Scholar]
- URBAIN J.L.C., VANTRAPPEN G., JANSSENS J., CUTSEM E.V., PEETERS T., ROO M.D. Intravenous erythromycin dramatically accelerates gastric emptying in gastroparesis diabeticorum and normals and abolishes the emptying discrimination between solids and liquids. J. Nucl. Med. 1990;31:1490–1493. [PubMed] [Google Scholar]
- WALSH J.H.Cholecystokinin Physiology of the Gastrointestinal Tract 19941New York: Raven Press; 49–67.ed. Johnson, L.R. [Google Scholar]
- YOSHIDA H., TSUNODA Y., OWYANG C. Diazepam-binding inhibitor33–50 elicits Ca2+ oscillation and CCK secretion in STC-1 cells via L-type Ca2+ channels. Am. J. Physiol. 1999;276:G694–G702. doi: 10.1152/ajpgi.1999.276.3.G694. [DOI] [PubMed] [Google Scholar]


