Abstract
Since protease-activated receptors (PARs) are distributed throughout the gastrointestinal tract, we investigated the role of PARs in modulation of the motility of the rat oesophageal muscularis mucosae.
Thrombin produced contraction of segments of the upper and lower part of the smooth muscle. Trypsin contracted both the muscle preparations only at high concentrations. SFLLR-NH2 and TFLLR-NH2 (PAR-1-activating peptides), but not the PAR-1-inactive peptide FSLLR-NH2, evoked a marked contraction. In contrast, the PAR-2 agonist SLIGRL-NH2 and the PAR-4 agonist GYPGKF-NH2 caused no or only a negligible contraction.
In oesophageal preparations precontracted with carbachol, thrombin produced a dual action i.e. relaxation followed by contraction. TFLLR-NH2 further contracted the precontracted preparations with no preceding relaxation. GYPGKF-NH2, but not the inactive peptide GAPGKF-NH2, produced marked relaxation. Trypsin or SLIGRL-NH2 caused no relaxation.
The PAR-1-mediated contraction was completely abolished in Ca2+-free medium and considerably attenuated by nifedipine (1 μM) and in a low Na+ medium. The PAR-4-mediated relaxation was resistant to tetrodotoxin (10 μM), apamin (0.1 μM), charybdotoxin (0.1 μM), L-NG-nitroarginine methyl ester (100 μM), indomethacin (3 μM), propranolol (5 μM) or adenosine 3′,5′-cyclic monophosphorothioate, 8-bromo, Rp-isomer (30 μM).
Thus, thrombin plays a dual role in modulating the motility of the oesophageal muscularis mucosae, producing contraction via PAR-1 and relaxation via PAR-4. The PAR-1-mediated effect appears to occur largely through increased Na+ permeability followed by activation of L-type Ca2+ channels and subsequent influx of extracellular Ca2+. Our data could provide evidence for a novel role of PAR-4 as opposed to PAR-1, although the underlying mechanisms are still open to question.
Keywords: Protease (proteinase)-activated receptor (PAR), oesophageal muscularis mucosae, motility modulation, thrombin, L-type Ca2+ channels, Na+ permeability
Introduction
Thrombin plays a variety of physiological and pathophysiological roles. In the haemostatic systems, thrombin causes plasma clotting and platelet aggregation. The activation of human platelets by thrombin is primarily mediated by a number of G protein-coupled, seven trans-membrane domain receptors that are now designated as protease-activated receptors (PARs) (Dery et al., 1998; Hollenberg, 1999; Kawabata & Kuroda, 2000; Vu et al., 1991). Thrombin activates this receptor by proteolytic unmasking of the N-terminal cryptic receptor-activating sequence, followed by an interaction between the exposed N-terminal tethered ligand and the body of the receptor itself. Synthetic peptides as short as five amino acids based on the amino acid sequence of the tethered ligand (i.e. SFLLR-NH2 for human PAR-1) can mimic the action of the agonist protease by directly binding to the body of the receptor. An additional three members of the PAR family have been cloned since the first cloning of PAR-1 (Vu et al., 1991; Nystedt et al., 1994; Ishihara et al., 1997; Kahn et al., 1998; Xu et al., 1998). PAR-3 and PAR-4 are activated by thrombin, whereas PAR-2 is activated by trypsin/tryptase but not by thrombin (Nystedt et al., 1994; Molino et al., 1997; Kawabata & Kuroda, 2000). PAR-1, PAR-2 and PAR-4 can be activated by synthetic peptides based on their receptor activating sequences, although no such peptides for PAR-3 have yet been found. PAR-3 is expressed in murine platelets, but not in human platelets, while PAR-4 is expressed in both human and murine platelets (Vu et al., 1991; Ishihara et al., 1997; Kahn et al., 1998; 1999).
PAR-1 and/or PAR-2 are widely distributed in various organs, especially in the digestive systems including the gastrointestical tract, salivary glands and pancreas and appear to be involved in a number of biological events such as inflammation, salivary and pancreatic glandular exocrine secretion, smooth muscle contraction and relaxation etc (Bohm et al., 1996; Cirino et al., 1996; Cocks et al., 1999a,1999b; Corvera et al., 1997; Hollenberg, 1999; Hollenberg et al., 1993; 1996; Kawabata et al., 1998; 1999a,1999b; 2000; Kawabata & Kuroda, 2000; Laniyonu & Hollenberg, 1995; Nguyen et al., 1999; Saifeddine et al., 1996; Vergnolle et al., 1998; 1999). Thrombin, as well as PAR-1-activating peptides, produce contraction and apamin-sensitive relaxation of gastric and duodenal longitudinal smooth muscle (Cocks et al., 1999b; Hollenberg et al., 1993; Kawabata et al., 1999b). Activation of PAR-1 also induces direct contraction and endothelial nitric oxide-mediated relaxation in vascular smooth muscle (Hollenberg et al., 1993; Laniyonu & Hollenberg, 1995). Similarly, PAR-1 also triggers contraction and epithelial prostanoid-dependent relaxation of airway smooth muscle (Cocks et al., 1999a). On the other hand, possible physiological/pathophysiological roles of PAR-3 and PAR-4 in tissues or cells other than platelets are poorly understood in spite of their wide distribution in varying tissues (Hollenberg, 1999; Ishihara et al., 1997; Kahn et al., 1998; Kawabata & Kuroda, 2000; Xu et al., 1998). PAR-4 is not involved in modulation of the motility of rat duodenal smooth muscle (Kawabata et al., 1999b). Most recently, Hollenberg et al. (1999) have found that PAR-4, as well as PAR-1, also mediates contraction of gastric longitudinal smooth muscle and nitric oxide-dependent relaxation of vascular smooth muscle. Likewise, PAR-4 appears to mimic the actions of PAR-1 in the airway smooth muscle (Lan et al., 2000).
Given the wide distribution of PARs in the digestive tracts and their demonstrated functions (Dery et al., 1998; Hollenberg, 1999; Kawabata & Kuroda, 2000), it is hypothesized that PARs might play key roles throughout the digestive systems, especially during inflammation and/or haemorrhage where thrombin and/or mast cell tryptase could become available as the endogenous agonists. In this context, using agonists specific for each PAR (Hollenberg et al., 1997; 1999; Kawabata et al., 1999c), we investigated the role of PARs in modulation of the motility of the rat oesophageal muscularis mucosae. Here we show that thrombin produces contraction via PAR-1 and relaxation via PAR-4 in this preparation, providing the first evidence for a role of PAR-4 as opposed to PAR-1.
Methods
Tissue preparation and isometric recording
Male Wistar rats weighing 250–350 g (Japan SLC. Inc., Japan) were killed by exsanguination under urethane (1.5 g kg−1) anaesthesia. The oesophagus between the cardia and thymus was excised and freed of fat and connective tissues in ice-cold Krebs-Henseleit buffer (pH 7.4) of the following composition (mM): NaCl, 118; KCl, 4.7; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; KH2PO4, 1.2; glucose 10. The oesophageal mucosae muscularis preparation was made by removing the outer muscle coat and was cut in the middle into upper and lower segments. Each cylindrical segment, the upper and lower ends of which were set with micro-pinch holders at the same side, was suspended in an organ bath containing 4 ml of gassed (95% O2/5% CO2) Krebs-Henseleit solution that was maintained at 37°C, and the tissue preparation was allowed to equilibrate for 1 h under a 3 mN load. The isometric tension of each segment was recorded through a force-displacement transducer (UL-10GR, Minebea Co., Ltd., Japan). The contractile responses to test compounds are expressed as a percentage (% KCl) of the contraction induced by a high K+ (50 mM)-containing solution, and the relaxation responses are represented as a percentage (% papaverine) of the relaxation to 100 μM papaverine.
Experimental protocol
After the equilibration period, the oesophageal muscularis mucosae was exposed to a high KCl (50 mM)-containing Krebs-Henseleit solution, and washed after the evoked contraction had peaked. This procedure was repeated a few times at 40 min intervals. Tissues were washed every 10–15 min during the resting period. Then, the responses of the preparations to thrombin, trypsin and PAR-related peptides were examined. Peptides tested were: SFLLR-NH2, a human PAR-1-derived PAR-1 agonist that also has weak agonistic activity toward PAR-2; TFLLR-NH2, a highly specific PAR-1 agonist analogue; SLIGRL-NH2, a murine PAR-2-derived specific PAR-2 agonist; GYPGKF-NH2, a murine PAR-4-derived specific PAR-4 agonist; FSLLR-NH2, a PAR-1-inactive control peptide; GAPGKF-NH2, a PAR-4-inactive control peptide. The assay of the activity of these peptides was always performed in the presence of 10 μM amastatin, an inhibitor of aminopeptidase (degrading enzyme of peptides), which was added to the organ bath 1 min before the challenge with each peptide. In preliminary experiments, amastatin itself at this concentration did not change the tension of the preparation (data not shown). When the relationship between concentration and contraction was examined, each agonist was added cumulatively to the organ bath. The relaxation responses to thrombin, trypsin and PAR-related peptides were determined in tissue preparations that had been precontracted with (0.3 μM) carbachol. Cumulative application of agonists was not employed to obtain the concentration-relaxation curve, because the carbachol-evoked contraction did not persist for a sufficiently long time to allow cumulative repeated addition of a relaxant. Thrombin and trypsin were applied only once to each tissue, because the response did not recover at all even after repeated washes for 3 h after its first application. In contrast, the response (contraction) of each segment to PAR-1-activating peptides recovered completely within 3 h of the first application. In inhibition experiments, therefore, after monitoring the response of each preparation to the PAR-1-activating peptide TFLLR-NH2 at 10 μM, the preparation was washed and, after 3 h, rechallenged with the same agonist 15 min following replacement of the medium with Ca2+-free and low Na+-containing Krebs-Henseleit buffers or addition of inhibitors/antagonists. The response (relaxation) to GYPGKF-NH2 recovered to a varying extent (10–80%) depending on the tissue 30–40 min after the first challenge, but did not completely recover even after repeated washing for 3 h. Thus, in inhibition experiments, GYPGKF-NH2 at 300 μM was applied only once to each tissue in the absence or presence of inhibitors/antagonists that had been added 10 min before carbachol, or in a 30 mM KCl-containing Krebs-Henseleit buffer. The control and test data obtained from distinct tissues were compared statistically. To prepare the high K+-containing solution, the required amount of KCl was added to the Krebs-Henseleit buffer in which the corresponding molar equivalent of NaCl was removed. The Ca2+-free buffer was prepared by adding 0.2 mM EGTA to the Krebs-Henseleit buffer containing no CaCl2. A low-Na+ Krebs-Henseleit solution contained 10 mM NaCl and 134.2 mM N-methyl-D-glucamine chloride (NMG-Cl). The inhibitors/antagonists employed were: nifedipine, an L-type Ca2+ channel inhibitor, at 1 μM; SK&F96365, a non-selective Ca2+ channel inhibitor, at 50 μM; GF109203X, a protein kinase C inhibitor, at 1 μM; genistein, a tyrosine kinase inhibitor, at 15 μM; wortmannin, a phosphatidyl inositol 3′-kinase (PI3K) inhibitor, at (0.1 μM); tetrodotoxin at 10 μM; indomethacin, a cyclo-oxygenase inhibitor, at 3 μM; apamin, a Ca2+-activated, small conductance K+ channel inhibitor, at 0.1 μM; charybdotoxin, a Ca2+-activated, large and intermediate conductance K+ channel inhibitor, at 0.1 μM; L-NG-nitroarginine methyl ester (L-NAME), a NO synthase inhibitor, at 100 μM; propranolol, a β-blocker, at 5 μM; adenosine 3′,5′-cyclic monophosphorothioate, 8-bromo-, Rp-isomer, sodium salt (Rp-8-Br-cAMPS), a protein kinase A inhibitor, at 30 μM.
Peptides and other chemicals employed
PARs-related peptides were prepared by a standard solid phase synthesis procedures by ourselves. The concentration, purity and composition of the peptides were determined by high-performance liquid chromatography, mass spectrometry and quantitative amino acid analysis. Human thrombin, porcine trypsin, nifedipine, L-NAME hydrochloride, apamin, tetrodotoxin and NMG were purchased from Sigma (U.S.A), GF109203X and genistein were obtained from Research Biochemicals International (U.S.A.), and SK&F96365 and Rp-8-Br-cAMPS were from Calbiochem (U.S.A.). Propranolol was provided from Tokyo Kasei (Japan), amastatin and charybdotoxin were purchased from Peptide Institute Inc. (Japan), and indomethacin and wortmannin were from Wako Pure Chemicals (Japan). Nifedipine was dissolved in ethanol and indomethacin in 5 mM Na2CO3 immediately before use. GF109203X, genistein and wortmannin were dissolved in DMSO and then diluted with distilled water. All other chemicals were dissolved in distilled water. In the control experiments an appropriate volume of each vehicle was added to the organ bath.
Statistics
Data obtained are expressed as mean±s.e.mean. Statistical significance in the inhibition experiments for the agonist-induced contraction was analysed by Student's paired t-test. The results in other experiments were evaluated statistically by Tukey's test. Significance was set at a P<0.05 level.
Results
Contractile effects of thrombin, trypsin and PARs-related peptides on the rat oesophageal muscularis mucosae
Thrombin in a dose range of 0.01–0.1 μM caused concentration-dependent contractile responses in the upper part as well as the lower part of the oesophageal muscularis mucosae segment isolated from the rat (Figures 1a and 2). SFLLR-NH2, a human PAR-1-derived peptide, at 0.3–30 μM also evoked similar contraction in both the upper and lower parts of the smooth muscle, while FSLLR-NH2, known to be a PAR-1-inactive control peptide, failed to mimic the action of SFLLR-NH2 (Figures 1b and 2). TFLLR-NH2, a more selective PAR-1 agonist, at the same dose range induced contractile responses in the preparations (Figures 1c and 2). The maximal responses elicited by the agonist peptides were much greater than those produced by thrombin (Figures 1 and 2; Table 1) which is in agreement with previous results using other smooth muscles (Kawabata et al., 1999b; Laniyonu & Hollenberg, 1995). On the other hand, GYPGKF-NH2, a PAR-4-activating peptide, at 300–1000 μM produced only a small contraction in both the lower and upper segments. SLIGRL-NH2, a PAR-2-activating peptide, was inactive in these smooth muscle preparations. Trypsin, considered primarily a PAR-2-activating enzyme but capable of activating PAR-4, and even PAR-1 at high concentrations (Kahn et al., 1998; Kawabata et al., 1999c; Xu et al., 1998), elicited contraction in both preparations at very high concentrations (Figure 2; Table 1). When the contractile responses to the agonists relative to KCl-induced contraction were compared between the upper and lower segments, the activity of the PAR-1-activating peptide TFLLR-NH2 in the upper part was significantly less than that in the lower part, and similar differences between the two parts were also seen in the activity of thrombin and another PAR-1 agonist SFLLR-NH2, but not trypsin. In contrast, carbachol at 0.3 μM produced almost the same magnitude of contraction in both the segments (Figure 2; Table 1).
Figure 1.

Representative recordings of contractile responses to thrombin and PAR-1-related peptides in the lower part segment of the rat oesophageal muscularis mucosae. Thrombin (a), and the PAR-1-activating peptide SFLLR-NH2 (b) or TFLLR-NH2 (c) were cumulatively applied to the preparations. SFLLR-NH2 was added without washing after no activity of the control peptide FSLLR-NH2 was confirmed (b). The contractile responses to SFLLR-NH2 was not altered by the preceding addition of FSLLR-NH2 (data not shown).
Figure 2.

Concentration-effect curves for the contraction exerted by thrombin, trypsin and PARs-related peptides in the upper and lower part segments of the rat oesophageal muscularis mucosae. Thrombin, trypsin, the PAR-1-activating peptide SFLLR-NH2 or TFLLR-NH2, the PAR-2-activating peptide SLIGRL-NH2 and the PAR-4-activating peptide GYPGKF-NH2 were cumulatively added to the organ bath, and the PAR-1-inactive analogue FSLLR-NH2, the PAR-4-inactive analogue GAPGKF-NH2 and carbachol were applied in a single concentration. Data represent the mean±s.e.mean from 4–6 distinct experiments.
Table 1.
Summary of the responses of the rat oesophageal muscularis mucosae to agonist enzymes and related peptides for PARs

Relaxant effects of thrombin, trypsin and PARs-related peptides on the rat oesophageal muscularis mucosae precontracted by carbachol
In the rat oesophageal muscularis mucosae precontracted with carbachol (0.3 μM) thrombin at a relatively low concentration (0.03 μM) further contracted the preparation, but produced no or only slight relaxation. However, thrombin at increasing concentrations (0.1–0.3 μM) elicited a dual response, relaxation followed by contraction, in both the upper and lower segments (Figures 3a and 4). The PAR-1-specific agonist TFLLR-NH2 at 30 μM produced additional contraction of the precontracted preparation, but had no relaxant activity (Figures 3b and 4). The PAR-4 agonist GYPGKF-NH2 at 300, 500 and 1000 μM, but not the control peptide GAPGKF-NH2 at 1000 μM, evoked remarkable relaxation, the maximal response being much greater than that due to thrombin (Figures 3c,d, and 4; Table 1). The PAR-2-activating peptide SLIGRL-NH2 had no relaxant activity, and, surprisingly, trypsin which is known to be capable of activating PAR-4 (Kahn et al., 1998; Xu et al., 1998) produced no relaxation even at high concentrations (Figure 4; Table 1). The relaxant activity of thrombin in the upper segment was significantly greater than that in the lower segment, and a similar tendency was observed for the activity of GYPGKF-NH2 (Figure 4; Table 1), which is opposite to the profile for the contractile activity of thrombin and PAR-1-activating peptides. (Figure 2; Table 1).
Figure 3.
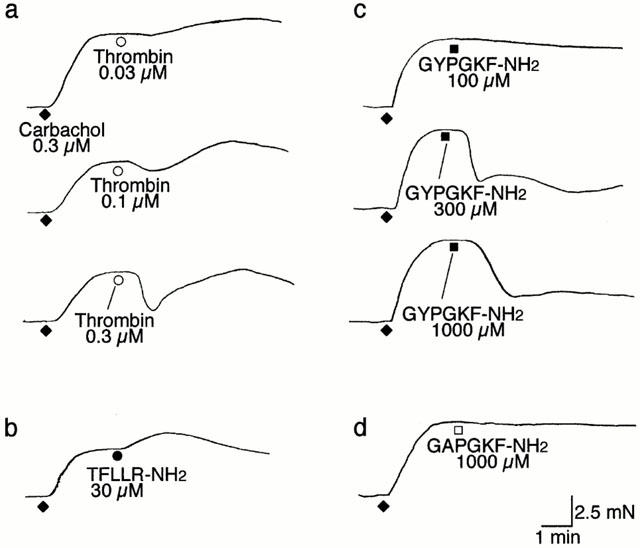
Representative recordings of responses to thrombin and receptor-activating peptides for PAR-1 and PAR-4 in the upper part segment of the rat oesophageal muscularis mucosae precontracted by carbachol. Thrombin (a), the PAR-1-activating peptide TFLLR-NH2 (b), the PAR-4-activating peptide GYPGKF-NH2 (c) and the PAR-4-inactive analogue GAPGKF-NH2 (d) were applied in a single concentration to the preparations precontracted by carbachol at 0.3 μM. Data represent the mean±s.e.mean from four distinct experiments.
Figure 4.

Concentration-effect curves for the relaxation exerted by thrombin, trypsin and PARs-related peptides in the upper and lower part segments of the rat oesophageal muscularis mucosae precontracted carbachol. Thrombin, trypsin, the PAR-1-activating peptide TFLLR-NH2, the PAR-2-activating peptide SLIGRL-NH2, the PAR-4-activating peptide GYPGKF-NH2 and the PAR-4-inactive analogue GAPGKF-NH2 were non-cumulatively applied to the preparations precontracted by carbachol at 0.3 μM. Data represent the mean±s.e.mean from 4–6 distinct experiments.
Characterization of the PAR-1 agonist-induced contractile response in the rat oesophageal muscularis mucosae
The contraction produced by the PAR-1 agonist TFLLR-NH2 at 10 μM was almost completely abolished by replacement of the organ solution with Ca2+-free buffer in both the upper and lower parts of the smooth muscle. The L-type Ca2+ channel inhibitor nifedipine at 1 μM greatly reduced the PAR-1-mediated contraction to approximately 20–30% of the control response in each preparation. The non-selective Ca2+ channel inhibitor SK&F96365 at 50 μM failed to abolish the residual contraction due to the PAR-1 agonist in the presence of nifedipine; the inhibitory rates by nifedipine alone and by SK&F96365 in combination with nifedipine were not significantly (P>0.05) different in either part segment. Lowering the extracellular Na+ concentration also attenuated the contractile response to PAR-1 activation, to an extent similar to that caused by nifedipine (Figure 5). Moreover, the PAR-1-agonist TFLLR-NH2-induced contraction was not significantly attenuated by tetrodotoxin at 10 μM, indomethacin at 3 μM, the protein kinase C inhibitor GF109203X at 1 μM, the tyrosine kinase inhibitor genistein at 15 μM or the PI3K inhibitor wortmannin at 0.1 μM; the contractile response (per cent control) in the presence of inhibitors in the lower part segment was 102.8±9.1, 97.2±14.9, 79.3±9.3, 82.8±8.0 and 106.0±1.8 (n=4), respectively.
Figure 5.

Effect of removal of extracellular Ca2+, Ca2+ channel inhibitors and lowering of extracellular Na+ on the PAR-1-mediated contraction in the rat oesophageal muscularis mucosae. After monitoring a control response to TFLLR-NH2 at 10 μM, the preparations were washed, and after 3 h, rechallenged with the same agonist 15 min following replacement of the medium with a Ca2+-free buffer or a low-Na+ buffer, or addition of nifedipine at 1 μM alone or in combination with SK&F96365 at 50 μM. Data represent the mean±s.e.mean as per cent control from six distinct experiments. **P<0.01 vs the control.
Characterization of the PAR-4 agonist-induced relaxation response in the rat oesophageal muscularis mucosae precontracted by carbachol
Involvement of Ca2+ in the production of the relaxation responses was not examined in the oesophageal smooth muscle preparations in the present study, because carbachol itself failed to evoke a tonic contraction adequate to evaluate the effect of relaxants in the Ca2+-free medium or in the presence of the L-type Ca2+ channel inhibitor nifedipine (data not shown). The relaxation by the PAR-4 agonist GYPGKF-NH2 at 300 μM in both the upper and lower segments precontracted by carbachol was resistant to tetrodotoxin at 10 μM, and was unaffected by apamin, an inhibitor of Ca2+-activated K+ channels, at 0.1 μM that has been shown to abolish the relaxation due to activation of PAR-1 or PAR-2 in the gastric and duodenal smooth muscle (Cocks et al., 1999b; Kawabata et al., 1999b). Neither charybdotoxin, an inhibitor of apamin-resistant, Ca2+-activated K+ channels, at 0.1 μM nor replacement of the bath medium with 30 mM K+-containing buffer abolished PAR-4-mediated relaxation responses (Figure 6). Also, the PAR-4 agonist GYPGKF-NH2-evoked relaxation was not significantly reduced by indomethacin at 3 μM, propranolol at 5 μM, the NO synthase inhibitor L-NAME at 100 μM or the protein kinase A inhibitor Rp-8-Br-cAMPS at 30 μM; the relaxant activity (per cent papaverine) of GYPGKF-NH2 in the upper part segment was 50.1±3.6 (n=6) in the absence (control) of inhibitors and 39.5±3.0 (n=6), 68.0±3.7 (n=4), 50.5±11.9 (n=6) and 55.5±12.6 (n=4) in the presence of inhibitors, respectively.
Figure 6.

Effect of tetrodotoxin, apamin, charybdotoxin and 30 mM K+ solution (K30) on the PAR-4-mediated relaxation in the rat oesophageal muscularis mucosae precontracted by carbachol. Each preparation was challenged only once with GYPGKF-NH2 at 300 μM alone as the control, or in the presence of tetrodotoxin at 10 μM, apamin at 0.1 μM or charybdotoxin at 0.1 μM, or in a 30 mM KCl-containing buffer. Addition of inhibitors or replacement of the medium were performed 10 min before addition of carbachol at 0.3 μM. Data represent the mean±s.e.mean from 10 control and 4–5 other experiments.
Discussion
Our data demonstrate that thrombin produces dual responses in the rat oesophageal muscularis mucosae, relaxation via PAR-4 followed by contraction via PAR-1, while PAR-2 does not participate in modulation of the motility of this smooth muscle. Further, our results indicate that the PAR-1-mediated contraction is largely dependent on an increase in Na+ permeability followed by influx of extracellular Ca2+ through L-type Ca2+ channels, and that the PAR-4-mediated relaxation is independent of activation of K+ channels, prostanoids and NO all of which are known to mediate relaxation of other smooth muscle tissues via PARs, and also resistant to tetrodotoxin, blockade of β-adrenoceptor and inhibition of protein kinase A. Although the mechanism responsible for the PAR-4-mediated relaxation has yet to be elucidated, the present study implies that the dual receptor system (PARs 1 and 4) for thrombin in the oesophageal smooth muscle does not simply provide redundancy as speculated from findings in platelets and in gastric, vascular or airway smooth muscle (Coughlin, 1999; Hollenberg et al., 1999; Kahn et al., 1998; 1999; Lan et al., 2000), leading to a hypothesis that PAR-4 might act to ease the excessive excitation of this smooth muscle through PAR-1 caused by thrombin possibly during inflammation and/or regional haemorrhage. Our work thus adds the dual modulation by PARs 1 and 4 of the motility of oesophageal muscularis mucosae to the list of possible roles for PARs in the digestive systems that currently consist of the salivary gland, stomach, intestine, pancreas, etc (Kawabata et al., 1999b; 2000; Cocks et al., 1999b; Corvera et al., 1997; Hollenberg et al., 1993; 1999; Nguyen et al., 1999; Saifeddine et al., 1996).
TFLLR-NH2 is considered to be an agonist which is highly specific for PAR-1, because this peptide has negligible agonist activity toward PAR-2 in receptor desensitization assays in human embryonic kidney (HEK) cells (Hollenberg et al., 1997; Kawabata et al., 1999c) and is incapable of producing aggregation of rodent platelets that express functional PARs 3 and 4 (unpublished data). The specificity of GYPGKF-NH2 as a PAR-4 agonist is supported by previous evidence that this peptide induces no Ca2+ signal in HEK cells that express functional PARs 1 and 2 (Hollenberg et al., 1999), and is incapable of producing aggregation of guinea-pig platelets that express functional PAR-1 (Nishikawa et al., 2000). It is also unlikely that GYPGKF-NH2 activates PAR-3, considering the extreme difference in its amino acid sequence from that of the tethered ligand of PAR-3 and the refractory property of PAR-3 to exogenously applied synthetic peptides (Ishihara et al., 1997). Taken together with the lack of activity of the control peptide analogues, FSLLR-NH2 and GAPGKF-NH2, the responses to TFLLR-NH2 and GYPGKF-NH2 in the present study are considered to result from activation of PAR-1 and PAR-4, respectively. That the effective concentration range of GYPGKF-NH2 for the induction of relaxation was much greater than that of TFLLR-NH2 for the production of contraction, is consistent with the potency of these peptides or their analogues in other assay systems (Kahn et al., 1998; 1999; Hollenberg et al., 1999; Xu et al., 1998). The finding that the induction of relaxation by thrombin occurred at relatively higher concentrations than those required for the production of contraction, is also in agreement with the previously described properties of PARs in human platelets that PAR-4 is less sensitive to thrombin than PAR-1 (Kahn et al., 1998; 1999). The reason why the maximum contractile responses to thrombin was much lower than those to PAR1-activating peptides is not known, although similar results have been reported in other smooth muscle preparations (Kawabata et al., 1999b; Laniyonu & Hollenberg, 1995). One possible explanation may be rapid metabolic degradation or endogenous inhibitors-mediated inactivation of thrombin. Alternatively, it may be hypothesized that the potential relaxant activity of thrombin via PAR-4 might reduce the PAR-1-mediated contraction, resulting in the lower maximum contractile responses. This possibility may be examined by measuring the response to thrombin after desensitization to PAR-4-activating peptides. However, this experiment could not be performed, because preliminary experiments showed that the PAR-4 agonist GYPGKF-NH2 (1 mM) did not remarkably reduce the relaxant response to the second application of the same agonist. Absence of desensitization might be due to the partial agonist property of GYPGKF as shown in a human platelet aggregation assay (Faruqi et al., 2000). Future experiments using a newly synthesized full agonist peptide (Faruqi et al., 2000) may be useful to explain the present results. Considering the previous evidence that PAR-4 can be activated by trypsin at concentrations equivalent to those of thrombin (Kahn et al., 1998; Xu et al., 1998), one could expect that trypsin should have had relaxant activity in the present study. One possible explanation is that an atypical PAR-4 that is resistant to trypsin might be present in the oesophageal muscularis mucosae. Alternatively, potential contractile activity of trypsin at low concentrations through an unknown mechanism, other than PAR-1, or via PAR-1 at high concentrations (Kawabata et al., 1999c) could mask its relaxant activity through PAR-4. The development of specific antagonists for each PAR may be necessary to explain this discrepancy. It still remains open to question whether functional PAR-3 is expressed and participates in the motility modulation by thrombin in the rat oesophageal muscularis mucosae, because agonists or antagonists specific for PAR-3 are not available at present.
The findings that the PAR-1-mediated contraction of the oesophageal smooth muscle was largely dependent on extracellular Ca2+ and activation of L-type Ca2+ channels, are consistent with the characteristics of the contraction via PAR-1 in the gastric or duodenal smooth muscle (Kawabata et al., 1999b; Zheng et al., 1998). Further, that the contraction via PAR-1 was reduced by lowering extracellular Na+ to an extent similar to inhibition by nifedipine, implies that L-type Ca2+ channels are activated by membrane depolarization due to enhanced Na+ permeability in response to PAR-1 activation. In addition, cyclo-oxygenase, protein kinase C, tyrosine kinase and PI3K that may be activated by PAR-1 agonists in other tissues (Kawabata et al., 1999b; Zheng et al., 1998), do not appear to participate in the PAR-1-mediated contraction of the rat oesophageal smooth muscle, since their inhibitors produced no effect in our study. Involvement of neuronal mechanisms in the evoked contraction is also unlikely because of its resistance to tetrodotoxin, although PAR-1 as well as PAR-2 are also expressed in peripheral nerves (Corvera et al., 1999).
Smooth muscle relaxation through activation of PARs is mediated by endothelial nitric oxide in blood vessels (Hollenberg et al., 1993; 1996; 1999; Saifeddine et al., 1996), by epithelial prostanoids in airways (Cocks et al., 1999a; Lan et al., 2000) and by activation of apamin-sensitive K+ channels in gastrointestinal tissues (Cocks et al., 1999b; Kawabata et al., 1999b). However, the PAR-4-mediated relaxation of the rat oesophageal muscularis mucosae was independent of these mechanisms in the present study. In addition, an involvement of β-adrenoceptors and protein kinase A was also ruled out by our experiments. Although the lack of effect of tetrodotoxin does not support such a speculation, prejunctional PAR-4, if any, might directly trigger the release of unknown relaxing substances from the peripheral nerve terminal. A study is now in progress in this laboratory to clarify the mechanisms by which thrombin relaxes oesophageal smooth muscle through PAR-4.
Of interest is the difference between the upper and lower part segments of the oesophageal muscularis mucosae in responsiveness to activation of PAR-1 and PAR-4. The larger contraction through PAR-1 in the lower segment than in the upper segment might predict that PAR-1 is more expressed in the lower part of oesophageal muscularis mucosae that is close to the stomach in which PAR-1 stimulation evokes a great contraction. On the other hand, the contractile responses to high concentrations of trypsin did not show this tendency, implying that the trypsin-induced contraction may involve mechanisms other than PAR-1 as predicted above. In contrast, the opposite profile for the PAR-4-mediated relaxation might suggest that PAR-4 is more expressed in the upper part of this smooth muscle than in the lower part. To elucidate the physiological significance of these profiles is an interesting topic for future research.
Thus, the dual receptor system (PAR-1 and PAR-4) for thrombin observed in the present study is very interesting, differing from the situation in platelets and other smooth muscle where PAR-4 just mimicked PAR-1 (Coughlin, 1999; Hollenberg et al., 1999; Kahn et al., 1998; 1999; Lan et al., 2000). We propose that, in response to thrombin under inflammation or haemorrhage, PAR-1, as a sensor, enhances the motility of the oesophageal muscularis mucosae, and, in turn, PAR-4 acts to ease the evoked excessive excitement of the muscle.
Abbreviations
- HEK
human embryonic kidney
- L-NAME
NG-nitro-L-arginine methyl ester
- NMG
N-methyl-D-glucamine
- PAR
protease-activated receptor
- PI3K
phosphatidyl inositol 3′-kinase
- Rp-8-Br-cAMPS
adenosine 3′,5′-cyclic monophosphorothioate, 8-bromo-
- Rp-isomer
sodium salt
References
- BOHM S.K., KONG W., BROMME D., SMEEKENS S.P., ANDERSON D.C., CONNOLLY A., KAHN M., NELKEN N.A., COUGHLIN S.R., PAYAN D.G., BUNNETT N.W. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem. J. 1996;314:1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRINO G., CICALA C., BUCCI M.R., SORRENTINO L., MARAGANORE J.M., STONE S.R. Thrombin functions as an inflammatory mediator through activation of its receptor. J. Exp. Med. 1996;183:821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKS T.M., FONG B., CHOW J.W., ANDERSON G.P., FARAUMAN A.G., GOLDIE R.G., HENRY P.J., CARR M.J., HAMILTON J.R., MOFFATT J.D. A protective role for protease-activated receptors in the airways. Nature (London) 1999a;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., SOZZI V., MOFFATT J.D., SELEMIDIS S. Protease-activated receptors mediate apamin-sensitive relaxation of mouse and guinea pig gastrointestinal smooth muscle. Gastroenterology. 1999b;116:586–592. doi: 10.1016/s0016-5085(99)70180-0. [DOI] [PubMed] [Google Scholar]
- CORVERA C.U., DERY O., McCONALOGUE K., BOHM S.K., KHITIN L.M., CAUGHEY G.H., PAYAN D.G., BUNNETT N.W. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J. Clin. Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORVERA C.U., DERY O., MCCONALOGUE K., GAMP P., THOMA M., AL-ANI B., CAUGHEY G.H., HOLLENBERG M.D., BUNNETT N.W. Thrombin and mast cell tryptase regulate guinea-pig myenteric neurons through proteinase-activated receptors-1 and -2. J. Physiol. 1999;517:741–756. doi: 10.1111/j.1469-7793.1999.0741s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUGHLIN S.R. Protease-activated receptors and platelet function. Thromb. Haemost. 1999;82:353–356. [PubMed] [Google Scholar]
- DERY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- FARUQI T.R., WEISS E.J., SHAPIRO M.J., HAUNG W., COUGHLIN S.R. Structure function analysis of protease activated receptor 4 tethered ligand peptides: Determinants of specificity and utility in assays of receptor function. J. Biol. Chem. 2000;275:19728–19734. doi: 10.1074/jbc.M909960199. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D. Protease-activated receptors: PAR4 and counting: how long is the course. Trends Pharmacol. Sci. 1999;20:271–273. doi: 10.1016/s0165-6147(99)01333-4. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D., LANIYONU A.A., SAIFEDDINE M., MOORE G.J. Role of the amino- and carboxyl-terminal domains of thrombin receptor-derived polypeptides in biological activity in vascular endothelium and gastric smooth muscle: evidence for receptor subtypes. Mol. Pharmacol. 1993;43:921–930. [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B. Proteinase-activated receptor-2 in rat aorta: Structural requirements for agonist activity of receptor-activating peptides. Mol. Pharmacol. 1996;49:229–233. [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B., GUI Y. Proteinase-activated receptor 4 (PAR4): action of PAR4-activating peptides in vascular and gastric tissue and lack of cross-reactivity with PAR1 and PAR2. Can. J. Physiol. Pharmacol. 1999;77:458–464. [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B., KAWABATA A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can. J. Physiol. Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A.J., ZENG D., KAHN M.L., ZHENG Y.W., TIMMONS C., TRAM T., COUGHLIN S.R. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature (London) 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- KAHN M.L., NAKANISHI-MATSUI M., SHAPIRO M.J., ISHIHARA H., COUGHLIN S.R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHN M.L., ZHENG Y.-W., HUANG W., BIGORNIA V., ZENG D., MOFF S., FARESE R.V., JR, TAM C., COUGHLIN S.R. A dual thrombin receptor system for platelet activation. Nature (London) 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R. Protease-activated receptor (PAR), a novel family of G protein-coupled seven trans-membrane domain receptors: activation mechanisms and physiological roles. Jpn. J. Pharmacol. 2000;82:74–77. doi: 10.1254/jjp.82.171. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., MINAMI T., KATAOKA K., TANEDA M. Increased vascular permeability by a specific agonist of protease-activated receptor-2 in rat hindpaw. Br. J. Pharmacol. 1998;125:419–422. doi: 10.1038/sj.bjp.0702063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NISHIKAWA H., ASAI T., KATAOKA K., TANEDA M. Enhancement of vascular permeability by specific activation of protease-activated receptor-1 in rat hindpaw: a protective role of endogenous and exogenous nitric oxide. Br. J. Pharmacol. 1999a;126:1856–1862. doi: 10.1038/sj.bjp.0702513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NISHIKAWA H., KAWAI K. Modulation by protease-activated receptors of the rat duodenal motility in vitro: possible mechanisms underlying the evoked contraction and relaxation. Br. J. Pharmacol. 1999b;128:865–872. doi: 10.1038/sj.bjp.0702755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., NISHIKAWA H., KURODA R., KAWAI K., HOLLENBERG M.D. Proteinase-activated receptor-2 (PAR-2): regulation of salivary and pancreatic exocrine secretion in vivo in rats and mice. Br. J. Pharmacol. 2000;129:1808–1814. doi: 10.1038/sj.bjp.0703274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., SAIFEDDINE M., AL-ANI B., LEBLOND L., HOLLENBERG M.D. Evaluation of proteinase-activated receptor-1 (PAR1) agonists and antagonists using a cultured cell receptor desensitization assay: activation of PAR2 by PAR1 targeted ligands. J. Pharmacol. Exp. Ther. 1999c;228:358–370. [PubMed] [Google Scholar]
- LAN R.S., STEWART G.A., HENRY P.J. Modulation of airway smooth muscle tone by protease activated receptor-1, -2, -3 and -4 in trachea isolated from influenza A virus-infected mice. Br. J. Pharmacol. 2000;129:63–70. doi: 10.1038/sj.bjp.0703007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANIYONU A.A., HOLLENBERG M.D. Vascular actions of thrombin receptor-derived polypeptides: structure-activity profiles for contractile and relaxant effects in rat aorta. Br. J. Pharmacol. 1995;114:1680–1686. doi: 10.1111/j.1476-5381.1995.tb14957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINO M., BARNATHAN E.S., NUMEROF R., CLARK J., DREYER M., CUMASHI A., HOXIE J.A., SCHECHTER N., WOOLKALIS M., BRASS L.F. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J. Biol. Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- NGUYEN T.D., MOODY M.W., STEINHOFF M., OKOLO C., KOH D.-S., BUNNETT N.W. Trypsin activates pancreatic duct epithelial cell ion channels. J. Clin. Invest. 1999;103:261–269. doi: 10.1172/JCI2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIKAWA H., KAWABATA A., KAWAI K., KURODA R. Guinea pig platelets do not respond to GYPGKF, a protease-activated receptor-4-activating peptide: a property distinct from human platelets. Blood Coag. Fibrinol. 2000;11:111–113. [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFEDDINE M., AL-ANI B., CHENG C.-H., WANG L., HOLLENBERG M.D. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br. J. Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., HOLLENBERG M.D., WALLACE J.L. Pro- and anti-inflammatory actions of thrombin: a distinct role for proteinase-activated receptor-1 (PAR1) Br. J. Pharmacol. 1999;126:1262–1268. doi: 10.1038/sj.bjp.0702408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., MACNAUGHTON W.K., AL-ANI B., SAIFEDDINE M., WALLACE J.L., HOLLENBERG M.D. Proteinase-activated receptor 2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7766–7771. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VU T.-K.H., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanisms of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- XU W.-F., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., GOSTER D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG X.-L., RENAUX B., HOLLENBERG M.D. Parallel contractile signal transduction pathways activated by receptors for thrombin and epidermal growth factor-urogastrone in guinea pig gastric smooth muscle: blockade by inhibitors of mitogen-activated protein kinase and phosphatidyl inositol-3′-kinase. J. Pharmacol. Exp. Ther. 1998;285:325–334. [PubMed] [Google Scholar]


