Abstract
The steady state levels of the messenger RNA (mRNA) of eight GABAA receptor subunits, five glutamate receptor subunits and seven enzymes involved in the synthesis of glutamate and GABA were measured in eight regions of rat brain in a recently developed animal model of ‘behavioural dependence' on ethanol.
‘Behavioural dependence' including loss of control was induced by offering the rats the choice between ethanol and water over a 9-month period (Group A). This group was compared with a group given the choice between ethanol and water for only 2 months (not yet ‘behaviourally dependent', Group B), a group forced to consume ethanol as sole fluid over a 9-month period (also not ‘behaviourally dependent', Group C) and ethanol-naive control rats (Group D). All groups were sacrificed 1 month after the ethanol was withdrawn.
The mRNA concentrations of all eight GABA receptor subunits, four out of the five subunits of different glutamate receptors and those of seven enzymes involved in GABA and glutamate production were reduced almost exclusively in the parieto-occipital cortex in Groups A and B, but not Group C.
These data suggest that the synthesis of glutamate and GABA and the activities of their respective neurons are selectively impaired in the parieto-occipital cortex in the groups having consumed ethanol in a free-choice design, in which its rewarding properties can better take effect than after forced administration.
As the parieto-occipital cortex is believed to contain emotional memory structures, it may be hypothesized that the glutamatergic and GABAergic neuronal systems in this area are involved in the development of memory for reward from ethanol. However, they are not specifically associated with ‘behavioural dependence'.
Keywords: Ethanol, rats, ‘behavioural dependence', GABA receptors, glutamate receptors, parieto-occipital cortex, brain
Introduction
Current strategies in the research of addiction focus mainly on work with cell cultures, forced drug administration and techniques of self-administration of the drug by the animals. In these designs ethanol and other drugs of abuse have been found to affect a wide variety of biochemical parameters and to induce processes of sensitization in experimental animals and cell cultures and long-term adaptational changes. However, these strategies do not yet enable us to answer the important question as to which of the numerous effects of the addictive drugs are directly related to the development of addiction. In other words, the rewarding properties and related biochemical effects of an addictive drug may explain why experimental animals and humans voluntarily consume the drug, but do not reveal why only a small percentage of subjects become irreversibly addicted and what underlying biochemical mechanisms are involved in the process of addiction. An animal model of ‘behavioural dependence' on ethanol was therefore developed in order to be able to study the biochemical changes directly related to addiction (Wolffgramm & Heyne, 1995). In this model rats are rendered ‘behaviourally dependent' on ethanol by offering them a choice between ethanol and water over a period of not less than 9 months. During this period rats of the Wistar strain, which voluntarily consume only small amounts of ethanol (less than 1 g kg−1 day−1) increase their drug intake in a manner somewhat similar to the corresponding process in humans. When re-exposed after 3 months of ‘forced abstinence' the rats immediately consume large amounts of ethanol (3–5 g kg−1 day−1). They do not markedly reduce their ethanol consumption even if the ethanol is adulterated with a highly aversive substance such as quinine hydrochloride. Since the preference for ethanol is irreversible after up to 9 months of abstinence and loss of control occurs (no effect of an aversive stimulus), it can be concluded that these rats show symptoms of behavioural dependence on ethanol.
The dopaminergic, GABAergic and glutamatergic transmitter systems are currently the focus of interest in research on addiction (Tabakoff & Hoffman, 1996; Wise 1996; Diamond & Gordon, 1997, Grobin et al., 1998; Harris, 1999).
Using the above animal model we recently measured the mRNA concentrations of the five cloned dopamine receptors in different rat brain areas. We found a decrease in mRNA concentrations of dopamine three receptors exclusively in the limbic forebrain in the rats which had been offered a free choice between ethanol and water, but not in those forced to drink ethanol over a 9-month period (Eravci et al., 1997).
In the last 15 years a large number of studies have produced most interesting results on the effects of ethanol on the characteristics of GABAergic and glutamatergic receptors (for reviews see Tabakoff & Hoffmann, 1996; Wise, 1996; Diamond & Gordon, 1997; Grobin et al., 1998; Harris, 1999, Mehta and Ticku 1999).
However, no studies on the relevance of the GABAergic and glutamatergic systems for the development of ‘behavioural dependence' on ethanol have as yet been published. Thus in order to investigate a possible role of these transmitter systems in general and of specific receptor subunits in particular, we measured the mRNA concentrations of eight GABA-A receptor subunits, six subunits of different glutamate receptors and seven enzymes involved in the production and recycling of glutamate and GABA. As the results of all these experiments clearly indicated an impairment of the production of both glutamate and GABA in the parieto-occipital cortex, we also measured the mRNA concentrations of several parameters involved in glycolysis, since glucose is the precursor of both neurotransmitters. The results of this study have already been reported (Eravci et al., 1999). Finally, as an impairment of glutamatergic and GABAergic neurotransmission may be associated with disturbed energy metabolism we determined the mRNA levels of parameters involved in mitochondrial energy production.
Methods
Materials
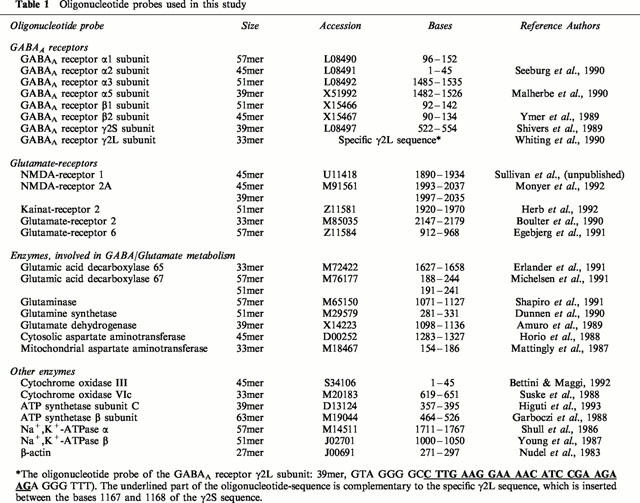
All chemicals and buffer reagents employed were of the highest purity or molecular biology grade and were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). T4 polynucleotide kinase and S1 nuclease were purchased from Gibco BRL (U.K.). γ32-ATP with a specific activity of >7000 Ci mmol−1 was purchased from ICN Biochemicals, Inc. (Costa Mesa, CA, U.S.A.). HPLC-purified oligonucelotide probes were obtained from TIB Molbiol (Berlin, Germany). The oligonucelotide sequences were chosen to be complementary to the part of the coding region with the least sequence homology with other sequences in the Genebank database. Details of the sequences of all oligonucelotide probes determined in this study are given in Table 1.
Table 1.
Oligonucleotide probes used in this study
Autoradiography was carried out with Hyperfilm MP plus Hyperscreen from Amersham (U.K.), and autoradiography cassettes from the Sigma Chemical Co. (St. Louis, MO, U.S.A.).
Methods
RNA isolation
Total RNA was isolated by the guanidium thiocyanate single step method described by Chomczynski & Sacchi (1987).
Multiple oligonucleotide solution hybridization
Multiple oligonucleotide hybridization (MOSH) was performed as described by O'Donovan et al. (1991). Briefly, oligonucleotide probes (TIB Molbiol, Berlin, Germany) were end-labelled with polynucelotide kinase by standard procedures, using γ32P-ATP. Probes and RNA were suspended in 30 μl hybridization buffer [probe concentration 1 pmol ml−1, 0.4 M NaCl, 40 mM pipes pH 6.4, 1 mM EDTA], heated at 90°C for 2 min and incubated at 65°C for 2 h. Excess probe was then removed by adding 300 μl S1 nuclease buffer (S1 nuclease 120 U ml−1, zinc sulfate 4.5 mM, sodium acetate 50 mM, pH 4.2, sodium chloride 0.3 M, 10 μg ml−1 single stranded DNA). Digestion was stopped after 15 min at 37°C. Three hundred μl of the reaction mixture were then transferred to a fresh tube and the reaction terminated by the addition of 48 μl 4 M ammonium acetate and 0.1 M EDTA. Double stranded hybrids were precipitated with ethanol, re-suspended in 10 μl formamide running buffer, denatured at 90°C for 2 min and subjected to acrylamide gel (10%) electrophoresis. The dried gels were analysed by autoradiography (exposure for 2 weeks) followed by scanning densitometry. The specific activity of each probe was adjusted empirically to give similar band intensities.
Quantification of mRNA
Each rat brain RNA sample was analysed individually. All bands measured on the autoradiographs were in the linear range of the film employed (Hyperfilm MP; Amersham, U.K.). The ratio of band intensities of each probe relative to the β-actin probe were calculated for each sample. β-actin is a structural gene commonly used as an internal standard for gene expression studies. Its expression is not affected by prolonged ethanol treatment (data not shown).
Animal treatments
Thirty-two adult male Wistar rats weighing roughly 100 g on arrival at our laboratory were employed throughout. Feed was available ad libitum and the rats were kept under a 12 h light/dark cycle (0600 to 1300 h). After allowing an adjustment period of 1 month the following experiments were performed in four groups each consisting of eight rats.
Group A–Induction of ‘behavioural dependence'
These rats were housed individually throughout the 15-month study period. They had free access to ethanol for a 9-month period during which they were given the choice between water and two different concentrations of ethanol (5% and 20% (v v−1)) in a three-bottle system. Two different concentrations of ethanol were offered as individual differences in the rats' preferences for the low and the high ethanol concentrations, respectively, have been noted. Furthermore, previous experiments had shown that rats which initially preferred the 5% ethanol solution sometimes later developed a preference for the 20% ethanol solution (Wolffgramm & Heyne, 1995). The positions of the bottles were changed weekly. Over the next 3 months (months 10–12) tap water only was provided. In months 13 and 14 the rats were again given a free choice between ethanol and water. In month 14, however, all the ethanol solutions were adulterated with 0.1 g l−1 quinine hydrochloride. Nothing was added to the tap water. During month 15 water only was provided.
Group B–‘Controlled consumers'
These rats were offered tap water only for the first 12 months. During this time they were housed four to a cage. It did not seem necessary to house them individually during this period as individual recording of each rat's ethanol consumption was not required. In months 13 and 14 the rats were housed individually and given a choice between water and two different concentrations of ethanol, as described for Group A. As for Group A the ethanol solutions were adulterated with quinine hydrochloride in month 14. In month 15 these rats all received tap water only. As these rats were given the choice between ethanol and water (without added quinine) for 1 month only, development of behavioural dependence seems unlikely. We therefore included this group in order to evaluate whether the consumption of ethanol in a free-choice situation per se would induce biochemical changes in any areas of the brain, even in animals not yet ‘behaviourally dependent'.
Group C–‘Forced consumers'
The rats in this group were housed four to a cage and received a 5% ethanol solution as sole fluid for the first 9 months. This was followed by a 3-month ‘abstinence period' with water as sole fluid. Re-exposition, quinine adulteration and abstinence period (months 13 –15) were the same as described for Groups A and B. In previous experiments rats forced to consume ethanol had not developed ‘behavioural dependence' (Wolffgramm & Heyne, 1995). We therefore included this group in order to differentiate between biochemical effects of ethanol consumed in a free choice design and those occurring after forced administration.
Group D–Drug-naive controls
These rats received tap water as sole fluid during the whole 15-month period.
All animal experiments were approved by the Berlin regional government (Senatsverwaltung für Gesundheit und Soziales, File No. G 0176/96). All rats were decapitated without anaesthesia at the end of month 15. Various areas of the brain were dissected according to Glowinski & Iversen (1966) and stored immediately at −70°C.
Statistical analysis
The data are given as means±s.e.mean. P-values of less than 0.05 were considered significant. Comparisons of the results for specific parameters and brain regions in the different groups of rats were performed by analysis of variance. Where significant main effects were found, individual rankings were calculated with the aid of the Newman-Keuls test.
Results
Ethanol consumption and induction of ‘behavioural dependence'
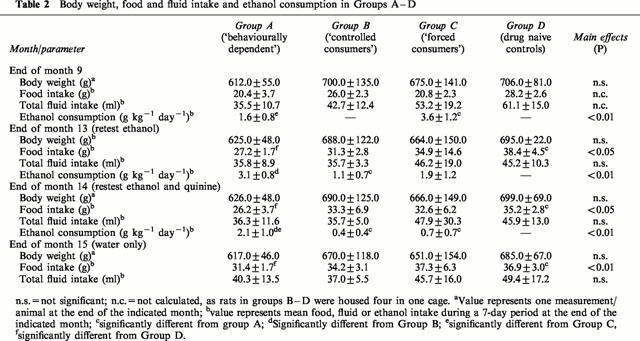
The data on ethanol consumption in Groups A, B and C are shown in Table 2. It can be seen from the table that the consumption of ethanol was significantly higher in Group C (forced consumers) than in Group A (‘behaviourally dependent' rats) during the first 9 months. Group B had a significantly lower ethanol consumption than Group A during the first month of re-exposition. The mean ethanol intake of Group A during the first week of re-exposition was significantly higher than that measured in the last week before ethanol withdrawal (P<0.001). In contrast to Group A the rats in Groups B and C drastically reduced their ethanol intake during the month of adulteration with quinine, thus the consumption of ethanol in Group A was significantly higher than in Groups B and C. During the period of alcohol intake, feed consumption was reduced by an amount equivalent to the caloric value of the ethanol, as already described previously (Wolffgramm & Heyne, 1995). However, there were no differences between either total fluid intake or body weight in Groups A, B and C at any of the four measuring times shown in Table 2. This lack of significant differences may in some cases be due to the large standard deviations found for individual groups (e.g. Group C at the end of month 14), for which we have no explanation.
Table 2.
Body weight, food and fluid intake and ethanol consumption in Groups A–D
According to our criteria a rat of the Wistar strain used in this study was considered ‘behaviourally dependent' when its ethanol consumption exceeded 2 g ethanol kg−1 day−1 during month 14 (ethanol re-exposure and quinine adulteration). On the basis of this criterion seven out of the eight rats in Group A were ‘behaviourally dependent'. One rat consumed on the average only 0.09 g ethanol kg−1 day−1 during month 14 and was therefore excluded from all biochemical investigations. In contrast, one of the eight rats in Group B consumed more than 1 g ethanol kg−1 day−1 during month 14 (mean 1.2 g ethanol kg−1 day−1). This rat was also excluded from further tests. In Group C two rats also consumed more than 1 g ethanol kg−1 day−1 during month 14 and were therefore considered ‘behaviourally dependent' and excluded from the biochemical investigations. Thus all statistical calculations on differences between the mRNA concentrations in the four different groups of rats were based on seven rats from Group A, seven rats from Group B, six rats from Group C and eight rats from Group D.
GABA receptor subunits
The mRNA concentrations of eight subunits of the GABA-A receptors were measured in between six and eight brain regions, depending on the areas in which they are expressed. The most pronounced effects of ethanol treatment were seen in the parieto-occipital cortex. In this area the mRNA levels of all eight GABA-A subunits were significantly reduced in the ‘behaviourally dependent' group (Group A, Figure 1). However, the changes in this group were specific only for the α1 and β1 subunits. The mRNA levels of the other six subunits were also significantly reduced in the ‘controlled consumers' (Group B), although the effects were was less marked than in Group A. Forced consumption of ethanol (Group C) induced a fall in the mRNA levels in the α3 subunit only (Figure 1). No effects of ethanol on mRNA levels were found for any subunit in the frontal cortex, hippocampus, amygdala, hypothalamus or cerebellum (data not shown). In the limbic forebrain, which, in our preparation technique (Glowinski & Iversen, 1966) includes the nucleus accumbens, the levels for the γ21 subunit were significantly reduced, but only in Group B. In the midbrain the mRNA levels in the α1 and γ21 subunits were significantly reduced in Groups A and C. The mRNA levels of all other subunits were unchanged (data not shown).
Figure 1.
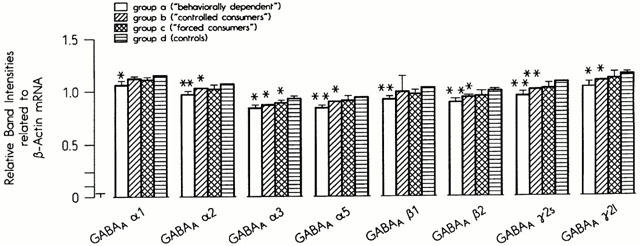
Steady-state mRNA concentrations of GABA2 receptor subunits in the parieto-occipital cortex in the four experimental groups.
Glutamate receptor subunits
The mRNA levels of the NMDA-R1 and NMDA-R2A receptors and GluR2, GluR6 and KA2 receptor were determined in six to eight brain regions. As the amount of available tissue was limited, particularly in small brain regions, we were unable to measure the mRNA concentrations of more glutamate receptor subunits.
As with the GABA-A receptor subunits, the most pronounced effects of ethanol on the mRNA levels of glutamate receptor subunits were seen in the parieto-occipital cortex (Figure 2). The mRNA concentrations in the NMDA-R1 subunit were significantly reduced in Group B, whereas the decreases seen in Group A failed to reach statistical significance (P=0.1). The mRNA levels of the NMDA-R2A and KA2 subunits were significantly lower than in the controls in Groups A and B, while the levels of GluR2 mRNA were reduced in Group B only (Figure 2). No significant changes in the mRNA concentrations of any of the five subunits were observed in the frontal cortex, hippocampus, hypothalamus or cerebellum (data not shown). The selective effect on the parieto-occipital cortex is demonstrated by the results for the KA2 receptor subunit in Figure 3. Only the mRNA levels of the NMDA-R1 subunits were affected by ethanol treatment in brain areas other than the parieto-occipital cortex. They were significantly reduced in the amygdala and midbrain in Groups A and B and enhanced in the limbic forebrain in all groups (Groups A, B and C, data not shown).
Figure 2.
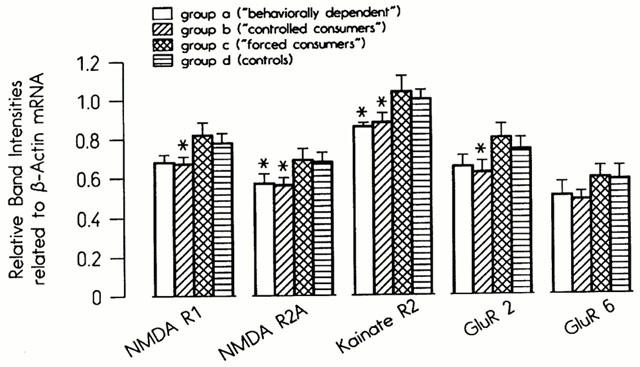
Steady-state mRNA concentrations of glutamate receptor subunits in the parieto-occipital cortex in the four experimental groups.
Figure 3.
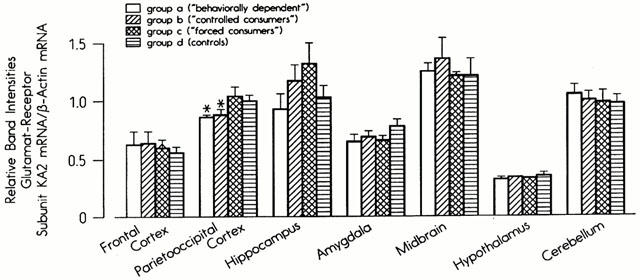
Steady-state mRNA concentrations of the kainate 2 receptor in seven brain regions in four experimental groups.
Enzymes involved in the synthesis and neuronal astrocytic shuttle of glutamate and GABA
The steady state levels of the mRNA of the two glutamate decarboxylase isoenzymes (GAD-65 and GAD-67) were investigated in eight brain regions. The mRNA levels of the GAD-65 subunit were significantly reduced in the parieto-occipital cortex in Group A and significantly enhanced in the limbic forebrain in Groups A and B (Figure 4). The mRNA concentrations of GAD-67 were selectively reduced in the parieto-occipital cortex (P=0.04) in Group A, but not affected in the hippocampus, limbic forebrain, midbrain or cerebellum (data not shown). The mRNA concentrations of the enzymes glutaminase, glutamate dehydrogenase, glutamine synthetase and aspartate aminotransferase were measured in the parieto-occipital cortex and limbic forebrain only. While in the latter area no significant effects of ethanol were found (data not shown), the mRNA levels of all five enzymes were significantly reduced in the parieto-occipital cortex in Groups A and B. The mRNA levels of glutaminase were reduced in this brain region in Group A only (Figure 5).
Figure 4.
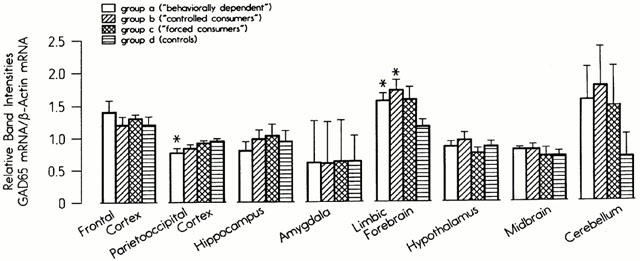
Steady-state mRNA concentrations of glutamate decarboxylase 65 in eight brain regions in four experimental groups.
Figure 5.
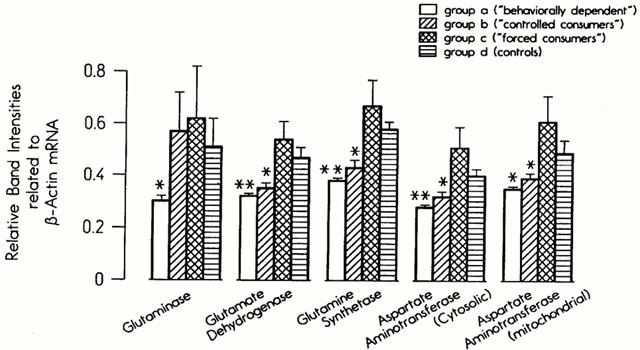
Steady-state mRNA concentrations of enzymes involved in glutamate and GABA production and recycling in the parieto-occipital cortex in four experimental groups.
Mitochondrial energy production
Changes in GABAergic and glutamatergic neurotransmission have been reported to be associated with changes in parameters of mitochondrial energy production such as cytochrome oxidase (Hendry et al., 1990; Wong-Riley, 1989). We therefore determined the mRNA concentrations of two subunits of cytochrome oxidase, the mitochondrially encoded subunit III and the nuclear genome encoded subunit VIc. The mRNA concentrations of two subunits of ATP synthetase, subunit β (of the catalytic sector F1) and subunit C (of the membrane sector F0) were also measured. Finally, Na+/K+ ATPase, like cytochrome oxidase, is a marker of neuronal activity (Hevner et al., 1992). We therefore also determined the mRNA concentrations of the α1 and β1 subunits of this enzyme. All these mRNA levels were determined in six to eight regions of the brain. In the parieto-occipital cortex the mRNA concentration of the β subunit of ATP synthetase was reduced in Groups A and B and the β1 subunit of Na+/K+ ATPase in Group A only (Figure 6). No effects of ethanol treatment occurred in any other brain region (data not shown).
Figure 6.
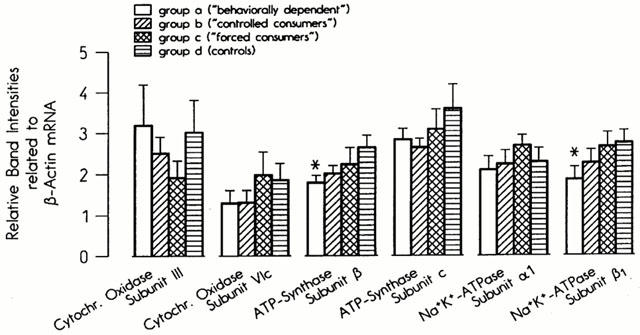
Steady-state mRNA concentrations of cytochrome oxidase subunits, ATP synthetase subunits and Na+/K+ ATPase subunits in the parieto-occipital cortex in the four experimental groups.
Discussion
The main results of the present study are a decrease in the expression in all eight subunits of the GABA-A receptor, four out of five subunits of the glutamate receptors and all seven enzymes involved in the production and recycling of glutamate and GABA in the parieto-occipital cortex in the rats in Groups A and B. These results raise three main questions: (1) What are the implications of the fact that the changes in mRNA concentrations occurred selectively in Groups A and B, but not in Group C?; (2) What role does the parieto-occipital cortex play in the development of addiction? and (3) What is the physiological significance of the effects mentioned above?
The above-mentioned reductions in mRNA concentrations were seen in ‘behaviourally dependent' (Group A) and non-dependent rats (Group B). The only common variable in these two groups was the fact that they were both able to choose freely between ethanol and water. In the free-choice situation the rats learned to consume as much ethanol as necessary to elicit a pleasant, reinforcing effect of the drug. Theoretically, when tolerance of the rewarding effects of ethanol develops, the rats can also learn to increase the dose correspondingly. In contrast, when rats are forced to drink a solution of ethanol as sole fluid, sedation and other unpleasant or toxic effects may predominate. Interestingly, the biochemical effects of ‘rewarding doses' of ethanol do differ widely from those induced by forced ethanol administration. It would seem striking that the consumption of approx. 1 g kg−1 day−1 in the free-choice situation during a 4-week period (Group B) induced numerous long-lasting effects on biochemical parameters, whereas after forced administration of 3.5 g kg−1 day−1 over a 9-month period (Group C, Table 2) hardly any persisting biochemical effects were found. The mechanisms underlying these different effects of forced and free-choice ethanol consumption are unknown. They may be in some way related to the factor ‘dose', since it is well known that low doses of ethanol often have the opposite effects of high doses (Pohorecky, 1977).
Some GABAergic parameters were selectively reduced in Group A, although trends towards a reduction were also seen in Group B. This was true for the GABA-A subunits α1 and β1, the GAD65 and 67 subunits and also for glutaminase. We cannot therefore completely exclude the possibility that these differences may reflect the transition to behavioural dependence.
At first glance it would seem surprising that reward-related changes in GABAergic and glutamatergic mRNA concentrations should occur in the parieto-occipital cortex. However, several studies have now reported that glucose metabolism, which is said to reflect neuronal activity, is most markedly reduced in parieto-cortical areas in abstinent alcoholics (e.g. Wik et al., 1988; Volkow et al., 1992). Furthermore, both Abi-Dargham et al. (1998) and Lingford-Hughes et al. (1998) reported a selective reduction of GABA-benzodiazepine binding sites in abstinent human alcoholics in the frontal, cingulate, parieto-occipital and temporal cortices. There thus seems to be a considerable overlap between the findings of studies in human alcoholics and our results measured in an animal model of behavioural dependence. Of special importance for the interpretation of our data are the findings that in humans, at least, the right hemisphere appears to contain a memory for emotion. The recognition of visually or auditively mediated emotions is severely impaired in patients with lesions of the right parieto-occipital cortex following stroke (Kolb, 1981; Bowers et al., 1991; Blonder et al., 1991). Further evidence for the representation of emotions in this region of the brain has been furnished by numerous reports that severe depressive symptoms may occur in patients following stroke-induced lesions of the right parieto-occipital cortex (e.g. Starkstein et al., 1989).
There is little conclusive data on the location of memory for reward. Arguments both in favour of and against the amygdala as locus of the memory for reward have been put forward (Aggleton & Passingham, 1982; Gaffan, 1992). Interestingly, associated learning is not possible when lesions of the connections between the amygdala and cortical sensory areas are present (Gaffan, 1992). Furthermore, the processing of complex emotional stimuli may involve circuits from the thalamus to the cortical sensory areas and amygdala (Rogan & LeDoux, 1996). It may therefore be hypothesized that circuits connecting the parieto-occipital cortex with the amygdala and probably also other brain regions such as the limbic forebrain and midbrain may process reward-induced emotional information and memory formation. It should therefore not be overlooked that discrete changes in GABAergic and glutamatergic parameters occurred also in the amygdala, limbic forebrain and midbrain in Groups A and B. However, we performed a large number of statistical calculations. The significant results found in the parieto-occipital cortex are so highly consistent with regard to brain area, groups of rats affected (Groups A and B only) and direction (decreases only) that we can definitely rule out the possibility that they are statistical artefacts due to the large number of calculations performed. The isolated significant results in the other brain regions, however, could well reflect statistical artefacts and must therefore be replicated in further experiments.
What is the functional significance of our findings? mRNA levels do not, of course, necessarily reflect protein concentrations or activities. However, our results are highly consistent in so far as we found only reductions in mRNA concentrations in 20 parameters of the glutamatergic and GABAergic systems. This makes it quite likely that the functioning of these transmitter systems is indeed impaired. Moreover, it has also been shown that the mRNA concentration of GAD65 may reflect GABAergic activity (Benson et al., 1991). Even if the reduction in all these mRNA concentrations should later prove not to be reflected by changes in the same direction in protein levels, the fact remains that offering ethanol to rats in a free-choice situation induces specific changes in the GABAergic and glutamatergic systems exclusively in the parieto-occipital cortex. However, the exact nature of these changes remains to be determined.
The functional implication of our results is that the production of GABA and glutamate and the activities of their cortical interneurons may have been reduced in Groups A and B. However, we cannot draw any conclusions regarding the glutamatergic efferents to other brain areas which may synthesis glutamate in their nerve terminals.
The combination of results obtained in our study is reminiscent of the findings reported in the visual cortex of the monkey after monocular deprivation. GABA immunoreactivity, receptor binding, GAD levels, mRNA concentrations and glutamate immunoreactivity have all been shown to be reduced (Hendry & Jones, 1986; Hendry et al., 1990; Carder & Hendry, 1994). The same manipulation has also been demonstrated to reduce the levels and activities of cytochrome oxidase (Wong-Riley & Carroll, 1984). This enzyme is of particular interest since it is said to be a marker of neuronal activity (Wong-Riley, 1989). The activity of cytochrome oxidase is also closely related to that of Na+/K+ ATPase, an enzyme that also plays a key role in neuronal activity (Hevner et al., 1992). However, in the present study no effects of ethanol on the mRNA levels of two subunits of cytochrome oxidase were observed. Decreases were seen in the β1 subunit of Na+/K+ ATPase in Group A and the β subunit of ATP synthetase in Groups A and B. These data are indicative of an impairment in both mitochondrial energy production and overall neuronal activity in the parieto-occipital cortex in the respective groups of rats. However, the data are much less consistent than those obtained for the GABAergic and glutamatergic systems and thus more information is needed before a sound interpretation can be made. Finally, as no effects of ethanol were found on the mRNA concentrations of the six parameters involved in glucose uptake and glycolysis, the decreased synthesis of glutamate and GABA is probably not due to an impairment of glycolytic flux (Eravci et al., 1999).
If we compare our results with those obtained by other groups it becomes apparent that forced acute and subchronic administration of ethanol and in vitro experiments produce results that are somewhat different from those obtained in a free-choice situation. For example, it was reported by Mhatre & Ticku (1992), that after 6 days' intoxication with ethanol, the levels of α1, α2 and α5 mRNAs in the cortex were reduced, while that of the α6 subunit in the cerebellum increased. The same authors (Mhatre & Ticku, 1994) found that chronic administration of ethanol induced a rise in the mRNA levels of the β1, β2 and β3 subunits in rat cortex. Devaud et al. (1995) found an increase in the concentrations in the α4, γ2s and γ1 subunits, but a fall in that of α1 subunit, and no change in that of the α5, β1, β2, β3, γ3 or δ subunits. In mouse cerebellar neurons Wu et al. (1995) reported a fall in the mRNA levels of the α1 subunits, an increase in those of the α6 and γ2 subunits, and no change in those of the β2 and β3 subunits following chronic administration of ethanol. Charlton et al. (1997) reported a reduction in the mRNA concentrations of the α1 subunit and an increase in those of the α5 unit in the hippocampus.
In summary, these results are somewhat contradictory, which may be attributable to the duration of ethanol application, the brain area under investigation, the sex of the experimental animals and the use of different cell culture systems.
Interestingly, the effects of forced ethanol administration have been shown to be completely reversible only 36 h after withdrawal (Mhatre & Ticku, 1992). Similarly, the upregulation of NMDA receptor subunit polypeptide levels after chronic ethanol administration has been found to be reversible 4 h after the last dose (Kalluri et al., 1998). These results may explain why we failed to find any changes in the mRNA levels of GABA-A or glutamate receptor subunits in the ‘forced consumers' (Group C) after 1 month of abstinence, apart from a decrease in mRNA in the GABA-A α3 subunit.
Our results for the subunits of the different glutamate receptors also differ from those obtained after forced administration of ethanol. The main difference is that prolonged ethanol administration was found to induce an upregulation of the mRNA levels, immunoreactivity and functioning of NMDA receptor subunits. Follesa & Ticku (1995) found no effect of chronic ethanol administration on the NMDA-R subunit-1 in the cortex, hippocampus or cerebellum. The NMDA-R subunits 2α and 2β were, however, increased in the cortex and hippocampus. Trevisan et al. (1994) also reported an increase in immunoreactivity of the NMDA-subunit 1 in the hippocampus, but not in the nucleus accumbens, cortex or striatum. No effects were seen on the glutamate receptor subunits 1 and 2. Kalluri et al. (1998) reported an upregulation of NMDA receptor subunit polypeptides in rat cortex and hippocampus after chronic ethanol administration.
In contrast to all the above results, the free-choice situation induced mainly a decrease in the mRNA levels in glutamate receptor subunits. Our data therefore indicate that the effects of ethanol on a given biochemical parameter observed in in vitro experiments and studies with forced administration of ethanol are not necessarily of relevance for the development of ‘behavioural dependence'.
The post-mortem investigation of the mRNA concentrations of GABA-A receptor subunit in the frontal cortex of human alcoholics has revealed that the expression of the α1 subunit mRNA and polypeptide remains unchanged (Mitsuyama et al., 1998). Again, these results differ from those of most studies performed with forced ethanol administration to rats (see above), but are in agreement with our results.
It therefore seems possible that the biochemical changes detected in the brains of animals rendered behaviourally dependent on ethanol may better reflect the situation in the brains of human alcoholics than forced ethanol administration.
In conclusion, our results showed that the GABAergic and glutamatergic transmitter receptors in rat parieto-occipital cortex remained affected by chronic consumption of rewarding doses of ethanol in a free-choice design even when the animals were sacrificed after 1 month of abstinence. In contrast, after forced administration of ethanol over a period of not less than 9 months no irreversible effects were demonstrated. The changes in the mRNA levels of receptors found in this study may therefore be somehow related to the development of the memory for reward. Ethanol affected all GABA and glutamate receptor subunits under investigation in a nonspecific manner and did not show specific effects on distinct subunits such as the GABA-A γ21 subunit. The lack of evidence that the changes in GABA or glutamate receptor mRNA were specifically related to ‘behavioural dependence' would seem to be of potential relevance. However, this is the very first study to investigate an involvement of the GABAergic and glutamatergic transmitter systems in the development of addiction. Future studies should clarify whether other parameters of these transmitter systems (e.g. polypeptide concentrations, functional experiments) arrive at the same results.
Acknowledgments
This study was supported by Deutsche Forschungsgemeinschaft (DFG grant Ba 932/6-1).
Abbreviations
- GABA
gamma-amino-n-butyric acid
- GAD
glutamate decarboxylase
- GluR
glutamate receptor
- KA
kainate
- MOSH
multiple oligonucleotide solution hybridization
- NMDA
n-methyl-D-aspartic acid
References
- ABI-DARGHAM A., KRYSTAL J.H., ANJILVEL S., SCANLEY B.E., ZOGHBI S., BALDWIN R.M., RAJEEVAN N., ELLIS S., PETRAKIS T.L., SEIBYL J.P., CHARNEY D.S., LARUELLE M., INNIS R.B. Alterations of benzodiazepine receptors in type II alcoholic subjects measured with SPECT and [123I]iomazenil. Am. J. Psychiat. 1998;155:1550–1555. doi: 10.1176/ajp.155.11.1550. [DOI] [PubMed] [Google Scholar]
- AGGLETON J.P., PASSINGHAM R.E. An assessment of the reinforcing properties of foods after amygdaloid lesions in rhesus monkeys. J. Comp. Physiol Psychol. 1982;1:71–77. doi: 10.1037/h0077861. [DOI] [PubMed] [Google Scholar]
- AMURO N., OOKI K., ITO A., GOTO Y., OKAZAKI T. Nucleotide sequence of rat liver glutamate dehydrogenase cDNA. Nucleic Acids Res. 1989;17:2356. doi: 10.1093/nar/17.6.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENSON D.L., ISACKSON P.J., GALL C.M., JONES E.G. Differential effects of monocular deprivation on glutamic acid decarboxylase and type II calcium-calmodulin-dependent protein kinase gene expression in the adult monkey visual cortex. J. Neurosci. 1991;11:31–47. doi: 10.1523/JNEUROSCI.11-01-00031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BETTINI E., MAGGI A. Estrogen induction of cytochrome c oxidase subunit III in rat hippocampus. J. Neurochem. 1992;58:1923–1929. doi: 10.1111/j.1471-4159.1992.tb10070.x. [DOI] [PubMed] [Google Scholar]
- BLONDER L.X., BOWERS D., HEILMAN K.M. The role of the right hemisphere in emotional communication. Brain. 1991;114:1115–1127. doi: 10.1093/brain/114.3.1115. [DOI] [PubMed] [Google Scholar]
- BOULTER J., HOLLMANN M., O'SHEA-GREENFIELD A., HARTLEY M., DENERIS E., MARON C., HEINEMANN S. Molecular cloning and functional expression of Glutamate receptor subunit genes. Science. 1990;249:1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- BOWERS D., BLONDER L.X., FEINBERG T., HEILMAN K.M. Differential impact of right and left hemisphere lesions on facial emotion and object imagery. Brain. 1991;114:2593–2609. doi: 10.1093/brain/114.6.2593. [DOI] [PubMed] [Google Scholar]
- CARDER R.K., HENDRY S.H.C. Neuronal characterization, compartmental distribution, and activity-dependent regulation of glutamate immunoreactivity in adult monkey striate cortex. J. Neurosci. 1994;14:242–262. doi: 10.1523/JNEUROSCI.14-01-00242.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANDLER L.J., HARRIS R.A., CREWS F.T. Ethanol tolerance and synaptic plasticity. Trends Pharmacol. Sci. 1998;19:491–495. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- CHARLTON M.E., SWEETNAM P.M., FITZGERALD L.W., TERWILLIGER R.Z., NESTLER E.J., DUMAN R.S. Chronic ethanol administration regulates the expression of GABAA receptor α1 and α5 subunits in the ventral tegmental area and hippocampus. J. Neurochem. 1997;68:121–127. doi: 10.1046/j.1471-4159.1997.68010121.x. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DEVAUD L.L., SMITH F.D., GRAYSON D.R., MORROW A.L. Chronic ethanol consumption differentially alters the expression of γ-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol. Pharmacol. 1995;48:861–868. [PubMed] [Google Scholar]
- DIAMOND I., GORDON A.S. Cellular and molecular neuroscience of alcoholism. Physiol. Rev. 1997;77:1–19. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- DUNNEN D.J.T., VAN NECK J.W., CREMERS F.P.M., LUBSEN N.H., SCHOENMAKERS J.G.G. Nucleotide sequence of the rat gamma-crystallin gene region and comparison with an orthologous human region. Gene. 1990;87:225–232. doi: 10.1016/0378-1119(89)90223-0. [DOI] [PubMed] [Google Scholar]
- EGEBJERG J., BETTLER B., HERMANS-BORGMEYER I., HEINEMANN S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991;351:745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- ERAVCI M., GROßPIETSCH T., PINNA G., SCHULZ O., KLEY S., BACHMANN M., WOLFFGRAMM J., GÖTZ E., HEYNE A., MEINHOLD H., BAUMGARTNER A. Dopamine receptor gene expression in an animal model of, behavioral dependence on ethanol. Mol. Brain Res. 1997;50:221–229. doi: 10.1016/s0169-328x(97)00188-5. [DOI] [PubMed] [Google Scholar]
- ERAVCI M., KLEY S., PINNA G., PRENGEL H., BRÖDEL O., HIEDRA L., MEINHOLD H., BAUMGARTNER A. Gene expression of glucose transporters and glycolytic enzymes in the CNS of rats behaviorally dependent on ethanol. Mol. Brain Res. 1999;65:103–111. doi: 10.1016/s0169-328x(98)00347-7. [DOI] [PubMed] [Google Scholar]
- ERLANDER M.G., TILLAKARATNE N.J.K., FELDBLUM S., PATEL N., TOBIN A.J. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- FOLLESA P., TICKU M.K. Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Mol. Brain Res. 1995;29:99–106. doi: 10.1016/0169-328x(94)00235-7. [DOI] [PubMed] [Google Scholar]
- GAFFAN D. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss, Inc; 1992. Amygdala and the memory of reward; pp. 471–483. [Google Scholar]
- GARBOCZI D.N., FOX A.H., GERRING S.L., PEDERSEN P.L. Beta subunit of rat liver mitochondrial ATP synthase: cDNA cloning, amino acid sequence, expression in Escherichia coli, and structural relationship to adenylate kinase. Biochemistry. 1988;27:553–560. doi: 10.1021/bi00402a008. [DOI] [PubMed] [Google Scholar]
- GLOWINSKI I., IVERSEN L.L. Regional studies of catecholamine metabolism in the rat brain. I. The disposition of [3H]dopamine and [3H]dopa in various regions of the brain. J. Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- GROBIN A.C., MATTHEWS D.B., DEVAUD L.L., MORROW A.L. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- HARRIS R.A. Ethanol actions on multiple ion channels: which are important. Alcohol Clin. Exp. Res. 1999;23:1563–1570. [PubMed] [Google Scholar]
- HENDRY S.H.C., FUCHS J., DEBLAS A.L., JONES E.G. Distribution and plasticity of immunocytochemically localized GABAA receptors in adult monkey visual Cortex. J Neurosci. 1990;10:2438–2450. doi: 10.1523/JNEUROSCI.10-07-02438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDRY S.H.C., JONES E. Reduction in number of immunostained GABAergic neurones in deprived-eye dominance columns of monkey area 17. Nature. 1986;320:750–753. doi: 10.1038/320750a0. [DOI] [PubMed] [Google Scholar]
- HIGUTI T., KUROIWA K., KAWAMURA Y., MORIMOTO K., TSUJITA H. Molecular cloning and sequence of cDNAs for the import precursors of oligomycin sensitivity conferring protein, ATPase inhibitor protein, and subunit c of H+-ATP synthase in rat mitochondria. Biochem. Biophys. Acta. 1993;1172:311–314. doi: 10.1016/0167-4781(93)90219-4. [DOI] [PubMed] [Google Scholar]
- HEVNER R.F., DUFF R.S., WONG-RILEY M.T.T. Coordination of ATP production and consumption in brain: parallel regulation of cytochrome oxidase and Na+, K(+)-ATPase. Neurosci. Lett. 1992;138:188–192. doi: 10.1016/0304-3940(92)90502-x. [DOI] [PubMed] [Google Scholar]
- HERB A., BURNASHEV N., WERNER P., SAKMANN B., WISDEN W., SEEBURG P.H. The KA-2 subunit of exitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- HORIO Y., TANAKA T., TAKETOSHI M., NAGASHIMA F., TANASE S., MORINO Y., WADA H. Rat cytosolic aspartate aminotransferase: molecular cloning of cDNA and expression in Escherichia coli. J. Biochem. 1988;103:797–804. doi: 10.1093/oxfordjournals.jbchem.a122349. [DOI] [PubMed] [Google Scholar]
- KALLURI H.S.G., MEHTA A.K., TICKU M.K. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Mol. Brain Res. 1998;58:221–224. doi: 10.1016/s0169-328x(98)00112-0. [DOI] [PubMed] [Google Scholar]
- KOLB B. Affective behavior in patients with localized cortical excisions: role of lesion site and side. Science. 1981;214:89–91. doi: 10.1126/science.7280683. [DOI] [PubMed] [Google Scholar]
- LINGFORD-HUGHES A.R., ACTON P.D., GACINOVIC S., SUCKLING J., BUSATTO G.F., BODDINGTON S.J., BULLMORE E., WOODRUFF P.W., COSTA D.C., PILOWSKY L.S., ELL P.J., MARSHALL E.J., KERWIN R.W. Reduced levels of GABA-benzodiazepine receptor in alcohol dependency in the absence of grey matter atrophy. Br. J. Psychiatry. 1998;173:116–122. doi: 10.1192/bjp.173.2.116. [DOI] [PubMed] [Google Scholar]
- MALHERBE P., SIGEL E., BAUR R., PERSOHN E., RICHARDS J.G., MOHLER H. Functional expression and sites of gene transcription of a novel alpha subunit of the GABAA receptor in rat brain. FEBS Lett. 1990;260:261–265. doi: 10.1016/0014-5793(90)80118-3. [DOI] [PubMed] [Google Scholar]
- MATTINGLY J.R., JR, RODRIGUEZ-BERROCAL F.J., GORDON J., IRIARTE A., MARTINEZ-CARRION M. Molecular cloning and in vivo expression of a precursor to rat mitochondrial aspartate aminotransferase. Biochem. Biophys. Res. Commun. 1987;149:859–865. doi: 10.1016/0006-291x(87)90487-6. [DOI] [PubMed] [Google Scholar]
- MEHTA A.K., TICKU M.K. An update GABAA receptors. Brain Res. Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- MHATRE M.C., TICKU M.K. Chronic ethanol administration alters γ-aminobutyric AcidA receptor gene expression. Mol. Pharmacol. 1992;42:415–422. [PubMed] [Google Scholar]
- MHATRE M.C., TICKU M.K. Chronic ethanol treatment upregulates the GABA receptor β subunit expression. Mol. Brain Res. 1994;23:246–252. doi: 10.1016/0169-328x(94)90231-3. [DOI] [PubMed] [Google Scholar]
- MICHELSEN B.K., PETERSON J.S., BOEL E., MOELDRUP A., DYRBERG T., MADSEN D. Cloning, characterization and autoimmune recognition of rat islet glutamic acid decarboxylase in insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. USA. 1991;88:8754–8758. doi: 10.1073/pnas.88.19.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUYAMA H., LITTLE K.Y., SIEGHART W., DEVAUD L.L., MORROW A.L. GABAA receptor α1, α4, and β3 subunit mRNA and protein expression in the frontal cortex of human alcoholics. Alcohol Clin. Exp. Res. 1998;22:815–822. [PubMed] [Google Scholar]
- MONYER H., SPRENGEL R., SCHOEPFER R., HERB A., HIGUCHI M., LOMELI H., BURNASHEV N., SAKMANN B., SEEBURG P.H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- NUDEL U., ZAKUT R., SHANI M., NEUMAN S., LEVY Z., YAFFEE D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983;11:1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'DONOVAN M.C., BUCKLAND P.R., MCGUFFIN P. Simultaneous quantification of several mRNA species by solution hybridisation with oligonucleotides. Nucleic Acids Res. 1991;19:3466. doi: 10.1093/nar/19.12.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POHORECKY L.A. Biphasic action of ethanol. Biobehav. Rev. 1977;1:231–240. [Google Scholar]
- ROGAN M.T., LEDOUX J.E. Emotion: systems, cells, synaptic plasticity. Cell. 1996;85:469–475. doi: 10.1016/s0092-8674(00)81247-7. [DOI] [PubMed] [Google Scholar]
- SEEBURG P.H., WISDEN W., VERDOORN T.A., PRITCHETT D., WERNER P., HERB A., LUEDDENS H., SPRENGEL R., SAKMANN B. The GABAA receptor family: molecular and functional diversity. Cold Spring Harb. Symp. Quant. Biol. 1990;55:29–40. doi: 10.1101/sqb.1990.055.01.006. [DOI] [PubMed] [Google Scholar]
- SHAPIRO R.A., FARRELL L., SRINIVASAN M., CURTHOYS N.P. Isolation, characterization, and in vitro expression of a cDNA that encodes the kidney isozyme of the mitochondrial glutaminase. J. Biol. Chem. 1991;266:18792–18796. [PubMed] [Google Scholar]
- SHIVERS B.D., KOLLISCH I., SPRENGEL R., SONTHEIMER H., KOEHLER M., SCHOFIELD P.R., SEEBURG P.H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989;3:327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- SHULL G.E., GREEB J., LINGREL J.B. Molecular cloning of three distinct forms of the Na+, K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986;25:8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- STARKSTEIN S.E., ROBINSON R.G., HONIG M.A., PARIKH R.M., JOSELYN J., PRICE T.R. Mood changes after right-hemisphere lesions. Brit. J. Psychiat. 1989;155:79–85. doi: 10.1192/bjp.155.1.79. [DOI] [PubMed] [Google Scholar]
- SUSKE G., ENDERS C., SCHLERF A., KADENBACH B. Organization and nucleotide sequence of two chromosomal genes for rat cytochrome c oxidase subunit VIc: a structural and a processed gene. DNA. 1988;7:163–171. doi: 10.1089/dna.1988.7.163. [DOI] [PubMed] [Google Scholar]
- TABAKOFF B., HOFFMAN P.L. Alcohol addiction: an enigma among us. Neuron. 1996;16:909–912. doi: 10.1016/s0896-6273(00)80113-0. [DOI] [PubMed] [Google Scholar]
- TREVISAN L., FITZGERALD L.W., BROSE N., GASIC G.P., HEINEMANN S.F., DUMAN R.S., NESTLER E.J. Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J. Neurochem. 1994;62:1635–1638. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- VOLKOW N.D., HITZEMANN R., WANG G.J., FOWLER J., BURR G., PASCANI K., DEWEY S.L., WOLF A.P. Decreased brain metabolism in neurologically intact healthy alcoholics. Am. J. Psychiatry. 1992;149:1016–1022. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- WHITING P., MCKERNAN R.M., IVERSEN L.L. Another mechanism for creating diversity in γ-aminobutyrate type A receptors: RNA splicing directs expression of two forms of γ2 subunit, one of which contains a protein kinase C phosphorylation site. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIK G., BORG S., SJÖGREN I., WIESEL F.A., BLOMQVIST G., BORG J., GREITZ T., NYBÄCK H., SEDVALL G., STONE-ELANDER S., WIDEN L. PET determination of regional cerebral glucose metabolism in alcohol-dependent men and healthy controls using 11C-glucose. Acta Psychiatr. Scand. 1988;78:234–241. doi: 10.1111/j.1600-0447.1988.tb06330.x. [DOI] [PubMed] [Google Scholar]
- WISE R.A. Neurobiology of addiction. Curr. Opin. Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- WOLFFGRAMM J., HEYNE A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav. Brain. Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- WONG-RILEY M.T.T. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Tins. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- WONG-RILEY M.T.T., CARROLL E.W. Effect of impulse blockage on cytochrome oxidase activity in monkey visual system. Nature. 1984;307:262–264. doi: 10.1038/307262a0. [DOI] [PubMed] [Google Scholar]
- WU C.H., FROSTHOLM A., DE BLAS A.L., ROTTER A. Differential expression of GABAA/Benzodiazepine receptor subunit mRNAs and ligand sites in mouse cerebellar neurons following in vivo ethanol administration: an autoradiographic analysis. J. Neurochem. 1995;65:1229–1239. doi: 10.1046/j.1471-4159.1995.65031229.x. [DOI] [PubMed] [Google Scholar]
- YMER S., SCHOFIELD P.R., DRAGUHN A., WERNER P., KOHLER M., SEEBURG P.H. GABAA receptor beta subunit heterogeneity: functional expression of cloned cDNAs. EMBO J. 1989;8:1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG R.M., SHULL G.E., LINGREL J.B. Multiple mRNAs from rat kidney and brain encode a single Na+, K+-ATPase beta subunit protein. J. Biol. Chem. 1987;262:4905–4910. [PubMed] [Google Scholar]


