Abstract
MT-7 (1–30 nM), a peptide toxin isolated from the venom of the green mamba Dendroaspis angusticeps and previously found to bind selectively to the muscarinic M1 receptor, inhibited the acetylcholine (ACh)-stimulated [35S]-guanosine-5′-O-(3-thio)triphosphate ([35S]-GTPγS) binding to membranes of Chinese hamster ovary (CHO) cells stably expressing the cloned human muscarinic M1 receptor subtype.
MT-7 failed to affect the ACh-stimulated [35S]-GTPγS binding in membranes of CHO cells expressing either the M2, M3 or M4 receptor subtype.
In N1E-115 neuroblastoma cells endogenously expressing the M1 and M4 receptor subtypes, MT-7 (0.3–3.0 nM) inhibited the carbachol (CCh)-stimulated inositol phosphates accumulation, but failed to affect the CCh-induced inhibition of pituitary adenylate cyclase activating polypeptide (PACAP) 38-stimulated cyclic AMP accumulation.
In both CHO/M1 and N1E-115 cells the MT-7 inhibition consisted in a decrease of the maximal agonist effect with minimal changes in the agonist EC50 value.
In CHO/M1 cell membranes, MT-7 (0.05–25 nM) reduced the specific binding of 0.05, 1.0 and 15 nM [3H]-N-methylscopolamine ([3H]-NMS) in a concentration-dependent manner, but failed to cause a complete displacement of the radioligand. Moreover, MT-7 (3 nM) decreased the dissociation rate of [3H]-NMS by about 5 fold.
CHO/M1 cell membranes preincubated with MT-7 (10 nM) and washed by centrifugation and resuspension did not recover control [3H]-NMS binding for at least 8 h at 30°C.
It is concluded that MT-7 acts as a selective noncompetitive antagonist of the muscarinic M1 receptors by binding stably to an allosteric site.
Keywords: Dendroaspis angusticeps toxin, muscarinic receptor subtypes, [35S]-GTPγS binding, phosphoinositide hydrolysis, cyclic AMP accumulation, [3H]-NMS binding, Chinese hamster ovary cells, N1E-115 cells, noncompetitive antagonism
Introduction
Muscarinic toxins are small peptides of 64–66 amino acids isolated from mamba snake venoms on the basis of their ability to bind to acetylcholine (ACh) muscarinic receptors (Adem et al., 1988; Karlsson et al., 1994; Jerusalinsky & Harvey, 1994; Adem & Karlsson, 1997). Nine muscarinic toxins (termed MT-1 to MT-7, ml-toxin and m2-toxin) have been isolated from the venom of the Eastern green mamba Dendroaspis angusticeps and three (termed MT-α, β and γ) from the venom of the black mamba Dendroaspis polylepsis (Adem & Karlsson, 1997; Carsi et al., 1999). Although the toxins generally display a high sequence homology and likely possess a similar ‘three-finger' structure (Segalas et al., 1995), they show notable differences in their selectivity for the distinct muscarinic receptor subtypes. For instance, muscarinic toxin 1 (MT-1), 4 (MT-4) and 5 (MT-5) bind with high affinity to both muscarinic M1 and M4 receptor subtypes but display low affinity for the M2, M3 and M5 subtypes (Adem & Karlsson, 1997). Muscarinic toxin 3 (MT-3) (Karlsson et al., 1994) shows high affinity for the M4, a low affinity for the M1 and a very low affinity for the M2, M3 and M5 receptor subtypes (Jolkkonen et al., 1994; Olianas et al., 1999), whereas m1-toxin binds with high affinity to the M1 and with a lower affinity to the M4 subtype (Max et al., 1993a; Potter et al., 1993). The recently isolated m2-toxin fully blocks radioligand binding to M2 receptors, has no effect on M4 receptors, and slightly increases radioligand binding to M1 receptors (Carsi et al., 1999). Important differences are also found in the functional activities of the muscarinic toxins. Thus, in an inhibitory learning task in rats MT-2 showed muscarinic agonist-like action (Jerusalinsky et al., 1993), and in different peripheral tissues MT-1 and MT-2 acted as selective agonists at M1 receptors (Jerusalinsky & Harvey, 1994). Conversely, MT-3 was found to behave as a competitive and reversible antagonist at the cloned and native M4 receptors (Olianas et al., 1996; 1999) whereas m1-toxin was reported to act as a pseudo-irreversible allosteric antagonist of the M1 receptor (Max et al., 1993b).
MT-7 is a 65 amino acid peptide toxin which has been identified as the first high-affinity and selective ligand for M1 receptors (Jolkkonen, 1996; Adem & Karlsson, 1997). In radioligand binding studies employing the human cloned muscarinic receptor subtypes expressed in Chinese hamster ovary (CHO) cells, MT-7 bound to the M1 subtype with a potency in the low nanomolar range and did not bind to the other receptor subtypes at micromolar concentrations (Adem & Karlsson, 1997).
To further characterize the pharmacological profile of MT-7, in the present study we have investigated the activity of the toxin in functional assays of the cloned human M1-M4 receptors expressed in CHO cells and of the native M1 and M4 receptors present in murine N1E-115 neuroblastoma cells. In addition, we have examined the nature of the toxin interaction with the M1 receptor by examining the toxin effects on [3H]-N-methylscopolamine ([3H]-NMS) binding. Part of this work has previously been presented in an abstract form (Onali et al., 1999).
Methods
CHO cell culture and membrane preparation
CHO cells stably expressing the cloned human M1-M4 receptors (CHO/M1-M4 cells) were kindly provided by Professor A.D. Strosberg (Institut Cochin de Genetique Moleculaire, Paris, France). The cells were grown as a monolayer culture in Ham's F-12 medium (GIBCO–BRL) supplemented with 10% foetal calf serum (GIBCO–BRL) in a humidified atmosphere (5% CO2) at 37°C. Cells were grown to ∼80 % confluency in plastic Petri dishes (Falcon), the medium was removed, and the cells were washed with ice-cold phosphate-buffered saline (PBS). The cells were then scraped into an ice-cold buffer containing 25 mM sodium phosphate buffer (pH 7.4) and 5 mM MgCl2 and lysed by the use of an Ultra-Turrax homogenizer. The cell lysate was centrifuged at 32,500×g for 30 min at 4°C and the pellet was resuspended in the same buffer at a protein concentration of ∼3 mg ml−1. The membrane preparations were either used immediately or stored at −75°C.
Assay of guanosine -5′-O-(3-[35S]-thio)triphosphate ([35S]-GTPγS) binding
CHO cell membranes were diluted 10 fold in an ice-cold buffer containing HEPES/NaOH (10 mM), EDTA (1 mM) (pH 7.4), centrifuged and resuspended in the same buffer supplemented with 0.1% bovine serum albumin (BSA). The binding of [35S]-GTPγS was assayed in a reaction mixture (final volume, 100 μl) containing (mM) HEPES/NaOH 25 (pH 7.4), MgCl2 10, EDTA 1, GDP (0.1 μM) for M1 and M3 and GDP (1 μM) for M2 and M4 receptor activities (Lazareno et al., 1993), NaCl 100, 10 kallikrein inhibitor units (KIU) of aprotinin. The membranes (1.5–2.0 μg of protein) were preincubated in the presence of the indicated concentrations of ACh and MT-7 at 30°C for 30 min. The samples were then placed on ice and the reaction was started by the addition of 10 μl of [35S]-GTPγS (final concentration, 1.0–1.5 nM). The samples were incubated for 45 min at 30°C. The reaction was stopped by the addition of 5 ml of ice-cold buffer containing HEPES/NaOH (10 mM) (pH 7.4) and MgCl2 (1 mM), immediately followed by rapid filtration through glass fibre filters (Whatman GF/C) presoaked in the same buffer. The filters were washed twice with 5 ml of buffer and the radioactivity trapped was determined by liquid scintillation spectrometry. Nonspecific binding was determined in the presence of 100 μM GTPγS. Assays were performed in duplicate.
Assay of [3H]-NMS binding
The binding of [3H]-NMS to CHO/M1 cell membranes was assayed in a buffer containing sodium phosphate (25 mM) (pH 7.4), MgCl2 (5 mM), 0.1% BSA, and 10–12 μg of membrane protein. The final assay volume was 1.0 ml. In competition experiments, the MT-7 concentration ranged from 50 pM to 30 nM and the [3H]-NMS concentrations were 0.05, 3.0 and 15 nM. The incubation was carried out at 30°C for 90 min. When the rate of dissociation of [3H]-NMS was studied, the membranes were incubated with 1.0 nM [3H]-NMS for 60 min before the addition of either vehicle or MT-7 (3 nM). After 20 min, atropine (10 μM) was added to each sample and the incubation was stopped at different time intervals after the atropine addition over a total period of 60 min. To investigate the stability of MT-7 binding to M1 receptors, CHO/M1 cell membranes were preincubated with either vehicle or MT-7 (100 nM) for 45 min at 30°C. Thereafter, the samples were centrifuged at 32,500×g for 20 min at 4°C and resuspended in fresh buffer. Aliquots of the membrane suspension were incubated in the presence of 1.5 nM [3H]-NMS for the indicated times over a total period of 8 h. The incubation was stopped by adding 4 ml of ice-cold buffer without BSA to each sample followed by immediate filtration through glass fibre filters presoaked in 0.1% polyethylenimine for at least 18 h. The filters were washed twice with the same buffer, dried and the bound radioactivity was counted by liquid scintillation spectrometry. Nonspecific binding was determined in the presence of 10 μM atropine. Assays were performed in triplicate.
N1E-115 neuroblastoma cell culture
Cells were obtained from European Collection of Cell Cultures (U.K.). The cells were grown in Dulbecco's modified Eagle's medium containing 2 mM glutamine and 10% foetal calf serum in 75-cm2 flasks (Falcon). The medium (20–30 ml) was changed on day 2 of subculture and every subsequent day. Confluent cell cultures (6–8 days postpassage) were used for the experiments.
Assay of [3H]-inositol phosphates ([3H]-IPs) accumulation
N1E-115 cells were prelabelled with myo-[3H]-inositol (1 μCi ml−1) in Dulbecco's modified Eagle medium for 24 h at 37°C in an incubator. The medium was then removed and the cells were washed twice with PBS. The cells were detached from the tissue culture flask by incubation in PBS containing EDTA (0.5 mM) for 5 min at 37°C followed by gentle agitation of the flask. The cell suspension was aspirated, mixed with an equal volume of PBS containing MgCl2 (1 mM) and centrifuged at 300×g for 1 min. The cells were resuspended in a freshly oxygenated Krebs-HEPES buffer containing (mM) HEPES/NaOH 25, MgSO4 1.2, KH2PO4 1.2, glucose 10, NaCl 110, KCl 3.8, CaCl2 1.2 and LiCl 10. Aliquots of the cell suspension were distributed into Bio-vials (Beckman, Ireland) and incubated for 30 min at 37°C in the presence and in the absence of MT-7. Thereafter, carbachol (CCh) was added as indicated and the incubation was continued for 45 min. The final incubation volume was 300 μl. The incubation was terminated by adding 940 μl of chloroform-methanol (1 : 2 v v−1). After the samples were shaken for 10 min, 310 μl aliquots of chloroform and water were added. The samples were centrifuged at 1000×g for 10 min and the upper aqueous phase was applied to a column of Dowex 1×8 in the formate form. The column was washed with 20 bed volumes of H2O, 20 bed volumes of 5 mM myo-inositol and 16 bed volumes of 5 mM sodium tetraborate in 60 mM sodium formate. [3H]-IPs were eluted by adding 6 bed volumes of 1 M ammonium formate in 0.1 M formic acid (Berridge et al., 1983). The radioactivity present in the eluate and in the organic phase was determined by liquid scintillation counting. For each sample the accumulation of [3H]-IPs was corrected for the amount of myo-[3H]-inositol incorporated in the organic phase. Assays were performed in triplicate.
Assay of [3H]-cyclic AMP accumulation
N1E-115 cells grown in 36-mm plastic dishes were incubated in Dulbecco's modified Eagle medium containing 2 μCi ml−1 of [3H]-adenine for 1 h at 37°C in an incubator. Thereafter, the medium was removed and the cells were incubated in an oxygenated Krebs-HEPES buffer containing 3-isobutyl-1-methylxanthine (1 mM) in the absence and in the presence of MT-7 for 30 min at 37°C. Pituitary adenylate cyclase activating polypeptide (PACAP) 38 and CCh were then added as indicated and the incubation was continued for 10 min. The incubation was stopped by the aspiration of the medium and the addition of an ice-cold solution containing 6% (w v−1) perchloric acid and 0.1 mM [14C]-cyclic AMP (∼3000 c.p.m.). After 30 min at ice-bath temperature, the solution was neutralized by the addition of ice-cold 0.6 M KOH and left on ice for additional 30 min. Following centrifugation at 15,000×g for 5 min, the supernatant was collected, and [3H]-cyclic AMP was isolated according to Salomon (1979). The recovery of [3H]-cyclic AMP from each sample was corrected on the basis of the recovery of [14C]-cyclic AMP. Assays were performed in triplicate.
Protein content was determined by the method of Bradford (1976), using BSA as a standard.
Statistical analysis
Results are given as mean±standard error of the mean (s.e.mean). Concentration–response curves were analysed by a least squares curve-fitting computer programme (GraphPAD Prism, San Diego, CA, U.S.A.). Statistical significance of the difference between means was determined by Student's t-test.
Materials
[35S]-GTPγS (1306 Ci mmol−1), [2,8-3H]-adenine (28.8 Ci mmol−1) and [8-14C]-cyclic AMP (45.1 mCi mmol−1) were obtained from New England Nuclear (Bad Homburg, Germany). [3H]-NMS (83 Ci mmol−1) and myo-[3H]-inositol with PT6-271 stabilizer (99 Ci mmol−1) were purchased from Amersham (U.K.). GTPγS was from Calbiochem (La Jolla, CA, U.S.A.). PACAP 38 was from Peninsula Laboratories (Merseyside, U.K.). MT-7 was purified from the venom of Dendroaspis angusticeps by gel filtration followed by ion-exchange and reverse phase-high performance liquid cromatographies (Adem & Karlsson, unpublished; Vandermeers et al., 1995). ACh chloride, CCh chloride, atropine sulphate and the other reagents used were from Sigma Chemical Company (St. Louis, MO, U.S.A.).
Results
Effects of MT-7 on [35S]-GTPγS binding to CHO/M1-M4 cell membranes
In membranes of CHO/M1 cells, MT-7 caused a concentration-dependent reduction of the maximal stimulation of [35S]-GTPγS binding elicited by ACh (Figure 1). At 1.0, 10 and 30 nM, MT-7 inhibited the maximum of agonist response by 15.7±1.5 (P<0.01), 50.5±3.1 and 72.8±1.7% (P<0.001), respectively. The EC50 values of ACh were 6.0±0.8, 6.8±0.5, 13.0±1.2 and 7.3±1.0 μM in the absence and in the presence of 1.0, 10 and 30 nM MT-7, respectively. Conversely, in membranes of CHO/M2, M3 and M4 cells MT-7 failed to affect the ACh- induced stimulation of [35S]-GTPγS binding (Figure 1).
Figure 1.
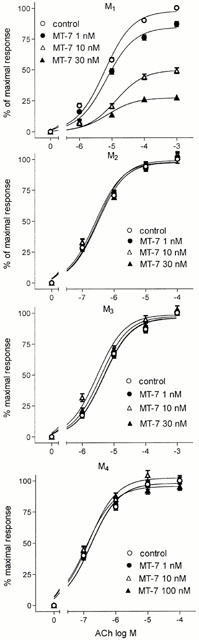
Effects of MT-7 on ACh stimulation of [35S]-GTPγS binding to membranes of CHO cells expressing the cloned human M1 to M4 receptors. The [35S]-GTPγS binding stimulated by ACh was measured in the absence (control) and in the presence of the indicated concentrations of MT-7. Data are the mean±s.e.mean of three experiments.
Effect of MT-7 on CCh-stimulated [3H]-IPs accumulation in N1E-115 cells
In N1E-115 mouse neuroblastoma cells, CCh increased [3H]-IPs accumulation by approximately 5 fold with an EC50 value of 33.8±4.0 μM (Figure 2A). The addition of MT-7 at 0.3, 1.0 and 3.0 nM progressively reduced the maximal agonist stimulation by 35.1±3.5, 69.3±2.1 and 84.9±1.3% (P<0.001), respectively. The agonist EC50 values were 54.0±6.0, 100±9.0 and 89.9±7.0 μM at 0.3, 1.0 and 3.0 nM MT-7, respectively.
Figure 2.
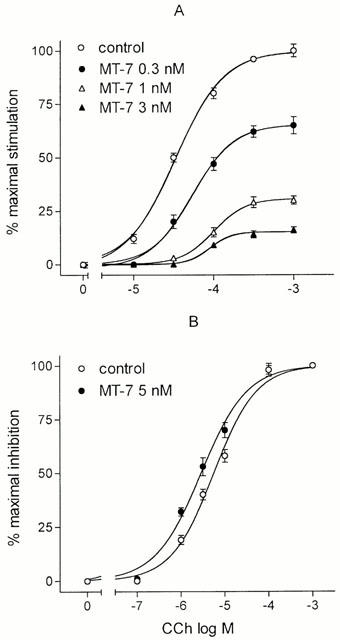
Effects of MT-7 on CCh-stimulated [3H]-IPs accumulation (A) and CCh-induced inhibition of PACAP38-stimulated cyclic AMP accumulation (B) in N1E-115 neuroblastoma cells. Cells were pretreated with the indicated concentrations of MT-7 for 30 min at 37°C and then exposed to the indicated concentrations of CCh. In (B), PACAP 38 was also added at the final concentrations of 10 nM. Data are the mean±s.e.mean of three experiments for both assays.
Effect of MT-7 on CCh inhibition of [3H]-cyclic AMP accumulation in N1E-115 cells
CCh caused a concentration-dependent inhibition of PACAP 38 (10 nM)-stimulated [3H]-cyclic AMP accumulation with a maximal effect corresponding to a 35.5±2.1% (n=5, P<0.001) reduction and an EC50 value of 5.6±0.8 μM. The addition of MT-7 (5 nM) failed to affect the CCh inhibitory effect (Figure 2B).
Effect of MT-7 on [3H]-NMS binding to CHO/M1 cell membranes
In competition experiments, MT-7 caused a concentration-dependent reduction of specific [3H]-NMS binding at different radioligand concentrations (Figure 3). However, the toxin consistently failed to cause a complete inhibition of [3H]-NMS binding. This behaviour was particularly evident at 3 and 15 nM [3H]-NMS, where the maximal inhibitions elicited by 25 nM MT-7 corresponded to ∼93 and 82% of total specific binding. The toxin IC50 values were 0.26±0.02, 0.27±0.03 and 0.48±0.05 nM at 0.05, 3 and 15 nM [3H]-NMS, respectively.
Figure 3.
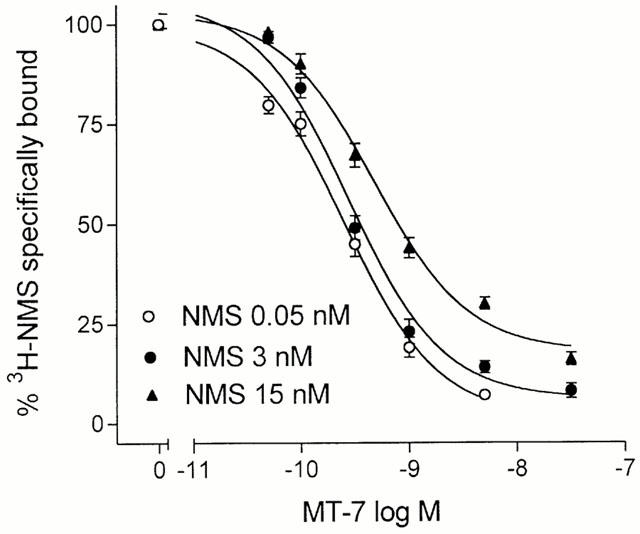
Inhibition of specific [3H]-NMS binding to CHO/M1 cell membranes by MT-7. Binding assays were performed at three concentrations of the radioligand in the presence of the indicated concentrations of MT-7. Data are expressed as per cent binding in the absence of MT-7 and are the mean±s.e.mean of three experiments.
In dissociation experiments, MT-7 (3 nM) markedly decreased the rate of atropine-induced dissociation of [3H]-NMS from M1 receptors (Figure 4). The estimated dissociation rate constants were 0.05 and 0.01 min−1 in control and toxin-treated membranes, respectively.
Figure 4.
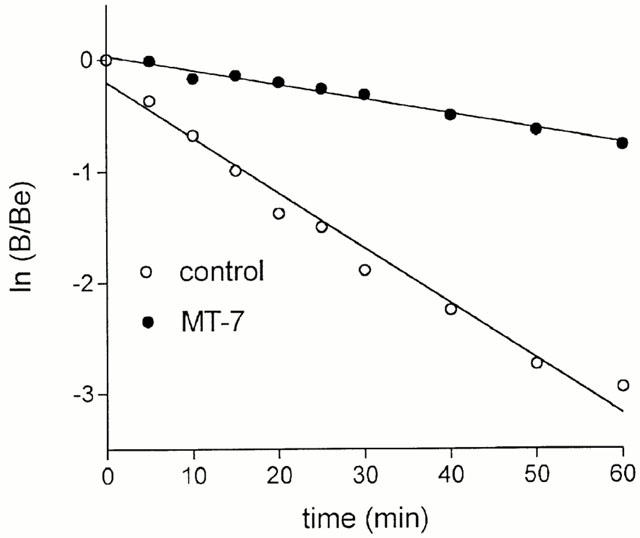
Effects of MT-7 on atropine-induced [3H]-NMS dissociation from M1 receptors. CHO/M1 cell membranes were incubated at 30°C first with 1 nM [3H]-NMS to equilibrium for 60 min and then with either vehicle (control) or 3 nM MT-7 for 20 min. The dissociation [3H]-NMS was started by the addition of 10 μM atropine and the incubation was stopped at the indicated time points. [3H]-NMS binding data are expressed as ln B/Be, where B is the amount of [3H]-NMS specifically bound at the indicated time and Be is the specific binding determined at zero time. Lines represent the least-squares linear regressions of the data. Values are the mean of three experiments.
To investigate the reversibility of MT-7 binding, CHO/M1 cell membranes were pretreated with either vehicle or MT-7 (100 nM), washed by centrifugation and resuspension and assayed for [3H]-NMS binding. As shown in Figure 5, in membranes pretreated with MT-7 there was a reduction in radioligand binding, which remained constant for at least 8 h.
Figure 5.
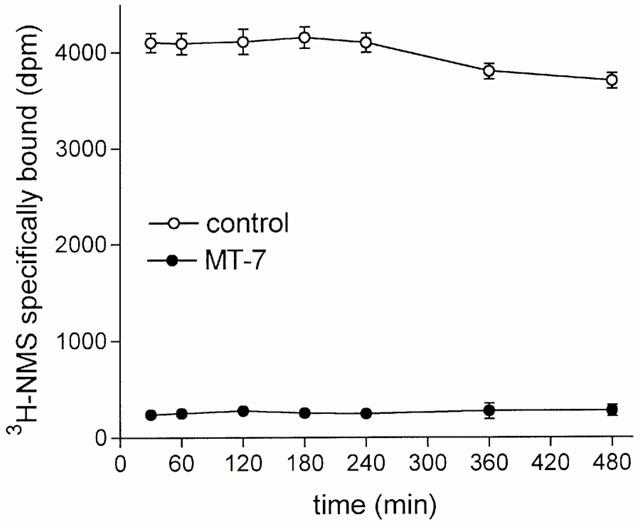
Stable inhibition of [3H]-NMS binding to M1 receptors by MT-7. CHO/M1 cell membranes were pretreated with either vehicle (control) or 100 nM MT-7, centrifuged and resuspended in fresh buffer. Aliquots of the membrane suspension were incubated in the presence of 1.5 nM [3H]-NMS at 30°C for the time periods indicated in abscissa. Data are the mean±s.e.mean of three experiments.
Discussion
MT-7 has recently been reported as a selective ligand for the muscarinic M1 receptor (Adem & Karlsson, 1997). In the present study, we have characterized the pharmacological activity of the toxin at different muscarinic receptor subtypes by using either CHO cells expressing the cloned human M1–M4 receptors or mouse N1E-115 neuroblastoma cells containing the native M1 and M4 receptor subtypes. In CHO/M1–M4 cells, we found that, at nanomolar concentrations MT-7 significantly inhibited the stimulation of [35S]-GTPγS binding elicited by ACh at M1 receptors but did not affect the response to the agonist when M2, M3 and M4 receptors were stimulated. At each receptor subtype, the toxin failed to stimulate basal [35S]-GTPγS binding, indicating a lack of agonist activity. Similarly, in N1E-115 neuroblastoma cells, MT-7 potently inhibited the CCh stimulation of [3H]-IPs accumulation, a response previously proposed to be mediated by endogenous M1 receptors (Kamba et al., 1990). In these cells, the toxin did not affect the CCh inhibition of PACAP 38-stimulated cyclic AMP accumulation, an effect mediated by M4 receptors (McKinney et al., 1991; Olianas et al., 1999). Collectively, these functional data indicate that MT-7 is an antagonist of the M1 receptor and confirm the subtype selectivity previously observed in radioligand binding studies (Adem & Karlsson, 1997).
In both CHO/M1 and N1E-115 cells the inhibitory effect of MT-7 consisted in a depression of the maximal agonist effect with minor changes in the agonist potency. This type of inhibition indicated that the toxin behaved as a noncompetitive antagonist. To further characterize the mode of toxin action on M1 receptors, radioligand binding studies were conducted in CHO/M1 cell membranes.
In competition experiments with [3H]-NMS, it was found that the toxin was not able to inhibit completely the binding of the radioligand and the fraction of [3H]-NMS not displaced by the toxin increased with increasing concentrations of the radioligand. In addition, the IC50 values of the toxin were little affected by large increases in the concentration of the radiolabelled ligand. These data are inconsistent with an action of the toxin as a competitive antagonist but rather indicate that it behaves as an allosteric antagonist which binds to a secondary allosteric site to decrease the affinity of the radioligand in a negative cooperative manner.
An important feature of allosteric ligands of muscarinic receptors is the ability to decelerate the dissociation of [3H]-NMS from the receptors (Nedoma et al., 1986; Waelbroeck, 1994; Lazareno & Birdsall, 1995). When this property was investigated, it was found that MT-7 markedly decreased the atropine-induced dissociation of [3H]-NMS from the muscarinic M1 receptor. At 3 nM, MT-7 decreased the [3H]-NMS dissociation rate by about 5 fold. This finding indicates that MT-7 is capable of binding to the M1 receptor also when the primary binding site is occupied by a competitive ligand and, by acting on the secondary allosteric site, can sterically regulate the accessibility of the primary binding site to competitive ligands.
Radioligand binding experiments also showed that MT-7 binds stably to the muscarinic M1 receptor. The binding withstood washing and resuspension of the membranes and was not reversible for at least 8 h at 30°C, as judged by the lack of recovery of [3H]-NMS binding to control values. A stable binding to muscarinic receptors has previously been observed for different muscarinic toxins. Thus, a large fraction of the binding of MT-1 and MT-2 to the cloned M1 receptor and to brain muscarinic receptors was found to be irreversible (Jerusalinsky & Harvey, 1994), whereas m1-toxin has been reported to bind pseudo-irreversibly to the M1 and reversibly to the M4 receptor (Max et al., 1993a). On the other hand, the blockade of the M4 receptor elicited by MT-3 appeared to be completely reversible (Olianas et al., 1996).
Several properties of MT-7 closely resemble those of m-1 toxin (Potter et al., 1993). Both toxins show high affinity for the m1 receptor subtype, act as allosteric modulators and bind tightly to the receptor. The two toxins display a high sequence homology, as they only differ by the fact that in position 28–29 MT-7 has a dipeptide Trp-Gln whereas m-1 toxin has His-Trp and lacks Lys in position 65 (Adem & Karlsson, 1997). Despite these similarities, MT-7 shows a higher selectivity for the M1 receptor than m-1 toxin. In fact, m-1 toxin also binds to the M4 receptor subtype at 5–65 fold higher concentrations (Max et al., 1993a), whereas MT-7 failed to affect the M4 receptor-induced [35S]-GTPγS binding at a concentration 100 fold higher than that significantly blocking the M1 receptor activity.
In conclusion, the present study shows that MT-7 behaves as a selective and noncompetitive antagonist of the cloned and native muscarinic M1 receptor subtype. These properties make MT-7 an unmatched tool for the identification and characterization of M1 receptors in different biological systems.
Acknowledgments
The authors thank Professor A.D. Strosberg (Institut Cochin de Genetique Moleculaire, Paris, France) for the gift of CHO cells transfected with the human muscarinic receptor genes. This work was supported by a grant from Italian Ministry of University and Scientific Research to P. Onali.
Abbreviations
- ACh
acetylcholine
- BSA
bovine serum albumin
- CCh
carbachol
- CHO
Chinese hamster ovary
- CHO/M1-M4 cells
CHO cells stably expressing the cloned human M1-M4 receptors
- GTPγS
guanosine-5′-O-(3-thio)triphosphate
- IPs
inositol phosphates
- KIU
kallikrein inhibitor unit
- MT-7
muscarinic toxin 7
- NMS
N-methylscopolamine
- PACAP
pituitary adenylate cyclase activating polypeptide
- PBS
phosphate buffered saline
References
- ADEM A., KARLSSON E. Muscarinic receptor subtype selective toxins. Life Sci. 1997;60:1069–1076. doi: 10.1016/s0024-3205(97)00049-0. [DOI] [PubMed] [Google Scholar]
- ADEM A., ASBLOM A., JOHANSSON G., MBUGUA P.M., KARLSSON E. Toxins from the venom of the green mamba Dendroaspis angusticeps that inhibit the binding of quinuclidinyl benzilate to muscarinic acetylcholine receptors. Biochim. Biophys. Acta. 1988;968:340–345. doi: 10.1016/0167-4889(88)90025-0. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J., DAWSON R.M.C., DOWNES C.P., HESLOP J.P., IRVINE R.F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem. J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CARSI J.M., VALENTINE H.H., POTTER L.T. m2-Toxin: A selective ligand for M2 muscarinic receptors. Mol. Pharmacol. 1999;56:933–937. doi: 10.1124/mol.56.5.933. [DOI] [PubMed] [Google Scholar]
- JERUSALINSKY D., HARVEY A.L. Toxins from mamba venoms: small proteins with selectivities for different subtypes of muscarinic acetylcholine receptors. Trends Pharmacol. Sci. 1994;15:424–430. doi: 10.1016/0165-6147(94)90092-2. [DOI] [PubMed] [Google Scholar]
- JERUSALINSKY D., CERVENANSKY C., WALZ R., BIANCHIN M., IZQUIERDO I. A peptide muscarinic toxin from the green mamba venom shows agonist-like action in an inhibitory avoidance learning task. Eur. J. Pharmacol. 1993;240:103–105. doi: 10.1016/0014-2999(93)90554-u. [DOI] [PubMed] [Google Scholar]
- JOLKKONEN M.Muscarinic toxins from Dendroaspis (mamba) venom Acta Univ. Uppsala 1996Uppsala, Sweden; Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 183 [Google Scholar]
- JOLKKONEN M., VAN GIERSBERGEN P.L.M., HELLMAN U., WERNSTEDT C., KARLSSON E. A toxin from the green mamba Dendroaspis angusticeps: Amino acid sequence and selectivity for muscarinic m4 receptors. FEBS Lett. 1994;352:91–94. doi: 10.1016/0014-5793(94)00933-3. [DOI] [PubMed] [Google Scholar]
- KAMBA S., KAMBA K.S., MCKINNEY M., PFENNING M., ABRAHAM R., NOMURA S., ENLOES L., MACKEY S., RICHELSON E. Desensitization of muscarinic M1 receptors of murine neuroblastoma cells (clone N1E-115) without receptor down-regulation and protein kinase C activity. Biochem. Pharmacol. 1990;40:1005–1014. doi: 10.1016/0006-2952(90)90486-5. [DOI] [PubMed] [Google Scholar]
- KARLSSON E., JOLKKONEN M., SATYAPAN N., ADEM A., KUMLIN E., HELLMAN U., WERNSTEDT C. Protein toxins that bind to muscarinic acetylcholine receptors. Ann. N.Y. Acad. Sci. 1994;710:153–161. doi: 10.1111/j.1749-6632.1994.tb26623.x. [DOI] [PubMed] [Google Scholar]
- LAZARENO S., BIRDSALL N.J.M. Detection, quantitation, and verification of allosteric interaction of agents with labeled and unlabeled ligands G protein-coupled receptors: Interaction of strychnine and acetylcholine at muscarinic receptors. Mol. Pharmacol. 1995;48:362–378. [PubMed] [Google Scholar]
- LAZARENO S., FARRIES T., BIRDSALL N.J.M. Pharmacological characterization of guanine nucleotide exchange reactions in membranes from CHO cells stably transfected with human muscarinic receptors M1-M4. Life Sci. 1993;52:449–456. doi: 10.1016/0024-3205(93)90301-i. [DOI] [PubMed] [Google Scholar]
- MAX S.I., LIANG J.-S., POTTER L.T. Purification and properties of m1-toxin, a specific antagonist of m1 muscarinic receptors. J. Neurosci. 1993a;13:4293–4300. doi: 10.1523/JNEUROSCI.13-10-04293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAX S.I., LIANG J.-S., POTTER L.T. Stable allosteric binding of m1-toxin to m1 muscarinic receptors. Mol. Pharmacol. 1993b;44:1171–1175. [PubMed] [Google Scholar]
- MCKINNEY M., ANDERSON D.J., VELLA-ROUNTREE L., CONNOLLY T., MILLER J.H. Pharmacological profiles for rat cortical M1 and M2 muscarinic receptors using selective antagonists: Comparison with N1E-115 muscarinic receptors. J. Pharmacol. Exp. Ther. 1991;257:1121–1129. [PubMed] [Google Scholar]
- NEDOMA J., TUCEK S., DANILOV A.F., SHELKOVNIKOV S.A. Stabilization of antagonist binding to cardiac muscarinic acetylcholine receptors by gallamine and other neuromuscular blocking drugs. J. Pharmacol. Exp. Ther. 1986;236:219–223. [PubMed] [Google Scholar]
- OLIANAS M.C., ADEM A., KARLSSON E., ONALI P. Rat striatal muscarinic receptors coupled to the inhibition of adenylyl cyclase activity: potent block by the selective m4 ligand muscarinic toxin 3 (MT3) Br. J. Pharmacol. 1996;118:283–288. doi: 10.1111/j.1476-5381.1996.tb15400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIANAS M.C., INGIANNI A., MAULLU C., ADEM A., KARLSSON E., ONALI P. Selectivity profile of muscarinic toxin 3 in functional assays of cloned and native receptors. J. Pharmacol. Exp. Ther. 1999;288:164–170. [PubMed] [Google Scholar]
- ONALI P., ADEM A., KARLSSON E., OLIANAS M.C. Functional activity of the selective muscarinic M1 receptor ligand MT-7 at cloned and native receptors. Proc. Soc. Neurosci. 1999;25:1725. [Google Scholar]
- POTTER L.T., HANCHETT-VALENTINE H., LIANG J.-S., MAX S.I., PURKERSON S.L., SILBERBERG H.A., STRAUSS W.L. m1-Toxin. Life Sci. 1993;52:433–440. doi: 10.1016/0024-3205(93)90299-i. [DOI] [PubMed] [Google Scholar]
- SALOMON Y. Adenylate cyclase assay. Adv. Cyclic Nucleotide Res. 1979;10:35–52. [PubMed] [Google Scholar]
- SEGALAS I., ROUMESTAND C., ZINN-JUSTIN S., GILQUIN B., MENEZ R., MENEZ A., TOMA F. Solution structure of a green mamba toxin that activates muscarinic acetylcholine receptors, as studied by nuclear magnetic resonance and molecular modeling. Biochemistry. 1995;34:1248–1260. doi: 10.1021/bi00004a019. [DOI] [PubMed] [Google Scholar]
- VANDERMEERS A., VANDERMEERS-PIRET M.-C., RATHE' J., WAELBROECK M., JOLKKONEN M., ORAS A., KARLSSON E. Purification and sequence determination of a new muscarinic toxin (MT4) from the venom of the green mamba (Dendroaspis angusticaps) Toxicon. 1995;33:1171–1179. doi: 10.1016/0041-0101(95)00057-s. [DOI] [PubMed] [Google Scholar]
- WAELBROECK M. Identification of drugs competing with d-tubocurarine for an allosteric site on cardiac muscarinic receptors. Mol. Pharmacol. 1994;46:685–692. [PubMed] [Google Scholar]


